Abstract
We assessed the importance of glycosaminoglycans and sulfur-mediated bonds for the mechanical properties of lens capsules by comparing the stress-strain responses from control and treated pairs of bovine source. No significant change in mechanical properties was observed upon reduction of disulfide bonds. However, removal of glycosaminoglycan chains resulted in a significantly stiffer lens capsule, whereas high concentrations of reducing agent, which is expected to reduce the recently reported sulfilimine bond of collagen IV, resulted in a significantly less stiff lens capsule. A comparison of the diffraction patterns of the control and strongly reduced lens capsules indicated structural rearrangements on a nanometer scale.
Introduction
Basement membranes (BMs) are supportive sheet-like structures that are found in almost every tissue of the human body. With few exceptions, these structures interface between cells on one face and extracellular matrix components on the other. Their roles are specialized and tissue-dependent, and include acting as a scaffold and selective filtration barrier, and regulating biological activities such as cell growth, differentiation, and migration (1).
The lens capsule is a multilayer BM. In addition to its interactions with cells, it plays an important role in the accommodative mechanism for vision by acting as a force distributor, enabling accurate adjustments to the shape and thickness of the lens for focusing (2–6). In doing so, it may experience strains of ∼10% (7). In light of this function, the mechanical properties of lens capsules and the contributions of each of its components to these properties are particularly relevant. These mechanical properties may also be important for the cell-signaling functions of lens capsules, as recent studies show that these properties impact regulation of growth, differentiation, and migration (8).
Previous studies into the mechanical properties of human lens capsules demonstrated age-related changes and correlated the tensile properties with loss of accommodative function (3,7,9), but it is not clear how individual components affect these overall mechanical properties.
The major protein components of lens capsule are collagen IV, laminin, nidogen, and perlecan (also known as heparan sulfate proteoglycan). Disulfide bonds are known to exist both within and between the chains of laminin (10,11) and in nidogen, where they are believed to prevent protein unfolding (12,13). Numerous cysteines are also predicted to participate in disulfide bonds in perlecan (14). The 7S and NC1 domains of collagen IV are responsible for the intermolecular interactions that give rise to the collagen IV network. Disulfide bonds found at the hexamer interface of the NC1 domains were once thought to stabilize intermolecular interactions (15), thereby stabilizing the collagen IV network. However, a more recent structural investigation (16) showed that the disulfide bonds of the NC1 domain are intramolecular. Instead, NC1 domains are cross-linked intermolecularly through a biologically rare sulfilimine bond formed via a methionine and a lysine residue (16–18). The extent to which any of these sulfur-containing bonds contribute to the mechanical properties of a lens capsule remains unclear.
In addition to the major protein components, lens capsules are abundant in glycosaminoglycans (GAGs). These long, unbranched polysaccharides play an important role in the mechanical properties of cartilage (19–21) and may also affect the tensile properties of a lens capsule. Recently, the effects of deletion of three GAG attachment sites on perlecan were investigated in genetically modified mice (22). The most notable biological effect was that these mice had smaller eyes than their wild-type counterparts, with lens capsules that lacked a lamellar substructure and leaked cellular material. The mechanical properties of these lens capsules were not tested.
In this work, we examined the contribution of reducible bonds and GAGs to the mechanical properties of bovine lens capsules (BLCs) by comparing the stress-strain responses of control samples with those of samples subjected to chemical reduction and enzymatic digestion, respectively.
Materials and Methods
Sample preparation
Bovine eyes from yearlings were obtained as matched pairs from individual animals from a local abattoir. All anterior lens capsules were dissected shortly after the animals were killed and then incubated for 18 h in 100 μl of either 1), 20 mM ammonium acetate, 5 mM calcium acetate, pH 7 (GAG+); 2), 20 mM ammonium acetate, 5 mM calcium acetate, pH 7.0, plus 10 μl each heparinase, heparatinase I and heparatinase II (0.5 units/ml; Seikagaku) (GAG−); 3), phosphate-buffered saline (PBS) plus 250 mM β-mercaptoethanol (BME; weakly reduced (WR)); or 4), PBS plus 10 M BME (strongly reduced (SR)). Experiments were carried out as pairs of samples, that is, for each GAG− sample, the paired lens capsule from the same animal was used as the GAG+ control and likewise for WR/SR and their PBS and SR/PBS control samples. For tensile testing, 10 pairs, 13 pairs, and 12 pairs of lens capsules were used to examine the effect of GAG removal (GAG+ versus GAG−), weak reduction (PBS versus WR), and strong reduction (PBS versus SR), respectively.
