Abstract
The phototaxis receptor sensory rhodopsin I (SRI) exists in two protein conformations, each of which is converted to the other by light absorption by the protein's retinylidene chromophore. One conformer inhibits a histidine-kinase attached to its bound transducer HtrI and its formation induces attractant motility responses, whereas the other conformer activates the kinase and its formation induces repellent responses. We performed Fourier transform infrared spectroscopy with temperature, pH, and mutation-induced shifts in the conformer equilibrium, and found that both conformers when present in the unphotolyzed dark state contain an all-trans retinal configuration that is photoisomerized to 13-cis, i.e., the same photoisomerization causes the opposite conformational change in the photointerconvertible pair of conformers depending on which conformer is present in the dark. Therefore, switching between the protein global conformations that define the two conformers is independent of the direction of isomerization. Insights into this phenomenon are gained from analysis of the evolution of the receptor from light-driven proton pumps, which use similar conformers for transport. The versatility of the conformational changes of microbial rhodopsins, including conformer interexchangeability in the photocycle as shown here, is likely a significant factor in the evolution of the diverse functionality of this protein family.
Introduction
Sensory rhodopsin I (SRI) has two conformers that respectively activate and inhibit a histidine-kinase attached to its tightly-bound transducer protein HtrI (1–3). Light absorption by the attractant receptor (AR) conformer produces an attractant signal, i.e., light-induced kinase inhibition. The AR is characterized by connection of the protonated Schiff base of the retinylidene chromophore to a cytoplasmic channel in the protein, and therefore photoactivation of the AR results in inward release of the Schiff base proton. The repellent receptor (RR) conformer, in which the Schiff base proton is photoreleased to anionic Asp-76 on the extracellular side of the protein, mediates photorepellent responses (light-induced kinase activation). In the wild-type (WT) SRI-HtrI complex (λmax = 587 nm), the inwardly connected AR conformer predominates and the cells are attracted to orange light. During the photocycle, the outwardly connected RR conformer accumulates in the M intermediate (λmax = 373 nm), sensitizing the orange-light-illuminated cells to react to near-UV light as a repellent. Hence, the cells are attracted to orange light only if it is not accompanied by near-UV wavelengths, resulting in the well-known color-discriminating phototaxis behavior mediated by SRI (4).
Several single mutations in the SRI-HtrI-complex (e.g., D201N in SRI, and E56Q in HtrI) invert the phototaxis response to orange-light excitation of the dark-adapted SRI-HtrI complex, i.e., cells expressing SRI-HtrIE56Q react to orange light as a repellent. The inverted signaling is attributable to the mutation shifting the conformer equilibrium in the dark from the inwardly connected AR conformer that dominates in the WT to the outwardly connected RR conformer that normally becomes dominant only during the photocycle. Investigators have most thoroughly demonstrated this altered conformational equilibrium in the complex SRI-HtrIE56Q, first by electrophysiologically measuring the direction of photorelease of the Schiff base proton (1), and then by confirming the alteration of the pKa of Asp-76 caused by its hydrogen bonding to the Schiff base proton (2).
Photoisomerization from the all-trans to the 13-cis configuration is the major cause of microbial rhodopsin protein conformational changes (5–8), and has been shown specifically to be responsible for attractant signaling by the SRI-HtrI complex (9,10). Titration data indicate that in the absence of ionized Asp-76, the WT and inverted (E56Q) mutant SRI-HtrI complexes exhibit indistinguishable absorption spectra, despite their greatly different proportions of attractant and repellent conformers (2). This result suggests that the two conformers do not differ in the isomeric configuration of the retinal around the C13 double bond, and that both therefore undergo all-trans to 13-cis photoisomerization. If this holds true, it would mean that the SRI-HtrI complex is, in terms of its global conformational changes, a protein analog of an electronic, two-state flip-flop, i.e., the same stimulus (all-trans to 13-cis photoisomerization) interconverts two states (the AR and RR protein conformers), producing the AR conformer from the RR, or the RR conformer from the AR, depending on which is present in the dark.
Materials and Methods
Plasmids and strains
SRI and HtrI protein fusion constructs were joined with a flexible peptide linker (ASASNGASA) between the C-terminal receptor and N-terminal transducer residues, and expressed under the bop promoter in the halobacterial plasmid vector pXP6 (11). Halobacterium salinarum strain Pho81Wr− (BR− HR− SRI− HtrI− SRII− HtrII−, carotenoid- and restriction-deficient) was used for transformation (11).
