Abstract
The immunoglobulin (Ig) constant CH2 domain is critical for antibody effector functions. Isolated CH2 domains are promising scaffolds for construction of libraries containing diverse binders that could also confer some effector functions. We have shown previously that an isolated human CH2 domain is relatively unstable to thermally induced unfolding, but its stability can be improved by engineering an additional disulfide bond (Gong, R., Vu, B. K., Feng, Y., Prieto, D. A., Dyba, M. A., Walsh, J. D., Prabakaran, P., Veenstra, T. D., Tarasov, S. G., Ishima, R., and Dimitrov, D. S. (2009) J. Biol. Chem. 284, 14203–14210). We have hypothesized that the stability of this engineered antibody domain could be further increased by removing unstructured residues. To test our hypothesis, we removed the seven N-terminal residues that are in a random coil as suggested by our analysis of the isolated CH2 crystal structure and NMR data. The resulting shortened engineered CH2 (m01s) was highly soluble, monomeric, and remarkably stable, with a melting temperature (Tm) of 82.6 °C, which is about 10 and 30 °C higher than those of the original stabilized CH2 (m01) and CH2, respectively. m01s and m01 were more resistant to protease digestion than CH2. A newly identified anti-CH2 antibody that recognizes a conformational epitope bound to m01s significantly better (>10-fold higher affinity) than to CH2 and slightly better than to m01. m01s bound to a recombinant soluble human neonatal Fc receptor at pH 6.0 more strongly than CH2. These data suggest that shortening the m01 N terminus significantly increases stability without disrupting its conformation and that our approach for increasing stability and decreasing size by removing unstructured regions may also apply to other proteins.
Keywords: Antibodies, Circular Dichroism (CD), Crystal Structure, NMR, Receptors, Fc Neonatal Receptor, Engineered Antibody Domains, Immunoglobulin, Nanoantibodies, Stability
Introduction
Monoclonal antibodies are now well established therapeutics and invaluable tools for biological research (1). A major problem for full-size monoclonal antibodies is their poor penetration into some tissues (e.g. solid tumors) and poor or absent binding to regions on the surface of some molecules (e.g. on the HIV envelope glycoprotein) that are accessible by molecules of smaller size. Antibody fragments, e.g. Fab fragments (∼60 kDa) or single-chain Fv fragments (20∼30 kDa), are significantly smaller than full-size antibodies (∼150 kDa) and have been used as imaging reagents and candidate therapeutics. Therefore, discovery of even smaller scaffolds, including engineered antibody domains, continues to be of major importance in the development of candidate therapeutics and imaging agents (2–4).
The second domain of the heavy chain constant regions, CH2, is unique among the other antibody domains in that it exhibits very weak carbohydrate-mediated interchain protein-protein interactions, in contrast to the extensive interchain interactions that occur between the other domains. The expression of mouse and human CH2 in bacteria, which does not support glycosylation, results in a monomeric domain (5, 6). We have proposed that the CH2 domain (CH2 of IgG, IgA, and IgD and CH3 of IgE and IgM) could be used as a scaffold and could offer additional advantages compared with engineered antibody domains based on other domains because it contains binding sites or portions of binding sites conferring effector and stability functions (7). Supporting this possibility is the finding that the half-life of human CH2 (∼70 h) in rabbits is much longer than that of CH3 and Fab (∼15 h), and CH2 might function to trigger the complement system (8, 9).
The native CH2 domain has significantly lower thermal stability compared with other small scaffolds such as the tenth type III domain of human fibronectin (5, 6, 10), which increases the probability of instability when engineering binding to antigens and enhanced effector functions. In the quest for a more stable CH2-based scaffold, we found previously that the stability of an isolated human IgG1 CH2 can be significantly increased by engineering an additional disulfide bond between the A and G strands (6). One of the newly developed mutants, denoted m01, exhibited significantly higher stability than wild-type CH2.
