Abstract
Nonphotochemical quenching (NPQ) is the fundamental process by which plants exposed to high light intensities dissipate the potentially harmful excess energy as heat. Recently, it has been shown that efficient energy dissipation can be induced in the major light-harvesting complexes of photosystem II (LHCII) in the absence of protein-protein interactions. Spectroscopic measurements on these samples (LHCII gels) in the quenched state revealed specific alterations in the absorption and circular dichroism bands assigned to neoxanthin and lutein 1 molecules. In this work, we investigate the changes in conformation of the pigments involved in NPQ using resonance Raman spectroscopy. By selective excitation we show that, as well as the twisting of neoxanthin that has been reported previously, the lutein 1 pigment also undergoes a significant change in conformation when LHCII switches to the energy dissipative state. Selective two-photon excitation of carotenoid (Car) dark states (Car S1) performed on LHCII gels shows that the extent of electronic interactions between Car S1 and chlorophyll states correlates linearly with chlorophyll fluorescence quenching, as observed previously for isolated LHCII (aggregated versus trimeric) and whole plants (with versus without NPQ).
Keywords: Bioenergetics, Biophysics, Photosynthesis, Photosynthetic Pigments, Protein Conformation, Protein Purification, Raman Spectroscopy, Nonphotochemical Quenching, Phototprotection
Introduction
The photosynthetic process starts with the absorption of incoming solar photons by specialized pigment-protein complexes. In plants, light energy is absorbed by the two multisubunit protein-cofactor complexes, photosystem I (PSI)3 and photosystem II (PSII), and the excitation energy is efficiently transferred to their reaction centers where photochemistry takes place. When these organisms are in low light environments, the process of light energy collection is extremely efficient. However, this process is regulated when the incoming light energy is above that which can be used in electron transport. In these latter conditions, the light-harvesting antenna is able to switch into a photoprotective mode, dissipating the excess energy as heat (1–3). This regulatory mechanism is measured as nonphotochemical quenching of chlorophyll (Chl) fluorescence (NPQ). In higher plants NPQ is a multicomponent process whose major component is called qE (energy-dependent quenching) (4), dependent upon the formation of the proton gradient (ΔpH) across the thylakoid membrane (which itself results from photosynthetic activity) (5). qE is facilitated by the deepoxidation of violaxanthin to zeaxanthin in the xanthophyll cycle (6). The PsbS protein also plays a crucial role in this process, possibly acting as a pH sensor (7, 8). A key characteristic of the qE process is that it is induced in seconds and thus cannot involve de novo protein synthesis, but rather corresponds to a reorganization of the existing photosynthetic architecture.
In the past decade a large amount of work has been performed to gain insight in the molecular mechanism(s) underlying this process, and several hypotheses have been put forward (9–12). Among these, it was in particular proposed that excitation energy quenching during qE involves charge transfer and would result from the formation of a carotenoid (Car) cation/Chl anion pair, which dissipates the excitation energy upon recombination to the ground state (10, 11, 13). Formation of a radical cation was reported in the reconstituted minor antenna complexes as well as in thylakoid membranes, but not in purified LHCII complexes, suggesting that the former proteins house the quenching site(s). However, other results suggest that this is not the case and that the quenching site is in LHCII. For instance, Bode et al. have observed a direct correlation between electronic Car S1-Chl interactions and quenching properties in both isolated LHCII and entire plants. The linear correlation observed between the Car S1-Chl a interactions in LHCII with NPQ indicates that the Car S1 state is involved in qE and, according to these results, the quenching results from a short lived Car-Chl excited state (9, 14). Recently, Holzwarth et al. have suggested that Cars are not directly involved in qE (15); rather, they propose that quenching in LHCII aggregates involves the formation of a Chl-Chl charge transfer state, characterized by weak far-red fluorescence emission (16). It was further proposed that qE may involve both quenching in LHCII aggregates and also at a separate site involving the PSII core and minor antenna complexes (12).
We originally proposed that trimeric LHCII is the site of qE (9, 17, 18) and that the formation of the quenching site involves a conformational change within this complex. Indeed, reversible fluorescence red shifts (up to 75 nm) have recently been observed in single trimeric LHCII complexes and were explained in terms of specific conformational dynamics of the protein scaffold (19). The conformational change is revealed by resonance Raman spectroscopy, a powerful and selective vibrational technique that gives access to the fine structure of photosynthetic chromophores. These conformational changes involve an alteration in Chl-protein interactions as well as in the planarity of the LHCII-bound neoxanthin (Neo) carotenoid. In isolated LHCII, the amplitude of the signal revealing this Neo structural change correlates linearly with the extent of quenching (9). Furthermore, the same signal was observed in intact chloroplasts and leaves after NPQ induction, and the correlation with the extent of quenching was maintained in these in vivo samples. The formation of NPQ is also associated with certain absorption changes that have been suggested to reflect a conformational change in LHCII, brought about by its protonation. The light − dark recovery absorption difference spectrum is characterized by a series of positive and negative bands, the best-known of which is ΔA535 (20). Using resonance Raman the origin of ΔA535 was shown to be a sub-population of red-shifted zeaxanthin molecules (21). In the absence of zeaxanthin (and antheraxanthin) a proportion of NPQ remains and the ΔA535 change is blue-shifted to 525 nm (ΔA525) (22). Using the Raman technique we have shown recently that the ΔA525 absorption change in Arabidopsis thaliana leaves lacking zeaxanthin belongs to a red-shifted sub-population of violaxanthin molecules formed during NPQ (23).
Transient absorption spectroscopy applied to purified LHCII in the aggregated, quenched state, has provided evidence that the pathway for energy dissipation may involve energy transfer from Chl a to the S1 excited state of Lutein 1 (Lut1). The short lifetime of this Car S1 state would ensure the efficient dissipation of the excitation energy into heat (9).