SRI that had been joined through the peptide linker to HtrI truncated at residue 147 (SRI-HtrI147) was cloned into an Escherichia coli expression vector pET21d (Novagen, Merck KgaA, Darmstadt, Germany) between NcoI and BamHI sites under the control of the T7 promoter, and introduced into BL21(DE3) as previously described (1). Expression in BL21(DE3) was induced by the addition of 1 mM isopropyl-β-D-thiogalactopyranoside and 5 μM all-trans-retinal.
Single-residue mutations were introduced by means of QuickChange site-directed mutagenesis kits with PfuTurbo polymerase (Stratagene, La Jolla, CA).
Cell motility measurements
Transformed H. salinarum cells were grown in complex medium (CM) containing 1 mg ml−1 mevinolin as previously described (11). Cultures at the end of their exponential growth phase were diluted 1:10 in fresh CM and incubated for 1 h at 37°C with agitation. For motility defined as in the dark, cell trajectories in nonactinic light at wavelengths >720 nm were captured as real-time AVI files with the use of a Flashbus Spectrim Lite Video Capture PCI Card on a Dell Dimension 8300 running VirtualDub 1.6.19.0 AVI encoder software for video capture (http://www.virtualdub.org). VirtualDub was set to record 10 frames per second during 30 s of cell swimming. We measured reversals of the cells by tracking each cell captured in the AVI files for 10 s. Illumination was delivered from a Nikon 100-W He/Xe short-arc lamp beam passed through a 590 ± 20 nm (orange-red light) interference filter.
Sample preparations for Fourier transform infrared spectroscopy
For Fourier transform infrared (FTIR) spectroscopy, we prepared membranes by sonicating E. coli cells expressing SRI with its C-terminus joined through a flexible peptide linker to HtrI truncated at position 147 (SRI-HtrI147) as previously described (12). After low-speed centrifugation to remove unbroken cells and cell debris, the membranes were pelleted for 1 h at 147,000 × g in an Optima L-100 XP ultracentrifuge (Beckman, Brea, CA) and suspended in loading buffer (4 M NaCl, 25 mM Tris (pH 6.8)). The membrane sample was solubilized with 1.0% n-dodecyl-β-D-maltoside (DDM), and SRI-HtrI complexes purified by Ni-NTA chromatography. We then reconstituted the samples into L-α-phosphatidylglycerol (PG) liposomes (SRI/PG) in a 1:50 molar ratio by removing the DDM with Bio-Beads SM-2 (Bio-Rad, Hercules, CA).
Light-induced attenuated total reflectance FTIR difference spectroscopy
The proteoliposome sample was placed on the surface of a diamond ATR crystal with nine effective internal reflections (DuraSamplIR II; Smiths Detection, Watford, UK). The sample was dried in a gentle stream of N2 and then immersed in buffer solutions containing 4 M NaCl at pH 5.5 (5 mM citrate) or pH 7.0 (5 mM phosphate) adjusted by addition of 1 M NaOH. Light-induced attenuated total reflectance (ATR)-FTIR difference spectra were recorded at 15, 32, and 42°C, and 2 cm−1 spectral resolution with a FTS-6000 spectrometer (Bio-Rad, Philadelphia, PA) equipped with a liquid-nitrogen-cooled MCT detector (13) The sample was illuminated by a 150 W halogen lamp (PHL-150; Sigma Koki, Japan) through an optical fiber, focusing lenses, and an optical filter cutting <480 nm wavelength. Four to eight spectra were averaged for each condition.
Results and Discussion
To test the intriguing bidirectional conformational coupling, we used FTIR spectroscopy, which produces well-established spectral changes in response to photoconversions between the all-trans and 13-cis retinal isomeric configurations in microbial rhodopsins. Our objective was to compare the WT SRI-HtrI complex, in which light produces attractant signals resulting from photoconversion from the AR to RR conformer, with the mutant SRI-HtrIE56Q complex, in which photorepellent responses result from the net RR-to-AR photoconversion. For the FTIR analysis, we take advantage of two conditions in which the SRI-HtrIE56Q inverted (repellent) signaling reverts to the WT-like attractant signaling, namely, low pH and low temperature (14,15). H. salinarum cells containing SRI-HtrIE56Q exhibit repellent responses to orange light at neutral pH at 37°C, the usual conditions of study, but at pH 5 the phototaxis response is a robust attractant one (Fig. 1). Similarly, at neutral pH at 25°C, the inverting effect of the E56Q mutation is suppressed and cells containing the mutant complex exhibit the WT attractant response (Fig. 1).
Figure 1.
