Abstract
Background
In Germany today, one-third of the 20 million women of child-bearing age use combined oral contraceptives (COCs). In this article, we summarize the current knowledge of the mode of action, wanted and unwanted side effects, and long-term risks of COCs. The levonorgestrel intrauterine device (IUD) and long-acting injectable or implantable monophasic progestogen preparations offer comparable contraceptive efficacy to COCs. Nonetheless, they are less frequently used in Germany than COCs, because of their propensity to cause breakthrough bleeding.
Method
Selective review of the literature.
Results
COCs suppress gonadotropin secretion and thereby inhibit follicular maturation and ovulation. Their correct use is associated with 0.3 pregnancies per 100 women per year, their typical use, with 1 pregnancy per 100 women per year (Pearl index). COCs have effects on the cardiovascular and hemostatic systems as well as on lipid and carbohydrate metabolism. When given in the presence of specific risk factors, they significantly increase the likelihood of cardiovascular disease and thromboembolism. Women with persistent human papilloma virus (HPV) infection who take COCs are at increased risk of developing invasive cervical cancer. On the other hand, COCs lower the cumulative incidence of endometrial and ovarian cancer by 30% to 50%, and that of colorectal cancer by 20% to 30%. Other malignancies seem to be unaffected by COC use.
Conclusion
As long as personal and familial risk factors are carefully considered, COCs constitute a safe, reversible, and well-tolerated method of contraception.
Hormonal contraceptives are among the most reliable reversible methods of contraception (Table 1) (1). Their composition, dosage and usage varies, leading to differing rates of risk, side effects and advantages. The wide range of available preparations offers individual choice, tailored to a woman’s priorities regarding effectiveness, cycle control, side effects and risks, as well as therapeutic and preventive effects.
Table 1. Incidence of unwanted pregnancy in the first year of use per 100 women with actual (Pearl-Index) and ideal use (theoretical Pearl-Index value) of various methods of contraception (1).
| Method | Adjusted Pearl Index (ideal use) | Pearl Index (typical use) |
| None | 85 | 85 |
| Female sterilization | 0.5 | 0.5 |
| Male sterilization | 0.1 | 0.15 |
| Oral ovulation inhibitorsl | 0.3 | 8 (2.2 *1, 2) |
| Contraceptive patch | 0.3 | 8 (1.2 *2) |
| Contraceptive vaginal ring | 0.3 | 8 (1.2 *2) |
| Progestagen only pill (POP) | 0.3 | 8 |
| POP with desogestrel | (0.14*2) | (0.41*2) |
| Depot progestagen (medroxyprogesterone acetate) | 0.3 | 3 |
| Gestagen implant | 0.05 | 0.05 |
| Copper IUD | 0.6 | 0.8 |
| Levonorgestrel IUD | 0.2 | 0.2 |
| Diaphragm with spermicide | 6 | 16 |
| Male condom (without spermicide) | 2 | 15 |
| Female condom (without spermicide) | 5 | 21 |
| Spermicide | 18 | 29 |
| Intravaginal sponge (nulliparous women) | 9 | 16 |
| Intravaginal sponge (parous women) | 20 | 32 |
| Coitus interruptus | 4 | 27 |
| Rhythm method | 3–5 | 25 |
It was 50 years ago in 1961 that the first hormonal contraceptive was introduced to the German market. Today, a third of Germany’s 20 million women of reproductive age take a combined pill (e1), while other hormonal methods such as the progestagen only pill (POP), the levonorgesterol-bearing intrauterine device (IUD), and transdermal and vaginal contraceptives, play a lesser role.
For this reason, this article will focus solely on combined oral contraceptives. It should however be noted at this point that the IUDs, long acting progestagen-only implants and injectables—containing 104 or 150 mg of medroxyprogesterone acetate, 200 mg norethisterone enantate or 68 mg etonorgestrel respectively—offer eaqually high contraceptive efficacy, at least in ideal use (Table 1). They are also associated with reduced thrombotic risk due to the absence of an estrogen component which reduces the effect on the clotting system, but bleeding abnormalities are commoner than with combined oral contraceptives, especially at the beginning of treatment.
Learning objectives
The learning objectives for the reader are:
to increase awareness of the mode of action and contraceptive efficacy of the combined pill
to understand and assess its risks and side effects
to be aware of the additional uses and indications for combined hormonal preparations.
Efficacy.
Hormonal contraceptives are among the most reliable reversible methods of contraception.
This review article is based on a selective review of the literature.
Composition of combined oral contraceptives
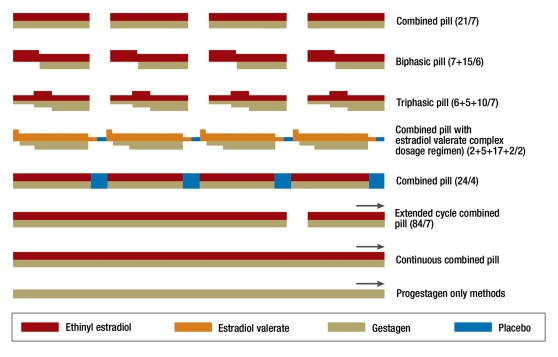
Combined oral contraceptives (COCs) consist of a combination of estrogen and progestagen. Most preparations are taken daily for 21 days, followed by a seven day break (hormone-free interval or pill-free interval, PFI) during which a withdrawal bleed occurs two to three days after the last tablet taken (conventional regimen, 21/7) (Figure 1). Ethinylestradiol (EE) is the estrogen used in almost all preparations, with the exception of one which uses estradiol valerate. These are combined with one of a variety of synthetic progestagens (eTable 1) (2) (e2– e4).
Figure 1.
Homonal contraception regimens (e4)
eTable 1. Ovulation inhibitory doses and spectra of activity for progestagens. These data are based largely on animal studies. The clinical effects of progestagens depend on both the binding affinity for the relevant steroid receptors and on hormone concentrate in target cells (2, e2– e4).
| Progestagen | OID (mg) | EST | A–E | AND | A–A | GLU | A–M |
| Progesterone | 300 | – | + | – | (+) | + | + |
| Chlormadinone acetate (CMA) | 1.7 | – | + | – | + | + | – |
| Cyproterone acetate (CPA) | 1.0 | – | + | – | + | + | – |
| Medroxyprogesterone acetate (MPA) | – | + | (+) | – | + | – | |
| Dienogest (DNG) | 1 | – | + | – | + | – | – |
| Norethisterone (NET) | 0.4 | (+) | + | + | – | – | – |
| Lynestrenol (LYN) | 2 | (+) | + | + | – | – | – |
| Levonorgestrel (LNG) | 0.06 | – | + | + | – | – | – |
| Norgestimate (NGM) | 0.2 | – | + | + | – | ? | ? |
| Norelgestromin (NGN) | – | + | + | – | ? | ? | |
| Desogestrel (DG) | 0.06 | – | + | + | – | (+) | – |
| Etonogestrel (EG) | 0.06 | – | + | + | – | (+) | – |
| Gestodene (GSD) | 0.04 | – | + | + | – | (+) | + |
| Drospirenone (DRSP) | 2 | – | + | – | + | – | + |
OID, Ovulation inhibition dose = minimal dose inhibiting ovulation in all women in daily use (without added estrogen). EST, estrogenic activity; A–E, antiestrogenic activity; AND, androgenic activity; A–A; antiandrogenic activity; GLU, glucocorticoid activity; A–M, antimineralocorticoid activity
To minimize the risk of serious side effects such as thromboembolic events, only low dose preparations, so-called micro-pills with 15–35 µg EE, or else the combination with estradiol valerate, should be prescribed routinely. Because the progestagens vary as to their efficacy and spectrum of action, the daily doses of these varies considerably (eTable 1).
Monophasic combined pills contain constant daily doses of contraceptive steroids, while biphasic or triphasic preparations have a daiy dose which varies in a stepwise fashion (Figure 1) (e4). During the pill-free interval, follicular maturation begins due to the loss of pituitary suppression via the contraceptive steroids. This is then suppressed once more in the course of the first week of the new pill cycle (e2, e4). The contraceptive vaginal ring and patch work like oral combined preparations, with a 21 day hormone phase and a seven day pill-free interval, during which a withdrawal bleed occurs. Their efficacy, side effect profile and hepatic effects are broadly equivalent to those of low dose oral contraceptives.
Combined oral contraceptives.
They consist of a combination of estrogen and progestagen. The majority of preparations are taken for 21 days, followed by a seven day pill-free interval, during which a withdrawal bleed occurs.
Method of action
The contraceptive effect of the COCs depends primarily on the suppression of gonadotrophin release. This leads to the arrest of follicular maturation, of the preovulatory luteinizing hormone surge, and of ovulation. Most COCs have a progestagen dose which is significantly above the threshold for ovulation suppression (eTable 1), so that even without the estrogen component, the contraceptive effect would be achieved. Additional effects include the peripheral action of progestagens on cervical mucus, tubular function and the endometrium. However, cycle control depends on the effects of the estrogen component (e2, e4).