Because no consensus has yet been found to explain the molecular mechanism of qE, it is clear that further studies are needed to solve the present controversy. The quenching mechanism in LHCII has recently been investigated using a different approach, involving a newly developed system where the protein is immobilized in a gel matrix. Quenching could be induced in isolated LHCII trimers without protein aggregation, indicating that aggregation per se is not the cause of excitation energy quenching, thus supporting the conclusion that quenching is rather due to a protein conformational change (24). Direct and formal evidence of such a conformational change was provided by resonance Raman spectroscopy. This method revealed perturbation in the environments of Neo and Chl b, two chromophores that are not directly involved in the process of energy quenching (9, 18, 25). If the Lut1 molecule is indeed directly involved in the quenching mechanism one would predict that a conformational change in LHCII would modify its structure, thereby tuning its electronic properties and/or its interactions with nearby pigments. In this work, we address the reorganization of the Lut1 binding domain upon induction of quenching in LHCII using resonance Raman spectroscopy and discuss the mechanistic implications for NPQ.
EXPERIMENTAL PROCEDURES
LHCII Isolation
LHCII from wild-type (WT) Arabidopsis and the npq2 mutant was isolated from unstacked thylakoid membranes according to the method described by Ruban et al. (26), with the exception that n-dodecyl α-d-maltoside was used rather than n-dodecyl β-d-maltoside, at a final concentration of 20 mm. LHCII was desalted in a PD10 desalting column (GE Healthcare) with a buffer containing 20 mm HEPES (pH 7.8) and 0.03% (w/v) n-dodecyl α/β-d-maltoside. Quenched LHCII was prepared by removal of detergent using SM-2 bioabsorbent beads (Bio-Rad), allowing for a 6-fold reduction in fluorescence yield as determined by a PAM-101 fluorometer (Heinz Walz). LHCII gel preparation and induction of quenching were performed as described previously (24).
Pigment Analysis
Pigment composition was determined by reverse-phase HPLC, using a LiChrospher 100 RP-18 column (Merck) and a Dionex Summit chromatography system, as described previously (27). The results of the analysis are summarized in Table 1. Chl concentration was determined according to the method of Porra et al. (28).
TABLE 1.
Pigment composition of isolated LHCII
Trimeric LHCII was prepared from WT and npq2 Arabidopsis plants (see “Experimental Procedures”). Data are mmol of Car/mol of Chl a + b, expressed as means ± S.E. from four replicates. Values in parentheses represent the Car content per monomer of protein (molar ratio), considering that one monomer has 14 Chls (8 Chl a and 6 Chl b (as in ref. 33). Vio, violaxanthin; Ant, antheraxanthin; Lut, lutein; Zea, zeaxanthin; Neo, neoxanthin.
| LHCII | Neo | Vio | Ant | Lut | Zea | Car/ Chl | Chl a/b |
|---|---|---|---|---|---|---|---|
| WT | 61 ± 1.1 (0.89 ± 0.01) | 12 ± 1.6 (0.18 ± 0.02) | 0 | 132 ± 2.6 (1.97 ± 0.03) | 0 | 0.22 | 1.35 ± 0.1 |
| npq2 | 0 | 0 | 0 | 131 ± 4.2 (1.87 ± 0.05) | 45 ± 6.6 (0.65 ± 0.09) | 0.18 | 1.35 ± 0.1 |
Resonance Raman Spectroscopy
Low temperature (77 K) resonance Raman spectra were obtained in a helium flow cryostat (Air Liquide) using a Jobin-Yvon U1000 Raman spectrophotometer equipped with a liquid nitrogen-cooled charge-coupled device detector (Spectrum One, Jobin-Yvon) (29). Excitation was provided by a Coherent Argon laser (Innova 100; 488.0, 496.5, 501.7 nm) and a Liconix helium-cadmium laser (441.6 nm). The choice of these wavelengths was determined by the absorption maxima of the pigments derived from low temperature absorption spectra, as described by Ruban et al. (30).
Two-photon Spectroscopy
The details of this method have been described in Ref. 14. Briefly, a 1188-nm excitation is used for the two-photon excitation of Cars in LHCII, with careful rejection of visible light from the signal beam, to avoid one-photon excitation of Chls. This excitation was steered into a confocal setup and focused, and two-photon fluorescence was detected by an ultrafast photodiode connected to a lock-in amplifier (EG&G, Dumberry). For one-photon excitation of LHCII, a conventional Pulse Amplitude Modulation fluorometer (FMS1, Hansatech) (PAM) was integrated into the confocal setup, using a beam of 594 nm to excite LHCII Chls directly. Fluorescence was measured on the same spot for both two-photon and one-photon excitation, thus, allowing the determination of changes in electronic interaction between Cars and Chls in different quenched states. To avoid collection of the fluorescence more than once from the same spot of the LHCII gels, a homemade rotating device supporting the gels was used.