We have hypothesized that the stability of m01 could be further increased by removing unstructured terminal residues such as the seven N-terminal residues that are in a random coil as suggested by our analysis of the isolated CH2 crystal structure and NMR data (6, 11). To test our hypothesis, we removed these residues and characterized the resulting shortened engineered CH2 (m01s). m01s was remarkably stable, with a melting temperature (Tm) of 82.6 °C, which is about 10 and 30 °C higher than those of the original stabilized CH2 (m01) and CH2, respectively. To detect possible conformational changes in m01s compared with CH2 and m01, a novel anti-CH2 antibody (m01m1) that recognizes a conformational epitope was identified. It bound to m01s significantly better (>10-fold higher affinity) than to CH2 and slightly better than to m01. We also expressed CH2 and m01s on the yeast cell surface and compared their binding to a soluble human neonatal Fc receptor (shFcRn)2 (12). Interestingly, we found that the binding of m01s was stronger than that of CH2 in a pH-dependent manner.
On the basis of these data, we suggest that m01s could be used as a scaffold for development of engineered antibody domains. These results also demonstrate for the first time that the stability of constant antibody domains can be further increased by decreasing their size. Importantly, although shortening the m01 N terminus significantly increases stability, it retains or increases some other properties of CH2 (e.g. binding to shFcRn). The increase in stability of isolated domains may result in an increase in stability of larger antibody fragments, e.g. Fc, and therefore could have implications as a general method for increasing antibody stability. It may also apply to other proteins as a method to increase stability and decrease size.
EXPERIMENTAL PROCEDURES
m01 Mutant Design and Plasmid Construction
To design the m01 mutant, we used the isolated CH2 crystal structure and NMR data (6, 11). The truncated m01 mutant (denoted m01s) with the absence of seven residues in the N terminus was cloned into pComb3X (provided by Dennis Burton, The Scripps Research Institute, La Jolla, CA). The clone was verified by direct sequencing and used for transformation of the Escherichia coli strain HB2151. m01s was expressed and purified similarly to wild-type CH2 (6).
Size Exclusion Chromatography
Purified m01s was loaded into a HiLoad 26/60 Superdex 75 HR 10/30 column (GE Healthcare) running on an ÄKTA BASIC pH/C chromatography system (GE Healthcare) to assess possible oligomer formation. PBS (pH 7.4) was selected as the mobile phase. Gel filtration of standards consisting of aldolase (158 kDa), bovine serum albumin (67 kDa), ovalbumin (44 kDa), chymotrypsinogen A (25 kDa), and ribonuclease A (13.7 kDa) was used to define the molecular mass.
Circular Dichroism
The secondary structure of m01s was determined by CD spectroscopy. The purified proteins were diluted in PBS at a final concentration of 0.54 mg/ml, and the CD spectra were recorded on Aviv Model 202 CD spectrometer. Wavelength spectra were recorded at 25 °C using a 0.1-cm path length cuvette for native structure measurements. To measure the Tm, m01s was diluted in PBS (pH 7.4) with 0, 3, 3.5, 4, and 5 m urea, respectively. Thermal stabilities under different conditions with 0, 3, 3.5, 4, and 5 m urea were measured at 216 nm (in the absence of urea) and 225 nm (in the presence of urea at different concentrations) by recording the CD signal at a temperature range of 25–90 °C with a heating rate of 1 °C/min. The temperature was recorded with an external probe sensor, and the temperature inside the microcuvette was calculated by calibration; it was ∼2–3 °C (range of 1.9–4.0 °C for temperatures from 20 to 90 °C) lower that the one measured by the external sensor.
Limited Proteolysis
The purified CH2, m01, and m01s proteins were subjected to trypsin digestion at 1:5 (w/w) trypsin/protein for 0, 1.5, and 3 h at 37 °C. After digestion, loading buffer was added immediately to the samples to terminate digestion, and all samples were examined by SDS-PAGE.
Conformational Changes in m01s Detected by Anti-CH2 Fab m01m1
ELISA was used for comparison of the structures of CH2, m01, and m01s. Briefly, CH2, m01, m01s, and human serum albumin (negative control) were coated on 96-well plates at a concentration of 2 μg/ml. Anti-CH2 Fab m01m1 identified from a human Fab naïve library (13) by panning against isolated CH2 expressed in E. coli, which recognized the conformational epitope of CH2 (data not shown), was added at concentrations of 0.064–200 μg/ml. HRP-conjugated anti-Fab (Sigma) was used as the secondary antibody. To confirm the result, a biotin-conjugated commercial mouse anti-human CH2 monoclonal antibody (AbD Serotec) was also used for ELISA at concentrations of 0–10 μg/ml. HRP-streptavidin (Sigma) was used for detection of biotinylated mouse anti-human CH2 antibody.
