Abstract
Immunoglobulin heavy chain (IgH) genes are formed, tested, and modified to yield diverse, specific, and high affinity antibody responses to antigen. The processes involved must be regulated, however, to avoid unintended damage to chromosomes. The 3′ regulatory region of the Igh locus plays a major role in regulating class-switch recombination (CSR), the process by which antibody effector functions are modified during an immune response. Loss of all known enhancer-like elements in this region dramatically impairs CSR, but individual element deletions have no effect on this process. In the present study, we explored the hypothesis that an underlying functional redundancy in the homologous elements hs3a and hs3b was masking the importance of either element to CSR. Several transgenic mouse lines were generated, each carrying a bacterial artificial chromosome transgene that mimicked Igh locus structure but in which hs3a was missing and hs3b was flanked by loxP sites. Matings to Cyclization Recombination Enzyme-expressing mice established “pairs” of lines that differed only in the presence or absence of hs3b. Remarkably, CSR remained robust in the absence of both hs3a and hs3b, suggesting that the remaining two elements of the 3′ regulatory region, hs1.2 and hs4, although individually dispensable for CSR, are, together, sufficient to support CSR.
Keywords: Antibodies, DNA Recombination, Gene Expression, Gene Regulation, Immunology, Lymphocyte, Transcription Enhancers, Class-switch Recombination, Immunoglobulin Genes, Transgenic Mice
Introduction
The immunoglobulin heavy and light chain (IgH and IgL) loci undergo multiple rounds of DNA recombination and somatic hypermutation as B lymphocytes form and then perfect their receptors for antigen. The process of heavy chain class-switch recombination (CSR)3 takes place upon antigen stimulation of the B cell and allows for the development of daughter clones that can produce antigen-reactive antibody with the appropriate properties to combat the pathogen in question (e.g. ability to traffic to appropriate sites in the body; ability to directly destroy the organism through complement-mediated lysis; etc.).
Although activation-induced cytidine deaminase has been identified as an essential protein, central to both CSR and the somatic hypermutation of variable-region encoding genes (1), the means by which activation-induced cytidine deaminase activity is recruited to the appropriate DNA targets within the Ig loci remains largely unknown. Several studies have shown that the 3′ regulatory region (3′RR) plays an important role in class-switch recombination, implicating it in this recruitment process (2–5). The 3′RR is a series of DNase hypersensitivity sites, initially identified as enhancer-like elements, that map downstream of the Ig heavy chain constant region genes (6–11) (see Fig. 1A). Analogous elements have been described downstream of each of the two Cα genes in the human Igh locus (12, 13), arguing that their function is evolutionarily conserved.
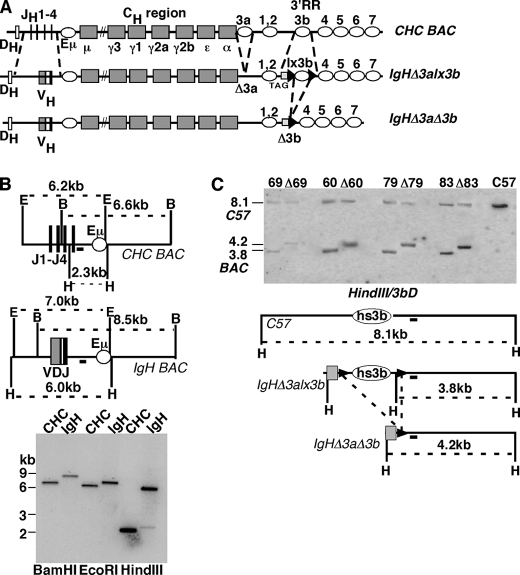
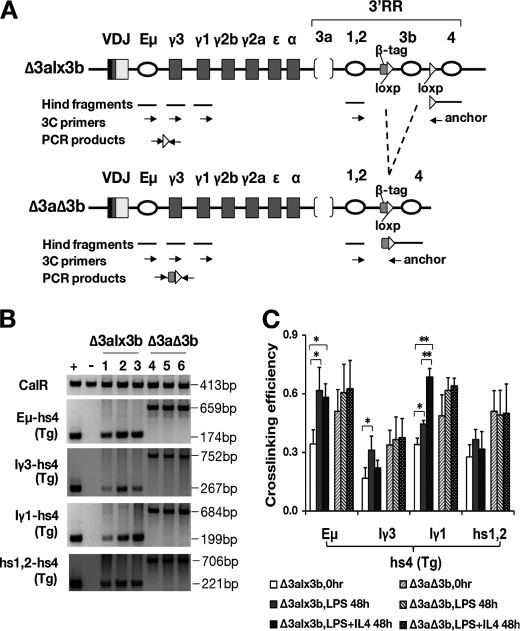
FIGURE 1.
Bacterial artificial chromosome construction and transgenesis. A, diagram of the IgHΔ3alx3b BAC before and after hs3b deletion. Upper map, CHC BAC, original BAC containing ∼230 kb derived from the Igh locus of the 129 mouse strain. DH, DHQ52 gene segment proximal to the JH region (JH1–4). Gray boxes denote the heavy chain constant region (CH) coding segments (μ, γ3, etc.); open ovals denote regulatory regions Eμ and 3′ regulatory region (3′RR). 3′RR consists of seven DNase I hypersensitivity regions (3a, 1,2, etc.) Maps not drawn to scale. Center map, IgHΔ3alx3b (JH gene segments replaced with B1–8 VH gene; hs3a deleted; black triangles denote loxP sites surrounding hs3b; 397-bp DNA tag (TAG)). Lower map, IgHΔ3aΔ3b (loxP-mediated deletion of hs3b). B, restriction enzyme maps and Southern blots of CHC and IgHΔ3alx3b BACs in the region of VH insertion. Maps are above, and Southern blot is below. J1-J4, JH gene region; open oval, Eμ regulatory region; VDJ, inserted VH region; IgH BAC, IgHΔ3alx3b. E = EcoRI, B = BamHI, H = HindIII. Black rectangle under each map and downstream of JH region denotes DNA probe used for Southern blot below. Southern blot is of CHC and IgHΔ3alx3b (IgH) BAC DNAs. Restriction enzymes are indicated below each pair of lanes. Molecular weight markers (in kb) are indicated to the left. Expected fragment sizes are: BamHI (6.6-kb CHC, 8.5-kb IgH), EcoRI (6.2-kb CHC, 7.0-kb IgH), HindIII (2.3-kb CHC, 6.0-kb IgH). C, BAC structure (hs3b region) in transgenic mice. Southern blot is above, and maps are below. Liver DNAs were digested with HindIII (H) and hybridized to the 3bD probe (black box below maps). C57BL/6J DNA was included as a control for genetic background (endogenous Igh loci of transgenic mice). 69, 60, 79, and 83 denote four transgenic lines prior to hs3b deletion; Δ69, etc. denote matched lines after hs3b deletion. Expected HindIII fragment sizes are: 8.1 kb (C57-derived hs3b fragment); 3.8 kb (from BAC, before hs3b deletion); 4.2 kb (from BAC, after hs3b deletion). In the maps, H = HindIII. Gray box denotes tag DNA; black arrowheads denote loxP sequences.
