Abstract
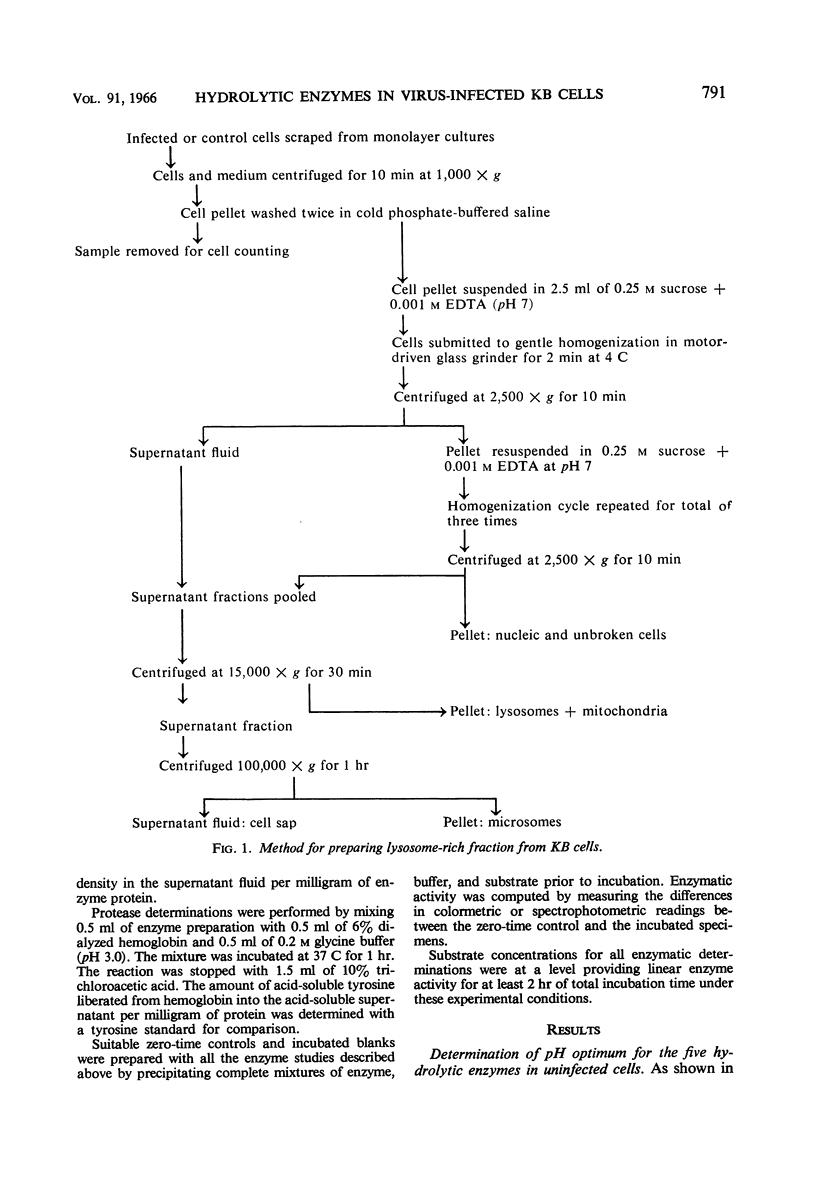
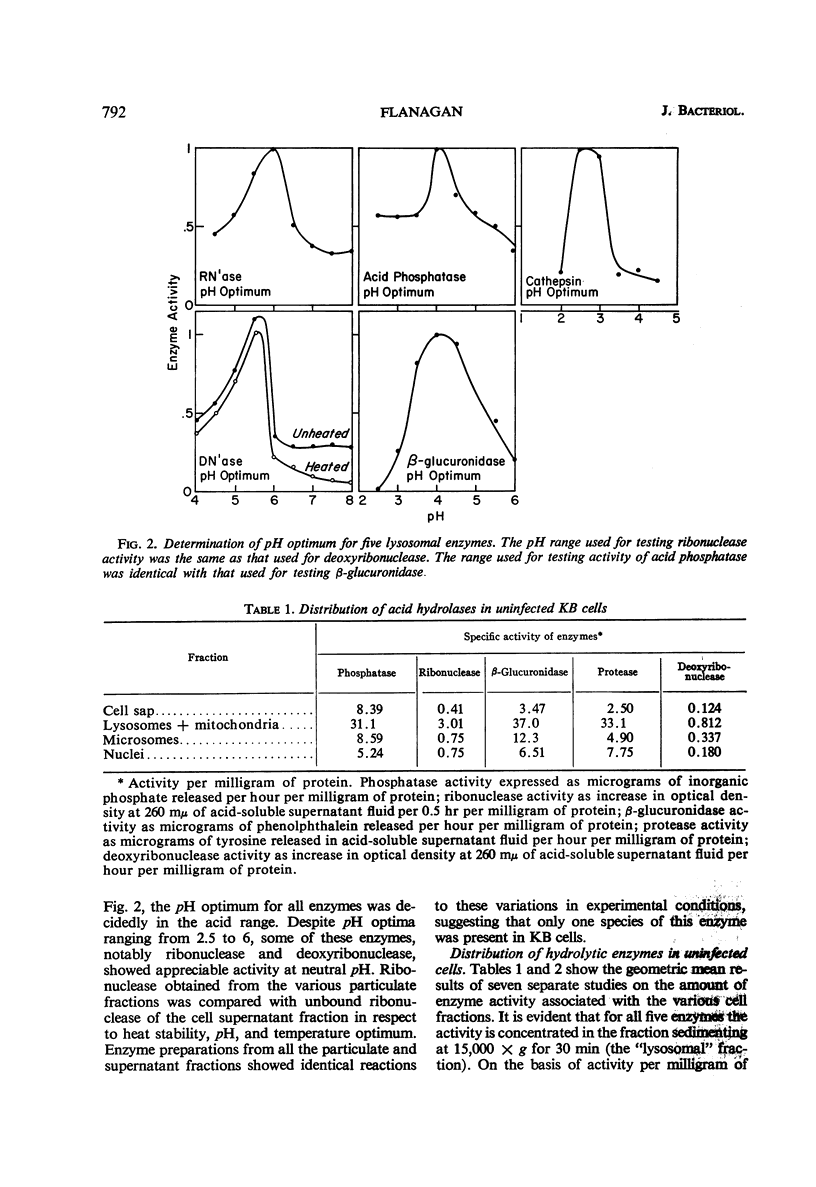
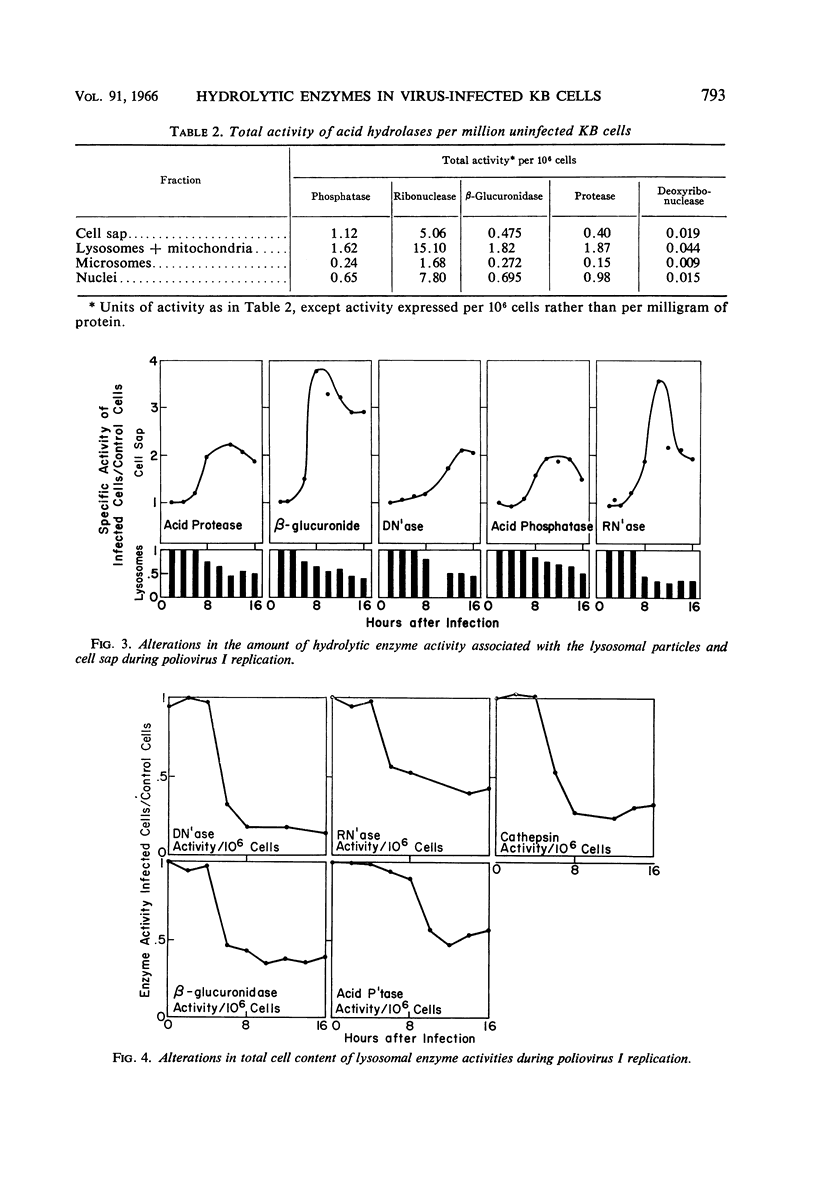
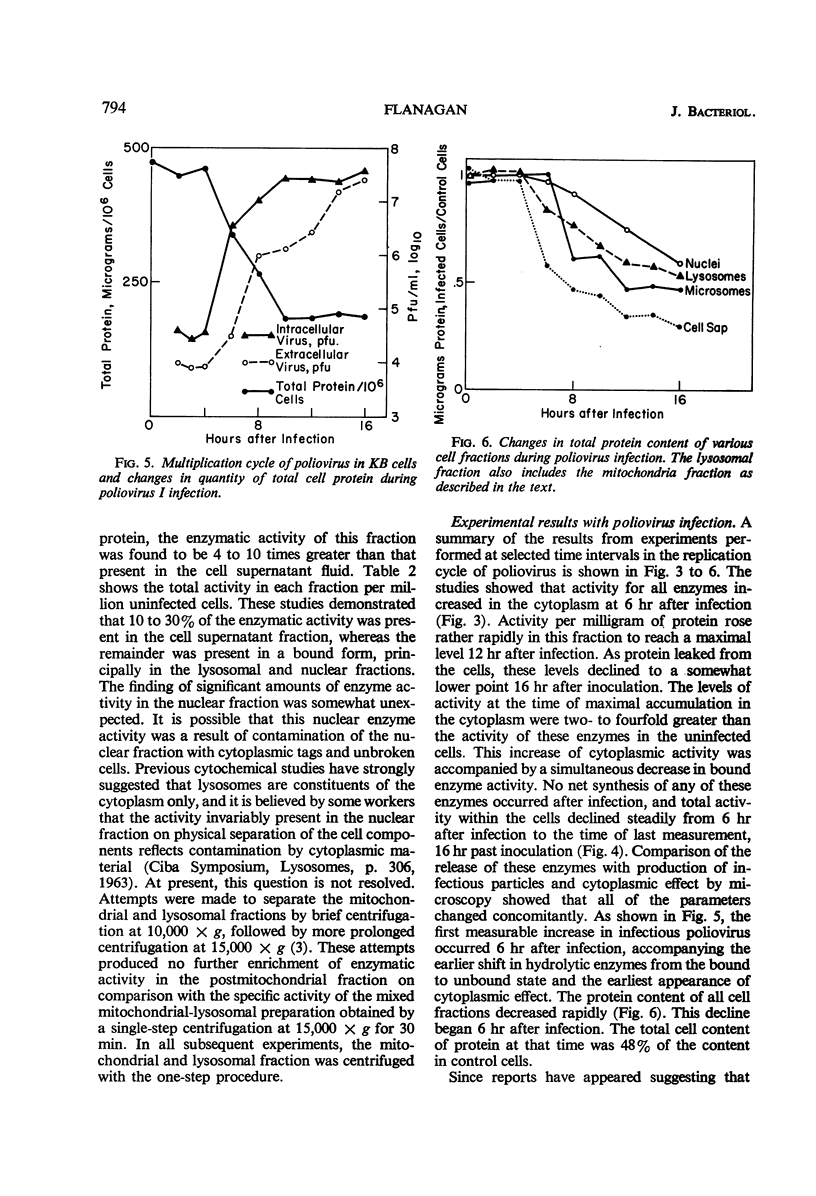
Flanagan, John F. (Duke University School of Medicine, Durham. N.C.). Hydrolytic enzymes in KB cells infected with poliovirus and herpes simplex virus. J. Bacteriol. 91:789–797. 1966.—The effect of poliovirus and herpes simplex virus infection on the activity of five hydrolytic enzymes was studied in tissue culture cells of KB type. During the course of poliovirus infection, the activity of β-glucuronidase, acid protease, acid ribonuclease, acid deoxyribonuclease, and acid phosphatase in the cytoplasm rose to levels two- to fourfold greater than the activity present in the cytoplasm of uninfected cells. The rise in cytoplasmic activity was accompanied by a concomitant decrease in enzymatic activity bound to cell particles. Shift of enzymatic activity from the particulate to soluble state was first detected at 6 hr after poliovirus infection, coinciding with the appearance of new infectious particles and virus cytopathic effect. No net synthesis of these enzymes after poliovirus infection was found. Hydrocortisone added to the culture medium failed to affect either the titer of virus produced in the cells or the release of hydrolytic enzymes from the particulate state. Herpes simplex infection produced minimal alterations in the state of these enzymes in KB cells. It is hypothesized that the breakdown of lysosomes