Construction of CH2, m01s, Fc, and CH3 for Yeast Surface Expression
CH2, m01s, Fc, and CH3 were cloned into the pYD7 vector, a modified version of pCTCON2 (14), allowing expression of fusion proteins with agglutinin, Aga2p, located at the C terminus. The clones were verified by direct sequencing. The constructs were transformed into EBY100 cells for surface expression according to the protocol described previously (14).
Flow Cytometry Assay
For measurement of the binding of CH2, m01s, Fc, and CH3 to shFcRn, yeast cells containing pYD7-CH2, pYD7-m01s, pYD7-Fc, and pYD7-CH3 were grown in SDCAA medium (20 g/liter dextrose, 6.7 g/liter Difco yeast nitrogen base w/o amino acid, 5 g/liter Bacto casamino acids, 5.4 g/liter Na2HPO4, and 8.56 g/liter NaH2PO4·H2O), and expression was induced in SGCAA medium (20 g/liter galactose, 20 g/liter raffinose, 1 g/liter dextrose, 6.7 g/liter Difco yeast nitrogen base w/o amino acid, 5 g/liter Bacto casamino acids, 5.4 g Na2HPO4, and 8.56 g/liter NaH2PO4·H2O) according to published protocols (14). For shFcRn binding, 5 × 105 yeast cells were harvested, washed with PBSA (PBS + 0.1% bovine serum albumin at pH 6.0), and resuspended in 50 μl of PBSA (pH 6.0) containing 100 nm biotin-conjugated shFcRn. The samples was kept on ice for 2 h, and the cells were washed again with PBSA (pH 6.0) and resuspended in 50 μl of PBSA (pH 6.0). 1 μl of R-phycoerythrin-streptavidin (PE-streptavidin; Invitrogen) was added to the resuspended cells. After 30 min of incubation on ice, the cells were washed with PBSA (pH 6.0) and resuspended in 0.5 ml of PBSA (pH 6.0) for flow cytometry measurement. The mouse anti-human CH2 monoclonal antibody described above and Alexa Fluor 488-conjugated goat anti-mouse IgG (Invitrogen) were used to test the expression of CH2, m01s, and Fc on the yeast, whereas FITC-conjugated goat anti-human Fc polyclonal antibody (Sigma) was used to test the expression of CH3 on the yeast. The same samples prepared at pH 7.4 were used as controls.
Competition Flow Cytometry Assay
To verify the specificity of binding of m01s to shFcRn, human IgG1 was used as a competitor. 5 × 105 yeast cells expressing m01s were harvested, washed with PBSA (pH 6.0), and resuspended in 50 μl of PBSA (pH 6.0) containing 100 nm biotin-conjugated shFcRn and human IgG1 at serial concentrations (0, 125, 250, 500, 1000, 2000, and 4000 nm). The sample prepared at pH 7.4 with 100 nm biotin-conjugated shFcRn only was used as a control. Binding was then analyzed by the same method described above. The mean value was taken to calculate the inhibition efficiency.
RESULTS
Analysis of an Isolated CH2 Crystal Structure and NMR Data
We have previously solved the crystal structure of an isolated γ1 CH2 domain (11). Analysis of the CH2 N terminus suggested that the first seven residues form a random coil. Our previously reported NMR data (6) indicate that the very N-terminal residues exhibit increased flexibility. Therefore, we have hypothesized that the disordered N terminus with increased dynamics may contribute to a decreased thermal stability and that its removal may result in increased stability.