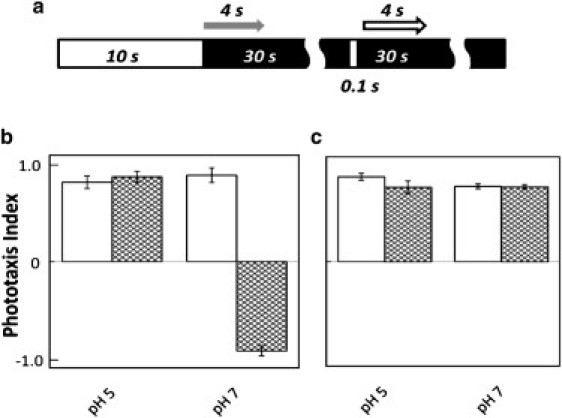
Phototaxis behavior of Pho81Wr− cells expressing SRI in complex with either WT HtrI (white bars) or mutated HtrIE56Q (hatched bars). (a) Scheme of illumination delivered to the cells imaged with nonactinic infrared light. A 10-s period of 590-nm light (white bar) was delivered to the cells, followed by 30 s dark (black bar) and a 100-ms pulse of 590-nm light. The fractions of cells that underwent a swimming reversal in the 4-s time window immediately after the step-down of the 10-s 590-nm light and after onset of the 100-ms light pulse (indicated by gray and white arrows, respectively) were measured. The phototaxis index is defined by subtracting the latter value from the former value; a positive index indicates an attractant response, and a negative index indicates a repellent response. The indices for WT SRI-HtrI and SRI-HtrIE56Q were measured at pH 5 and 7, and at 37°C (b) and 25°C (c).
These effects are likely attributable to shifts in the AR–RR dark equilibrium toward AR at low pH and low temperature. Spectroscopic pH titrations revealed that WT SRI-HtrI is dominated by the AR conformer with the pKa of Asp-76 at ∼8.5, whereas the E56Q mutation in HtrI increases the population of the RR conformer of SRI in which Asp-76, the Schiff base counterion on the extracellular side, has a pKa of ∼7.0 (2). Therefore, as expected from the lower dielectric of the environment of Asp-76 when the Schiff base proton is connected to the cytoplasmic side of the protein, its protonation is favored by the AR conformer. Evidently, protonation of Asp-76 by low pH reciprocally favors the AR conformer, since low pH restores the signal-inverted mutant to normal attractant behavior. The effect of temperature on the phototaxis responses is not unexpected, because metastable conformer equilibria are generally temperature-dependent. A priori, it may appear that the temperature effect is due to postreceptor processes in the phototaxis system, but the results provided below show that the conformer equilibrium is indeed shifted toward the AR conformer at low temperature.
In the RR conformer, the outwardly directed photocurrent observed is attributable to the proton transfer from the Schiff base to Asp-76 during the formation of M (also called S373), whereas in the AR conformer an inwardly directed photocurrent results from transfer of the Schiff base proton toward the cytoplasmic side (1) and does not result in protonation of a carboxylate (16). Therefore, the light-induced protonation of Asp-76, which is readily seen by light-minus-dark (L-D) difference FTIR spectroscopy, provides an internal measurement in the L-D spectrum of the presence of the AR conformer.
We obtained FTIR spectral measurements of SRI-HtrI in L-α-phosphatidylglycerol proteoliposomes on the surface of a diamond ATR crystal soaked in perfusion buffer solution at desired pH values with and without continuous illumination with >480 nm light. We then calculated the L-D difference ATR-FTIR spectra by subtracting the spectrum in the dark from the spectrum of the illuminated sample.
In Fig. 2, L-D FTIR spectra between 1800 and 850 cm−1 measured at 42°C of WT SRI-HtrI and SRI-HtrIE56Q are compared. At pH 5.5, where both of the receptor-transducer complexes elicit attractant responses, most of the spectral changes between the dark state and the M state are superimposable in the two samples, as is evident from the double difference spectrum (Fig. 2, upper trace). There are some differences in the 1700–1500 cm−1 region, where strong absorption by water and peptide vibrations in the absolute spectra can potentially cause spectral distortions, and changes in interaction in the amide group of the substituted Asn-56 upon M formation may contribute difference bands. Overall, the extensive identity between the two L-D spectra shows that the E56Q mutation in HtrI exerts almost no change in the structure of SRI-HtrI at pH 5.5. Therefore, the minimal spectral differences are in agreement with the absence of phenotypic behavioral changes caused by E56Q at low pH. Furthermore, there is no difference in the two L-D spectra in the region between 1720 and 1760 cm−1, where carboxyl perturbations have been assigned (16,17). Each L-D spectrum shows small changes that are typical of perturbations rather than carboxylate protonation.