Contraceptive efficacy
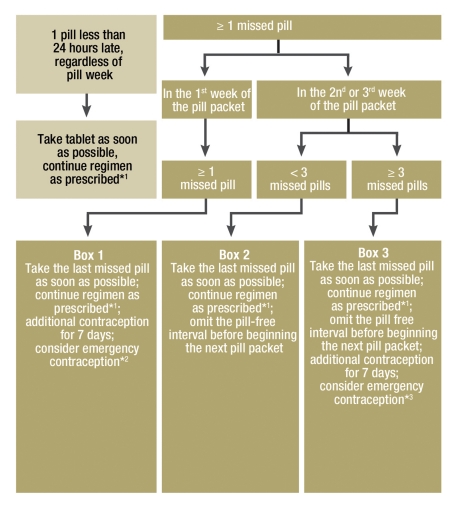
The efficacy of a method of contraception is represented using the Pearl Index (PI), i.e. the number of unwanted pregnancies per 1300 cycles of use (100 woman-years) (e5). User errors and compliance problems lead to an increase in the Pearl Index. With ideal use (no missed pills), the PI is around 0.3 (Table 1) (1, 3). However, half of women miss at least one and a quarter at least two pills per cycle (actual use), which raises the PI (4). The failure rate of COCs appears to be somewhat lower (PI around 1) in Europe than the USA (PI around 8) (Table 1) (3, 5). This may relate to more careful instruction in reliable use, or to differing attitudes to safe contraception between European and American women (e6). The risk of pregnancy is highest when pills are missed at the beginning of the first week of pills, or the end of the last one, because these prolong the pill-free interval during which follicular maturation resumes. Appropriate measures to prevent unwanted pregnancy where pills have been missed are presented in Figure 2 (6, e7). These measures apply only to monophasic combined preparations taken conventionally, i.e. for 21 days with a 7 day pill-free interval. With mistakes using the following preparations, these measures must be adapted accordingly (6):
Figure 2.
Measures to manage mistakes in pill taking with monophasic COCs (modified from [6]).
| Box 1: | One or more missed pills during the first pill week could result in the extension of the hormone free interval to >7 days and hence to follicle maturation and ovulation. |
| Box 2: | If fewer than 3 consecutive pills are missed in weeks 2 or 3, the hormone-free interval is shortened and this can lead to breakthrough bleeding. Contraceptive efficacy is however unaffected, as long as regular COC intake was maintained for at least 7 days prior to missed pills. |
| Box 3: | If 3 or more consecutive pills are missed in week 3, contraception is very likely to be compromised as the hormone-free interval is continuous with the third pill week and is prolonged by the number of days for which pills have been missed. For this reason it is recommended to omit the pill-free interval, in other words to commence the next pill packet immediately following the previous one. To simplify the recommended measures, the same recommendation is made for pills missed in week two, even though in some cases the scheduled pill free interval would have no effect on contraception. |
*1This measure means that in some cases two pills will be taken on one day.
*2If unprotected sex has occurred in the last 5 days,
*3Where several pills have been missed, or there has been a greater than 7 day period with no pills taken
where the pill-free interval is shorter than seven days
where the preparation uses placebo tablets in the pill-free interval
where the route of administration is vaginal or transdermal
with progestagen only methods.
COCs with 20 µg EE per day are as effective as ≥ 30 µg preparations, since ovulation inhibition depends primarily on the progestagen component (e8). Extending the pill cycle from 21 to 24 days (24/4) increases the suppression of follicular maturation and hence allows for a dose reduction in the contraceptive steroids (3). The use of extended cycle regimens with a seven day PFI after for example 84 days (84/7), or continuous COC use, suppress ovarian function even more effectively. The number of withdrawal bleeds is also reduced, which many women view positively (Figure1) (7, e9).
Mode of action.
The contraceptive efficacy of the COC relies primarily on the suppression of gonadotrophin release.
The efficacy of the COC can be compromised by gastrointestinal disturbance or antibiotic use (e10). Although a recently published study found no influence of antibiotics (e11), the recommendation is still to use additional, alternative methods of contraception (barrier methods) during antibiotic use and for a further seven days.
Intercurrent use of liver enzyme inducing medications such as rifampicin and rifabutin can reduce the efficacy of hormonal contraceptives. By contrast, oral contraceptives can also influence the efficacy of other medications. Hence COC administration can reduce serum levels of lamotrigine resulting in increased seizure risk, with a subsequent rise in the pill-free interval (8, e12). Extended cycle or continuous use of the COC can avoid these fluctuations.
Side effects and risks
Hormonal contraceptives affect the whole body and some of these effects are beneficial or therapeutic (Box) (9, e13). On the other hand, unwanted drug effects can represent a health risk, depending on a woman’s pre-existing risk factors. The majority of side effects reported during COC use are equivalent in frequency to those reported in placebo groups (e14– e16).
BOX. Non-contraceptive indications for and other beneficial effects of the combined pill*1.
Cycle regulation
Bleeding disorders with or without iron deficiency anemia
Polymenorrhea
Menorrhagia
Dysmenorrhea
Induction of amenorrhea via extended cycle or continuous use to improve quality of life
PMS and PMDD
Prevention of menstrual migraine
Reduction in incidence of benign ovarian tumors and cysts
Reduction of ovarian and endometrial cancer risk
Acne
Hirsutism
Preservation of bone mineral density in perimenopausal women
Endometriosis related chronic pelvic pain
Fibroid related bleeding disorders
PMS, premenstrual syndrome; PMDD, premenstrual dysphoric disorder; *1 modifed from (9, e13)
Cardiovascular disease
Hormonal contraceptives affect cardiac function, the circulation, blood pressure, fat and carbohydrate metabolism and blood clotting, and have direct effects on the vascular wall. In the presence of pre-existing cardiovascular risk factors, the COC can increase the risk of vascular disease.
The incidence of venous thromboembolic disease (VTE) in healthy women of reproductive age is very low, at around 4 to 5/10 000 women per year (10). COC use alters clotting factors and fibrinolysis via its effect on liver metabolism, and can lead to a reversible activate protein C resistance (e17). These effects contribute to an increased VTE risk with COC use of 9 to 10/10 000 woman years, a risk which is proportionate to the estradiol dose, and highest at the beginning of treatment. By comparison, this risk during pregnancy is around 29/10 000, and in the postpartum period, around 300–400/10 000 woman years (e18– e20).
Unwanted effects.
The majority of unwanted side effects are no more frequent among COC users than in placebo groups.
Cardiovascular disease.
In the presence of risk factors, the COC can increase the risk of cardiovascular disease.
There is a suggestion that the progestagen component modifies the estradiol related VTE risk. Desogestrel, gestodene and cyproterone acetate are associated with a higher risk than levonorgestrel-based COCs (11). There are however contradictory data on this point (e21). Additional factors which further increase the risk of VTE during COC use include age, high BMI, immobility, vasculitis and smoking, while moderate alcohol consumption reduces the risk (e22, e23). The contraceptive patch carries a higher VTE risk than other contraceptives, presumably due to its heavier estradiol burden on the liver, while the vaginal ring carries similar risks to those of oral preparations (e4, e24). The presence of a positive family or personal history of thromboembolic disease, or of genetic or acquired thrombophilia, increases baseline VTE risk significantly, a risk which is increased still further with COC use. Hence a woman with a heterozygous Factor-V-Leiden-Mutation (prevalence of around 5%) or a perthrombin mutation has a 7- to 10-fold VTE risk with COC use (12). Nevertheless, general screening for these conditions has not been found to be cost effective (1, e17, e25, e26). In order to prevent one venous thromboembolic event, around 8000 women would need to undergo screening, of whom 400 would stop taking the COC (13, e27).
Combined pills.
COCs alter clotting and fibrinolysis via the hepatic effects of ethinyl estradiol.
Where surgical procedures with a high thromboembolic risk are planned, the COC should be discontinued four to six weeks preoperatively in order to normalize clotting. By contrast with the COC, progestagen only methods (progestagen only pill/POP containing levonorgestrel or desogestrel, or the levonorgestrel-bearing IUD), appear not to increase this risk (1, 11, e28).
The increased risk of myocardial infarction in young women taking the COC is marginal. Additional risk factors such as age, hypertension, smoking, diabetes, hyperlipidemia, and high BMI must also be taken into account (14, e4, e26, e29) (Table 2). Progestagen only methods appear not to affect the risk of myocardial infarction. (e30).
Table 2. Effect of COCs on the incidence of and mortality from cardiovascular diseases (per 100000 per year)*1.
| Age | Incidence | Mortality | ||||
| Without COC | Non-smoker with COC | Smoker with COC | Without COC | Non-smoker with COC | Smoker with COC | |
| Myocardial infaction | ||||||
| 20–24 | 0.01 | 0.03 | 0.27 | 0.004 | 0.01 | 0.08 |
| 30–34 | 0.17 | 0.42 | 3.39 | 0.05 | 0.13 | 1.02 |
| 40–44 | 2.13 | 5.32 | 42.60 | 0.64 | 1.60 | 12.80 |
| Ischemic stroke | ||||||
| 20–24 | 0.60 | 1.51 | 3.03 | 0.15 | 0.38 | 0.75 |
| 30–34 | 0.98 | 2.46 | 4.92 | 0.25 | 0.61 | 1.23 |
| 40–44 | 1.60 | 4.01 | 8.02 | 0.40 | 1.00 | 2.01 |
*1modified using data from (14)
The risk of ischemic stroke is extremely small in young women, but rises with a history of focal migraine (15, e26, e31, e32). COC use doubles this pre-existing risk, and smoking increases it seven fold (e33) (table 2). COC does not affect the risk of hemorrhagic stroke.