Design and Generation of an N-terminal Truncated Engineered CH2 Domain
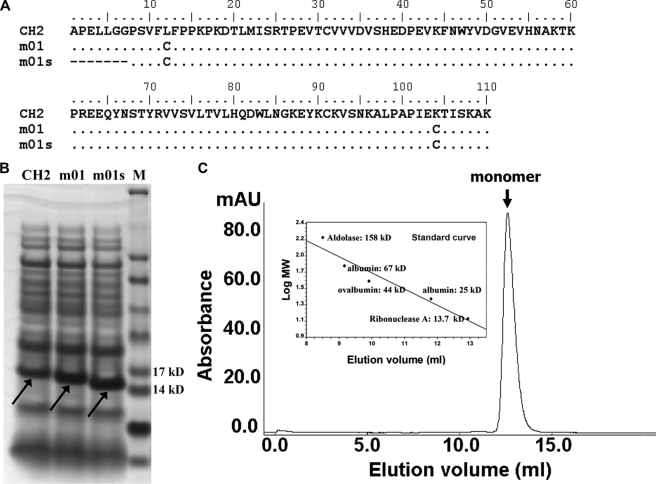
To test our hypothesis, we used a previously developed engineered CH2 domain (m01) in which an additional disulfide bond was introduced between the A and G strands, resulting in increased stability (6). The first seven N-terminal residues of m01 were deleted, resulting in a shorter variant of m01 termed m01s (Fig. 1A). m01s was expressed in E. coli at a higher level than that observed for either CH2 or m01 (Fig. 1B). m01s was completely monomeric in PBS at pH 7.4 as determined by size exclusion chromatography (Fig. 1C).
FIGURE 1.
Design, expression, and estimation of oligomer formation of m01s. A, amino acid sequence alignment of wide-type CH2 (NCBI accession number J00228), m01, and m01s. B, comparison of the expression of CH2, m01, and m01s. M, marker. C, size exclusion chromatography of m01s. The inset is a standard curve. mAU, milli-absorbance units.
m01 Is Significantly More Stable than CH2 and m01
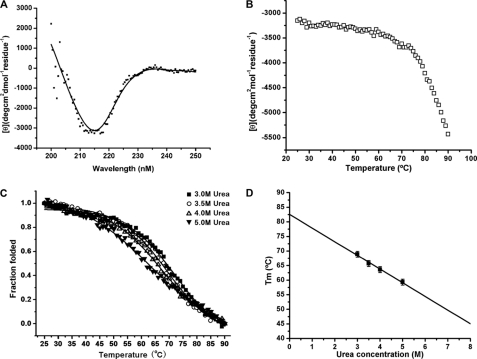
The secondary structure and thermodynamic stability of m01s were measured by CD. The CD spectra of m01s showed that it has high β-sheet content at 25 °C (Fig. 2A). The β-sheet structure was gradually disrupted, and the protein unfolded as the temperature increased (Fig. 2B). However, even at the highest temperature measured (90 °C), part of the protein was still in a folded state, and there was no second transition point (Fig. 2B). Therefore, we used an indirect approach to estimate the melting temperature at which 50% of the protein is in the folded state (Tm) using increasing concentrations (3, 3.5, 4, and 5 m) of urea to decrease the Tm and extrapolate to 0 m urea (Fig. 2C). The sigmoidal curves were fitted by a two-state model that was used previously (5). Tm was calculated at each urea concentration and fitted to a linear equation according to a previously described method (15): Tm = 82.6 − 4.7 Uc, where Uc is the urea concentration (Fig. 2D). Therefore, at Uc = 0, Tm equals 82.6 °C. This value is significantly higher than those previously measured by the same method for CH2 (54.1 °C) and m01 (73.4 °C) (6).
FIGURE 2.
Stability of m01s. A, CD spectra of m01s measured at 25 °C. m01s displayed a maximum negative peak between 210 and 225 nm, indicating a typical β-helical secondary structure. deg, degrees. B, thermo-induced unfolding of m01s in PBS without urea. The change in mean residue ellipticities was monitored at 216 nm. No second transition point was observed up to the highest temperature measured (90 °C). C, thermo-induced unfolding of m01s in PBS with different concentrations of urea (3, 3.5, 4, and 5 m). The fraction folded of the protein (ff) was calculated as ff = ([θ] − [θM])/([θT] − [θM]), where [θT] and [θM] are the mean residue ellipticities at 225 nm of the folded state at 25 °C and the unfolded state at 90 °C. The Tm values (68.9, 65.7, 63.6, and 59.3 °C correspond to 3, 3.5, 4, and 5 m urea, respectively) from CD were determined by the first derivative (d(fraction folded)/dT) with respect to temperature (T). D, calculation of the m01s Tm (82.6 °C) at 0 m urea.