Figure 2.
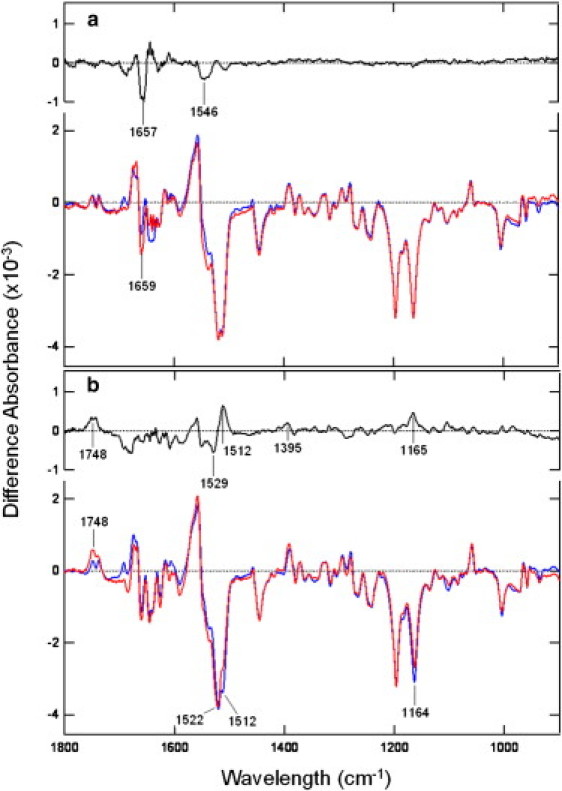
L-D difference FTIR spectra recorded at 42°C of SRI-HtrI (blue) and SRI-HtrIE56Q (red) at pH 5.5 (a) and 7.0 (b). Upper traces in each panel (black) are double difference spectra of the L-D difference spectra of SRI-HtrIE56Q minus SRI-HtrI of the same panel.
In contrast, at pH 7.0, more significant changes throughout the L-D spectra are seen between SRI in complex with E56Q HtrI as opposed to WT HtrI (Fig. 2, lower trace). The appearance of the 1748-cm−1 band previously assigned to Asp-76 protonation in SRI (12,16) in the mutant, but not in the WT, indicates the light-induced appearance of the kinase-activating AR conformer in a substantial amount in SRI in complex with HtrIE56Q, and is consistent with the repellent signaling by SRI-HtrIE56Q at pH 7.0 at 37°C (Fig. 1). In Fig. 3 (top panels), the expanded 1800–1700 cm−1 regions of the L-D spectra of SRI complexed with WT or HtrIE56Q measured at 15, 32, and 42°C are shown. SRI-HtrIE56Q at pH 7.0, but not at pH 5.5, shows temperature-dependent increases in the fraction of Asp-76 protonation upon M formation, whereas WT shows no such increases at either pH. This result confirms a temperature-dependent increase in the fraction of the deprotonated Asp-76 (a measure of the RR conformer) in the dark state, in agreement with the temperature-dependent increase in repellent signaling of the HtrIE56Q mutant (14,15) observed at 37°C at pH 7.0 but not at pH 5.5 (Fig. 1). We can assess the proportion of the species with unprotonated Asp-76 (i.e., the RR conformer) in the measured population by comparing the ratio of the increased portion of the intensities of the 1748 vs. 1197 cm−1 bands with that in SRI-HtrI with 100% unprotonated Asp-76 measured at pH 9.5, since the intensity of the 1197 cm−1 band (assigned to the chromophore C14-C15 stretching mode) is pH-independent (12). The proportion of the deprotonated Asp-76 thus obtained is 52%, which is larger than the estimated proportion of the deprotonated species (28%) based on the population of RR in SRI-HtrIE56Q (∼55% measured at 22-24°C) and pKa of Asp-76 at 7.0 (2). The temperature-dependent trend of the Asp-76 protonation band upon M formation indicates a temperature dependence of either the pKa of Asp-76 of RR, which would be expected to increase the RR/AR ratio, or other temperature-dependent processes that would increase the RR/AR ratio.
Figure 3.