Class-switch recombination is preceded by transcription from “intronic” promoters that lie upstream of the constant region genes. These transcripts are termed “germline” (GLT) as they are generated prior to the DNA rearrangement between S (switch) regions that juxtaposes an assembled VH with a new CH (e.g. between Sμ and Sγ2a to place the VH upstream of Cγ2a). Because of G/C-rich sequences in S regions, RNA transcripts remain associated with the template strand, displacing the G-rich, non-template strand (R loop formation), thereby providing a single-stranded DNA substrate for activation-induced cytidine deaminase (14, 15). T lymphocytes “instruct” B cells to switch to particular heavy chain isotypes through the secretion of cytokines that, in turn, signal the activation of particular intronic promoters and the production of germline transcripts (e.g. IL-4 prompts γ1 and ϵ GLTs and a switch to IgG1 or IgE) (reviewed in Ref. 16).
The first indication that the 3′RR played a role in class-switch recombination arose from an analysis of mice in which the hs1.2 region (see Fig. 1A, 1,2) was replaced with a selectable marker gene (neoR). In B cells carrying this modified Igh locus, germline transcripts from the γ2a, γ2b, γ3, and ϵ constant regions genes were reduced or undetectable, and class-switch recombination to these isotypes was significantly impaired (17). Although this defect turned out to be due to marker gene insertion and not to hs1.2 deletion (18), subsequent work supported the underlying suggestion that the 3′RR was important to the CSR process and that perturbation of the 3′RR interrupted proper functioning of that process. Most notably, deletion of the pair of elements hs3b and hs4 (Fig. 1A, 3b and 4), had a profound effect on CSR to all classes except IgG1, and deletion of the 28 kb spanning hs3a, hs1.2, hs3b, and hs4 dramatically reduced CSR to IgG1 as well (2–5). Chromosome conformation capture techniques have revealed a cytokine-induced, tripartite interaction involving the Igμ transcription unit, the relevant intronic promoter for germline transcripts, and the 3′RR, providing physical evidence for the role of 3′RR in the class-switch recombination process (19).
In the present study, we have sought to extend analysis of the 3′RR to more precisely determine which elements are expendable for CSR and, reciprocally, which are sufficient to maintain it. Others have recently shown that although deletion of both hs3b and hs4 profoundly reduces CSR to most IgH classes, either element deleted individually has no such effect (20, 21). Because hs3b is an inverse replicate of hs3a, however, we thought it possible that the importance of these two elements to CSR was masked in single-element deletions because of an underlying functional redundancy. Notably, there is only one hs3-like element in the human 3′RR.
To address this hypothesis, we generated several independent transgenic mouse lines carrying a large segment of the Igh locus as transgene. In all the mouse lines, the Igh locus transgene lacked hs3a, which was shown previously to be individually dispensable for CSR (18). The Igh locus transgene also carried a loxP-flanked version of hs3b so that each mouse line could also generate a paired “sister” line in which both hs3a and hs3b were missing. Remarkably, we found that class-switch recombination took place efficiently within the bacterial artificial chromosome (BAC), whether or not the second hs3 element (hs3b) was present. The remaining DNA sequences, which included the 3′RR elements hs1.2 and hs4, were sufficient for robust class-switch recombination, although hs1.2 and hs4 are each individually dispensable for CSR (18, 21). These findings demonstrate a remarkable flexibility in the structure/composition of the 3′RR capable of supporting class-switch recombination. Moreover, as discussed in more detail below, these findings point toward a new approach for further unraveling the mode of action of the 3′RR in this important process.
EXPERIMENTAL PROCEDURES
IgHΔ3alx3b BAC Construction
A BAC that carried the immunoglobulin heavy chain constant region genes of the murine Igh locus (129 mouse strain) was modified to create a functional IgH gene, delete the 3′RR sequence hs3a, and flank the 3′RR sequence hs3b with loxP sequences. The BAC was designed so that after loxP-mediated deletion of hs3b, it would lack the regions of homology shared by hs3a and hs3b (nt 1192–2154 and nt 24019–24989, respectively; GenBankTM accession number AF450245). Development of the BAC was achieved through a sequence of homologous recombination events in bacterial cells (described below). Primer sequences used to clone specific fragments of DNA are provided in the supplemental data.
CHC BAC
CHC BAC is a BAC containing ∼230 kb of DNA from the murine Igh locus. It begins 11 kb 5′ of the JH cluster and extends 35 kb 3′ of hs4.
VH Insertion
A 2.2-kb DNA segment containing the assembled B1–8 VH gene was cloned from the plasmid pIVHB1–8L2neor (22). The encoded VH corresponds to that found in the (4-hydroxy-3-nitrophenyl) acetyl-binding antibody B1–8. The B1–8 VH gene used in the current studies carries a silent point mutation (codon 92) that inactivates the internal recombination signal sequence, eliminating the possibility of VH replacement (22).
The B1–8 VH gene fragment replaced the JH gene region (inserted between nt 1000 and 2350, GenBank accession number J00440). Upstream and downstream homology sequences were joined to the 2.2-kb fragment containing B1–8 VH using a PCR strategy (23) and inserted into the pSV1-RecA shuttle vector for homologous recombination with CHC BAC (methods of Yang et al. (24)). The resulting BAC, which lacks the JH region, was designated VDJCμ.
hs3a Deletion
hs3a was deleted by homologous recombination, removing nt 1054–2171 (GenBank accession number AF450245). The resulting BAC was VDJCμΔ3a (which carries the B1–8 VH and is missing hs3a).
hs3b Deletion
hs3b was deleted first and then reinserted with loxP sites and a DNA “tag.” Deletion included nt 23601–25205, GenBank accession number AF450245. The resulting BAC, VDJCμΔ3aΔ3b BAC, carries the B1–8 VH and is missing both hs3a (1.1-kb deletion, 1054–2171, GenBank accession number AF450245) and hs3b (1.6-kb deletion, nt 23601–25205, GenBank accession number AF450245). These deletions fully encompass the regions of homology shared by hs3a and hs3b.
hs3b Reinsertion
A 1.2-kb XbaI fragment of hs3b (24023–25205, GenBank accession number AF450245) was flanked by two loxP sites derived from ploxP2neor (25), and a 397-bp segment of human β-globin cDNA was added upstream (nt 1–397, GenBank accession number V00497). The β-globin tag sequence was PCR-amplified from pCR2-βs plasmid, a gift from Dr. Karl Drlica (Public Health Research Institute, New York, NY). After homologous recombination, the hs3b region that had been deleted (1.6 kb) in VDJCμΔ3aΔ3b BAC was replaced with a “tagged” and loxP-flanked hs3b sequence (∼2.3 kb), generating the BAC used for transgenesis, IgHΔ3alx3b (see Fig. 1A).
BAC constructs were analyzed at each stage of the modification process, both for the presence of the expected changes and for the absence of any other, unwanted modifications. One method used to screen for unwanted modifications was digestion with BamHI, EcoRI, and HindIII, respectively, and comparisons of the ethidium bromide-stained fragment profiles (data not shown). Expected changes induced by homologous recombination were confirmed by Southern blot (see “Results” and supplemental Fig. 1).