and release of hydrolytic enzymes accompanying poliovirus infection is produced by alterations in cell membrane permeability during the course of virus replication and by the consequent change in the ionic content of the cell sap.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., SANDELIN K. Activation of lysosomal enzymes in virus-infected cells and its possible relationship to cytopathic effects. J Exp Med. 1963 Jun 1;117:879–887. doi: 10.1084/jem.117.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON P. J., COHEN S., BARKA T. Hepatic injury. A histochemical study of intracytoplasmic globules occurring in liver injury. Arch Pathol. 1961 Jan;71:89–95. [PubMed] [Google Scholar]
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., CHOPPIN P. W. Attachment and penetration of influenza virus. Virology. 1962 Nov;18:489–493. doi: 10.1016/0042-6822(62)90041-7. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- GINSBERG H. S., BADGER G. F., DINGLE J. H., JORDAN W. S., Jr, KATZ S. Etiologic relationship of the RI-67 agent to acute respiratory disease (ARD). J Clin Invest. 1955 Jun;34(6):820–831. doi: 10.1172/JCI103137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S. Biological and biochemical basis for cell injury by animal viruses. Fed Proc. 1961 Jul;20:656–660. [PubMed] [Google Scholar]
- GINSBERG H. S., DIXON M. K. Nucleuc acid synthesis in types 4 and 5 adenovirus-infected HeLa cells. J Exp Med. 1961 Feb 1;113:283–299. doi: 10.1084/jem.113.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. INHIBITION OF HOST CELL MACROMOLECULAR SYNTHESIS BY HIGH MULTIPLICITIES OF POLIOVIRUS UNDER CONDITIONS PREVENTING VIRUS SYNTHESIS. J Mol Biol. 1964 Apr;8:574–581. doi: 10.1016/s0022-2836(64)80012-7. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., PETERSON J. A. NUCLEIC ACID AND PROTEIN SYNTHESIS DURING POLIOVIRUS INFECTION OF HUMAN CELLS. J Mol Biol. 1964 Apr;8:556–575. doi: 10.1016/s0022-2836(64)80011-5. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E. Vegetative bacteriophage and the maturation of the virus particles. Adv Virus Res. 1961;8:1–61. doi: 10.1016/s0065-3527(08)60682-x. [DOI] [PubMed] [Google Scholar]
- KOENIG H., JIBRIL A. Acidic glycolipids and the role of ionic bonds in the structure-linked latency of lysosomal hydrolases. Biochim Biophys Acta. 1962 Dec 17;65:543–545. doi: 10.1016/0006-3002(62)90469-9. [DOI] [PubMed] [Google Scholar]
- LUCY J. A., DINGLE J. T., FELL H. B. Studies on the mode of action of excess of vitamin A. 2. A possible role of intracellular proteases in the degradation of cartilage matrix. Biochem J. 1961 Jun;79:500–508. doi: 10.1042/bj0790500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B. Electron microscopy: cytology of cell fractions. Science. 1956 Nov 16;124(3229):969–972. doi: 10.1126/science.124.3229.969. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C., WALKER B. M. DEGRADATION OF DEOXYRIBONUCLEIC ACID AND ALTERATION NUCLEIC ACID METABOLISM IN SUSPENSION CULTURES OF L-M CELLS INFECTED WITH EQUINE ABORTION VIRUS. J Bacteriol. 