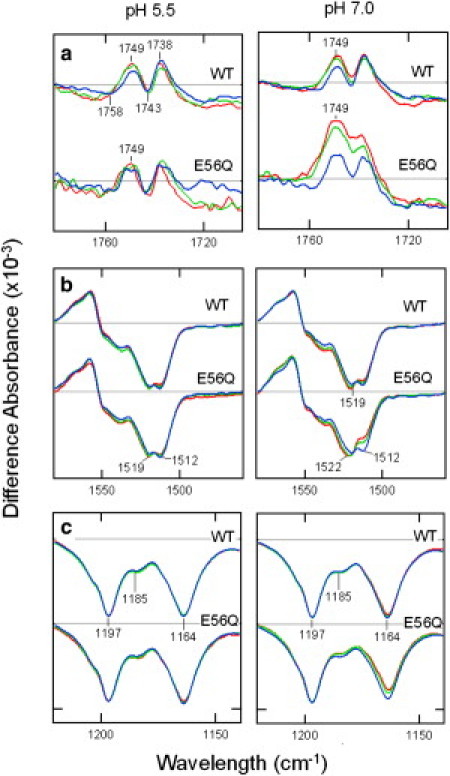
L-D difference FTIR spectra of SRI-HtrI (upper traces in each panel) and SRI-HtrIE56Q (lower traces in each panel). In each pair of panels in a–c, the scale is magnified to show the particular spectral region, and the scale is the same for all traces within the two panels. Absolute amplitude changes across the full spectra for the 42°C case are shown in Fig. 2. Frequency ranges: (a) 1780–1705 cm−1, (b) 1580–1460 cm−1, and (c) 1220–1140 cm−1. Samples were obtained at 15°C (blue), 32°C (green), and 42°C (red).
The spectral differences between the L-D spectra of WT and E56Q HtrI-complexed SRI at pH 7.0, 42°C, as shown in the double difference FTIR spectra in Fig. 2 b should therefore contain changes that differentiate between the opposite-signaling AR and RR conformers. The changes in the chromophore ethylenic stretching mode that appears near 1500 cm−1 (Fig. 2) are due to the frequency upshift of the mode from 1512 to 1522 cm−1 in the dark state of SRI-HtrIE56Q compared with that in WT, or to the measurement of SRI-HtrIE56Q at lower temperature (15°C; see Fig. 3 b). This shift in the ethylenic stretching mode is to be expected on the basis of the empirical inverse-correlation of this vibrational mode and the shift of the absorption maximum (λmax) of the chromophore, which is blue-shifted upon Asp-76 deprotonation (2,11,18,19).
Another prominent change in the double difference spectrum is the 1164 cm−1 band due to the decrease in intensity of the C10-C11 stretching mode of the chromophore in the dark state of SRI-HtrIE56Q at pH 7.0 and 42°C compared with that in the WT, or with that obtained at lower temperature or pH (Fig. 3 c). This intensity change at the C10-C11 stretching mode has been observed in other microbial rhodopsins with all-trans retinylidene chromophore upon protonation changes of the counterion Asp (12). Therefore, the changes in intensity of this mode are likely due to the Asp-76 deprotonation as well. The proportion of the molecules that underwent the intensity change in SRI-HtrIE56Q at pH 7.0 and 42°C (Fig. 2 b), as assessed by comparing the ratio of the 1164 and 1197 cm−1 bands with those in SRI with fully deprotonated or protonated Asp-76 (12), is 51%. The excellent match to the proportion of 52% obtained from the intensity of the carboxyl stretching mode of Asp-76 indicates that the intensity changes at 1164 cm−1 are fully explained as being caused by the deprotonation of Asp-76, and are not a result of other changes such as configurational differences of the chromophore.
The L-D difference spectra in the 1220–1140 cm−1 region are dominated by the C-C stretching modes of the chromophore with a protonated Schiff base, which are sensitive to the isomeric state. All-trans retinylidene chromophores, such as those in WT SRI-HtrI complex (9,10), are characterized by bands near 1164 and 1197 cm−1 (C10-C11 and C14-C15 stretching, respectively), whereas 13-cis chromophores are characterized by the appearance of a strong band near 1180 cm−1 assigned to the C10-C11 stretching in infrared and resonance Raman spectroscopy (20–22). The absence of differences in the spectral intensity near 1180 cm−1 between the spectra of SRI-HtrIE56Q at pH 7.0 and WT SRI-HtrI at 42°C (Fig. 2 b), or between spectra of SRI-HtrIE56Q at pH 7.0 measured at different temperatures (Fig. 3 c), rules out the possibility of different isomeric conversions of the chromophore between the two conformers of SRI-HtrIE56Q. Therefore, we conclude that both the attractant- and repellent-signaling forms of the SRI-HtrI complex, which are dominated by AR and RR conformers, respectively, when present in unphotolysed dark states have all-trans chromophores and like typical microbial rhodopsins undergo all-trans to 13-cis photoisomerization.