Measurement of GAG content and free sulfhydryls
We used the fluorophore-assisted carbohydrate electrophoresis (FACE) technique as described previously (23) to measure levels of heparan sulfate (HS), chondroitin sulfate (CS), and hyaluronan (HA), and to verify the removal of HS GAG in GAG− lens capsules. The free sulfhydryl content of two PBS and two WR lens capsules was compared with the use of Ellman's reagent (5,5′-dithiobis-(2-nitrobenzoic acid; Pierce, Rockford, IL) (24). Ellman's reagent buffer consists of 0.1 M sodium phosphate, pH 8.0, and 1 mM EDTA. Each lens capsule was cut into eight similar-sized pieces that were rinsed and patted dry before they were placed into a 96-well plate and incubated for 15 min in 100 μl Ellman's reagent (0.08 mg/ml in Ellman's reagent buffer). Absorbances at 412 nm were measured with a Biotek PowerWave XS plate reader (Biotek, Winooski, VT). The average absorbance from eight wells containing Ellman's reagent without lens capsules was subtracted from the absorbances from wells containing lens capsules.
Tensile testing
We measured the thickness of each lens capsule with a micrometer and cut it into a dumbbell shape (Fig. S1 in the Supporting Material) using a stainless-steel die with a gauge length of 4 mm and width of 3 mm. The undeformed cross-sectional dimensions were used for stress determinations at a given stretch. The lens capsule dumbbells were placed onto an open-sided plastic guide frame (polyethylene terephthalate film) and loaded between lightweight lever-action clamps with surface clamp area 6 mm × 6 mm. The closed side of the plastic guide frame was cut after loading to prevent its interference with data collection. All samples were kept wet until immediately before testing, and all tests were performed at 20°C and 65% relative humidity on an Instron tensile tester (model 5500R) with Merlin software. The lens capsules were stretched at a constant rate of 3 mm/min with continuous recording of elongation and load using a 2.5 N load cell. To ensure that the origin of each stress-strain curve was at 0% stretch, with no contribution from slackness or pretension, each lens capsule was loaded in a slightly slack position. The sample was not considered to be at 0% stretch until a stress of 10−4 MPa was achieved. The length discrepancy (fractions of millimeters) between the start of testing and the application of 10−4 MPa was added to the sample length of 4 mm to obtain the total length of the sample for calculation of % stretch (25). Hereafter, when the term “strain” is used, it refers to the % stretch of the sample.
Diffraction
Five matched pairs of BLCs (PBS and SR) were stretched and dried. Two-dimensional (2D) diffraction patterns were obtained at the SAXS/WAXS beamline of the Australian Synchrotron with the use of a Pilatus-1M detector (Dectris, Baden, Switzerland). The detector was placed slightly off-center at a distance of 563 mm from the sample and a wavelength of 0.62 Å was used, yielding a q-range of 0.05–2.3 Å−1. SAXS15id (26) was used to obtain meridional one-dimensional (1D) traces by radial averaging over two radially opposed 60° area masks (120° total) along the meridian. All 1D profiles were then averaged with the use of Primus software (27). Equatorial 1D traces were obtained in the same manner.
Results and Discussion
We assessed the GAG content of the BLCs using FACE. The BLCs contained HS GAG with unsulfated, monosulfated, and disulfated disaccharides, but not trisulfated disaccharides (Fig. 1 A). In contrast, HA and CS were not detected in BLC (Fig. 1 B). A band in the position of ΔDi6S appears in the BLC sample; however, the intensity and position of this band are equal to those observed in the fluorescence reaction control (2-aminoacridone (AMAC) reaction buffer) and are not due to the presence of CS (Fig. 1 B). Because FACE is a quantitative technique, these results show that HS is the predominant GAG in BLC. Fig. 2 shows that after combined treatment with heparinase, heparatinase I, and heparatinase II, the lens capsule no longer has an appreciable amount of HS. Ellman's test was used to measure free sulfhydryls. The average absorbances for nonreduced and WR lens capsules were 0.05 (±0.04) and 1.16 (±0.36), respectively, confirming a dramatic increase in the number of free sulfhydryls present after treatment with 250 mM BME.
Figure 1.