Risk factors for venous thrombosis during COC use.
Age
High BMI
Smoking
Immobility
Vasculitis
Benign tumors
Benign liver tumors
Focal nodular hyperplasia (FNH) is a rare, benign disease (with a prevalence of 0.4% to 3% in the general population), which is usually diagnosed in asymptomatic women and is rarely associated with complications (e34– e36). It is questionable whether COC affects the severity or prevalence of FNH (e37, e38). If FNH is diagnosed during treatment with a low dose COC, it is reasonable to continue treatment with regular monitoring of the lesion(s) (Table 3) (1, e38).
Table 3. Recommendations on the use and safety of different contraceptives for women with baseline risks*1.
| COC | POP | D/NE | ETG | Copper IUD | LNG IUD | ||||||||
| High BMI: | |||||||||||||
| a) BMI: ≥ 30 kg/m 2 | 2 | 1 | 1 | 1 | 1 | 1 | |||||||
| b) Menarche <18 and bmi: ≥ 30 kg/m 2 | 2 | 1 | D=2, NE=1 | 1 | 1 | 1 | |||||||
| Deep vein thrombosis (DVT) / pulmonary embolus (PE) | |||||||||||||
| a) history of DVT/PE | 4 | 2 | 2 | 2 | 1 | 2 | |||||||
| b) acute DVT/PE | 4 | 3 | 3 | 3 | 1 | 3 | |||||||
| c) DVT/PE on anticoagulants | 4 | 2 | 2 | 2 | 1 | 2 | |||||||
| d) family history (1st degree relatives) | 2 | 1 | 1 | 1 | 1 | 1 | |||||||
| e) major surgery | |||||||||||||
| (i) prolonged immobilization | 4 | 2 | 2 | 2 | 1 | 2 | |||||||
| (ii) brief immobilization | 2 | 1 | 1 | 1 | 1 | 1 | |||||||
| f) minimal surgical procedures without immobilization | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Thrombophilia | 4 | 2 | 2 | 2 | 1 | 2 | |||||||
| Systemic lupus erythematosus (SLE): Women with SLE are at increased risk of ischemic heart disease, stroke and venous thromboembolism. The above recommendations assume that none of the above risks is present at the time of prescribing. Michael: bitte horziontalen Strich unter dieser Zeile entfernen | |||||||||||||
| a) positive (or unknown) antiphospholipid antibody status | 4 | 3 | 3 | 3 | 1 | 3 | |||||||
| b) severe thrombocytopenia | 2 | 2 | 3*2 2*3 | 2 | 3*2 2*3 | 2 | |||||||
| c) immunosuppressive therapy | 2 | 2 | 2 | 2 | 2*2 1*3 | 2 | |||||||
| d) none of the above | 2 | 2 | 2 | 2 | 1 | 2 | |||||||
| Viral hepatitis | |||||||||||||
| a) acute or reactivated | 3/4 | 1 | 1 | 1 | 1 | 1 | |||||||
| b) carrier | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| c) chronic | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Liver cirrhosis | |||||||||||||
| a) mild (compensated) | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| b) severe (decompensated) | 4 | 3 | 3 | 3 | 1 | 3 | |||||||
| Liver tumors | |||||||||||||
| a) benign | |||||||||||||
| (i) focal nodular hyperplasia | 2 | 2 | 2 | 2 | 1 | 2 | |||||||
| (ii) hepatocellular adenoma | 4 | 3 | 3 | 3 | 1 | 3 | |||||||
| b) malignant | 4 | 3 | 3 | 3 | 1 | 3 | |||||||
| Age | |||||||||||||
| a) menarche to <18 | 1 | 1 | 2 | 1 | 2 | 2 | |||||||
| a) 18 to <40 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| b) ≥ 40 | 2 | 1 | 2 | 1 | 1 | 1 | |||||||
| Smoking | |||||||||||||
| a) age <35 | 2 | 1 | 1 | 1 | 1 | 1 | |||||||
| b) age ≥ 35 | |||||||||||||
| (i) <15 cigarettes/day | 3 | 1 | 1 | 1 | 1 | 1 | |||||||
| (ii) ≥ 15 cigarettes/day | 4 | 1 | 1 | 1 | 1 | 1 | |||||||
| Hypertension | |||||||||||||
| a) systolic 140–159 or diastolic 90–99 mmHg | 3 | 1 | 2 | 1 | 1 | 2 | |||||||
| b) systolic ≥160 or diastolic ≥ 100 mmHg | 4 | 2 | 3 | 2 | 1 | 2 | |||||||
| c) with vascular damage | 4 | 2 | 3 | 2 | 1 | 1 | |||||||
| Category 1: Use unrestricted. | |||||||||||||
| Category 2: Benefits generally outweigh risks | |||||||||||||
| Category 3: Risks generally outweigh benefits | |||||||||||||
| Category 4: This method represents an unacceptable health risk | |||||||||||||
*1From the WHO Medical Eligibility Criteria for Contraceptive Use, 4th edition, 2010 (1); COC, combined oral, transdermal, or vaginal contraceptive (ethinyl estradiol or estradiol valerate + progestagen); POP, oral progestagen only preparation (progestagen only pill); D, depot medroxyprogesterone acetate; NE, Norethisterone enantate (depot progestagens); ETG, etonogestrel implant (progestagen implant); IUD, intrauterine device; LNG, levonorgestrel;
*2initial onset of treatment;
*3continued treatment
Hepatocellular adenomas are extremely rare in young women, including long term low dose COC users (prevalence: 3 to 4/100 000) (16, e34, e39). The risk appears to depend on duration of use and the EE dose (e40, e41). If hepatocellular adenoma is diagnosed during treatment with a COC, COC treatment should be discontinued (Table 3). No association has been found between COC use and the incidence of meningioma and prolactinoma.
Carcinomas and mortality
Reproductive factors can influence the incidence of a number of malignancies. For example late menarche, early menopause and prolonged breastfeeding are associated with a reduced risk of breast cancer. Two large cohort studies have shown that the COC—with the exception of invasive cervical cancer—are not associated with any increased risk of cancer (eTable 2) (17, 18).
eTable 2. The effect of COCs on cancer risk.
| Carcinoma | Royal College of General Practitioners—Study 2007*1 RR (95% CI) | Oxford Family Planning Association Study 2006*2 RR (95% CI) |
| Colorectal | 0.72 (0.58–0.90) | 0.8 (0.6–1.2) |
| Hepatobiliary | 0.55 (0.26–1.17) | No data |
| Lung | 1.05 (0.82–1.35) | 1.4 (0.9–2.1) |
| Melanoma | 0.92 (0.65–1.29) | 0.8 (0.5–1.2) |
| Breast | 0.98 (0.87–1.10) | 1.0 (0.8–1.1) |
| Cervical | 1.33 (0.92–1.94) | 4.2 (1.8–12.0) |
| Endometrial and uterine body | 0.58 (0.42–0.79) | 0.3 (0.2–0.6) |
| Ovar | 0.54 (0.40–0.71) | 0.5 (0.3–0.7) |
| CNS/pituitary | 1.34 (0.73–2.47) | No data |
| Gastrooesophageal | No data | 0.6 (0.3–1.3) |
| Renal/bladder | No data | 0.8 (0.5–1.5) |
| Lymphoma/leukemia | No data | 1.1 (0.7–1.6) |
| All cancers | 0.88 (0.83–0.94) | No data |
Hepatocellular adenomas.
The risk of hepatocellular adenomas appears to depend on the length of COC use and the dose of EE. If a hepatocellular adenoma is diagnosed during COC treatment, the pill should be discontinued.
Numerous recent studies have concluded that the COC has either no, or a marginal, influence on breast cancer risk (eTable 3) (17– 20, e42– e46). The absolute risk is very small. For example, if women aged 25 use the COC for five years, there will be just five extra breast cancers per 10 000 women during the following 10-year period (49 instead of 44). Most of these would be local, non-metastasized tumors. The increased risk disappears within ten years of discontinuing the pill (19). Even in women with BRCA1 and BRCA2 mutations, either no risk increase or an extremely small risk increase was found (20, e47, e48). Progestagen only methods (progestagen only pill, depot-medroxypergesteronacetate and the levonorgestrel bearing IUD) also have but a small effect (e49).
eTable 3. The effect of combined oral contraceptives on breast cancer risk*1.
| Study | Number | Relative risk | 95% CI | |
| Cases | Controls | |||
| Oxford reanalysis, 1996 (19) | 53 297 | 100 239 | Current/previous COC: 1.24 | 1.15–1.33 |
| Nurses’ Health Study, 1997 (cohort study) (e43) | 3383 | 1.11 COC >5 years: 0.96 | 0.94–1.32 | |
| 0.65–1.43 | ||||
| Women’s CARE Study, 2002 (e44) | 4575 | 4682 | 1.0 | 0.8–1.1 |
| Women’s Lifestyle and Health Study, 2002 (cohort study) (e45) | 103 027 | Previous COC: 1.2 C | 1.1–1.4 | |
| urrent/previous COC: 1.6 | 1.2–2.0 | |||
| Mayo Clinic (metaanalysis), 2006 (e46) | Premenopausal breast cancer | Current/previous COC: 1.19 | 1.09–1.29 | |
| Oxford Family Planning Association Study, 2006 (cohort study) (18) | 17 032 | 1.0 | 0.8–1.1 | |
| Royal College of General Practitioners’ Study. 2007 (cohort study) (17) | 46 000 (744 000 woman years) | 0.98 | 0.87–1.1 | |
COC, combined preparations; CI, confidence interval;
*1data from (20)
Breast cancer.