1963 Jul;86:138–146. doi: 10.1128/jb.86.1.138-146.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall C. C., Gafford L. G., Gentry G. A., Lawson L. A. Lability of host-cell DNA in growing cell cultures due to Mycoplasma. Science. 1965 Sep 3;149(3688):1098–1099. doi: 10.1126/science.149.3688.1098. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D., MUNYON W. On the replication of vaccinia virus. Cold Spring Harb Symp Quant Biol. 1962;27:237–244. doi: 10.1101/sqb.1962.027.001.023. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN S. C., MARCUS P. I. EARLY STAGES OF NEWCASTLE DISEASE VIRUS-HELA CELL INTERACTION: AN ELECTRON MICROSCOPIC STUDY. Virology. 1964 Jul;23:370–380. doi: 10.1016/0042-6822(64)90259-4. [DOI] [PubMed] [Google Scholar]
- STONE A. B., BURTON K. Studies on the deoxyribonucleases of bacteriophage-infected Escherichia coli. Biochem J. 1962 Dec;85:600–606. doi: 10.1042/bj0850600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. Studies on lysosomes. I. The effects of endotoxin, endotoxin tolerance, and cortisone on the release of acid hydrolases from a granular fraction of rabbit liver. J Exp Med. 1962 Oct 1;116:433–450. doi: 10.1084/jem.116.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF D. A., BUBEL H. C. THE DISPOSITION OF LYSOSOMAL ENZYMES AS RELATED TO SPECIFIC VIRAL CYTOPATHIC EFFECTS. Virology. 1964 Nov;24:502–505. doi: 10.1016/0042-6822(64)90196-5. [DOI] [PubMed] [Google Scholar]