RESULTS
Evidence for Lut1 Changes in Quenched LHCII
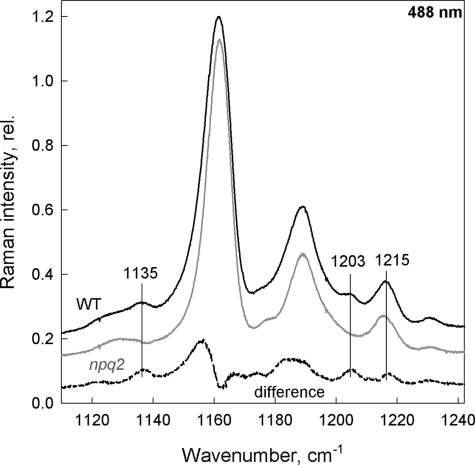
As stated above, no Raman evidence of lutein structural changes in LHCII upon quenching has been reported up to now. However, selectivity between the different LHCII Cars cannot be fully achieved in resonance Raman due to their overlapping absorption bands (note that the absorption maxima of Lut1 and Neo are only 7 nm apart, at 495 and 488 nm, respectively; Ref. 30). Because the magnitude of any Raman changes seen is directly related to the extent of the Car structural change involved, the large Neo distortion occurring upon quenching could impair the observation of any lutein changes, if they are smaller. We therefore studied LHCII from the npq2 mutant of Arabidopsis plants, which lack Neo (Table 1). Excitation at 488 nm favors contributions of Neo in WT LHCII (30). Using this excitation, spectra of the WT complexes display fingerprint bands of the 9-cis configuration of Neo molecules, at 1135, 1203, and 1215 cm−1 (Fig. 1, black trace (31). In spectra of npq2 LHCII at the same excitation, these bands are missing (Fig. 1, gray trace; see also the WT − npq2 difference spectrum, dashed trace).
FIGURE 1.
υ2 region of resonance Raman spectra obtained at 488.0 nm. Isolated trimeric LHCII from WT (black), npq2 (gray) Arabidopsis plants and the associated WT − npq2 difference (dashed) highlighting the absence of cis-modes (vertical lines) in the npq2 samples.
In the ν4 region of resonance Raman spectra, between 900 and 1000 cm−1, bands arising from C-H out-of-plane wagging modes contribute to the spectrum. For strictly planar Car molecules, these modes are not coupled with the S0-S2 transition, and thus bands in this region of the spectrum are extremely weak. However, these bands may gain in intensity when the molecules are distorted around C-C bonds, so they constitute reliable markers of the Car configuration (29). In resonance Raman spectra of LHCII from npq2 plants, bands at 956, 966, and 971 cm−1 clearly gain intensity (Fig. 2) when these complexes are quenched, and the change is most pronounced when 496.5-nm excitation was used (Fig. 2B). In the absence of Neo in these samples, this indicates that one lutein undergoes a small but significant change in configuration and that the lutein involved is the blue-absorbing one (495 nm), i.e. Lut1.
FIGURE 2.
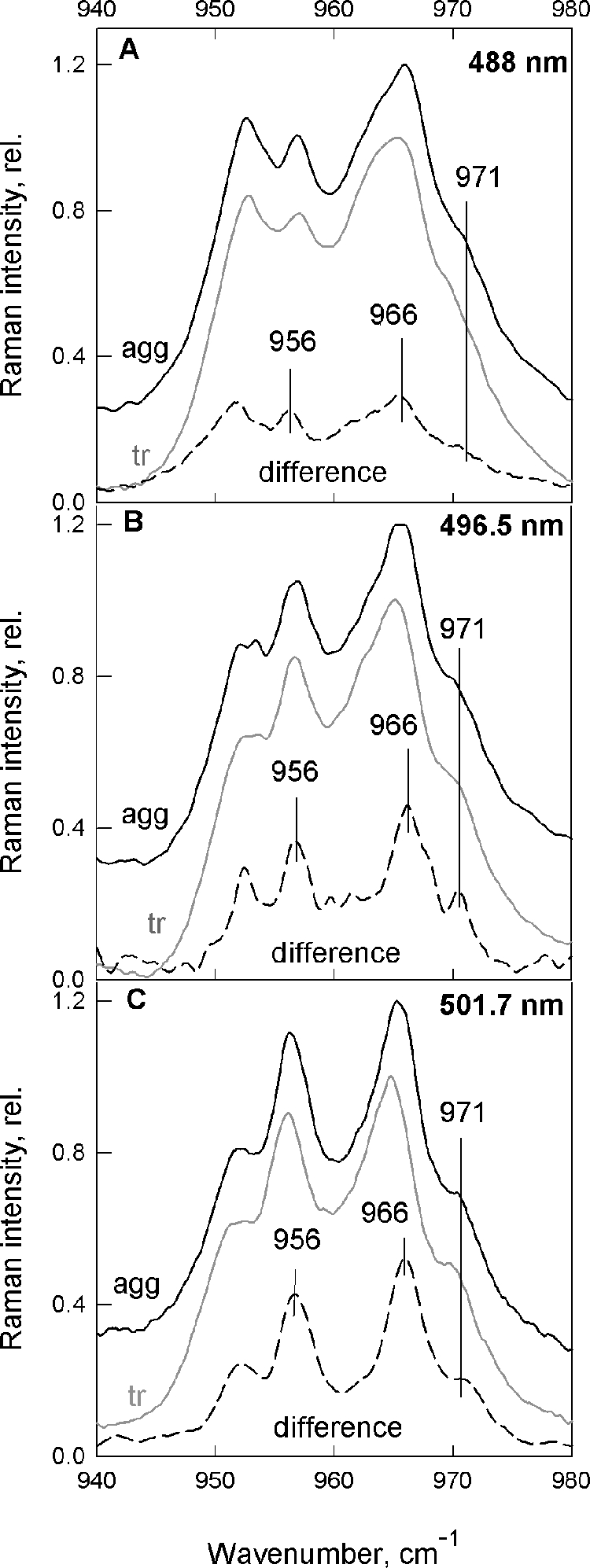
υ4 region of resonance Raman spectra recorded at 488.0, 496.5, and 501.7 nm (A–C, respectively). Trimeric (gray), aggregated (black) LHCII isolated from Arabidopsis npq2 plants and the associated difference spectra (aggregated − trimeric) are shown for each excitation wavelength (dashed; normalization at 1003 cm−1).
Do These Changes Exist in WT LHCII?
A change in the Lut1 configuration is thus observed upon quenching in LHCII purified from npq2 plants. However, it could be argued that this change might only occur when Neo is not present, due to perturbation of the protein structure caused by an empty binding locus.