Numerous recent studies have concluded that the COC has either no influence or a very small influence on breast cancer.
The cumulative incidence of ovarian cancer in women under 75 is 1.2% and is reduced by the COC in proportion to the length of treatment by 30% to 50%. This protective effect persists for many years after discontinuation (eTable 2) (17, 18, 21, e50, e51). The same is true for women with BRCA1 and BRCA2 mutations (e47). The incidence of endometrial cancer is also reduced with COC use to a half. The risk reduction correlated with the duration of use and persists after discontinuation for at least 15 years (e52).
The COC does not increase the risk of any of the following.
Thyroid, bronchial, esophageal, gastric or pancreatic cancer, cholangiocarcinoma, renal cell carcinoma, neuroblastoma, melanoma, and Hodgkin’s and Non-Hodgkin’s lymphoma.
COCs increase the risk of cervical intraepithelial neoplasia (CIN), in proportion to the duration of use, with a doubling of risk after five years and a quadrupling after 10 years. However, only women with persistent HPV infection are affected (e53). It appears that the progestagen component promotes the progression of cervical dysplasia. Regular cervical cytology allows early detection of these CIN lesions, while immunization, especially prior to first sexual intercourse, provides protection from HPV. HPV infection is also involved in the development of vulval and vaginal cancer, but the COC appears to have no influence on these.
COC use significantly reduces the risk of colorectal cancer by 20% to 30% (16, 17, e54, e55). The potential role of COC in the development of hepatocellular carcinoma is as yet not established, but the absolute risk of this illness is extremely small (16, e56).
No effect was found for COC use on thyroid, bronchial, esophageal, gastric, pancreatic carcinoma, or on cholangiocarcinoma, renal cell carcinoma, neuroblastoma, melanoma or Hodgkin’s’ and Non-Hodgkin’s’ lymphoma (16– 18, 22, e52, e57, e58).
Mortality is by no means increased in women who have taken the COC, if anything it is reduced (22, 23). The positive effect is largely due to a reduction in cancer related mortality and ischemic heart disease (eTable 4). There was however an increase in mortality in pill users under 45 due to vascular disease. (31 versus 14 cases per 100 000 women per year). This emphasizes the relevance of taking a careful history including family history of vascular risks prior to prescribing the COC.
eTable 4. The effect of COCs on mortality*2.
| Cause of death | Never COC— number per 100 000 woman years | Ever COC— number per 100 000 woman years | Adjusted relative risk*1 | 95% CI |
| All cause mortality | 417.45 | 365.51 | 0.88 | 0.82–0.93 |
| All cancers | 194.55 | 165.45 | 0.85 | 0.78–0.93 |
| – colorectal | 20.05 | 12.41 | 0.62 | 0.46–0.83 |
| – hepatobiliary | 3.12 | 2.03 | 0.65 | 0.30–1.39 |
| – lung | 26.08 | 31.70 | 1.22 | 0.96–1.53 |
| – melanoma | 2.67 | 1.95 | 0.73 | 0.33–1.61 |
| – breast | 43.91 | 39.41 | 0.90 | 0.74–1.08 |
| – cervical | 4.02 | 5.38 | 1.34 | 0.74–2.44 |
| – endometrial | 4.47 | 1.94 | 0.43 | 0.21–0.88 |
| – ovarian | 18.04 | 9.47 | 0.53 | 0.38–0.72 |
| – gynecological cancers | 26.51 | 16.80 | 0.63 | 0.49–0.82 |
| All vascular diseases | 115.18 | 99.15 | 0.86 | 0.77–0.96 |
| – ischemic heart disease | 57.41 | 42.85 | 0.75 | 0.63–0.88 |
| – cerebrovascular disease | 27.86 | 29.19 | 1.05 | 0.84–1.30 |
| Violent death – suicide | 12.86 | 19.20 | 1.49 | 1.09–2.05 |
| 4.79 | 6.03 | 1.26 | 0.73–2.18 |
COC, combined oral contraceptives; CI, confidence interval;
*1adjustied for age, parity, smoking, and socioeconomic status;
*2data from Hannaford et al. 2010 (23)
Mortality during COC use.
Mortality is reduced overall in women who have been COC users.
Implications for practice
COC use does not overall increase cancer risk. On the contrary, two cohort studies found that in pill users, 10 to 45 fewer women developed cancers overall, and mortality was reduced by 52 cases per 100 000 women per year (17, 23).
Additional effects and indications for oral contraceptive use
In addition to their contraceptive effect, COCs have numerous additional benefits which can be used therapeutically (Box). Around 10 % of women of reproductive age suffer from menorrhagia (uterine blood loss of >80 mL/period), affecting particularly older women (e59, e60). In these women, blood loss is reduced by at least 50% (e61). For this reason the pill has long been used as a treatment for menorrhagia. At the end of last year, “treatment of heavy periods” was added as an indication for a specific COC preparation in Germany.
Up to 90% of all young women suffer from dysmenorrhea, which is usually caused by strong uterine contractions (e62). The use of the combined pill leads to a significant improvement in up to 80% of women (e13, e63– e66). A particularly useful strategy is to use an extended cycle or continuous regimen—omitting the pill free interval—to produce amenorrhea. Progestagen implants and the levonorgestrel bearing IUD also have a beneficial effect on dysmenorrhea, although they can be associated with irregular bleeding (e67– e71).
Additional indications for COC use.
Oral contraceptives may be used to treat
menorrhagia, dysmenorrhea
endometriosis
uterine fibroids
premenstrual syndrome
At least 10 % of all women of reproductive age and a large proportion of patients with chronic pelvic pain have endometriosis. The associated symptoms can be treated using continuous or extended cycle use of monophasic COCs (e72). After surgical treatment of endometriotic lesions, COC treatment can offer a long term reduction in disease and symptom recurrence (7, e72– e74).
Women with a fibroid uterus, many of whom suffer menstrual irregularities and dysmenorrhea, often benefit from COC use, particularly extended cycle use. Fears about promoting fibroid growth with COC use appear to be unfounded (e75– e79), although there is a suggestion that COC use in early adolescence (age 13–16) can lead to an increase in their incidence (e80).
COCs are frequently used to treat acne and hirsutism. Their beneficial effect is primarily due to a reduction in ovarian and adrenal androgen production, an increase in sex hormone binding globulin (SHBG) levels, and a reduction in free testosterone. The effect of antiandrogenic progestagens is probably responsible for only a small part of the effect.
Premenstrual syndrome (PMS) is characterized by affective disturbances (irritability, anxiety, aggression, low mood), which are often associated with vegetative symptoms (abdominal distension, headache), weight gain and fluid retention. These symptoms arise in the mid to late luteal phase, and disappear in the first days of menstruation (e81). COC use can reduce the intensity and frequency of individual symptoms, especially with a shortened pill-free interval (24/4), or using an extended or continuous regimen (e82, e83).
Dysmenorrhea.
The COC leads to noticeable improvements in up to 80% of women.
Smoking.
Women over 35 who smoke over 15 cigarettes a day should not take the COC.
Hypertension.
At systolic pressures of 160 mm Hg or over, and diastolic pressures of 100 mm Hg or over, the COC should not be used.
Contraception in women over 40
Although fertility is reduced in women over 40, effective contraception is still needed at this age where most women have completed their families, particularly given the increased miscarriage and anomaly risk, as well as increased maternal risks, in this age group (e26). Healthy, low risk women can use all forms of contraception until the menopause (Table 3) (9, e26, e61, e84). Many perimenopausal symptoms can also be improved by COC use. Examples include irregular cycles and other menstrual problems, vasomotor symptoms, loss of bone mineral density, PMS, functional ovarian cysts, bleeding disturbances, or dysmenorrhea (e73, e85). Before commencing treatment, a careful individual risk assessment must be made in order to minimize the risk of harms in this age group already at increased risk (e22, e26). In the presence, for example, of high BMI, smoking, hypertension, diabetes or migraine the COC is contraindicated, and alternative methods should be used.
Fertility following COC discontinuation
The cumulative pregnancy rates of 83% within six months and to 94 % within a year are similar for the COC to figures found with the offset of barrier methods (e86). Long term COC use appears to improve fertility, possibly via protection against ascending genital infection.
New developments
Estradiol pill
There have long been efforts to develop and estradiol based pill, as this estrogen has significantly reduced hepatic effects compared to ehinyl estradiol. However, these attempts have mostly failed due to poor cycle control, as estradiol is broken down in the endometrium far more rapidly than ethinyl estradiol, leading to a higher rate of breakthrough bleeding. Since 2009, the first contraceptive with estradiol valerate has been licensed. Using a complex dosage regimen, efficacy and breakthrough bleeding rates comparable with those of a 20 µg EE pill have been achieved (Figure 1). However, there is an approximately 20% rate of so-called silent periods—i.e. the lack of a withdrawal bleed (e87– e89). It is possible that fewer serious complications will arise due to the reduced hepatic effects of estradiol, however as long term data are lacking, the contraindications currently remain as for other COCs.