Conclusions
The data presented here show that in two SRI-HtrI complexes (the WT and an inverted signaling mutant), the same chemical event (all-trans to 13-cis photoisomerization of a protonated retinylidene chromophore) interconverts two conformationally distinct states of SRI (the AR and RR protein conformers), producing the AR conformer from the RR, or the RR conformer from the AR, depending on which is present in the dark. In the WT SRI-HtrI complex, the AR conformer is more thermally stable than the RR conformer, and therefore dominates in the dark. All-trans to 13-cis photoisomerization of its protonated retinylidene chromophore by orange light produces a transient, near-UV-absorbing intermediate state in which the proportion of RR conformer increases and then thermally decays to the prestimulus dark state, completing a photocycle. In the inverted mutant, the RR rather than the AR conformer predominates in the dark, and the all-trans to 13-cis photoisomerization produces a transient photocycle state in which the AR intermediate predominates. In other words, the photoisomerization is coupled in the opposite way to the conformational change. The mechanism by which the same light-induced isomeric configuration change of the retinylidene chromophore results in either-way conversions between two conformers is intriguing as a unique example in a protein of a bidirectional switch that is to some extent analogous to an electronic flip-flop. The analogy is not complete, however, because the conversion is expected to change the isomeric configuration of the retinal and the structure of the photoactive site. This means that switching between the protein global conformations that define the AR and RR conformers is independent of the isomerization direction, but the photoactive site change in atomic structure depends, at least in the immediate environment of the retinylidene chromophore, on the isomerization direction.
Under normal physiological conditions of cells swimming in light-intensity gradients, changes in light intensity result in changes in the quasi-steady-state ratio of the AR/RR conformer. This in turn results in changes in his-kinase activity, which controls the probability that the cells will reverse their swimming direction. Increases in orange light result in an increased proportion of RR conformer, which suppresses kinase activity and hence swimming reversals (an attractant response). Note that in the WT complex and in natural conditions, the predominant form of the RR conformer will be in the M state, i.e., a near-UV-absorbing (deprotonated chromophore) 13-cis form. Photoactivation of the M state, which normally consists predominantly of this 13-cis RR conformer, results in a rapid photoconversion back to the SRI-HtrI dark state dominated by the AR conformer, resulting in increased kinase activity and consequently causing swimming reversals (a repellent response).
Some clues to the nature of this mechanism may come from evolutionary considerations, which also may explain the existence of the novel bidirectional coupling of isomerization to conformational changes in SRI. Phylogenetic analysis strongly suggests that sensory rhodopsins evolved from interactions of light-driven proton pumps with existing signal transduction machinery in the cell (23). The biochemical properties of the phototaxis receptors in haloarchaea, the SRI and sensory rhodopsin II (SRII) proteins, fit this notion very well because they closely resemble the light-driven proton pump BR, and their Htr transducers have extensive homology with chemotaxis receptors. Both SRI and SRII exhibit light-driven proton pumping when they are separated from their transducers (HtrI and HtrII, respectively) in the membrane (18,24,25). In addition to being highly homologous to chemotaxis receptors, the Htr proteins interact with the chemotaxis cytoplasmic components, and in H. salinarum HtrII actually functions as both a phototaxis transducer and a chemotaxis receptor for serine (26). Finally, it requires only three mutations in BR to convert the pump into a robust SRII-like repellent phototaxis receptor when complexed with HtrII, and even a single mutation confers detectable signaling activity (27).
The predominant dark conformer of WT SRII has a structure and outward Schiff base connectivity similar to those of BR (28), and its light-induced M conformer has an outward displaced helix F (29,30) and inward Schiff base connectivity (24,25) as does BR M. Therefore, SRII and BR mutants, which generate repellent signals when photoactivated, each couple all-trans to 13-cis retinal to conversion from a conformer that is closely similar to the RR of SRI to a conformer that is closely similar to AR. Therefore, the RR-to-AR photoconversion in the mutant SRI-HtrIE56Q, which like SRII and the mutant BR produces a repellent signal, simply reproduces the same photoisomerization/conformer coupling generated by its presumably recent BR-like (and SRII-like) ancestor. It remains to be explained how this coupling was altered by evolution to allow the WT SRI to operate in reverse, i.e., to couple the AR conformation to all-trans retinal in the dark. From our data, we can conclude that the energy difference between the all-trans retinal AR and all-trans retinal RR conformers is small, and their equilibrium is readily shifted by temperature, pH, and single mutations. However, the detailed mechanism of the photoisomerization/conformer coupling is not clear, and the atomic structures of both conformers may be needed to resolve it.