Lutein Changes in Aggregated WT LHCII
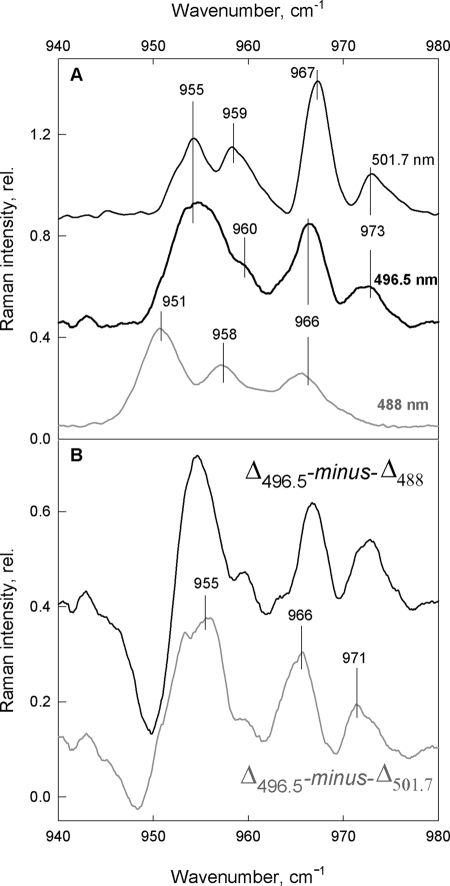
To test whether the changes in lutein configuration occur also when Neo is present, we repeated these experiments on aggregated LHCII isolated from WT Arabidopsis plants. Fig. 3A displays the aggregation-induced differences in Car spectra attributable to Neo, which peak at 488.0 nm excitation and still dominate the differential traces at redder excitations (496.5 and 501.7 nm). As discussed above, this is why it has been difficult to ascertain the presence of changes in lutein structure prior to this report. Nevertheless, as the Neo bands lose intensity at the higher excitation wavelengths, smaller additional bands are indeed seen in the differences (Fig. 3A) which may correspond to those observed in the absence of Neo (see Fig. 2). However, these features only represent minor contributions, and their exact frequencies are difficult to determine under the stronger Neo bands. Because the dominating bands from Neo are maximal at this Car absorption maximum, i.e. at 488.0 nm, while at longer wavelengths these contributions diminish but should not be shifted in frequency to any large extent, it is possible to extract the additional (non-Neo) bands by calculating double differences between the difference spectra. Thus, the traces Δ496.5 − Δ488 and Δ496.5 − Δ501.7, calculated with an arbitrary normalization factor designed to minimize the dominating Neo bands, give an approximation of the underlying spectral differences peaking at 496.5 nm. Surprisingly, the results are very similar, apart from a slight up-shift of the Δ496.5 − Δ488 (black trace) with respect to Δ496.5 − Δ501.7 (gray trace) (Fig. 3B). Both double-difference spectra show the same bands as present in the single-difference spectra for npq2 samples, in the absence of Neo (i.e. 955, 966, and 971 cm−1; see Fig. 2). This is a very good indication that we have successfully isolated the differential bands of Lut1, peaking at 496.5 nm ((0,0) transition of Lut1 is at 495 nm as in Ref. 30) and confirms the distortion of this pigment upon aggregation-induced quenching of LHCII.
FIGURE 3.
Difference-associated (aggregated minus trimeric) resonance Raman spectra. A, ν4 region of WT LHCII obtained with 488 nm (gray), 496.5 nm (thick black), and 501.7 nm (thin black) excitation lines. B, double-difference spectra Δ496.5 − Δ488 (black) and Δ496.5 − Δ501.7 (gray) showing Lut1 changes (vertical lines) peaking at 496.5 nm (for details, see “Results”).
Quenching in LHCII Gels
It has recently been shown that quenching can be produced in LHCII trimers in the absence of aggregation (24). This LHCII gel system has two advantages over aggregated LHCII for studying the quenching mechanism: the lack of aggregation results in samples of better optical quality because light scattering is almost entirely absent, and the samples can be quenched easily and to a greater extent. Thus, it should be possible to obtain better quality difference spectra for the observed changes in Lut1 even in the presence of Neo by using this new gel system. However, the structural changes associated with this aggregation-free quenching mechanism have not yet been characterized. It is therefore necessary to verify that the same structural mechanism is responsible for quenching in the gel system before using it to characterize further the structural changes involved.
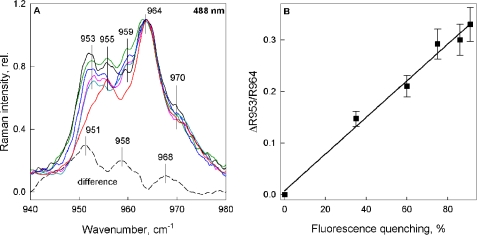
Resonance Raman experiments were therefore performed on LHCII embedded in gels, at several excitations. 488.0 nm excitation favors Neo contributions, and, upon aggregation-induced quenching, or in LHCII crystals (33) (where LHCII are always quenched; Refs. 18, 47), a strong resonance Raman signal in the ν4 is observed, indicating that Neo undergoes a change in configuration. Fig. 4A shows the same ν4 region for 488.0 nm excitation of LHCII gels in different quenching states (see figure legend for details). The spectrum of the unquenched LHCII gel (red trace) shows two typical bands at 955 and 964 cm−1, as observed previously for trimeric LHCII in solution (9, 18, 25). Upon induction of fluorescence quenching the enhancement of several vibrational modes is observed, the most prominent being the 953 cm−1 band. The increase in this vibrational mode relative to 964 cm−1 has previously been used to quantify the distortion of the Neo molecule, and this parameter was found to be linearly related to the quenching strength in aggregated LHCII as well as in vivo (9). For the quenched LHCII gels measured here, the same linear relationship is observed (Fig. 4B).