Generation of Transgenic Mouse Lines
IgHΔ3alx3b BAC was digested with NotI, and the insert was purified before transfer to the Mouse Genetics Core Facility, Memorial Sloan Kettering Cancer Center (New York, NY) for microinjection. Transgenic founders were identified in PCR screens, using primer pairs specific for B1–8 VH and for loxP-flanked hs3b (lx3b), respectively (primer sequences are provided in the supplemental data). Animals were bred and maintained in animal facilities at Hunter College (Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited), City University of New York, and all mouse experiments were approved by the Hunter College Institutional Animal Care and Use Committee.
Developing Matched Mouse Lines with (Δ3alx3b) and without (Δ3aΔ3b) hs3b
Transgenic mouse lines 60, 69, 79, and 83 were mated to EIIa-cre mice (C57BL/6J background) (26) to induce deletion of hs3b. Each pair of lines (eight total mouse lines) was later bred to the IghJtm1Cgn/J mouse line, which lacks the JH gene region, and Eμ (The Jackson Laboratory, stock number 2438, recovered from cryopreservation) (27). PCR screens for hs3b deletion used primers 5′-pβ: CAACGTGCTGGTCTGTGTGCTG and 3′-hs3bD: CAGTCGACCCACATAATATCCTATTGACC (sequence downstream of hs3b, underlined nucleotides = nt 25465–25445, GenBank accession number AF450245). Before loxP-mediated deletion of hs3b, the primers were >2.3 kb apart; after deletion, they generated a 385-bp product (data not shown).
For genomic Southern screens for hs3b deletion, DNA was digested with BamHI and hybridized to hs3b (1.2-kb XbaI fragment from HS3.4 plasmid) (10). A 0.9-kb BamHI fragment was lost upon hs3b deletion. Genomic DNA was digested with HindIII and hybridized to hs3bD, a sequence that lies downstream of hs3b. A HindIII fragment detected by hs3bD increased from 3.8 to 4.2 kb upon hs3b deletion.
Checking BAC Integrity in Transgenic Mouse Lines
Methods for checking the integrity of the VH region and hs3b region were described above (“Generation of Transgenic Mouse Lines”). For other regions (Sγ2a, Sγ2b, hs4, hs1.2, Cϵ), the strategy of Dunnick et al. (28) was followed (supplemental Fig. 3 and data not shown). The presence of functional constant region genes was further confirmed by expression (e.g. Igμa in mice with Ighb genetic background) and/or presence of allele-specific germline transcripts (see below). DNA probes used in supplemental Fig. 3 are as follows: Sγ2b, 5.2-kb XbaI fragment from pBR1.4 plasmid (25, 29); Hs4, 2.4-kb PstI/HindIII fragment from HS3.4 plasmid (10).
Southern Blot Analyses
Southern blot analyses were performed as described previously, using ∼10–30 μg of mouse tail-tip DNA or liver DNA (30).
Enzyme-linked Immunosorbent Assays (ELISA)
Mouse sera were assayed for immunoglobulin isotype levels, using a sandwich ELISA and standard methods. Most antisera were supplied by BD Biosciences (see supplemental data for a list of all antisera and monoclonal antibodies used).
All sera were diluted in duplicate, and each dilution was analyzed in duplicate (four tests/serum sample). Individual readings generally deviated less than 10% from the mean. Standard curves for each assay were generated with purified mouse immunoglobulin of the relevant class. Plates were read at wavelength 405 nm, using a PowerWave HT microplate spectrophotometer (BioTek, Winooski, VT), and data were analyzed by the software Gen5 (BioTek). Statistical tests for serum concentration differences among the mouse lines were performed with the GraphPad Prism® software (version 5.0c).
In Vitro Stimulation of Splenic B Cells
Resting splenic B cells were enriched by negative selection (antibody to CD43), using a B cell isolation kit (catalog number 130-090-862; Miltenyi Biotec, Bergisch Gladbach, Germany). Experiments were performed in parallel on cells isolated from WT, IgHΔ3alx3b, and IgHΔ3aΔ3b transgenic mice. ∼3 × 106 B cells were cultured in each of the following conditions to induce class-switching to specific isotypes.
IgG3/IgG2b
RPMI 1640 medium supplemented with 20% fetal calf serum (FCS), 50 μm β-mercaptoethanol, and 25 μg/ml LPS (Sigma-Aldrich, catalog number L6511) was used. Cells harvested and analyzed on day 4.
IgG1/IgE
Conditions were as above but with the addition of IL-4 (10 ng/ml) (PeproTech, catalog number 214-14). Cells were harvested and analyzed on day 4.
IgG2a
Conditions were as for IgG3/IgG2b but with the addition of IFNγ (100 ng/ml) (PeproTech catalog number 315-05) and BAFF (10 ng/ml) (PeproTech catalog number 310-13). Cells were harvested and analyzed on day 5.
IgA
Conditions were as for IgG1/IgE but with the addition of TGFβ (1 ng/ml) (PeproTech catalog number 100-21), IL-5 (1.5 ng/ml) (PeproTech catalog number 215-15), and anti-δ(IgD)-dextran (3 ng/ml) (Fina Biosolutions LLC). These conditions are as described (31). Cells were harvested and analyzed on day 5.
Flow Cytometry
Methods were essentially as described previously (32). For expression of IgM, spleen cells were stained with antibodies to B220, IgMa, and/or IgMb. B cells stimulated to undergo class-switch recombination were stained with antibodies for B220 and the relevant IgH class. A list of the reagents used for all flow cytometry experiments is provided in the supplemental data.
In each staining experiment of stimulated B cells, an isotype-matched control antibody (with no specificity for murine cells or Ig) was included. For example, the FITC-conjugated rat IgG2a,κ monoclonal antibody specific for murine IgG3 (BD553403) was matched with a control rat IgG2a,κ monoclonal antibody with irrelevant specificity (BD554688).
Reverse Transcription-Polymerase Chain Reactions (RT-PCRs) to Detect Germline Transcripts
RNAs from stimulated splenic B cells were extracted with an RNeasyTM mini prep kit (Qiagen, Valencia, CA, catalog number 74106), and RT-PCR was performed with a OneStep RT-PCR kit (Qiagen, catalog number 210212). Primers specific for the germline transcripts from individual CH regions were as described (28) and are shown in supplemental Tables 1 and 2. Supplemental Table 1 provides restriction enzymes used to distinguish RT-PCR products from “a” allotype and “b” allotype transcripts, respectively. For IgA, germline transcripts from both alleles were first amplified with consensus primers (Iαf and ALPHMURCH2). The products from this reaction were used as template with primers specific for the Igha allotype (Iαf and ALPHMURCH2A), generating a secondary (or nested) PCR product only when α-germline transcripts from the Igha chromosome were present.