The versatility of the conformational changes of microbial rhodopsins, which are now shown to include exchangeability of conformers in the photocycle, is likely a significant factor in the evolution of the diverse functionality of this protein family.
Acknowledgments
We thank Dr. Hartmut Luecke (University of California, Irvine) for suggesting the analogy to an electronic flip-flop.
This work was supported by the National Institutes of Health (grant R37GM27750), the Department of Energy (grant DE-FG02-07ER15867), the Robert A. Welch Foundation (endowed chair AU-0009 to J.L.S.), and the Ministry of Education, Culture, Sports, Science and Technology (19042013 and 19045015 to Y.F., and 20108014 and 22247024 to H.K.).
Footnotes
Yuji Furutani's present address is Institute for Molecular Science, Okazaki, Japan.
References
- 1.Sineshchekov O.A., Sasaki J., Spudich J.L. A Schiff base connectivity switch in sensory rhodopsin signaling. Proc. Natl. Acad. Sci. USA. 2008;105:16159–16164. doi: 10.1073/pnas.0807486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sineshchekov O.A., Sasaki J., Spudich J.L. Attractant and repellent signaling conformers of sensory rhodopsin-transducer complexes. Biochemistry. 2010;49:6696–6704. doi: 10.1021/bi100798w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki J., Tsai A.L., Spudich J.L. Opposite displacement of helix F in attractant and repellent signaling by sensory rhodopsin-Htr complexes. J. Biol. Chem. 2011 doi: 10.1074/jbc.M110.200345. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spudich J.L., Bogomolni R.A. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 1984;312:509–513. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haupts U., Tittor J., Oesterhelt D. General concept for ion translocation by halobacterial retinal proteins: the isomerization/switch/transfer (IST) model. Biochemistry. 1997;36:2–7. doi: 10.1021/bi962014g. [DOI] [PubMed] [Google Scholar]
- 6.Furutani Y., Kandori H. Internal water molecules of archaeal rhodopsins (Review) Mol. Membr. Biol. 2002;19:257–265. doi: 10.1080/0968768021000035069. (Review) [DOI] [PubMed] [Google Scholar]
- 7.Klare J.P., Chizhov I., Engelhard M. Microbial rhodopsins: scaffolds for ion pumps, channels, and sensors. Results Probl. Cell Differ. 2008;45:73–122. doi: 10.1007/400_2007_041. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki J., Spudich J.L. Signal transfer in haloarchaeal sensory rhodopsin-transducer complexes. Photochem. Photobiol. 2008;84:863–868. doi: 10.1111/j.1751-1097.2008.00314.x. [DOI] [PubMed] [Google Scholar]
- 9.Yan B., Nakanishi K., Spudich J.L. Mechanism of activation of sensory rhodopsin I: evidence for a steric trigger. Proc. Natl. Acad. Sci. USA. 1991;88:9412–9416. doi: 10.1073/pnas.88.21.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haupts U., Eisfeld W., Oesterhelt D. Sensory rhodopsin I photocycle intermediate SRI380 contains 13-cis retinal bound via an unprotonated Schiff base. FEBS Lett. 1994;356:25–29. doi: 10.1016/0014-5793(94)01226-1. [DOI] [PubMed] [Google Scholar]
- 11.Chen X., Spudich J.L. Demonstration of 2:2 stoichiometry in the functional SRI-HtrI signaling complex in Halobacterium membranes by gene fusion analysis. Biochemistry. 2002;41:3891–3896. doi: 10.1021/bi015966h. [DOI] [PubMed] [Google Scholar]
- 12.Furutani Y., Takahashi H., Kandori H. Structural changes of sensory rhodopsin I and its transducer protein are dependent on the protonated state of Asp76. Biochemistry. 2008;47:2875–2883. doi: 10.1021/bi702050c. [DOI] [PubMed] [Google Scholar]
- 13.Kitade Y., Furutani Y., Kandori H. Proton release group of pharaonis phoborhodopsin revealed by ATR-FTIR spectroscopy. Biochemistry. 2009;48:1595–1603. doi: 10.1021/bi801984u. [DOI] [PubMed] [Google Scholar]
- 14.Jung, K.-H. 1999. Protein-protein interaction between phototaxis receptor sensory rhodopsin I and its transducer HtrI. PhD thesis. University of Texas Graduate School of Biomedical Sciences, Houston, TX.