FIGURE 4.
ν4 region of the resonance Raman spectra for Neo excitation (488.0 nm). A, LHCII gels in the unquenched state (red) and with increasing levels of quenching (cyan, 35%; magenta, 60%; blue, 75%; green, 86%; black, 91%). Spectra were normalized at 964 cm−1. The dotted line displays the difference spectrum quenched − unquenched for maximum quenching (91%), after normalization in the ν3 region. Vertical lines indicate the vibrational modes discussed under “Results.” B, relationship between the change in 953 cm−1 Raman intensity (relative to 964 cm−1 intensity) from A and the extent of fluorescence quenching, expressed as a percentage of fluorescence in the unquenched LHCII gel. The error bars in B are the calculated amplitude of noise on the spectrum obtained using Datamax GRAMS32 Galactic software (Instruments SA).
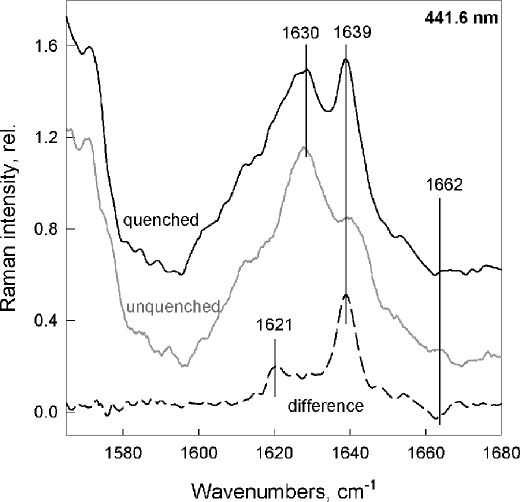
Comparison of the resonance Raman signal of LHCII trimers and aggregates at 441.6 nm excitation, which favors Chl b contributions, has shown that the formyl group of at least one Chl b (and possibly two) enters into a stronger interaction with its environment upon aggregation-induced quenching. This strong H bond was also observed in quenched LHCII in crystals (18). Again, the same changes are observed when quenching is induced in LHCII gels (Fig. 5). Upon quenching, a 1639 cm−1 band gains intensity, indicating the presence of strongly interacting formyl groups which, in unquenched samples, were free from interactions. The full width at half maximum of this additional band (6–7 cm−1) represents a high degree of homogeneity in bonding strength for this new H bond in the quenched state, indicating an interaction that is tightly controlled by the protein (Raman bands of single populations are usually approximately 13 cm−1 full width at half maximum in biological samples) (25, 34). Note that this was again the case for aggregation-induced quenching and in quenched LHCII crystals (18, 25, 47).
FIGURE 5.
Chl b resonance Raman spectra of the unquenched (gray) and quenched (black) LHCII gels at 441.6 nm excitation. The difference spectrum (quenched − unquenched; dashed trace) shows the typical, narrow 1639 cm−1 band (full width at half maximum = 6–7 cm−1) assigned to H bond formation (18, 20). Quenching in gels was 91%.
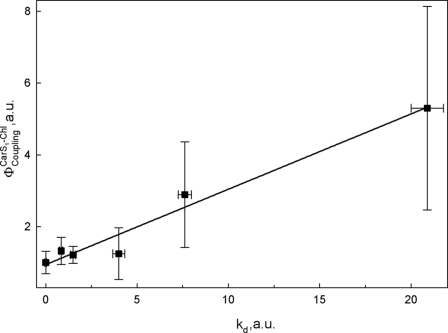
Several studies have provided evidence that electronic interactions between Car S1 and Chls may play a key role in the dissipation of excess excitation energy (3, 9, 35). To test the involvement of the Car S1 state in the quenching process in LHCII gels, two-photon excitation spectroscopy was performed, similar to that reported in Ref. 14 on LHCII trimers, aggregates, and whole plants. According to optical selection rules, direct electronic transition from S0 to S1 is forbidden, but this transition is, however, two-photon allowed (36, 37), and energy transfer from the S1 state of carotenoid to Chl can thus be probed by Chl fluorescence upon two-photon excitation. Comparing two-photon Chl fluorescence with fluorescence upon direct one-photon Chl excitation allows quantification of the electronic interaction between Cars and Chls (38). In isolated LHCII, as well as in intact plants, this interaction correlates linearly with fluorescence quenching (14, 39, 40). In Fig. 6 this correlation is shown for LHCII gels at various different quenching levels (see legend). Here, kd was calculated from the fluorescence measured with a PAM fluorometer (as described under “Experimental Procedures”), where Funq and Fq are fluorescence intensities of unquenched and quenched samples, respectively.
 |
This linear correlation confirms that quenching in LHCII in the absence of aggregation shares the same properties and that it thus involves the same mechanism(s) as for aggregation-induced quenching.
FIGURE 6.
Correlation of φCouplingCar S1 − Chl with fluorescence quenching (presented as kd), for quenched LHCII in the gel system. Fluorescence quenching was induced as in Ilioaia et al. (24). Samples were 10, 27, 40, 60, and 78% quenched compared with the unquenched LHCII gel (0%). This corresponds to 0.11, 0.37, 0.67, 1.5, and 3.55 kd values, respectively. Error bars, S.E.