Chromatin Conformation Capture Assays
Methods were as described previously (33). Briefly, isolated B cells were harvested either before culture or after stimulation for 48 h in LPS or in LPS+IL-4. Harvested cells were incubated in formaldehyde to cross-link interacting proteins, nuclei were prepared, and non-cross-linked protein was removed by SDS. Triton X-100 was added to sequester the SDS, after which samples were digested with HindIII. Digested nuclei were subjected to intramolecular ligation and then treated with proteinase K and RNase A, after which DNA was purified. One hundred nanograms of ligated DNA were analyzed by 32 cycles of PCR (94 °C/30 s, 56 °C/30 s, and 72 °C/30 s) in a 25-μl reaction (PCR amplifications were confirmed to be in the linear range). A positive control template consisted of a mixture of the IgHΔ3alx3b BAC with two calreticulin (CalR) fragments in equal molar ratios. The mixture was subjected to HindIII digestion and then DNA ligation and was used to normalize PCR efficiency among the different primer pairs. The negative control template was treated in the same way but consisted of a BAC containing unmodified, endogenous Igh locus sequences mixed with CalR fragments. loxP sequences and a β-globin tag sequence unique to the IgHΔ3alx3b BAC were used to design a chromatin conformation capture primer that specifically tracked the hs4 segment of the BAC transgenic allele (see supplemental Table 3). As expected, this transgene-specific 3′ primer generated PCR products when used with the various 5′ primers (for Eμ, Iγ1, Iγ3, and hs1.2) on the positive control but not on the negative control template (confirming its specificity).
PCR products were size-fractionated by electrophoresis (2% agarose gel), and gel pictures were analyzed by the SYNGENE GeneGenius bioimaging system. Cross-linking efficiency between two HindIII fragments was calculated as described previously (the ratio of the signal for PCR product from the IgH locus/signal for PCR product from CalR in a given cell type, divided by this ratio in the positive control template).
RESULTS
Construction of IgHΔ3alx3b BAC
In a previous study, it was shown that CSR was unimpaired in B cells from mice lacking the 3′RR element hs3a (18). More recently, deletion of hs3b alone was also shown to have no effect on CSR (20). To test the possibility that these nearly identical elements are functionally redundant, we established several, independent mouse lines with a fully functional, but hs3a-deficient, Igh locus transgene (BAC) and then tested whether subsequent loss of hs3b affected locus expression and/or CSR.
A bacterial artificial chromosome containing much of the murine Igh locus (CHC BAC) (28) was first modified to replace the JH region with an assembled VH gene (Fig. 1A). In subsequent steps, hs3a of the 3′ regulatory region was deleted, hs3b was flanked by loxP sites, and a short human DNA sequence (397-bp β-globin cDNA segment) was inserted to uniquely tag this region of the BAC (Fig. 1A, TAG). hs3a deletion corresponded to the nucleotides shared by hs3a and hs3b, and, reciprocally, the hs3b region between loxP sites included all nucleotide sequences shared by these two 3′RR elements. The resulting BAC (IgHΔ3alx3b) extended from a site ∼11 kb upstream of the JH cluster to a site ∼35 kb downstream of hs4. Included in the BAC were DQ52, the DH gene segment most proximal to the JH region, and all of the 3′RR elements thus far identified (hs1–7) (11) (Fig. 1A). Deletion of the JH region precluded DQ52-JH recombination events on the BAC.
BAC modifications involved several sequential rounds of homologous recombination in bacterial cells, using the methods of Yang et al. (24). Southern blots were performed to confirm that the appropriate modifications had occurred. For example, the DNA probe JHD (Fig. 1B, black rectangle downstream of the JH region) revealed a 6.6-kb BamHI band in the original (CHC) BAC, which was replaced with an 8.5-kb BamHI band in IgHΔ3a,lx3b BAC (Fig. 1B). Expected changes in the EcoRI and HindIII fragments detected with this probe further confirmed that the VH insertion had occurred as planned. The modifications involved in deleting hs3a and flanking hs3b with loxP sites and tag DNA were also confirmed by Southern blot analyses of the BACs (supplemental Fig. 1). A broader analysis of BAC DNA, using ethidium bromide-stained gels of enzyme-cleaved BACs, revealed no unexpected changes (data not shown). As described below, several regions along the BAC were further checked for integrity in transgenic mice.
Generation of IgHΔ3alx3b Transgenic Mice
Twenty-two founder mice with apparently intact IgHΔ3alx3b BAC transgenes were recovered, and seven of these (lines 24, 60, 69, 79, 81, 83, and 111) were maintained and mated to C57BL/6J for further study. As will be described below, all seven of the transgenic lines showed robust expression of the IgHΔ3alx3b BAC. Five of the lines (60, 69, 79, 81, and 83) were further analyzed to confirm intact BAC structure in regions mapping from the assembled VH gene through to the loxP-flanked hs3b (supplemental Figs. 2 and 3). Allelic differences between the BAC that carried Igha sequences derived from the 129 mouse strain and the endogenous Ighb loci (C57BL/6-derived) aided in these analyses (3). Genomic Southern blots also provided an estimate of BAC copy number in each of the lines (60, 69, 79, and 83 had 1–3 copies of the BAC, whereas 81 had many) (supplemental Fig. 3 and data not shown).
Allelic Exclusion of the Endogenous (Ighb) Loci in IgHΔ3alx3b Transgenic Mice
Expression of the IgHΔ3alx3b BAC was first analyzed by flow cytometry in transgene-positive mice. Allotype-specific reagents demonstrated that all seven lines analyzed (lines 24, 60, 69, 79, 81, 83, and 111) expressed transgenic Igμa in the spleen but little or no Igμb (Fig. 2A, and data not shown), suggesting that the functional IgHΔ3alx3b BAC was effectively inhibiting assembly and expression of IgH genes within the endogenous Ighb loci.
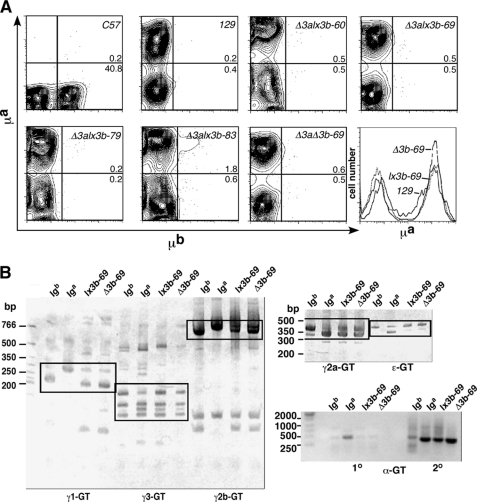
FIGURE 2.