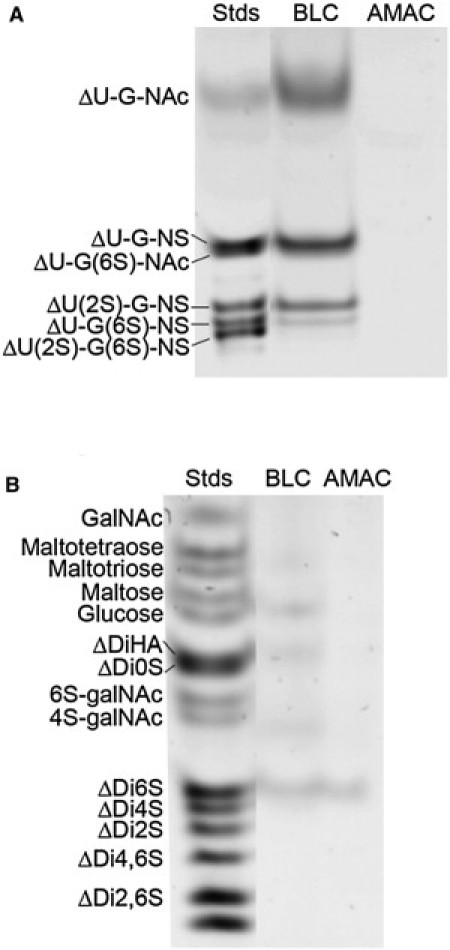
FACE gels for determination of (A) HS and (B) HA and CS content. Lane 1: standard; lane 2: BLC; lane 3: AMAC reaction buffer. Band positions for ΔU-G-NAc ([ΔUA-glcNAc]), ΔU-G-NS ([ΔUA-glcNAc(2-N-sulfate)]), ΔU-G(6S)-NAc ([ΔUA-glcNAc(6-O-sulfate)]), ΔU(2S)-G-NS ([ΔUA(2-O-sulfate)-glcNAc(6-O-sulfate)]), ΔU-G(6S)-NS ([ΔUA-glcN(2-N-, 6-O-sulfate)]), and ΔU(2S)-G(6S)-NS ([ΔUA(2-O-sulfate)-glcN(2-N-, 6-O-sulfate)]) are indicated beside the gel in panel A. Band positions for ΔDiHA ([ΔUA-glcNAc]), ΔDi0S ([ΔUA-galNAc]), ΔDi6S ([ΔUA-galNAc(6-O-sulfate)]) and ΔDi4S ([ΔUA-galNAc(4-O-sulfate)]), ΔDi2S ([ΔUA(2-O-sulfate)-galNAc]), ΔDi4,6S ([ΔUA-galNAc(4-O-, 6-O-disulfate)]), and ΔDi2,6S ([ΔUA(2-O-sulfate)-galNAc(6-O-sulfate]) are indicated beside the gel in panel B.
Figure 2.
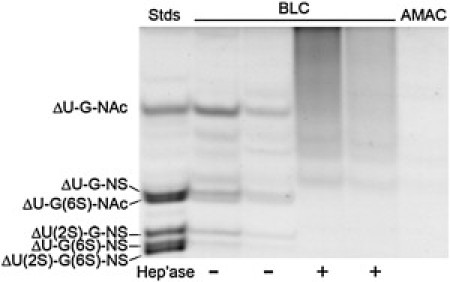
Confirmation of HS digestion. Lane 1: standard; lane 2: GAG+ BLC; lane 3: GAG+ BLC; lane 4: GAG− BLC; lane 5: GAG− BLC; lane 6: AMAC. Band positions for ΔU-G-NAc ([ΔUA-glcNAc]), ΔU-G-NS ([ΔUA-glcNAc(2-N-sulfate)]), ΔU-G(6S)-NAc ([ΔUA-glcNAc(6-O-sulfate)]), ΔU(2S)-G-NS ([ΔUA(2-O-sulfate)-glcNAc(6-O-sulfate)]), ΔU-G(6S)-NS ([ΔUA-glcN(2-N-, 6-O-sulfate)]), and ΔU(2S)-G(6S)-NS ([ΔUA(2-O-sulfate)-glcN(2-N-, 6-O-sulfate)]) are indicated beside the gel.
All stress-strain curves were J-shaped, as is commonly found for animal tissues (25,28), and therefore a single Young's modulus could not be fitted to the curves. Instead, we examined stresses at the toe region (low-strain region) using strains of 10% due to the physiological relevance of this region (7,25) and at 70% strain. At 70% strain, tangents to the stress-strain curves were appreciably steeper than those of the toe region.
The average stresses obtained for untreated (PBS) BLCs (Table 1) differ from those previously reported for human lens capsules (3,7,29). This likely reflects differences not only in the mechanical properties of the species but also in the testing procedures used. Indeed, values for human lens capsule stresses vary substantially according to the testing method employed, including uniaxial and multiaxial tests, traditional load cell instruments, fluorescent marker monitoring, and inflation systems.
Table 1.
Average stress (MPa) at 10% and 70% strain
| Strain (%) | GAG+ | GAG− | PBS | WR | PBS | SR |
|---|---|---|---|---|---|---|
| 10 | 0.018 | 0.022 | 0.013 | 0.011 | 0.014 | 0.018 |
| 70 | 0.708 | 0.959 | 0.629 | 0.462 | 0.745 | 0.304 |
Significantly different (confidence interval > 95%) average stresses between pairs are indicated by shading. Unshaded average stresses had confidence intervals < 95%.