We have thus established that quenching in the LHCII gel system has all of the same spectral characteristics as that in aggregation-induced quenching, and so we can now use this system to investigate the changes in Lut1 structure further. The ν4 region of resonance Raman spectra obtained with excitation at 496.5 and 501.7 nm of unquenched and quenched LHCII complexes in gels are displayed in Fig. 7, B and C, along with their associated quenched − unquenched differences. The 488 nm spectra are also plotted for comparison, together with the associated difference spectrum (Fig. 7A, taken from Fig. 4). The fingerprint of Neo distortion is again present at 488 nm excitation (Fig. 7A) and is still strong at 496.5 nm (Fig. 7B). However, at 501.7 nm (Fig. 7C) this Neo contribution is reduced significantly, and as a result the emergence of the three new bands at 955, 966, and 973 cm−1 is clearly seen. Note that at 496.5 nm, these bands can also be observed, on top of the dominant Neo contributions (951, 958, and 968 cm−1) indicated by arrows. These new bands, which correspond exactly to those observed in the LHCII aggregation experiments (though less clearly in the presence of Neo), must reflect the distortion of the same Car, i.e. Lut1 (Lut 620 from the crystal structure of Liu et al.; Ref. 33).
FIGURE 7.
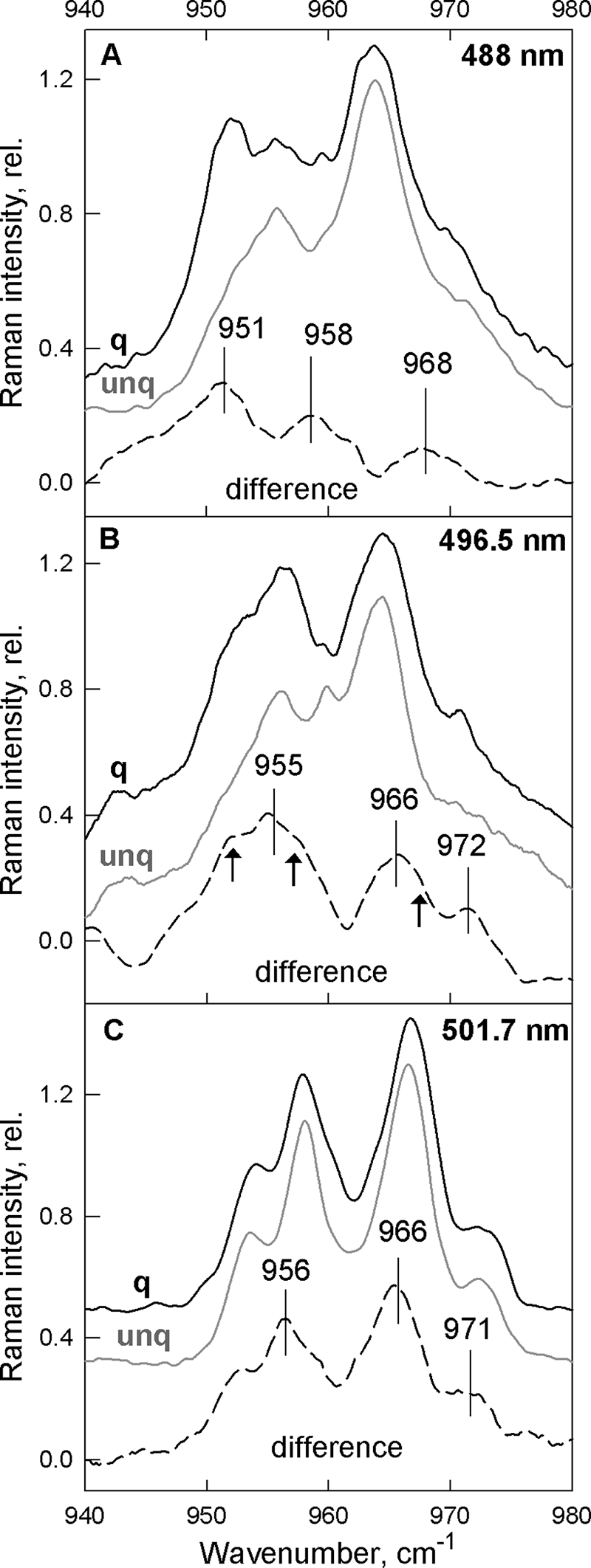
ν4 region of the resonance Raman spectra recorded at 488.0, 496.5, and 501.7 nm (A–C, respectively) for LHCII gels. Unquenched (gray) and quenched (black) LHCII gels are presented together with the difference-associated spectra (quenched − unquenched), calculated for each excitation (dashed), showing the presence of the new vibrational modes. Arrows in B indicate the Neo vibrations present as shoulders at 496.5-nm excitation.
DISCUSSION
In this study we have shown that, along with the known changes in pigment configuration that accompany fluorescence quenching in aggregates of LHCII complexes (involving Neo, Chl a, and Chl b) (9, 18, 25), there is also a change in the Lut1 binding domain. Lut1 undergoes a distortion during the transition of LHCII into the quenched state. We also show here that LHCII quenching in the absence of protein aggregation, in a recently developed gel system (24), displays all of the spectral signatures observed previously in LHCII aggregates. This indicates that fluorescence quenching in the LHCII gels occurs by the same mechanism(s) as aggregation-induced quenching. The absence of scattering artifacts in this new model system results in spectra of much higher quality, allowing us to confirm the small but significant changes in Raman signature of the Lut1 molecule.
The structural change in Lut1 may reflect its specific activation by the LHCII conformational change as the excitation energy quencher, either by increasing Chl a–Lut1 coupling interactions (14, 41) or by mediating more efficient energy transfer from Chl a to the Lut1 S1 state (9), or both. Alternatively, the twisting of Lut1 observed here could alter its excited state properties influencing the likelihood of energy transfer between Chl a and the Lut1 S1 state (42). Indeed, spectral changes in the terminal emitter chlorophylls, a610, a611, and a612, that lie in van-der-Waals contact with Lut1 (33), have also been observed during quenching in isolated LHCII and in vivo (43), providing further evidence of a change in this domain. The two-photon excitation spectroscopy data reported here and our preliminary results from transient absorption spectroscopy on quenched LHCII gels upon selective Chl a excitation lend support to the view that a Car S1 excited state is transiently populated from the excited Chl in a way similar to that observed in artificial photosynthetic antennas (44) and aggregated LHCII (9).