Allelic exclusion and cytokine-induced germline transcription in the absence of both hs3a and hs3b. A, contour plots of spleen cells incubated with anti-μa (y axis) and anti-μb (x axis) antibodies. C57 and 129 spleen cells serve as controls for μb- and μa-expressing cells, respectively. Remaining plots are of spleen cells from mice of the indicated transgenic mouse lines. BAC (Iga) transgenes are on the C57BL/6J (Igb/Igb) background. The Δ3aΔ3b-69 mouse carries the BAC after loxP-mediated deletion of hs3b (compare Δ3alx3b-69 and Δ3aΔ3b-69). Histogram in lower right corner compares mean fluorescence of μa-expressing cells in a 129 mouse (natural Igha locus, indicated by 129), a Δ3alx3b-69 mouse (lx3b-69), and a Δ3aΔ3b-69 mouse (Δ3b-69). B, cytokine-induced GLTs from BAC transgenes, before and after removal of hs3b; RT-PCRs were used to identify GLTs from endogenous loci and BAC transgenes. Heavy chain constant regions are identified below each blot. For each Ig isotype, B cells from four mouse genotypes were compared (equal amounts of RNA as template). Iga denotes strain 129 B cells; Igb = C57BL/6J B cells; BAC (Iga) transgenes are on the C57BL/6J (Igb/Igb) background in both Δ3alx3b-69 (lx3b-69) and Δ3aΔ3b-69 (Δ3b-69) mice. White boxes enclose the areas of allelic difference (strategy from Ref. 3). For Ig α-GLTs, a primary RT-PCR (1°) was followed by a second PCR with allele-specific nested primers (2°); only α-GLTs from the Iga allele generate a strongly amplified product in the second reaction (3).
The level of surface IgM expression on splenic B cells closely matched that seen in normal mice expressing an Igha allele. As shown in the histogram in Fig. 2A, for example, spleen cells in the transgenic line carrying one copy of the BAC (lx3b-69) expressed μa at the same level as spleen cells from a 129P2/Olahsd (129) mouse.
It should be pointed out that the site-independent expression of IgHΔ3alx3b was not dependent upon the introduction of heterologous insulator sequences. In studies by others, the chicken β-globin locus-derived insulator was inserted at the 5′ end of an Igh BAC to assist in generating integration site-independent expression (4). The authors noted, however, that another BAC modification that may have explained better expression of this BAC relative to earlier versions was removal of an upstream DH element, preventing deletion of the assembled VH by DH-JH recombination. As described above, IgHΔ3alx3b was designed to preclude B1–8VH deletion by DH-JH rearrangement as well, suggesting that this, rather than the presence of the β-globin locus-derived insulator, improved expression in the prior study (4). Notably, CTCF-associated insulator sequences lie just downstream of hs4 (11).
Deletion of hs3b within Four Independent Transgenic Mouse Lines
Having confirmed that lines 60, 69, 79, and 83 carried one or more copies of the intact BAC transgene, we bred these lines (each assumed to carry the BAC at a unique integration site) to mice carrying the bacterial cre gene under control of the adenovirus EIIa promoter (expressed in early embryos) (26). In those progeny that inherit both the BAC and the cre gene, the hs3b element within the BAC transgene should undergo loxP-mediated deletion (Fig. 1A, diagram). Progeny were screened for hs3b deletion by PCR (see “Experimental Procedures,” data not shown). hs3b deletion within the BAC was further confirmed by Southern blot (Fig. 1C and supplemental Fig. 2B). As maps of this region show (Fig. 1C), a probe downstream of hs3b (3bD; black rectangle under maps) should anneal to the BAC both before and after hs3b deletion but revealing HindIII fragments of distinguishable sizes (3.8 and 4.2 kb, respectively). An example of such analyses for four independent transgenic lines (60, 69, 79, and 83), before and after hs3b deletion, is shown in Fig. 1C. Using the 8.1-kb HindIII fragment derived from the endogenous (C57BL/6-derived) Igh loci for normalization, we confirmed that BAC copy number was unchanged upon hs3b deletion. After hs3b deletion, the BAC lacks all the sequences shared by hs3a and hs3b (see “Experimental Procedures”). As shown in Fig. 2A, deletion of hs3b in combination with the absence of hs3a did not affect allelic exclusion (see contour plot for Δ3aΔ3b-69), nor did it affect surface IgM levels (compare lx3b-69 and Δ3b-69 in the histogram).
Class-switch Recombination in the Absence of Both hs3a and hs3b
Resting, splenic B cells were isolated from pairs of transgenic mice and assayed for class-switch recombination by flow cytometry (“Experimental Procedures”). As noted above, BAC copy number was not changed upon hs3b deletion, and in these and in all subsequent experiments, transgenic mice were kept hemizygous for the BAC transgene (carrying only one transgene allele) to ensure that matched pairs always carried the same BAC copy number.
Initially, the single-copy transgenic line 69 on the C57BL/6J background was assayed for CSR, and it was found that the percentage of switched cells did not change significantly upon hs3b deletion (representative results, supplemental Fig. 4). Consistent with these flow cytometry data, germline transcripts emanating from each of the BAC heavy chain constant region genes (a necessary precursor to CSR) were detectable in appropriately stimulated B cells, whether or not the BAC retained hs3b (Fig. 2B). Allelic differences permitted distinction between BAC-derived and wild-type Igh loci-derived transcripts (3).
We extended our flow cytometry analyses of CSR in the presence and absence of hs3b after back-crossing all four pairs of transgenic mouse lines to the IghJtm1Cgn/J mouse line (cited here as ΔJH/ΔJH). The ΔJH chromosome lacks all of the JH genes and the intronic enhancer (Eμ), rendering it incapable of VH gene assembly (27). In the BAC transgenic ΔJH/ΔJH mice, therefore, the BAC transgene is the only source of Ig heavy chain and is responsible both for development of B cells and for surface Ig expression in stimulated B cell cultures.
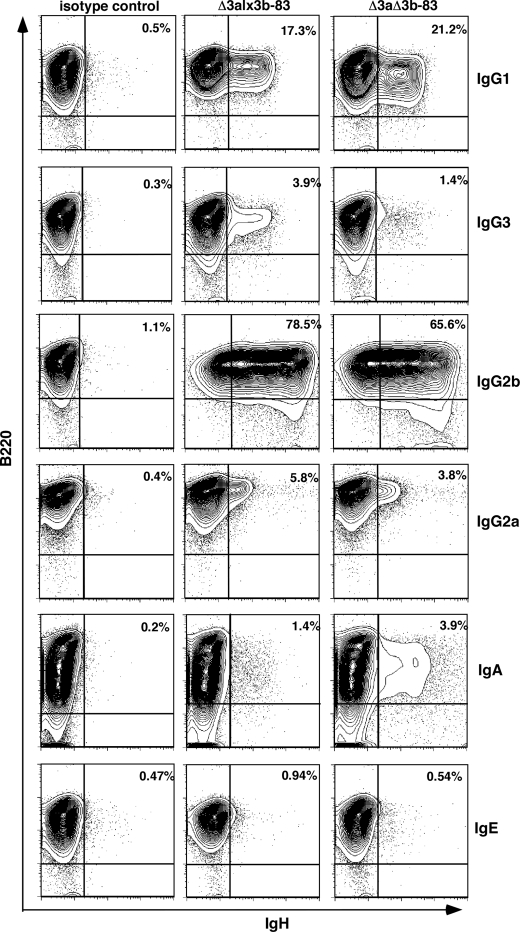
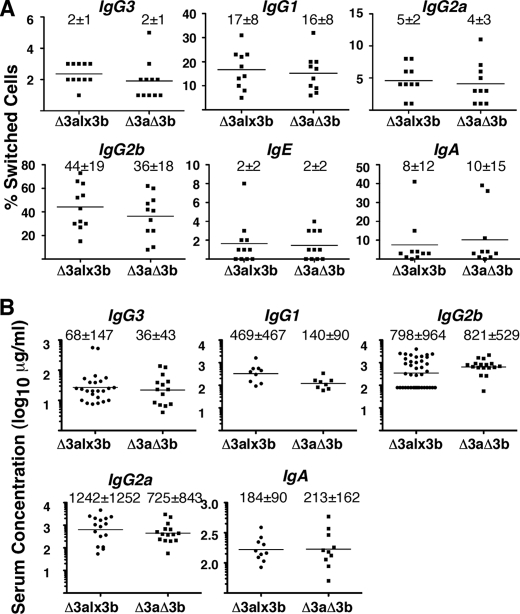
Representative flow cytometry data for CSR-stimulated B cells from the lx3b-83/Δ3b-83 pair on this ΔJH/ΔJH background are shown in Fig. 3. Class-switch recombination was cytokine-dependent and robust in these animals, whether hs3a was the only 3′RR element missing (Δ3alx3b-83) or both hs3a and hs3b were absent (Δ3aΔ3b-83) (Figs. 3 and 4A and supplemental Fig. 5). As summarized in Fig. 4A, this proved true for all of the transgenic mouse lines; with the exception of switching to IgG2b, there was no significant difference in CSR between the matched pairs, regardless of the BAC integration site. In the case of switching to IgG2b, the difference was statistically significant (paired, two-tailed t test; p < 0.02) but small as the percentage of cells switching to this isotype was reduced by only ∼20% when hs3b was removed (means = 44 versus 36).