m01s and m01 Are More Resistant to Protease (Trypsin) Digestion than CH2 but Are Equally Stable in Human Serum in Vitro
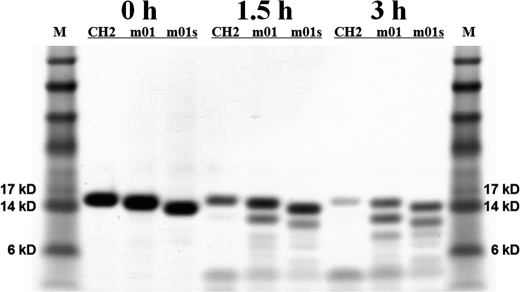
To evaluate the stabilities of CH2, m01, and m01s against digestion by a typical protease, trypsin was used as an example. Significantly larger amounts of CH2 were digested compared with m01 and m01s in 3 h (Fig. 3). We also estimated their stability in human serum by incubation at 37 °C for 9 days (see supplemental “Experimental Procedures”). No significant degradation was observed (supplemental Fig. S1). These data suggest that the stabilized variants of CH2 are more resistant to protease (trypsin) digestion than CH2 but are equally stable in human serum in vitro.
FIGURE 3.
Limited proteolysis of CH2, m01, and m01s by trypsin. After 3 h of digestion, m01 and m01s were partially digested, whereas CH2 was almost completely digested. M, marker.
Conformational Changes in m01s and m01 Demonstrated by a Newly Identified Anti-CH2 Fab, m01m1
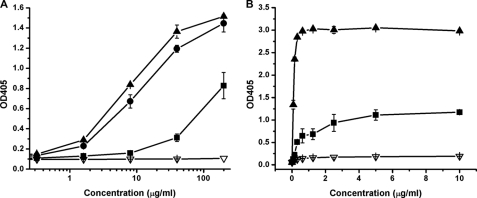
To determine whether the CH2 engineering resulted in conformational changes, we developed a human Fab, m01m1, specific for CH2. Fab m01m1 was identified by panning and screening of a Fab library against purified CH2 as described under “Experimental Procedures.” It was expressed in E. coli and purified. m01m1 bound to CH2 with relatively low affinity (EC50 > 1305 nm) (Fig. 4A). Interestingly, the addition of a disulfide bond in m01 and m01s resulted in a significant increase in binding: EC50 = 181 and 129 nm, respectively (Fig. 4A). The shortening of m01 led to a slight increase in m01m1 affinity (Fig. 4A). Similar results were obtained with a commercial mouse anti-human CH2 monoclonal antibody that binds a conformational epitope (Fig. 4B). These results indicate that some conformational epitopes are largely conserved in CH2, m01, and m01s, but these conformational epitopes are more exposed in the engineered CH2 domains than they are in CH2.
FIGURE 4.
Binding of CH2 (■), m01(●), m01s (▴), and human serum albumin (▿) to anti-human CH2 Fab m01m1 selected from a human naïve Fab library (A) and commercial mouse anti-human CH2 IgG (B). The EC50 values of Fab m01m1 for CH2, m01, and m01s were >1305, 181, and 129 nm, respectively, whereas the EC50 values of commercial mouse anti-human CH2 IgG for CH2 and m01s were 4.9 and 0.59 nm, respectively. In both cases, m01s could be better recognized by the antibodies (Fab and mouse IgG) compared with CH2.
Binding of shFcRn at pH 6.0 to m01s Is Stronger Than That to CH2
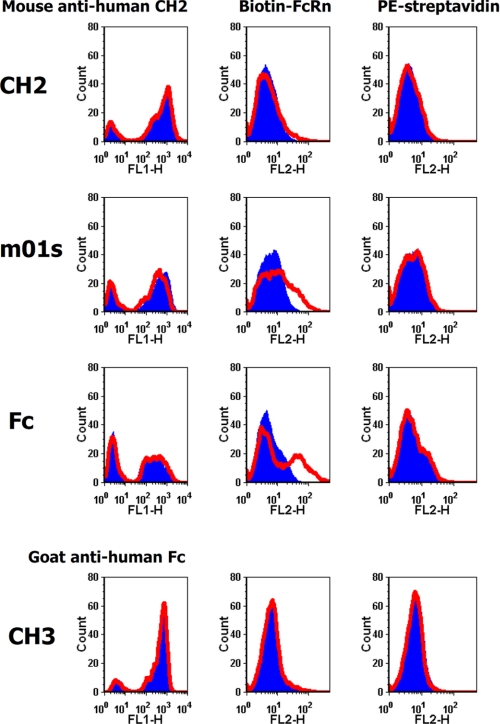
Recently, we developed a method to produce shFcRn in mammalian cells with high yield (12). We used this shFcRn to test its binding at pH 6.0 to CH2 and m01s expressed on yeast cells (Fig. 5). The fluorescence intensity shift for m01s was larger than that for CH2, indicating stronger binding of m01s to shFcRn compared with CH2. The largest fluorescence intensity shift in the case of Fc indicated that binding of shFcRn at pH 6.0 to Fc on yeast cells was stronger than that to m01s on yeast cells. No fluorescence intensity shift was observed for CH3 at pH 6.0, indicating that CH3 makes a minor contribution to the binding of Fc to shFcRn.