Contraception in women over 40.
Many perimenopausal symptoms can be improved by COC use.
Long cycles
Continuous use of the monophasic COC without a pill-free interval can defer withdrawal bleeding indefinitely. This benefits not only women suffering menstruation-related symptoms, but also women who wish to avoid bleeding for long periods of time for quality of life reasons (7, e9, e90). Although there is in Europe no licensed long term preparation, there is widespread experience with off label long term use (24).
Pill with folate
The prevalence of fetal neural tube defects (NTD), in Germany around 3/10 000 live births, or 9/10 000 pregnancies (e91, e92), can be reduced using preconceptual folate. It is recommended that all women planning pregnancy should take folate (0.4 mg/day) from several weeks prior to conception. The use of a COC with metafolin increases long term folate levels, providing protection in the event of conception shortly after discontinuing the COC. Modelling studies suggest that this might prevent around 600 cases per year, albeit there is currently no folate based pill available in Europe (e93).
Emergency contraception (interception)
Interception is a short acting method of preventing pregnancy where regular contraception has failed or on other particular circumstances. Post coital oral contraceptives are progestagen only methods, containing levonorgestrel (LNG), or a selective progestagen receptor modulator, ulipristal acetate (UPA).
The postcoital contraceptive with 1.2 mg LNG should be used as soon as possible, but at the latest 72 hours after unprotected sex. Their use reduces the unwanted pregnancy rate by 75% to 85%, but the contraceptive efficacy reduces with increasing time interval from unprotected sex (e94– e97). Its use at the time of implantation has no further contraceptive effect, and it does not interrupt an implanted pregnancy (25, e98, e99). The only absolute contraindication is pregnancy, although no teratogenic effects have been demonstrated to date (e100).
The postcoital pill with 30 mg UPA should also be used as soon as possible, but at the latest five days (120 hours) after unprotected sex. Compared with 1.5 mg LNG, 30 mg UPA was found in a meta-analysis to be associated with a significantly reduced pregnancy rate (1.4% versus 2.2%) (e101, e102). The frequency of side effects is comparable for both preparations.
Fertility after discontinuing the COC.
The cumulative pregnancy rates of 83% 6 months, and 94% a year after discontinuing the COC are comparable to those for barrier methods.
Reliable interception can also be achieved via the insertion of a copper bearing intrauterine device (IUD) within five days of intercourse, which also offers ongoing contraceptive protection (off-label-use) (e4, e103). The pregnancy rarte is around 0.2% (e104).
Pill containing folate.
The addition of metafolin to the COC increases long term folate levels, giving protection should conception occur shortly after discontinuing the pill.
Emergency contraception.
1.5 mg levonorgestrel or 30 mg ulipristal acetate should be taken orally as soon as possible, but up to 72 (levonorgestrel) or 120 (ulipristal acetate) hours after coitus.
Further information on CME. This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: cme.aerzteblatt.de
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in issue 37/2011. The CME unit “The Differential Diagnosis of Hearing Loss” (issue 25/2011) can be accessed until 5 August 2011.
For issue 33/2011, we plan to offer the topic “Hereditary Cancers.”
Solutions to the CME questionnaire in issue 21/2011:
Trappe HJ, Gummert J: Current Pacemaker and Defibrillator Therapy.
Solutions:: 1d, 2b, 3a, 4c, 5a, 6e, 7c, 8b, 9e, 10e
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is meant by the term micro-pill in the context of hormonal ovulation inhibitors?
a preparation containing the progestagen levonorgestrel at a daily dose of 30 µg
a preparation containing estradiol valerate as the estrogen component
a preparation causing only minimal withdrawal bleeding— ≤35mL, in the pill-free interval
a preparation taken continuously with no breaks
a preparation containing a daily ethinyl estradiol dose of between 15 und 35 µg.
Question 2
Which of the following increase the contraceptive efficacy of combined oral contraceptives?
extension of the pill-free interval from 7 to 10 days
continuous administration of a monophasic ovulation inhibitor
the co-administration of rifampicin
the co-administration of antibiotics
taking a micro-pill in the evening while consuming alcohol
Question 3
When is the thrombotic risk particularly increased during COC use?
in the first months of use
in early adolescence (age 13–16)
in the presence of moderate alcohol consumption
when a woman suffers from acne
where there is a diagnosis of uterine fibroids
Question 4
How high is the Pearl Index value, if the COC is used correctly?
<1
8
15
85
100
Question 5
For which cancers are the risks increased with monophasic ovulation inhibitors?
Colon cancer
Ovarian cancer
Cervical cancer
Non Hodgkin’s lymphoma
Endometrial cancer
Question 6
Which of the following is to be expected following discontinuation of a monophasic combined contraceptive?
prolongation of the first six cycles (>35 days)
a low probability of achieving pregnancy in the following 12 months
an increased rate of fetal malformations if pregnancy occurs
an increased risk of twin pregnancy
a withdrawal bleed
Question 7
Which of the following measures is appropriate for emergency contraception following unprotected sex?
1.5 mg levonorgestrel up to 5 days following coitus
the insertion of a copper IUD up to 5 days following coitus
2 tablets of 0.75 mg ethinyl estradiol within 3 days of coitus
the insertion of a levonorgestrel bearing IUD within a week of coitus
10 mg of cyproterone acetate within 3 days of coitus
Question 8
Which of the following are side effects of taking combined oral contraceptives?
hirsutism
hyperandrogenemia
hyperprolactinemia
heavier uterine bleeding
a reduction in dysmenorrhea
Question 9
Which side effect occurs commonly during continuous treatment (without a pill-free interval) with monophasic combined pills?
galactorrhea
endometriotic cysts
adnexitis
amenorrhea
dysmenorrhea
Question 10
The contraceptive efficacy of parenteral combined preparations (transdermal patch, vaginal ring) is equivalent to which other contraceptive method?
the rhythm or periodic abstinence method
condoms
spermicides
combined oral preparations
coitus interruptus
Acknowledgments
Translated from the original German by Dr. Sandra Goldbeck-Wood.
Footnotes
Conflict of interest statement
Perf. Wiegratz has received honoraria for consultancy services from Bayer Healthcare. She has received travel and congress attendance expenses from Serono, Essex and Jenapharm. She has received honoraria for giving scientific lectures and attending expert meetings from Jenapharm GmbH & Co. KG, Bayer Schering Pharma AG, Dr. Kade/Besins Pharma GmbH, Essex Pharma GmbH, and Merck Serono GmbH. She has received honoraria to support clinical studies from Jenapharm and Bayer Healthcare.
Prof. Thaler has received honoraria for consultancy services from Wyeth, Pfizer, MSD and Bayer Healthcare. He has received honoraria for services as a reviewer from MSD and Sandoz. He has received payments in respect of travel and accommodation expenses from MSD, Wyeth, Jenapharm, Bayer Healthcare, Merck-Serono, and Ferring. Prof. Thaler has received honoraria for conducting clinical studies from Pfizer. He has received financial support for research projects into a designated account, from MSD, Merck-Serono, Baxter, and Ferring.