FIGURE 3.
Class-switch recombination to multiple IgH classes in the absence of the hs3a/hs3b enhancer pair. Contour plots of stimulated B cells, stained with antibody to B220 (y axis) and to the individual IgH classes (x axis; class names indicated to right of plots), are shown. Δ3alx3b-83 and Δ3aΔ3b-83 denote BAC carried on ΔJH/ΔJH genetic background. Left-most plots, control stains with an isotype-matched antibody. Upper right corner of each plot, the percentage of switched cells (B220+, IgH+ cells). Specificity of switching was confirmed by staining LPS-stimulated and LPS+IL-4-stimulated cells for IgG1, IgG2b, and IgG3 (supplemental Fig. 5).
FIGURE 4.
Class-switch recombination and serum Ig levels in mice dependent on the BAC for B cell development and Ig expression. A, graphs comparing in vitro class-switch recombination efficiency before and after hs3b deletion. Plots show the percentage of switched cells obtained in a total of 11 CSR experiments (two or more with each transgenic line). Mice were homozygous for JH region and Eμ deletion on the endogenous Igh loci (ΔJH/ΔJH), preventing IgH gene assembly within these loci. Horizontal lines denote mean; S.D. is provided above each plot. In every case, IgHΔ3alx3b (Δ3alx3b) and IgHΔ3aΔ3b (Δ3aΔ3b) mice were tested in parallel. Data were analyzed by paired, two-tailed t test (GraphPad Prism 5.0). p < 0.05 for IgG2b (20% reduction in the percentage of switched cells in IgHΔ3aΔ3b mice). B, graphs comparing serum Ig levels in mice expressing the IgH BAC with or without hs3b. Plots are of serum levels of IgH isotypes measured in 40 individual IgHΔ3alx3b mice (Δ3a) and 17 individual IgHΔ3aΔ3b mice (Δ3aΔ3b). As in A, BAC transgenes were carried on the ΔJH/ΔJH genetic background. Data were analyzed by two-tailed t test (GraphPad Prism 5.0); no significant differences were found. Mean concentration, with S.D., is provided above each scattered dot plot.
In all these experiments, B cells from wild-type animals (siblings of the BAC transgenic mice) or from mice carrying an endogenous locus knock-in of the same VH gene used in the BACs (VHEμ) (22, 32) were used as controls to ensure that culture conditions were adequate to induce class-switch recombination. The percentage of cells switching in wild-type or VH knock-in animals was similar to that in the BAC transgenic mice (generally differing by no more than 2-fold), indicating that CSR on the BACs was very efficient (data not shown).
Ig Serum Levels Remain the Same, before and after hs3b Deletion
As another measure of class-switch recombination, we compared serum levels of the various Ig heavy chain classes in mice carrying either the IgHΔ3alx3b or the IgHΔ3aΔ3b BAC (on the ΔJH/ΔJH background). As shown in Fig. 4B, the mean concentrations did not differ significantly between these two groups of mice. Paired comparisons of the mice from a single line (e.g. line 60 mice with or without hs3b) were also done, again with no significant difference detected (data not shown). The serum phenotypes of these mice, therefore, are in concordance with the in vitro CSR results; CSR proceeds equivalently in IgHΔ3alx3b (no hs3a) and IgHΔaΔ3b (no hs3a or hs3b) transgenic mice.
Physical Interactions between I Region Promoters and 3′RR Persist in the Absence of hs3a and hs3b
Prior studies have shown that the 3′RR physically associates with the IgH transcription unit in resting B cells and shows a cytokine-enhanced association with the intronic promoters upstream of the various heavy chain constant region genes (19). Given that CSR continued unabated on the IgHΔ3aΔ3b BAC, we looked for evidence of physical association between this truncated 3′RR (retaining only hs1,2 and hs4) and upstream regions involved in CSR. Matched pairs from two independent mouse lines (79 and 83) were analyzed in two sets of experiments. As shown in Fig. 5, the 3′RR of the IgHΔ3alx3b BAC mimicked the behavior of a normal Igh locus: strong association prior to stimulation among the 3′RR elements (hs1.2 and hs4) and between the 3′RR and the IgH transcription unit (Eμ-region) and cytokine-dependent increases in the association of the 3′RR with the intronic promoters of Cγ1 and Cγ3 in an isotype-specific manner (i.e. maximum cross-linking efficiency between Iγ3 and hs4 when stimulated by LPS and between Iγ1 and hs4 when stimulated by LPS+IL-4; Fig. 5, B and C). When hs1,2 and hs4 were the only remaining elements in the 3′RR (IgHΔ3aΔ3b), all of these associations still took place. In fact, cross-linking efficiency among the elements was generally higher in nuclei isolated from these cells, even without cytokine. Interestingly, the associations showed no isotype-specific response to cytokine. As described above in the flow cytometry studies, however, the B cells of IgHΔ3aΔ3b mice continued to show an isotype-specific response to cytokine with respect to class-switch recombination.
FIGURE 5.