Various fiber-reinforced models are available for tissues that consist of fibril-forming collagens (30–32); however, these models are inadequate for the tissues of network-forming collagens, such as the lens capsule (33). Models that were previously applied to lens capsule measurements, including linear elastic (3) and Fung hyperelastic (34) models, cannot provide satisfactory representations of mechanical behavior in both uniaxial and biaxial loading measurements. The mechanical responses of lens capsules cannot be adequately addressed for both uniaxial and biaxial testing conditions without the use of a rather complex model that combines the constitutive behavior of individual collagen elements with the basic geometry of the network and its interaction with the surrounding matrix (33). In a computational study, Burd (33) attempted to fit the mechanical data of lens capsules to such a model using a simplified and approximated 2D model for the 3D geometry of the lens capsule, with some success. However, the author indicated that a better model would include a more complex hyperelastic model as well as a more complex topological form for the network structure of the tissue.
As observed by x-ray diffraction, unstretched, air-dried BLCs consist of networks of randomly oriented collagen. Upon stretching, these networks become reoriented such that fibers align parallel to the direction of applied stress (35). Under uniaxial mechanical stress, the collagen IV network geometry is thought to initially reorganize in the low-strain region, whereas under biaxial loads the collagen IV network does not appear to experience this initial alignment. Therefore, it is likely that a biaxial testing method would report stiffer mechanical properties than those observed in our investigation.
In this study, we used uniaxial testing to compare only untreated and treated BLCs and assess the relative contributions of sulfur-mediated bonds and GAGs to the mechanical properties of the tissue. In previous studies (3,9,29,33,36), investigators performed more-detailed assessments of the mechanical properties of untreated lens capsules, as opposed to an analysis of the contributions of chemical components to these properties.
Fig. 3, A–C, show the tensile properties of two typical pairs of lens capsules for comparison after weak reduction, strong reduction, and GAG digestion, respectively. Variation between individual animals was observed, most likely associated with variations in age, sex, and condition (9). Weak reduction (250 mM BME) did not cause any significant changes in the tensile properties of the lens capsule at any region of the stress-strain curve (Fig. 3 A). Neither strong reduction (10 M BME) nor GAG digestion affected the stiffness of the lens capsules in the toe region of the curve (Fig. 3, B and C, respectively). However, both treatments altered the mechanical response at higher strains, with strong reduction resulting in significantly less stiff lens capsules (Fig. 3 B). In contrast, removal of the HS GAGs gave rise to significantly stiffer lens capsules (Fig. 3 C). The average stresses at 10% and 70% strain from 10 paired GAG+ and GAG−, 13 paired PBS and WR and 12 paired PBS and 13 SR lens capsules are provided in Table 1. An analysis of variance for stresses at 70% strain in GAG+ and GAG− samples yielded a >98% confidence interval (p = 0.017) indicating that differences observed were due to the digestion of GAGs rather than intraspecies variation (Table S1). For PBS and SR samples, the p-value was 2.3 × 10−6, providing a confidence level of nearly 100% (Table S2). We did not compare the maximum stress and extensibility of the samples at failure, because these values were variable due to the difficult nature of testing these very soft tissues.
Figure 3.
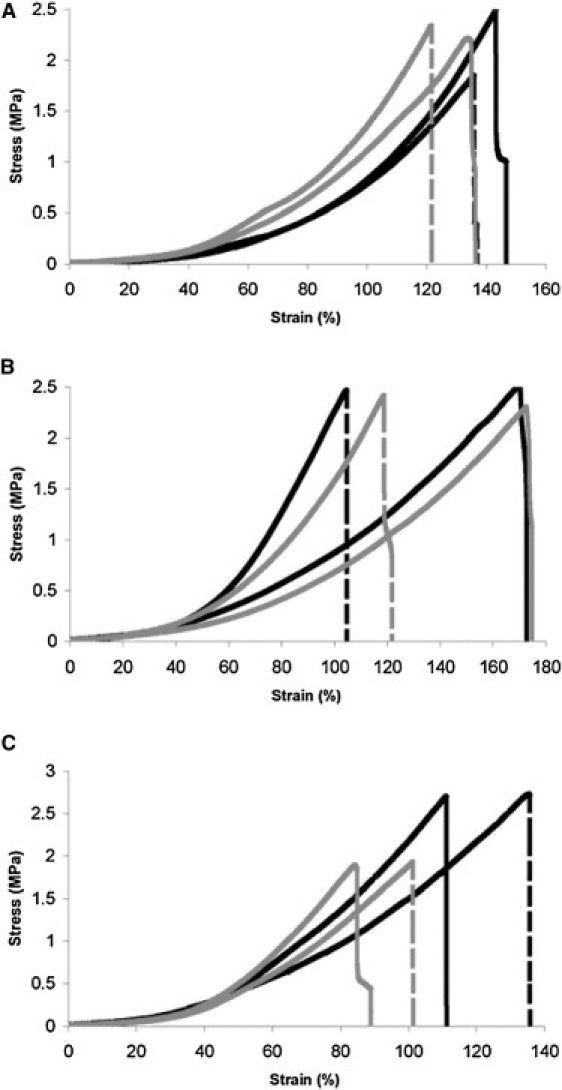
Mechanical response curves for two typical pairs of lens capsules for each comparison. Curves for pair 1 are presented in black and those for pair 2 are in gray. (A) Control lens capsules (PBS) are shown with dashed lines, and WR lens capsules (250 mM BME) are shown with solid lines. (B) Control lens capsules (PBS) are shown with dashed lines, and SR lens capsules (10 M BME) are indicated by solid lines. (C) Control lens capsules (GAG+) are shown with dashed lines, and GAG-digested lens capsules (GAG−) are shown with solid lines.