Early work on xanthophyll biosynthetic mutants of Arabidopsis lacking lutein showed that the absence of this pigment reduced NPQ by ∼30% (45). In these lutein-deficient plants, lutein is replaced at the Lut1 and Lut2 sites by a mixture of violaxanthin, antheraxanthin, and zeaxanthin that, unlike the “normal” xanthophyll cycle pigments in the WT were not available for (de-)epoxidation (45, 46). Lokstein et al. thus argued that, because zeaxanthin was known to be important for NPQ, the lack of any increase in the capacity for NPQ in these mutants indicated that the xanthophylls in the Lut1 and Lut2 sites are not directly involved in quenching (46). They postulated that the decrease in NPQ capacity in the lutein mutants was in fact due to the modified structure of LHCII (46). However, this interpretation neglects the fact that such structural changes may negatively affect the interactions between the xanthophyll bound at the Lut1 site and the terminal emitter chlorophylls. Thus, zeaxanthin may actually be a weaker quencher than lutein at this site, explaining the reduction in NPQ in the lutein-deficient plants.
Alternatively, it is possible that the structural changes in Lut1, and indeed those previously reported in Neo (9, 18, 25), Chl b (18, 25), zeaxanthin (21) and violaxanthin (23) may simply reflect the conformational change in the LHCII that engage Chl-Chl quenching interactions as suggested by Holzwarth and co-workers (15, 16). Indeed, in such an interpretation the disrupted LHCII structure in mutants lacking lutein could directly affect the Chl-Chl interactions resulting in the observed reduction in NPQ.
Irrespective of whether xanthophylls are directly involved in the quenching mechanism, the observed changes in Lut1 contribute to our growing knowledge of the microscopic alterations in LHCII conformation that occur upon transition to the quenched state. Our growing insight into the finer details of these changes will allow us to form a more complete picture of how and why changes in LHCII conformation result in quenching.
This work was supported by The Netherlands Organization for Scientific Research via the Foundation of Earth and Life Sciences (to C. I. and R. v. G.), the European Union FP7 Marie Curie Reintegration Grant ERG 224796 (to C. I.), research and equipment grants from the United Kingdom Biotechnology and Biological Sciences Research Council and Engineering and Physical Sciences Research Council (to A. V. R. and M. P. J.), the German Science Foundation (to P.-N. L. and P. J. W.), Blaise Pascal Chair from the Fondation of the Ecole Normale Supérieure (to R. v. G.), a European Union FP7 Marie Curie HARVEST Network grant and European Research Council Grant PhotProt (to B. R. and R. v. G.).
- PSI
- photosystem I
- PSII
- photosystem II
- Car
- carotenoid
- Car S1
- Car S1 (dark) state
- Chl
- chlorophyll
- ΔpH
- proton gradient across the thylakoid membrane
- kd
- quenching constant used to define quenching of LHCII gel samples
- LHCII
- light harvesting antenna complex of photosystem II
- Lut1
- lutein 1 Car bound to LHCII
- Neo
- neoxanthin Car bound to LHCII
- NPQ
- nonphotochemical Chl fluorescence quenching
- qE
- rapidly reversible component of NPQ.
REFERENCES
- 1. Horton P., Ruban A. V., Walters R. G. (1996) Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684 [DOI] [PubMed] [Google Scholar]
- 2. Niyogi K. K. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 333–359 [DOI] [PubMed] [Google Scholar]
- 3. Holt N. E., Fleming G. R., Niyogi K. K. (2004) Biochemistry 43, 8281–8289 [DOI] [PubMed] [Google Scholar]
- 4. Müller P., Li X. P., Niyogi K. K. (2001) Plant Physiol. 125, 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briantais J. M., Vernotte C., Picaud M., Krause G. H. (1979) Biochim. Biophys. Acta 548, 128–138 [DOI] [PubMed] [Google Scholar]
- 6. Demmig-Adams B., Adams W. W., Heber U., Neimanis S., Winter K., Krüger A., Czygan F. C., Bilger W., Björkman O. (1990) Plant Physiol. 92, 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li X. P., Björkman O., Shih C., Grossman A. R., Rosenquist M., Jansson S., Niyogi K. K. (2000) Nature 403, 391–395 [DOI] [PubMed] [Google Scholar]
- 8. Li X. P., Gilmore A. M., Caffarri S., Bassi R., Golan T., Kramer D., Niyogi K. K. (2004) J. Biol. Chem. 279, 22866–22874 [DOI] [PubMed] [Google Scholar]
- 9. Ruban A. V., Berera R., Ilioaia C., van Stokkum I. H., Kennis J. T., Pascal A. A., van Amerongen H., Robert B., Horton P., van Grondelle R. (2007) Nature 450, 575–578 [DOI] [PubMed] [Google Scholar]
- 10. Holt N. E., Zigmantas D., Valkunas L., Li X. P., Niyogi K. K., Fleming G. R. (2005) Science 307, 433–436 [DOI] [PubMed] [Google Scholar]
- 11. Ahn T. K., Avenson T. J., Ballottari M., Cheng Y. C., Niyogi K. K., Bassi R., Fleming G. R. (2008) Science 320, 794–797 [DOI] [PubMed] [Google Scholar]