Long range interactions between the 3′RR, IgH transcription unit, and CH region promoters. A, maps of BAC transgenes before (Δ3alx3b) and after (Δ3aΔ3b) hs3b deletion. 5′ primers for Eμ, the Iγ3 promoter, Iγ1 promoter, and 3′RR element hs1.2 are indicated as arrows, as is the transgene-specific 3′ primer near hs4 (anchor). VDJ, inserted VH region. 3C, chromatin conformation capture. B, PCR products generated by chromatin conformation capture (see “Experimental Procedures”) with the indicated primer pairs (left of gel). Interactions between fragments within the CalR gene served as a positive control (top panel). + = positive control template (mixture of CalR fragments and the IgHΔ3alx3b BAC); − = negative control template (mixture of CalR fragments and a BAC with sequences equivalent to the wild-type, endogenous Igh loci). Cell source of template is shown above the lanes (Δ3alx3b and Δ3aΔ3b B cells). Lanes 1 and 4 are from B cells at time 0 (resting B cells); lanes 2 and 5 are from B cells 48 h after LPS stimulation, and lanes 3 and 6 are from B cells 48 h after LPS+IL-4 stimulation. C, summary of cross-linking efficiency data. Cross-linking was measured between the hs4 region of the BAC transgene (hs4(Tg) and the indicated regions upstream (see “Experimental Procedures” for details). Two independent CSR experiments were performed using two different paired mouse lines, and PCRs were performed at least twice with each template. The first three bars in each set of six (for each primer pair) are the results with IgHΔ3alx3b B cells; the second three bars are with IgHΔ3aΔ3b B cells (see legend). * = p < 0.05; ** = p < 0.01.
DISCUSSION
In the present study, we tested the hypothesis that an essential role for the hs3a/hs3b elements in class-switch recombination was being masked by their sequence identity and underlying functional redundancy. Deletions were designed to fully encompass the regions of shared identity (95%) between hs3a and hs3b (nt 1192–2154 and 24019–24989, GenBank accession number AF450245). The IgHΔ3alx3b BAC introduced into animals already carried the deletion of hs3a as earlier studies had shown that this deletion had no substantial effect on IgH gene expression or class-switch recombination (18). Consistent with those findings, the IgHΔ3alx3b transgene was expressed efficiently in all of the transgenic lines analyzed. When on the C57BL/6 background (Ighb), the BAC (Igha) was expressed to the exclusion of the endogenous locus and at wild-type levels, as judged by surface IgM expression in splenic B cells (Fig. 2A). When on the ΔJH/ΔJH background, B cell development was normal, demonstrating that heavy chain gene expression from the hs3a-deficient BAC was sufficient to drive this process (data not shown).
Remarkably, these observations were duplicated in mice carrying the BAC that lacked both hs3a and hs3b. Class-switch recombination, as measured in vitro, and serum levels of the various Ig isotypes were also indistinguishable, whether B cells were dependent upon the IgHΔ3alx3b or the IgHΔ3aΔ3b transgene for Ig expression. Although it is possible that some of the IgH expressed by the B cells in these mice resulted from trans-allelic switching (VH on the BAC, switching to the CH genes within the ΔJH/ΔJH or Igb/Igb endogenous loci), it has been demonstrated that such switching is rare between a BAC and an endogenous Igh chromosome when assayed in culture (4). Moreover, when the transgenes were on the C57BL/6 background (Igb/Igb), germline transcripts from the BACs could be specifically examined. These BAC-derived GLTs were detected in both IgHΔ3alx3b and IgHΔ3aΔ3b B cell cultures, supporting the flow cytometry data (Fig. 2B).
Consistent with the ability of the IgHΔ3aΔ3b transgene to undergo CSR, its “mini”-3′RR (hs1.2 and hs4) could be seen to physically associate with both the IgH transcription unit and the CH region promoters. In contrast, earlier studies showed that deletion of both hs3b and hs4 (leaving only hs3a and hs1.2) resulted in a dramatic reduction in these associations, along with diminished CSR to all isotypes except IgG1 (2, 19) Similarly, a “technical knock-out” of the 3′RR in a cell line resulted in loss of its association with the IgH transcription unit and loss of IgH gene expression (33, 34). The present studies, therefore, support a developing model of CSR in which long range interactions involving the 3′RR play an essential role.
Interestingly, the interaction between hs1.2 and hs4 increases upon loss of both hs3 elements, and this increased interaction extends to that between the 3′RR and the IgH transcription unit (Eμ) and the Iγ3 and Iγ1 promoters. The increased interaction between the 3′RR and Iγ3 and Iγ1 promoters in IgHΔ3aΔ3b B cells matches the levels seen only after cytokine stimulation in the IgHΔ3alx3b B cells. Importantly, CSR itself remains cytokine-dependent, demonstrating that augmented 3′RR/intronic promoter interactions do not supplant the role of cytokine. This is most likely because the Iγ3 and Iγ1 promoters themselves require cytokine-induced activation of promoter-specific transcription factors. The finding that the hs1.2/hs4 interaction is increased upon loss of both hs3a and hs3b further emphasizes the dynamic interplay taking place among elements in the 3′RR region, allowing for the kind of functional flexibility that underlies the functional redundancy revealed by single and (in the present study) double element deletion.
To summarize the experimental findings to date on the role of the 3′RR in class-switch recombination, deletion of all the enhancer-like elements (deletions of hs3a through hs4) impedes CSR to all IgH classes (3–5). A much smaller deletion covering only hs3b and hs4 (4.6-kb deletion) impedes CSR to all IgH classes except γ1 (2). Remarkably, the dramatic effects of this relatively small deletion can be reversed by leaving intact either the 2000 bp surrounding hs3b or the 1400 bp surrounding hs4 (20, 21). The restored sequences (hs3b and hs4) do not share obvious sequence homology, and in fact, follow distinct time lines for chromatin modification during development (11), yet each can work in synergy with the remaining elements of the 3′RR to effect normal CSR activity.
These previous studies showed that more than one combination of three enhancer-like elements from the 3′RR could support normal CSR (hs3a, hs1.2, and hs4 versus hs3a, hs1.2, and hs3b). Notably, each set of three included one or more of the hs3 elements, suggesting a possible requirement for one or the other. The current study disproves that hypothesis, demonstrating that the hs3a and hs3b elements can be removed simultaneously without significant effect on CSR to the various heavy chain classes. More generally, this finding reveals that functional redundancy in this system is not based on sequence homology. These findings prompt an adjustment in approach to further analyses of this important regulatory region. It is now clear that there is more than one way to “build” a CSR regulatory region. The present study identifies the smallest number of building blocks to date (hs1.2 and hs4).
Given the multiple combinations capable of activity, however, it is clear that discerning the nature of the mechanistic cooperation among these elements is critical. Perhaps there is an overall structure required that can be achieved by more than one combination of elements (e.g. providing a required density of transcription factor-binding sites and associated activities; a tether to a nuclear subregion; the scaffold for interaction with the IgH transcription unit and the CH region promoters, etc.). Alternatively, it is possible that each of these elements preferentially supports a subset of CSR events, with each of these subsets partially overlapping the others. These overlapping preferences would be masked by the individual element deletion approach employed to date. To answer these questions of mechanism, future studies will need to aim toward identification of the CSR-related activities supported by each individual element of the 3′RR (knowing that none is absolutely essential for this process). Comparing the activity of individual elements with that displayed by distinct combinations of elements can reveal the combinatorial logic that apparently provides many mechanistic routes to complete CSR activity.