One might expect the reduction of sulfur-mediated bonds to increase the thickness of the lens capsule by allowing tight structures to relax, and removal of GAGs to result in a thinner lens capsule. The thickness of all lens capsules was measured after treatment and was found to vary between individuals, likely resulting from variations in age, sex, and condition. The average thickness for GAG− lens capsules (45.7 μm) was slightly less than that for GAG+ samples (46.0 μm), as might be expected; however, a variance analysis showed that the difference was not significant in this sample size (Table S3). Similarly, SR samples, with an average thickness of 47.9 μm, were found to be somewhat thicker than paired PBS controls (45.7 μm), but not significantly so (Table S4). The fact that mechanical differences were significant but thickness differences were not suggests that decreased thickness after GAG removal and increased thickness upon strong reduction were not the major cause of mechanical differences.
The lack of change in stress-strain response after weak reduction, combined with evidence from Ellman's test showing appreciable disulfide reduction, suggests that the disulfide bonds of collagen IV, which is thought to be the major structural protein of the BM, do not play a significant role in the tissue's mechanical properties. The intramolecular bonds of the NC1 domain may instead serve to capture necessary intermediates in the folding pathway of the collagen. Once folded, the NC1 domain may be stabilized by other interactions that are not susceptible to reduction. Alternatively, some unfolding of this domain may not cause an appreciable effect on the mechanical properties of the collagen IV network, provided that intermolecular cross-links are maintained. The 7S domain is additionally stabilized by lysine-derived cross-links (37), and these bonds may provide compensatory stability upon reduction of disulfides. This result also indicates that disulfide bonds found in laminin, perlecan core protein, and nidogen do not play a significant role in the mechanical properties of the lens capsule.
It is not clear what conditions are necessary for cleavage of a sulfilimine bond in vivo. Although information about some of the chemical properties of sulfilimines has been available for more than three decades (38), there is still conjecture regarding the character of the bond (39). Vanacore and colleagues (17) were able to partly reduce this bond at room temperature with 100 mM dithiothreitol, and achieved complete reduction by additional heating to 80°C; however, they achieved this reduction on isolated peptides rather than an intact collagen IV network. Access to the NC1 hexamer interface and hence the sulfilimine bond may be more limited when it is part of an intact collagen IV network. Although the sulfilimine bond had not yet been identified, a previous investigation (40) showed that whereas 5% (v/v; ∼0.7 M) BME was insufficient to separate the monomers of the NC1 hexamer, 40–60% (v/v; ∼6–9 M) BME enabled complete separation. It is plausible that these observations were based on disruption of the sulfilimine bond only at higher concentrations of BME. For this reason, it seems likely that the sulfilimine bond remained intact in WR lens capsules (250 mM BME), whereas strong reduction with 10 M BME would be sufficient to reduce these bonds. Therefore, the decreased stiffness observed in SR samples may result from the separation of the NC1 hexamer interface of the collagen IV network. Of note, although SR lens capsules were only ∼40% as stiff as their paired controls, they remained intact, indicating either that other nonreducible interactions, perhaps including lysine-derived cross-links (37), also serve to stabilize the intermolecular networks of the lens capsule, or that in intact lens capsule, some sulfilimine bonds persisted in the presence of 10 M BME.
We used diffraction to assess any changes in the structural periodicities of the lens capsule upon strong reduction. The diffraction patterns from all five of the SR lens capsules had lower overall intensity (average reduction 36%). Averaged 1D curves for meridional and equatorial reflections are shown in Fig. 4, and typical 2D patterns are provided in Fig. S2.
Figure 4.