References
- 1.WHO. http://www.who.int/reproductivehealth/publications/family_planning/9789241563888/en/ 4th. Geneva: WHO; 2009. Medical elegibility criteria for contraceptive use. [Google Scholar]
- 2.Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- 3.Dinger J, Minh TD, Buttmann N, Bardenheuer K. Effectiveness of oral contraceptive pills in a large US. cohort comparing progestogen and regimen. Obstet Gynecol. 2011;117:33–40. doi: 10.1097/AOG.0b013e31820095a2. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg M, Waugh MS. Causes and consequences of oral contraceptive noncompliance. Am J Obstet Gynecol. 1999;180:276–279. doi: 10.1016/s0002-9378(99)70718-0. [DOI] [PubMed] [Google Scholar]
- 5.Mansour D, Inki P, Gemzell-Danielson K. Efficacy of contraceptive methods: a review of the literature. Eur J Contracept Reprod Health Care. 2010;15:4–16. doi: 10.3109/13625180903427675. [DOI] [PubMed] [Google Scholar]
- 6.Guilbert E, Black A, Dunn S, et al. Missed hormonal contraceptives: new recommendations. J Obstet Gynaecol Can. 2008;30:1050–1062. doi: 10.1016/S1701-2163(16)33001-8. [DOI] [PubMed] [Google Scholar]
- 7.Wiegratz I, Kuhl H. Long-cycle treatment with oral contraceptives. Drugs. 2004;64:2447–2462. doi: 10.2165/00003495-200464210-00006. [DOI] [PubMed] [Google Scholar]
- 8.Contin M, Albani F, Ambrosetto G, et al. Variation in lamotrigine plasma concentrations with hormonal contraceptive monthly cycles in patients with epilepsy. Epilepsia. 2006;47:1573–1575. doi: 10.1111/j.1528-1167.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 9.ESHRE Capri Workshop Group. Female contraception over 40. Hum Reprod Update. 2009;15:265–277. doi: 10.1093/humupd/dmp020. [DOI] [PubMed] [Google Scholar]
- 10.Heinemann LAJ, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328–336. doi: 10.1016/j.contraception.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339 b2890. doi: 10.1136/bmj.b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism - pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86:809–816. [PubMed] [Google Scholar]
- 13.Hannaford PC. Epidemiology of the contraceptive pill and venous thromboembolism. Thromb Res. 2011;127(Suppl 3):30–34. doi: 10.1016/S0049-3848(11)70009-3. [DOI] [PubMed] [Google Scholar]
- 14.Farley TM, Collins J, Schlesselman JJ. Hormonal contraception and risk of cardiovascular disease. An international perspective. Contraception. 1998;57:211–230. doi: 10.1016/s0010-7824(98)00019-5. [DOI] [PubMed] [Google Scholar]
- 15.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330 doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Vecchia C, Bosetti C. Oral contraceptives and neoplasms other than breast and female genital tract. Eur J Cancer Prev. 2009;18:407–411. doi: 10.1097/CEJ.0b013e32832caaca. [DOI] [PubMed] [Google Scholar]
- 17.Hannaford PC, Selvaraj S, Elliott A, Angus V, Iversen L, Lee AJ. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioners’ oral contraception study. BMJ. 2007;335 doi: 10.1136/bmj.39289.649410.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vessey M, Painter R. Oral contraceptive use and cancer. Findings in a large cohort study, 1968-2004. Br J Cancer. 2006;95:385–389. doi: 10.1038/sj.bjc.6603260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 20.Cibula D, Gompel A, Mueck AO, et al. Hormonal contraception and risk of cancer. Hum Reprod Update. 2010;16:631–650. doi: 10.1093/humupd/dmq022. [DOI] [PubMed] [Google Scholar]
- 21.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 22.Vessey M, Yeates D, Flynn S. Factors affecting mortality in a large cohort study with special reference to oral contraceptive use. Contraception. 2010;82:221–229. doi: 10.1016/j.contraception.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ. 2010;340 c927 doi: 10.1136/bmj.c927. doi:10.1136/bmj.c927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiegratz I, Kuhl H. Stuttgart: Thieme; 2010. Langzyklus; pp. 1–76. [Google Scholar]
- 25.Piaggio G, Kapp N, von Hertzen H. Effect on pregnancy rates of the delay in the administration of levonorgestrel for emergency contraception: a combined analysis of four WHO trials. Contraception. 2011 doi: 10.1016/j.contraception.2010.11.010. article in press. [DOI] [PubMed] [Google Scholar]
- e1.Skouby SO. Contraceptive use and behavior in the 21st century: a comprehensive study across five European countries. Eur J Contracept Reprod Health Care. 2010;15(Suppl 2):42–53. doi: 10.3109/13625187.2010.533002. [DOI] [PubMed] [Google Scholar]
- e2.Kuhl H, Jung-Hoffmann C. Stuttgart: Thieme; 1999. Kontrazeption; pp. 16–171. [Google Scholar]
- e3.Speroff L, Fritz MA. Oral contraception. In: Speroff L, Fritz MA, editors. Clinical gynecologic endocrinology and infertility. 7th edition. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 861–942. [Google Scholar]
- e4.Wiegratz I. Hormonale Kontrazeption. In: Leidenberger F, Strowitzki T, Ortmann O, editors. Klinische Endokrinologie für Frauen-ärzte. 4th edition. Heidelberg: Springer; 2009. pp. 251–301. [Google Scholar]
- e5.Pearl R. Contraception and fertility in 2,000 women. Hum Biol. 1932;4:363–407. [Google Scholar]
- e6.Dinger JC, Cronin M, Möhner S, Schellschmidt I, Minh TD, Westhoff C. Oral contraceptive effectiveness according to body mass index, weight, age, and other factors. Am J Obstet Gynecol. 2009;201 doi: 10.1016/j.ajog.2009.03.017. e1-9. [DOI] [PubMed] [Google Scholar]
- e7.Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit (FFPRHC) Faculty statement from the CEU on a new publication: WHO selected practice recommendations for contraceptive use update. Missed pills: new recommendations. J Fam Plann Reprod Health Care. 2005;31:153–155. doi: 10.1783/1471189053629572. [DOI] [PubMed] [Google Scholar]
- e8.Gallo MF, Nanda K, Grimes DA, Lopez LM, Schulz KF. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2011;1 doi: 10.1002/14651858.CD003989.pub4. CD003989. [DOI] [PubMed] [Google Scholar]
- e9.Wiegratz I, Hommel HH, Zimmermann T, Kuhl K. Attitude of -German women and gynecologists towards long-cycle treatment with oral contraceptives. Contaception. 2004;69:37–42. doi: 10.1016/j.contraception.2003.09.004. [DOI] [PubMed] [Google Scholar]
- e10.Dickinson BD, Altman RD, Nielsen NH, Sterling ML. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853–860. doi: 10.1016/s0029-7844(01)01532-0. [DOI] [PubMed] [Google Scholar]
- e11.Toh S, Mitchell AA, Anderka M, de Jong-van den Berg LTW, Hernandez-Diaz S. Antibiotics and oral contraceptive failure - a case cross-over study. Contraception. 2011;83:418–425. doi: 10.1016/j.contraception.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48:484–489. doi: 10.1111/j.1528-1167.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- e13.The American College of Obstetrics and Gynecologists. Noncontraceptive uses of hormonal contraceptives Practice Bulletin No 110. Obstet Gynecol. 2010;115:206–218. doi: 10.1097/AOG.0b013e3181cb50b5. [DOI] [PubMed] [Google Scholar]
- e14.Redmond G, Godwin AJ, Olson W, Lippman J. Use of placebo controls in an oral contraceptive trial: methodological issues and adverse event incidence. Contraception. 1999;60:81–85. doi: 10.1016/s0010-7824(99)00069-4. [DOI] [PubMed] [Google Scholar]
- e15.Aznar-Ramos R, Ginger-Velazquez J, Lara-Ricalde R, Martinez-Manautou J. Incidence of side effects with contraceptive placebo. Am J Obstet Gynecol. 1969;105:1144–1149. doi: 10.1016/0002-9378(69)90142-2. [DOI] [PubMed] [Google Scholar]
- e16.Goldzieher JW, Moses LE, Averkin E, Scheel C, Taber BZ. A -placebo-controlled double-blind crossover investigation of the side effects attributed to oral contraceptives. Fertil Steril. 1971;22:609–623. [PubMed] [Google Scholar]
- e17.Tchaikovski S, Tans G, Rosing J. Venous thrombosis and oral contraceptives: current status. Womens Health. 2006;2:761–772. doi: 10.2217/17455057.2.5.761. [DOI] [PubMed] [Google Scholar]
- e18.Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ., 3rd Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143:697–706. doi: 10.7326/0003-4819-143-10-200511150-00006. [DOI] [PubMed] [Google Scholar]
- e19.Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJ. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6:632–637. doi: 10.1111/j.1538-7836.2008.02921.x. [DOI] [PubMed] [Google Scholar]
- e20.Reid R, Leyland N, Wolfman W, et al. Oral contraceptives and the risk of venous thromboembolism: an update. J Obstet Gynaecol Can. 2010;252:1192–1197. doi: 10.1016/S1701-2163(16)34746-6. [DOI] [PubMed] [Google Scholar]
- e21.Heinemann LA, Dinger JC, Assmann A, Minh TD. Use of oral contraceptives containing gestodene and risk of venous thromboembolism: outlook 10 years after the third-generation „pill scare“. Contraception. 2010;81:401–407. doi: 10.1016/j.contraception.2009.12.014. [DOI] [PubMed] [Google Scholar]
- e22.Nightingale AL, Lawrenson RA, Simpson EL, Williams TJ, MacRae KD, Fermer RD. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Health Care. 2000;5:265–274. doi: 10.1080/13625180008500402. [DOI] [PubMed] [Google Scholar]