- 15.Jung K.-H., Spudich J.L. Protonatable residues at the cytoplasmic end of transmembrane helix-2 in the signal transducer HtrI control photochemistry and function of sensory rhodopsin I. Proc. Natl. Acad. Sci. USA. 1996;93:6557–6561. doi: 10.1073/pnas.93.13.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rath P., Spudich E., Rothschild K.J. Asp76 is the Schiff base counterion and proton acceptor in the proton-translocating form of sensory rhodopsin I. Biochemistry. 1996;35:6690–6696. doi: 10.1021/bi9600355. [DOI] [PubMed] [Google Scholar]
- 17.Bergo V., Spudich E.N., Rothschild K.J. FTIR analysis of the SII540 intermediate of sensory rhodopsin II: Asp73 is the Schiff base proton acceptor. Biochemistry. 2000;39:2823–2830. doi: 10.1021/bi991676d. [DOI] [PubMed] [Google Scholar]
- 18.Bogomolni R.A., Stoeckenius W., Spudich J.L. Removal of transducer HtrI allows electrogenic proton translocation by sensory rhodopsin I. Proc. Natl. Acad. Sci. USA. 1994;91:10188–10192. doi: 10.1073/pnas.91.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs M.P., Spudich E.N., Spudich J.L. Rapid high-yield purification and liposome reconstitution of polyhistidine-tagged sensory rhodopsin I. Protein Expr. Purif. 1995;6:780–788. doi: 10.1006/prep.1995.0009. [DOI] [PubMed] [Google Scholar]
- 20.Ames J.B., Fodor S.P., Mathies R.A. Bacteriorhodopsin's M412 intermediate contains a 13-cis, 14-s-trans, 15-anti-retinal Schiff base chromophore. Biochemistry. 1989;28:3681–3687. doi: 10.1021/bi00435a009. [DOI] [PubMed] [Google Scholar]
- 21.Fodor S.P., Pollard W.T., Mathies R.A. Bacteriorhodopsin's L550 intermediate contains a C14-C15 s-trans-retinal chromophore. Proc. Natl. Acad. Sci. USA. 1988;85:2156–2160. doi: 10.1073/pnas.85.7.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fodor S.P., Ames J.B., Mathies R.A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988;27:7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A.K., Spudich J.L., Doolittle W.F. Microbial rhodopsins: functional versatility and genetic mobility. Trends Microbiol. 2006;14:463–469. doi: 10.1016/j.tim.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Schmies G., Engelhard M., Bamberg E. Electrophysiological characterization of specific interactions between bacterial sensory rhodopsins and their transducers. Proc. Natl. Acad. Sci. USA. 2001;98:1555–1559. doi: 10.1073/pnas.031562298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudo Y., Iwamoto M., Kamo N. Photo-induced proton transport of pharaonis phoborhodopsin (sensory rhodopsin II) is ceased by association with the transducer. Biophys. J. 2001;80:916–922. doi: 10.1016/S0006-3495(01)76070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou S., Brooun A., Alam M. Sensory rhodopsin II transducer HtrII is also responsible for serine chemotaxis in the archaeon Halobacterium salinarum. J. Bacteriol. 1998;180:1600–1602. doi: 10.1128/jb.180.6.1600-1602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudo Y., Spudich J.L. Three strategically placed hydrogen-bonding residues convert a proton pump into a sensory receptor. Proc. Natl. Acad. Sci. USA. 2006;103:16129–16134. doi: 10.1073/pnas.0607467103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luecke H., Schobert B., Spudich J.L. Crystal structure of sensory rhodopsin II at 2.4 angstroms: insights into color tuning and transducer interaction. Science. 2001;293:1499–1503. doi: 10.1126/science.1062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wegener A.A., Chizhov I., Steinhoff H.J. Time-resolved detection of transient movement of helix F in spin-labelled pharaonis sensory rhodopsin II. J. Mol. Biol. 2000;301:881–891. doi: 10.1006/jmbi.2000.4008. [DOI] [PubMed] [Google Scholar]
- 30.Bordignon E., Klare J.P., Steinhoff H.J. Analysis of light-induced conformational changes of Natronomonas pharaonis sensory rhodopsin II by time resolved electron paramagnetic resonance spectroscopy. Photochem. Photobiol. 2007;83:263–272. doi: 10.1562/2006-07-05-RA-960. [DOI] [PubMed] [Google Scholar]