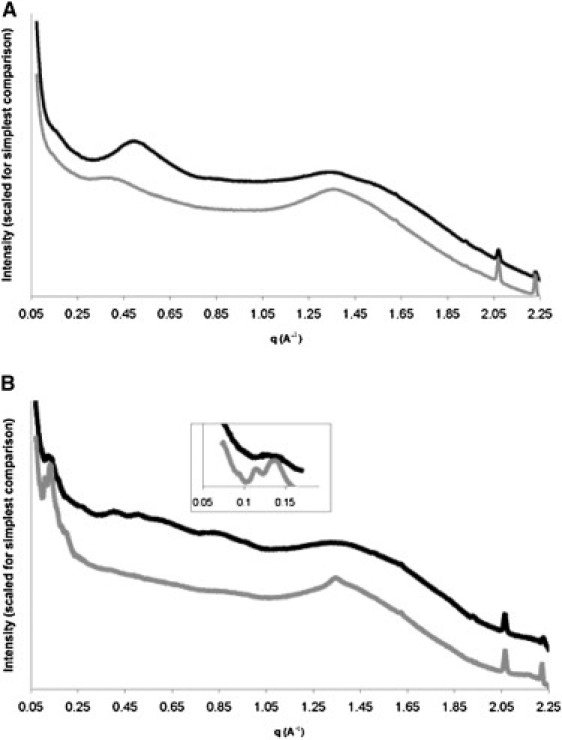
Structural comparison of control and SR BLCs. (A) Averaged equatorial diffraction for PBS (black) and SR (gray) lens capsules. (B) Averaged meridional diffraction for PBS (black) and SR (gray) lens capsules. Data have been rescaled to simplify viewing comparison.
The equatorial profile for SR lens capsules had a dramatically reduced reflection at q = 0.5 Å−1 compared with that for PBS samples (Fig. 4 A). This peak corresponds to a real-space distance of 12.5 Å, suggesting that the lateral associations of collagen IV (41) may be disrupted. The broad, weak peak centered around q = 0.4 Å−1 (∼16 Å) may simply be more readily observed in the SR profile than in the PBS profile due to the absence of neighboring peaks; alternatively, this peak may represent disrupted, looser lateral associations of collagen IV. Along the axis of stretch, greater structural differences were observed (Fig. 4 B). Of note, the ripples observed in the PBS profile at q = 0.26, 0.39, and 0.52 Å−1 (indices 2, 3, and 4 of 4.8 nm) were abated, and the small peak at q = 0.13 Å−1 (index 1 of 4.8 nm) was replaced by two sharper peaks at q = 0.115 and 0.14 Å−1. It is likely that these two sharper peaks are more readily observed after removal of the 4.8 nm periodicity. The decrease in the 4.8 nm reflections for SR samples suggests that this periodicity relies on intact sulfur-mediated bonds. These structural changes are likely involved in the decreased stiffness observed for SR lens capsules.
It has been suggested that reflections at q = 0.115 and 0.138 Å−1 index to a 22 nm periodicity originating at the overlap of 7S regions (41). If this assignment is accepted, our data would indicate that the interactions of 7S domains are not appreciably disrupted by the breaking of sulfur-containing bonds. This suggestion is commensurate with notion that lysine-mediated cross-links in the 7S region may serve to stabilize these interactions upon reduction. The SR meridional profile also showed a sharper peak at q = 1.36 (4.6 Å). The cause of this change is not clear.
When considering the functional significance of alterations to the mechanical properties of lens capsule caused by both GAG removal and strong reduction, it is important to note that the significant effects of these treatments were observed well above the toe region of the response curves. The role of the lens capsule as a force distributor would not be expected to be affected by this change. During normal accommodative function, the lens capsule experiences strains of ∼10%, whereas cataract surgery may induce strains of up to 40% (7). Nevertheless, the decreased elasticity in the case of GAG-digested lens capsules and the decreased stiffness in the case of SR lens capsules may affect the ability of the lens capsule to perform its cell-signaling functions as a BM. Several investigations have indicated that the elasticity of the extracellular matrix can have a major influence on cell behaviors such as migration (42,43), apoptosis (44), and proliferation (45). Furthermore, an extensive investigation covering a wide range of equivalent substrates with various Young's moduli showed that elasticity directly affects the ability of stem cells to self-renew and differentiate (46). Therefore, even though GAGs and reducible bonds are not essential for force distribution function, they may play an important role in the ability of the lens capsule to act as a BM. In light of the reduced elasticity of GAG-digested BLCs, it is possible that, in addition to the loss of direct binding interactions with HS chains, a decrease in BM elasticity may contribute to the ocular abnormalities observed in mice lacking the HS chains of perlecan.
Considering the suggestions of Burd (33) regarding the interpretation of lens capsules mechanics, it is of interest to note that neither sulfilimine bond reduction nor GAG removal would be expected to affect the constitutive behavior of the individual collagen networks. Rather, one would expect sulfilimine bond reduction to affect the collagen IV network geometry, and GAG removal to affect the interaction of the network with its surrounding matrix.