- e23.Pomp ER, Rosendaal FR, Doggen CJ. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am J Hematol. 2008;83:97–102. doi: 10.1002/ajh.21059. [DOI] [PubMed] [Google Scholar]
- e24.Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339–346. doi: 10.1097/01.AOG.0000250968.82370.04. [DOI] [PubMed] [Google Scholar]
- e25.Wu O, Robertson L, Langhorne P, et al. Oral contraceptives, hormone replacement therapy, thrombophilias and risk of venous thromboembolism: a systematic review. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) Study. Thromb Haemost. 2005;94:17–25. doi: 10.1160/TH04-11-0759. [DOI] [PubMed] [Google Scholar]
- e26.Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262–1270. doi: 10.1056/NEJMcp0708481. [DOI] [PubMed] [Google Scholar]
- e27.Vandenbroucke JP, van der Meer FJ, Helmerhorst FM, Rosendaal FR. Factor V Leiden: should we screen oral contraceptive users and pregnant women? BMJ. 1996;313:1127–1130. doi: 10.1136/bmj.313.7065.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e28.Gomes MPV, Deitcher SR. Risk of venous thromboembolic -di-sease associated with hormonal contraceptives and hormone replacement therapy: a clinical review. Arch Intern Med. 2004;164:1965–1976. doi: 10.1001/archinte.164.18.1965. [DOI] [PubMed] [Google Scholar]
- e29.Baillargeon JP, McClish DK, Essah PA, Nestler JE. Association between the current use of low-dose oral contraceptives and cardiovascular arterial disease: a meta-analysis. J Clin Endocrinol Metab. 2005;90:3863–3870. doi: 10.1210/jc.2004-1958. [DOI] [PubMed] [Google Scholar]
- e30.Chakhtoura Z, Canonico M, Gompel A, Scarabin PY, Plu-Bureau G. Progestogen-only contraceptives and the risk of acute myo-cardial infarction: a meta-analysis. J Clin Endocrinol Metab. 2011;96:1169–1174. doi: 10.1210/jc.2010-2065. [DOI] [PubMed] [Google Scholar]
- e31.Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830–833. doi: 10.1136/bmj.310.6983.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e32.Kruit MC, van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291:427–434. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]
- e33.MacClellan LR, Giles W, Cole J, et al. Probable migraine with -visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438–2445. doi: 10.1161/STROKEAHA.107.488395. [DOI] [PubMed] [Google Scholar]
- e34.Maillette de Buy Wenninger L, Terpstra V, Beuers U. Focal nodular hyperplasia and hepatic adenoma: epidemiology and pathology. Dig Surg. 2010;27:24–31. doi: 10.1159/000268404. [DOI] [PubMed] [Google Scholar]
- e35.Karhunen PJ. Benign hepatic tumours and tumour-like conditions in men. J Clin Pathol. 1986;39:183–188. doi: 10.1136/jcp.39.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e36.Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular no-dules. Hepatology. 1990;11:787–797. doi: 10.1002/hep.1840110512. [DOI] [PubMed] [Google Scholar]
- e37.Scalori A, Tavani A, Gallus S, La Vecchia C, Colombo M. Oral contraceptives and the risk of focal nodular hyperplasia of the liver: a case-control study. Am J Obstet Gynecol. 2002;186:195–197. doi: 10.1067/mob.2002.120277. [DOI] [PubMed] [Google Scholar]
- e38.Mathieu D, Kobeiter H, Cherqui D, Rahmoundi A, Dhumeaux D. Vol. 352. Lancet: 1998. Oral contraceptive intake in women with focal nodular hyperplasia of the liver; pp. 1679–1680. [DOI] [PubMed] [Google Scholar]
- e39.Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85–89. doi: 10.1136/gut.2010.222109. [DOI] [PubMed] [Google Scholar]
- e40.Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644–648. [PubMed] [Google Scholar]
- e41.Heinemann LA, Weimann A, Gerken G, Thiel C, Schlaud M, DoMinh T. Modern oral contraceptive use and benign liver tumors: the German Benign Liver Tumor Case-Control Study. Eur J Contracept Reprod Health Care. 1998;3:194–200. doi: 10.3109/13625189809167253. [DOI] [PubMed] [Google Scholar]
- e42.Wingo PA, Austin H, Marchbanks PA, et al. Oral contraceptives and the risk of death from breast cancer. Obstet Gynecol. 2007;110:793–800. doi: 10.1097/01.AOG.0000284446.22251.6e. [DOI] [PubMed] [Google Scholar]
- e43.Hankinson SE, Colditz GA, Manson JE, et al. A prospective study of oral contraceptive use and risk of breast cancer (Nurses’ Health Study, United States) Cancer Causes Control. 1997;8:65–72. doi: 10.1023/a:1018435205695. [DOI] [PubMed] [Google Scholar]
- e44.Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025–2032. doi: 10.1056/NEJMoa013202. [DOI] [PubMed] [Google Scholar]
- e45.Kumle M, Weiderpass E, Braaten T, Persson I, Adami HO, Lund E. Use of oral contraceptives and breast cancer risk: The Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Cancer Epidemiol Biomarkers Prev. 2002;11:1375–1381. [PubMed] [Google Scholar]
- e46.Kahlenborn C, Modugno F, Potter DM, Severs WB. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc. 2006;81:1290–1302. doi: 10.4065/81.10.1290. [DOI] [PubMed] [Google Scholar]
- e47.Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta–analysis. Eur J Cancer. 2010;46:2275–2284. doi: 10.1016/j.ejca.2010.04.018. [DOI] [PubMed] [Google Scholar]
- e48.Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372–380. doi: 10.1016/j.contraception.2009.04.010. [DOI] [PubMed] [Google Scholar]
- e49.McNaught J, Reid RL, et al. SOGC/GOC Joint Ad Hoc Committee on Breast Cancer. Progesterone-only and non-hormonal contraception in the breast cancer survivor: Joint review and committee opinion of the Society of Obstetricians and Gynaecologists of Canada and the Society of Gynecologic Oncologists of Canada. J Obstet Gynaecol Can. 2006;28:616–639. doi: 10.1016/S1701-2163(16)32195-8. [DOI] [PubMed] [Google Scholar]
- e50.La Vecchia C, Bosetti C. Benefits and risks of oral contraceptives on cancer. Eur J Cancer Prev. 2004;13:467–470. doi: 10.1097/00008469-200412000-00001. [DOI] [PubMed] [Google Scholar]
- e51.La Vecchia C. Oral contraceptives and ovarian cancer: an update, 1998-2004. Eur J Cancer Prev. 2006;15:117–124. doi: 10.1097/01.cej.0000179274.24200.9d. [DOI] [PubMed] [Google Scholar]
- e52.International Agency for Research on Cancer (IARC) Monographs on the Evaluations of Carcinogenic risks to humans. Combined estrogen-progestogen contraceptives and combined estrogen-progestin menopausal therapy. http://monographs.iarc.fr/ENG/Monographs/PDFs/index.php. 2007;Vol 91 [PMC free article] [PubMed] [Google Scholar]
- e53.Moreno V, Bosch FX, Munoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- e54.Bosetti C, Bravi F, Negri E, La Vecchia C. Oral contraceptives and colorectal cancer risk: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:489–498. doi: 10.1093/humupd/dmp017. [DOI] [PubMed] [Google Scholar]
- e55.Fernandez E, La Vecchia C, Balducci A, Chatenoud L, Franceschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722–727. doi: 10.1054/bjoc.2000.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e56.Maheshwari S, Sarraj A, Kramer J, El-Serag HB. Oral contracep-tion and the risk of hepatocellular carcinoma. J Hepatol. 2007;47:506–513. doi: 10.1016/j.jhep.2007.03.015. [DOI] [PubMed] [Google Scholar]
- e57.Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet. 2003;362:185–191. doi: 10.1016/S0140-6736(03)13907-4. [DOI] [PubMed] [Google Scholar]
- e58.Meinhold CL, Berrington de Gonzales A, Bowman ED, et al. Reproductive and hormonal factors and the risk of nonsmall cell lung cancer. Int J Cancer. 2011;128:1404–1413. doi: 10.1002/ijc.25434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e59.Burkman RT, Collins JA, Shulman LP, Williams JK. Current perspectives on oral contraceptive use. Am J Obstet Gynecol. 2001;185:4–12. doi: 10.1067/mob.2001.117416. [DOI] [PubMed] [Google Scholar]
- e60.Petitti DB. Combination estrogen-progestin oral contraceptives. N Engl J Med. 2003;349:1443–1450. doi: 10.1056/NEJMcp030751. [DOI] [PubMed] [Google Scholar]
- e61.ESHRE Capri Workshop Group. Noncontraceptive health benefits of combined oral contraception. Hum Reprod Update. 2005;11:513–525. doi: 10.1093/humupd/dmi019. [DOI] [PubMed] [Google Scholar]
- e62.Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol. 1996;87:55–58. doi: 10.1016/0029-7844(95)00360-6. [DOI] [PubMed] [Google Scholar]
- e63.Milsom I, Lete I, Bjertnaes A, et al. Effects on cycle control and body weight of the combined contraceptive ring, NuvaRing, versus an oral contraceptive containing 30 µg ethinyl estradiol and 3 mg drospirenone. Hum Reprod. 2006;21:2304–2311. doi: 10.1093/humrep/del162. [DOI] [PubMed] [Google Scholar]
- e64.Hendrix SL, Alexander NJ. Primary dysmenorrhea treatment with a desogestrel-containing low-dose oral contraceptive -Contraception. 2002;66:393–399. doi: 10.1016/s0010-7824(02)00414-6. [DOI] [PubMed] [Google Scholar]
- e65.Winkler UH, Ferguson H, Mulders JA. Cycle control, quality of life, and acne with two low-dose oral contraceptives containing 20 microg ethinylestradiol. Contraception. 2004;69:469–476. doi: 10.1016/j.contraception.2003.12.017. [DOI] [PubMed] [Google Scholar]