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr. Wesley Dunnick (University of Michigan Medical School, Ann Arbor, MI) for providing unmodified CHC BAC, Dr. Klaus Rajewsky (Harvard Medical School, Boston, MA) for the cloned B1–8VH gene, Dr. F. W. Alt (Harvard Medical School, Boston, MA) for the loxP vector used to flank hs3b with loxP sites, Dr. Karl Drlica (Public Health Research Institute, New York, NY) for human β-globin plasmid, and Dr. Heiner Westphal (NICHD, National Institutes of Health) for EIIa-cre mice. We thank Joon Kim of the Hunter College Flow Cytometry Facility for expert help with cell sorting and Jodi-Ann Powell and Cheng Peng for expert technical support in mouse genotyping. Finally, we thank Dr. Benjamin Ortiz (Hunter College, The City University of New York) for helpful discussions and critical reading of the manuscript. The infrastructure and instrumentation at Hunter College are supported in part by a Research Centers in Minority Institutions award from the National Institutes of Health (RR003037).
This work was supported, in whole or in part, by National Institutes of Health Grants AI30653 (to L. A. E.) and AI13509 (to B. K. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1–3, Figs. 1–5, and Supporting Information.
- CSR
- class-switch recombination
- 3′RR
- IgH 3′ regulatory region
- BAC
- bacterial artificial chromosome
- GLT
- germline transcripts
- nt
- nucleotides
- CalR
- calreticulin.
REFERENCES
- 1. Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. (2000) Cell 102, 553–563 [DOI] [PubMed] [Google Scholar]
- 2. Pinaud E., Khamlichi A. A., Le Morvan C., Drouet M., Nalesso V., Le Bert M., Cogné M. (2001) Immunity 15, 187–199 [DOI] [PubMed] [Google Scholar]
- 3. Dunnick W. A., Shi J., Graves K. A., Collins J. T. (2005) J. Exp. Med. 201, 1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunnick W. A., Collins J. T., Shi J., Westfield G., Fontaine C., Hakimpour P., Papavasiliou F. N. (2009) J. Exp. Med. 206, 2613–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vincent-Fabert C., Fiancette R., Pinaud E., Truffinet V., Cogné N., Cogné M., Denizot Y. (2010) Blood 116, 1895–1898 [DOI] [PubMed] [Google Scholar]
- 6. Dariavach P., Williams G. T., Campbell K., Pettersson S., Neuberger M. S. (1991) Eur. J. Immunol. 21, 1499–1504 [DOI] [PubMed] [Google Scholar]
- 7. Lieberson R., Giannini S. L., Birshtein B. K., Eckhardt L. A. (1991) Nucleic Acids Res. 19, 933–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthias P., Baltimore D. (1993) Mol. Cell. Biol. 13, 1547–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michaelson J. S., Giannini S. L., Birshtein B. K. (1995) Nucleic Acids Res. 23, 975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Madisen L., Groudine M. (1994) Genes Dev. 8, 2212–2226 [DOI] [PubMed] [Google Scholar]
- 11. Garrett F. E., Emelyanov A. V., Sepulveda M. A., Flanagan P., Volpi S., Li F., Loukinov D., Eckhardt L. A., Lobanenkov V. V., Birshtein B. K. (2005) Mol. Cell. Biol. 25, 1511–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen C., Birshtein B. K. (1997) J. Immunol. 159, 1310–1318 [PubMed] [Google Scholar]
- 13. Mills F. C., Harindranath N., Mitchell M., Max E. E. (1997) J. Exp. Med. 186, 845–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu K., Chedin F., Hsieh C. L., Wilson T. E., Lieber M. R. (2003) Nat. Immunol. 4, 442–451 [DOI] [PubMed] [Google Scholar]
- 15. Shinkura R., Tian M., Smith M., Chua K., Fujiwara Y., Alt F. W. (2003) Nat. Immunol. 4, 435–441 [DOI] [PubMed] [Google Scholar]
- 16. Manis J. P., Tian M., Alt F. W. (2002) Trends Immunol. 23, 31–39 [DOI] [PubMed] [Google Scholar]
- 17. Cogné M., Lansford R., Bottaro A., Zhang J., Gorman J., Young F., Cheng H. L., Alt F. W. (1994) Cell 77, 737–747 [DOI] [PubMed] [Google Scholar]
- 18. Manis J. P., van der Stoep N., Tian M., Ferrini R., Davidson L., Bottaro A., Alt F. W. (1998) J. Exp. Med. 188, 1421–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wuerffel R., Wang L., Grigera F., Manis J., Selsing E., Perlot T., Alt F. W., Cogne M., Pinaud E., Kenter A. L. (2007) Immunity 27, 711–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bébin A. G., Carrion C., Marquet M., Cogné N., Lecardeur S., Cogné M., Pinaud E. (2010) J. Immunol. 184, 3710–3717 [DOI] [PubMed] [Google Scholar]
- 21. Vincent-Fabert C., Truffinet V., Fiancette R., Cogné N., Cogné M., Denizot Y. (2009) J. Immunol. 182, 6926–6932 [DOI] [PubMed] [Google Scholar]
- 22. Sonoda E., Pewzner-Jung Y., Schwers S., Taki S., Jung S., Eilat D., Rajewsky K. (1997) Immunity 6, 225–233 [DOI] [PubMed] [Google Scholar]
- 23. Misulovin Z., Yang X. W., Yu W., Heintz N., Meffre E. (2001) J. Immunol. Methods 257, 99–105 [DOI] [PubMed] [Google Scholar]
- 24. Yang X. W., Model P., Heintz N. (1997) Nat. Biotechnol. 15, 859–865 [DOI] [PubMed] [Google Scholar]
- 25. Shi X., Eckhardt L. A. (2001) Int. Immunol. 13, 1003–1012 [DOI] [PubMed] [Google Scholar]
- 26. Lakso M., Pichel J. G., Gorman J. R., Sauer B., Okamoto Y., Lee E., Alt F. W., Westphal H. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gu H., Zou Y. R., Rajewsky K. (1993) Cell 73, 1155–1164 [DOI] [PubMed] [Google Scholar]
- 28. Dunnick W. A., Shi J., Graves K. A., Collins J. T. (2004) J. Immunol. 173, 5531–5539 [DOI] [PubMed] [Google Scholar]
- 29. Zaller D. M., Eckhardt L. A. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 5088–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang B., Alaie-Petrillo A., Kon M., Li F., Eckhardt L. A. (2007) J. Immunol. 178, 6297–6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McIntyre T. M., Kehry M. R., Snapper C. M. (1995) J. Immunol. 154, 3156–3161 [PubMed] [Google Scholar]
- 32. Li F., Eckhardt L. A. (2009) J. Exp. Med. 206, 153–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ju Z., Volpi S. A., Hassan R., Martinez N., Giannini S. L., Gold T., Birshtein B. K. (2007) J. Biol. Chem. 282, 35169–35178 [DOI] [PubMed] [Google Scholar]
- 34. Lieberson R., Ong J., Shi X., Eckhardt L. A. (1995) EMBO J. 14, 6229–6238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.