Conclusions
The structural changes that occur upon strong reduction of the lens capsule, which is expected to break the recently reported sulfilimine bond between NC1 domains, indicate that sulfur-mediated bonds are important for packing geometries within the BM. Although sulfur-mediated bonds and GAGs are not essential for keeping the lens capsule intact and do not appear to play a major role in accommodative function, both contribute to the mechanical properties of this BM and thus may have an impact on its cell-signaling roles.
Acknowledgments
The authors thank David Alexander for advice regarding statistical analyses, and Mickey Huson for his critique of the manuscript.
Supporting Material
References
- 1.Kefalides N., Borel J. Elsevier; Amsterdam: 2005. Basement Membranes: Cell and Molecular Biology. [Google Scholar]
- 2.Fisher R.F. The significance of the shape of the lens and capsular energy changes in accommodation. J. Physiol. 1969;201:21–47. doi: 10.1113/jphysiol.1969.sp008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher R.F. Elastic constants of the human lens capsule. J. Physiol. 1969;201:1–19. doi: 10.1113/jphysiol.1969.sp008739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koretz J.F., Handelman G.H. A model for accommodation in the young human eye: the effects of lens elastic anisotropy on the mechanism. Vision Res. 1983;23:1679–1686. doi: 10.1016/0042-6989(83)90183-9. [DOI] [PubMed] [Google Scholar]
- 5.Koretz J.F., Handelman G.H. Model of the accommodative mechanism in the human eye. Vision Res. 1982;22:917–927. doi: 10.1016/0042-6989(82)90028-1. [DOI] [PubMed] [Google Scholar]
- 6.Koretz J.F., Handelman G.H. Modeling age-related accommodative loss in the human eye. Math. Model. 1986;7:1003–1014. [Google Scholar]
- 7.Krag S., Olsen T., Andreassen T.T. Biomechanical characteristics of the human anterior lens capsule in relation to age. Invest. Ophthalmol. Vis. Sci. 1997;38:357–363. [PubMed] [Google Scholar]
- 8.Guilak F., Cohen D.M., Chen C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krag S., Andreassen T.T. Mechanical properties of the human lens capsule. Prog. Retin. Eye Res. 2003;22:749–767. doi: 10.1016/s1350-9462(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 10.Kalkhof S., Haehn S., Sinz A. Determination of disulfide bond patterns in laminin β1 chain N-terminal domains by nano-high-performance liquid chromatography/matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:1933–1940. doi: 10.1002/rcm.3576. [DOI] [PubMed] [Google Scholar]
- 11.Timpl R., Rohde H., Martin G.R. Laminin—a glycoprotein from basement membranes. J. Biol. Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- 12.Hopf M., Göhring W., Hohenester E. Crystal structure and mutational analysis of a perlecan-binding fragment of nidogen-1. Nat. Struct. Biol. 2001;8:634–640. doi: 10.1038/89683. [DOI] [PubMed] [Google Scholar]
- 13.Takagi J., Yang Y., Springer T.A. Complex between nidogen and laminin fragments reveals a paradigmatic β-propeller interface. Nature. 2003;424:969–974. doi: 10.1038/nature01873. [DOI] [PubMed] [Google Scholar]
- 14.Kallunki P., Tryggvason K. Human basement membrane heparan sulfate proteoglycan core protein: a 467-kD protein containing multiple domains resembling elements of the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J. Cell Biol. 1992;116:559–571. doi: 10.1083/jcb.116.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siebold B., Deutzmann R., Kühn K. The arrangement of intra- and intermolecular disulfide bonds in the carboxyterminal, non-collagenous aggregation and cross-linking domain of basement-membrane type IV collagen. Eur. J. Biochem. 1988;176:617–624. doi: 10.1111/j.1432-1033.1988.tb14321.x. [DOI] [PubMed] [Google Scholar]
- 16.Than M.E., Henrich S., Bode W. The 1.9-A crystal structure of the noncollagenous (NC1) domain of human placenta collagen IV shows stabilization via a novel type of covalent Met-Lys cross-link. Proc. Natl. Acad. Sci. USA. 2002;99:6607–6612. doi: 10.1073/pnas.062183499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanacore R., Ham A.J., Hudson B.G. A sulfilimine bond identified in collagen IV. Science. 2009;325:1230–1234. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanacore R.M., Friedman D.B., Hudson B.G. Identification of S-hydroxylysyl-methionine as the covalent cross-link of the noncollagenous (NC1) hexamer of the α1α1α2 collagen IV network: a role for the post-translational modification of lysine 211 to hydroxylysine 211 in hexamer assembly. J. Biol. Chem. 2005;280:29300–29310. doi: 10.1074/jbc.M502752200. [DOI] [PubMed] [Google Scholar]
- 19.Rieppo J., Töyräs J., Helminen H.J. Structure-function relationships in enzymatically modified articular cartilage. Cells Tissues Organs (Print) 2003;175:121–132. doi: 10.1159/000074628. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M.B., Mow V.C., Eyre D.R. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J. Orthop. Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 21.Zhu W., Mow V.C., Eyre D.R. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J. Orthop. Res. 1993;11:771–781. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 22.Rossi M., Morita H., Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nigro J., White J.F., Werkmeister J.A. The effect of bovine endosteum-derived particles on the proliferation of human mesenchymal stem cells. Biomaterials. 2010;31:5689–5699. doi: 10.1016/j.biomaterials.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 24.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Hukins D.W.L., Aspden R.M., Sikoryn T.A. Definition of strain and stiffness in mechanical testing of animal tissues. J. Mater. Sci. Lett. 1991;10:535–536. [Google Scholar]
- 26.Synchrotron. http://www.synchrotron.org.au/index.php/aussyncbeamlines/saxswaxs/saxs-data-a-processing. Accessed January 2, 2010.