- e66.Milsom I, Sundell G, Andersch B. The influence of different combined oral contraceptives on the prevalence and severity of dysmenorrhea. Contraception. 1990;42:497–506. doi: 10.1016/0010-7824(90)90078-a. [DOI] [PubMed] [Google Scholar]
- e67.Hohmann H, Creinin MD. The contraceptive implant. Clin Obstet Gynecol. 2007;50:907–917. doi: 10.1097/GRF.0b013e318159c2f6. [DOI] [PubMed] [Google Scholar]
- e68.Funk S, Miller MM, Mishell DR, Jr, et al. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing -etonogestrel. Contraception. 2005;71:319–326. doi: 10.1016/j.contraception.2004.11.007. [DOI] [PubMed] [Google Scholar]
- e69.Croxatto HB. Clinical profile of Implanon: a single-rod etonogestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000;5(Suppl 2):21–28. [PubMed] [Google Scholar]
- e70.Varma R, Sinha D, Grupta JK. Non-contraceptive uses of levonorgestrel-releasing hormone-system (LNG-IUS)—a systematic enquiry and overview. Eur J Obstet Gynecol Reprod Biol. 2006;125:9–28. doi: 10.1016/j.ejogrb.2005.10.029. [DOI] [PubMed] [Google Scholar]
- e71.Rodriguez MI, Darney PD. Non-contraceptive applications of the levonorgestrel intrauterine system. Int J Womens Health. 2010;2:63–68. doi: 10.2147/ijwh.s6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e72.Vercellini P, Somigliana E, Viganò P, Abbiati A, Barbara G, Crosignani PG. Endometriosis: current therapies and new pharmacological developments. Drugs. 2009;69:649–675. doi: 10.2165/00003495-200969060-00002. [DOI] [PubMed] [Google Scholar]
- e73.Maia HJ, Casoy J. Non-contraceptive health benefits of oral contraceptives. Eur J Contracept Reprod Health Care. 2008;13:17–24. doi: 10.1080/13625180701712745. [DOI] [PubMed] [Google Scholar]
- e74.Deutsche Gesellschaft für Gynäkologie und Geburtshilfe e. V. (DGGG) Aktuelle Leitlinien, Diagnostik und Therapie der Endometriose (S1) http://www.dggg.de/leitlinien/ 2010 [Google Scholar]
- e75.Parazzini F, Negri E, La Vecchia C, Fedele L, Rabaiotti M, Luchini L. Oral contraceptive use and risk of uterine fibroids. Obstet Gynecol. 1992;79:430–433. doi: 10.1097/00006250-199203000-00021. [DOI] [PubMed] [Google Scholar]
- e76.Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk association with oral contraceptives. Br Med J (Clin Res Ed) 1986;293:359–362. doi: 10.1136/bmj.293.6543.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e77.Friedman AJ, Thomas PP. Does low-dose combination oral contraceptive use affect uterine size or menstrual flow in premenopausal women with leiomyomas? Obstet Gynecol. 1995;85:631–635. doi: 10.1016/0029-7844(95)00007-E. [DOI] [PubMed] [Google Scholar]
- e78.Chiaffarino F, Parazzini F, La Vecchia C, Marsico S, Surace M, Ricci E. Use of oral contraceptives and uterine fibroids: results from a case-control study. Br J Obstet Gynaecol. 1999;106:857–860. doi: 10.1111/j.1471-0528.1999.tb08409.x. [DOI] [PubMed] [Google Scholar]
- e79.Wise LA, Palmer JR, Harlow BL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159:113–123. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e80.Marshall LM, Spiegelman D, Goldman MB, et al. A prospective study of reproductive factors and oral contraceptive use in rela-tion to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–439. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]
- e81.Grady-Weliky TA. Premenstrual dysphoric disorder. N Engl J Med. 2003;348:433–438. doi: 10.1056/NEJMcp012067. [DOI] [PubMed] [Google Scholar]
- e82.Coffee AL, Kuehl TJ, Willis S, Sulak PJ. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311–1319. doi: 10.1016/j.ajog.2006.05.012. [DOI] [PubMed] [Google Scholar]
- e83.Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson-Landers C, Rapkin A. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol. 2005;106:492–501. doi: 10.1097/01.AOG.0000175834.77215.2e. [DOI] [PubMed] [Google Scholar]
- e84.Faculty of Family Planning and Reproductive Health Care FFPRHC Guidance (January 2005) contraception for women aged over 40 years. J Fam Plann Reprod Health Care. 2005;31:51–63. doi: 10.1783/0000000052973086. [DOI] [PubMed] [Google Scholar]
- e85.Gebbie AE, Hardman SM. Contraception in the perimenopause - old and new. Menopause Int. 2010;16:33–37. doi: 10.1258/mi.2010.010013. [DOI] [PubMed] [Google Scholar]
- e86.Wiegratz I, Mittmann K, Dietrich H, Zimmermann T, Kuhl H. Fertility after discontinuation of treatment with an oral contraceptive containing 30 mcg of ethinyl estradiol and 2 mg of dienogest. Fertil Steril. 2006;85:1812–1819. doi: 10.1016/j.fertnstert.2005.11.052. [DOI] [PubMed] [Google Scholar]
- e87.Ahrendt HJ, Makalova D, Parke S, Mellinger U, Mansour D. -Bleeding pattern and cycle control with an estradiol-based oral contraceptive: a seven-cycle, randomized comparative trial of -estradiol valerate/dienogest and ethinylestradiol/levonorgestrel. Contraception. 2009;80:436–444. doi: 10.1016/j.contraception.2009.03.018. [DOI] [PubMed] [Google Scholar]
- e88.Palacios S, Wildt L, Parke S, Machlitt A, Römer T, Bitzer J. Efficacy and safety of a novel oral contraceptive based on oestradiol (oestradiol valerate/dienogest): a phase III trial. Eur J Obstet Gynecol Reprod Biol. 2010;149:57–62. doi: 10.1016/j.ejogrb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- e89.Endrikat J, Parke S, Trummer D, Schmidt W, Duijkers I, Klipping C. Ovulation inhibition with four variations of a four-phasic estradiol valerate/dienogest combined oral contraceptive: results of two prospective, randomized, open-label studies. Contraception. 2008;78:218–225. doi: 10.1016/j.contraception.2008.05.004. [DOI] [PubMed] [Google Scholar]
- e90.Bundeszentrale für gesundheitliche Aufklärung (BZGA) Verhütungsverhalten Erwachsener. Ergebnisse der Repräsentativbefragung. http//www.bzga.de/botmed_13317100.html. 2007.
- e91.European network of population-based registries for the epidemiologic surveillance of congenital anomalies (EUROCAT) http://www.eurocat-network.eu/; Prevalence Tables Daten von. 2005-2009.
- e92.Rißmann A, Hoyer-Schuschke J, Köhn A, Vogt C, Götz D. Jahresbericht des Bundeslandes Sachsen-Anhalt zur Häufigkeit von congenitalen Fehlbildungen und Anomalien sowie genetisch bedingten Erkrankungen. www.angeborene-fehlbildungen.com. 2009 [Google Scholar]
- e93.Taylor T, Farkouh R, Graham J, et al. Potential impact of using folate-fortified oral contraceptives on risk reduction in neural tube defects in the United States and Germany. Eur J Contracept Reprod Health Care. 2010;15(Suppl.1):128–129. [Google Scholar]
- e94.Piaggio G, von Hertzen H Grimes, DA Van Look, PF on behalf of the Task Force on Postovulatory Methods of Fertility Regulation: Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353 doi: 10.1016/s0140-6736(98)05718-3. [DOI] [PubMed] [Google Scholar]
- e95.Von Hertzen H, Piaggio G, Van Look PF. Emergency contraception with levonorgestrel or the Yuzpe regimen. Task Force on Postovulatory Methods of Fertility Regulation. Lancet. 1998;352 doi: 10.1016/s0140-6736(05)60440-0. [DOI] [PubMed] [Google Scholar]
- e96.Von Hertzen HG, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803–1810. doi: 10.1016/S0140-6736(02)11767-3. [DOI] [PubMed] [Google Scholar]
- e97.Ho PC, Kwan MS. A prospective randomized comparison of levonorgestrel with the Yuzpe regimen in post-coital contraception. Hum Reprod. 1993;8:389–392. doi: 10.1093/oxfordjournals.humrep.a138057. [DOI] [PubMed] [Google Scholar]
- e98.Lalitkumar PG, Lalitkumar S, Meng CX, et al. Mifepristone, but not levonorgestrel, inhibits human blastocyst attachment to an in vitro endometrial three-dimensional cell culture model. Hum -Reprod. 2007;22:3031–3037. doi: 10.1093/humrep/dem297. [DOI] [PubMed] [Google Scholar]
- e99.Meng CX, Andersson KL, Bentin-Ley U, Gemzell-Danielsson K, Lalitkumar PG. Effect of levonorgestrel and mifepristone on endometrial receptivity markers in a three-dimensional human cell culture model. Fertil Steril. 2009;91:256–264. doi: 10.1016/j.fertnstert.2007.11.007. [DOI] [PubMed] [Google Scholar]
- e100.Zhang L, Chen J, Wang Y, Ren F, Yu W, Cheng L. Pregnancy outcome after levonorgestrel-only emergency contraception failure: a prospective cohort study. Hum Reprod. 2009;24:1605–1611. doi: 10.1093/humrep/dep076. [DOI] [PubMed] [Google Scholar]
- e101.Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108:1089–1097. doi: 10.1097/01.AOG.0000239440.02284.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e102.Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555–562. doi: 10.1016/S0140-6736(10)60101-8. [DOI] [PubMed] [Google Scholar]
- e103.Cheng L, Gülmezoglu AM, Piaggio G, Ezcurra E, Van Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;2 doi: 10.1002/14651858.CD001324.pub3. CD001324. [DOI] [PubMed] [Google Scholar]
- e104.Bastianelli C, Farris M, Benagiano G. Emergency contraception: a review. Eur J Contracept Reprod Health Care. 2008;13:9–16. doi: 10.1080/13625180701781096. [DOI] [PubMed] [Google Scholar]