- 12. Holzwarth A. R., Miloslavina Y., Nilkens M., Jahns P. (2009) Chem. Phys. Lett. 483, 262–267 [Google Scholar]
- 13. Avenson T. J., Ahn T. K., Zigmantas D., Niyogi K. K., Li Z., Ballottari M., Bassi R., Fleming G. R. (2008) J. Biol. Chem. 283, 3550–3558 [DOI] [PubMed] [Google Scholar]
- 14. Bode S., Quentmeier C. C., Liao P. N., Hafi N., Barros T., Wilk L., Bittner F., Walla P. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 12311–12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Müller M. G., Lambrev P., Reus M., Wientjes E., Croce R., Holzwarth A. R. (2010) Chem. Phys. Chem. 11, 1289–1296 [DOI] [PubMed] [Google Scholar]
- 16. Miloslavina Y., Wehner A., Lambrev P. H., Wientjes E., Reus M., Garab G., Croce R., Holzwarth A. R. (2008) FEBS Lett. 582, 3625–3631 [DOI] [PubMed] [Google Scholar]
- 17. Horton P., Wentworth M., Ruban A. V. (2005) FEBS Lett. 579, 4201–4206 [DOI] [PubMed] [Google Scholar]
- 18. Pascal A. A., Liu Z., Broess K., van Oort B., van Amerongen H., Wang C., Horton P., Robert B., Chang W., Ruban A. V. (2005) Nature 436, 134–137 [DOI] [PubMed] [Google Scholar]
- 19. Krüger T. P., Novoderezhkin V. I., Ilioaia C., van Grondelle R. (2010) Biophys. J. 98, 3093–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson M. P., Pérez-Bueno M. L., Zia A., Horton P., Ruban A. V. (2009) Plant Physiol. 149, 1061–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruban A. V., Pascal A. A., Robert B., Horton P. (2002) J. Biol. Chem. 277, 7785–7789 [DOI] [PubMed] [Google Scholar]
- 22. Noctor G., Ruban A. V., Horton P. (1993) Biochim. Biophys. Acta 1183, 339–344 [Google Scholar]
- 23. Ilioaia C., Johnson M. P., Duffy C. D., Pascal A. A., van Grondelle R., Robert B., Ruban A. V. (2011) J. Biol. Chem. 286, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ilioaia C., Johnson M. P., Horton P., Ruban A. V. (2008) J. Biol. Chem. 283, 29505–29512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruban A. V., Horton P., Robert B. (1995) Biochemistry 34, 2333–2337 [DOI] [PubMed] [Google Scholar]
- 26. Ruban A. V., Lee P. J., Wentworth M., Young A. J., Horton P. (1999) J. Biol. Chem. 274, 10458–10465 [DOI] [PubMed] [Google Scholar]
- 27. Johnson M. P., Havaux M., Triantaphylidès C., Ksas B., Pascal A. A., Robert B., Davison P. A., Ruban A. V., Horton P. (2007) J. Biol. Chem. 282, 22605–22618 [DOI] [PubMed] [Google Scholar]
- 28. Porra R. J., Thompson W. A., Kriedemann P. E. (1989) Biochim. Biophys. Acta 975, 384–394 [Google Scholar]
- 29. Robert B., Lutz M. (1986) Biochemistry 25, 2303–2309 [Google Scholar]
- 30. Ruban A. V., Pascal A. A., Robert B. (2000) FEBS Lett. 477, 181–185 [DOI] [PubMed] [Google Scholar]
- 31. Ruban A. V., Pascal A. A., Robert B., Horton P. (2001) J. Biol. Chem. 276, 24862–24870 [DOI] [PubMed] [Google Scholar]
- 32. Robert B., Horton P., Pascal A. A., Ruban A. V. (2004) Trends Plant Sci. 9, 385–390 [DOI] [PubMed] [Google Scholar]
- 33. Liu Z., Yan H., Wang K., Kuang T., Zhang J., Gui L., An X., Chang W. (2004) Nature 428, 287–292 [DOI] [PubMed] [Google Scholar]
- 34. Lutz M. (1984) in Advances in IR and Raman Spectroscopy (Clark R. J. H., Hetser R. E. eds) pp. 211–300, John Wiley and Sons Inc., New York [Google Scholar]
- 35. Frank H. A., Bautista J. A., Josue J. S., Young A. J. (2000) Biochemistry 39, 2831–2837 [DOI] [PubMed] [Google Scholar]
- 36. Wehling A., Walla P. J. (2005) J. Phys. Chem. B 109, 24510–24516 [DOI] [PubMed] [Google Scholar]
- 37. Hilbert M., Wehling A., Schlodder E., Walla P. J. (2004) J. Phys. Chem. B 108, 13022–13030 [Google Scholar]
- 38. Frähmcke J. S., Walla P. J. (2006) Chem. Phys. Lett. 430, 397–403 [Google Scholar]
- 39. Liao P.-N., Bode S., Wilk L., Hafi N., Walla P. J. (2010) Chem. Phys. 373, 50–55 [Google Scholar]
- 40. Bode S., Quentmeier C. C., Liao P.-N., Barros T., Walla P. J. (2008) Chem. Phys. Lett. 450, 379–385 [Google Scholar]
- 41. van Amerongen H., van Grondelle R. (2001) J. Phys. Chem. B 105, 604–617 [Google Scholar]
- 42. Dreuw A. (2006) J. Phys. Chem. A 110, 4592–4599 [DOI] [PubMed] [Google Scholar]
- 43. Johnson M. P., Ruban A. V. (2009) J. Biol. Chem. 284, 23592–23601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berera R., Herrero C., van Stokkum I. H., Vengris M., Kodis G., Palacios R. E., van Amerongen H., van Grondelle R., Gust D., Moore T. A., Moore A. L., Kennis J. T. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5343–5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pogson B. J., Niyogi K. K., Björkman O., DellaPenna D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lokstein H., Tian L., Polle J. E., DellaPenna D. (2002) Biochim. Biophys. Acta 1553, 309–319 [DOI] [PubMed] [Google Scholar]
- 47. van Oort B., Maréchal A., Ruban A. V., Robert B., Pascal A. A., de Ruiter N., van Grondelle R., van Amerongen H. (2011) Phys. Chem. Chem. Phys., in press [DOI] [PubMed] [Google Scholar]