- 27.EMBL Hamburg. http://www.embl-hamburg.de/biosaxs/software.html. Accessed January 2, 2010.
- 28.Kendall K., Fuller K.N.G. J-shaped stress/strain curves and crack resistance of biological materials. J. Phys. D Appl. Phys. 1987;20:1596–1600. [Google Scholar]
- 29.Pedrigi R.M., David G., Humphrey J.D. Regional mechanical properties and stress analysis of the human anterior lens capsule. Vision Res. 2007;47:1781–1789. doi: 10.1016/j.visres.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Lanir Y. Constitutive equations for fibrous connective tissues. J. Biomech. 1983;16:1–12. doi: 10.1016/0021-9290(83)90041-6. [DOI] [PubMed] [Google Scholar]
- 31.Pinsky P.M., van der Heide D., Chernyak D. Computational modeling of mechanical anisotropy in the cornea and sclera. J. Cataract Refract. Surg. 2005;31:136–145. doi: 10.1016/j.jcrs.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 32.Sacks M.S. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar-collagenous tissues. J. Biomech. Eng. 2003;125:280–287. doi: 10.1115/1.1544508. [DOI] [PubMed] [Google Scholar]
- 33.Burd H.J. A structural constitutive model for the human lens capsule. Biomech. Model. Mechanobiol. 2009;8:217–231. doi: 10.1007/s10237-008-0130-5. [DOI] [PubMed] [Google Scholar]
- 34.Fung Y.C. Structure and stress-strain relationship of soft tissues. Am. Zool. 1984;24:13–22. [Google Scholar]
- 35.Roveri N., Ripamonti A., Giro M.G. X-ray diffraction study of bovine lens capsule collagen. Biochim. Biophys. Acta. 1979;576:404–408. doi: 10.1016/0005-2795(79)90415-x. [DOI] [PubMed] [Google Scholar]
- 36.Heistand M.R., Pedrigi R.M., Humphrey J.D. Multiaxial mechanical behavior of the porcine anterior lens capsule. Biomech. Model. Mechanobiol. 2005;4:168–177. doi: 10.1007/s10237-005-0073-z. [DOI] [PubMed] [Google Scholar]
- 37.Bailey A.J., Sims T.J., Light N. Cross-linking in type IV collagen. Biochem. J. 1984;218:713–723. doi: 10.1042/bj2180713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilchrist T.L., Moody C.J. The chemistry of sulfilimines. Chem. Rev. 1977;77:409–435. [Google Scholar]
- 39.Pichierri F. Theoretical characterization of the sulfilimine bond: double or single? Chem. Phys. Lett. 2010;487:315–319. [Google Scholar]
- 40.Reddy G.K., Hudson B.G., Noelken M.E. Reductive cleavage of the disulfide bonds of the collagen IV noncollagenous domain in aqueous sodium dodecyl sulfate: absence of intermolecular nondisulfide cross-links. Biochem. Biophys. Res. Commun. 1993;190:277–282. doi: 10.1006/bbrc.1993.1042. [DOI] [PubMed] [Google Scholar]
- 41.Gathercole L.J., Barnard K., Atkins E.D. Molecular organization of type IV collagen: polymer liquid crystal-like aspects. Int. J. Biol. Macromol. 1989;11:335–338. doi: 10.1016/0141-8130(89)90004-4. [DOI] [PubMed] [Google Scholar]
- 42.Guo W.H., Frey M.T., Wang Y.L. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelham R.J., Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H.B., Dembo M., Wang Y.L. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 45.Hadjipanayi E., Mudera V., Brown R.A. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regen. Med. 2009;3:77–84. doi: 10.1002/term.136. [DOI] [PubMed] [Google Scholar]
- 46.Saha K., Keung A.J., Healy K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