FIGURE 5.
Binding of yeast-expressed CH2, m01s, Fc, and CH3 to shFcRn at pH 6 (red) and pH 7.4 (blue). A very slight fluorescence intensity shift occurred in the case of CH2, indicating very weak binding to shFcRn. A modest fluorescence intensity shift was observed in the case of m01s, indicating modest binding to shFcRn. The largest fluorescence intensity shift was observed in the case of Fc, indicating strong binding to shFcRn. CH3 did not bind to shFcRn in a pH-dependent manner; there was no observable fluorescence intensity shift. The expression of CH2, m01s, Fc, and CH3 was tested by the corresponding antibodies. PE-streptavidin was used as negative control.
Binding of m01s to shFcRn Is Inhibited by Human IgG1
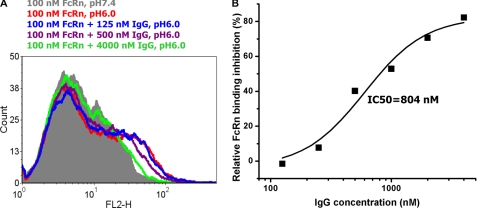
To test the specificity of binding of m01s to shFcRn at pH 6.0, IgG1 was used as a competitor in a flow cytometry assay. With an increase in the IgG1 concentration, the fluorescence intensity shift decreased (Fig. 6A). The half-maximal binding inhibition concentration (IC50) was 804 nm (Fig. 6B), which is consistent with a previous report (16). Therefore, as expected, m01s and IgG1 share the same binding site on shFcRn; this result also indicates that the binding of m01s to shFcRn at pH 6.0 is specific.
FIGURE 6.
Inhibition of yeast-expressed m01s binding to shFcRn by IgG1. A, fluorescence intensity shift in the presence of IgG1 at different concentrations. B, inhibition curve. Percent inhibition = ((meanmax at pH 6.0 − mean at pH 6.0)/(meanmax at pH 6.0 − mean at pH 7.4)) × 100, whereas meanmax at pH 6.0 is the mean value of the fluorescence intensity measured at pH 6.0 in the absence of IgG1, mean at pH 7.4 is the mean value of the fluorescence intensity measured at pH 7.4 in the absence of IgG1, and mean at pH 6.0 is mean value of the fluorescence intensity measured at pH 6.0 with different IgG1 concentrations. The binding decreased with an increase in the IgG1 concentration; IC50 = 804 nm.
DISCUSSION
The major findings of this study are that shortening an isolated unglycosylated engineered human γ1 CH2 domain significantly increased its stability without affecting some other properties and the identification of a new anti-CH2 Fab, m01m1, which is sensitive to CH2 conformational changes induced by an additional disulfide bond. The measured m01s Tm is the highest achieved for isolated CH2-based domains and comparable with that of the tenth type III domain of human fibronectin (10). We have not investigated possible molecular mechanisms that determine the higher stability of m01s compared with CH2 and m01. One can speculate that decreased dynamics at the N terminus is a major factor of the increased stability. The decreased size could also contribute. These results also indicate that the stability of other proteins could be increased by removing unstructured regions and decreasing size. The size dependence of folding and stability is in agreement with a previous analysis (17). The increased thermal stability combined with high solubility and expression levels and resistance to proteases are highly desirable properties for a candidate therapeutic protein based on the m01s scaffold. In addition, shortening CH2 in intact Fc and Ig could also result in an increase in their stability, and ongoing experiments are testing this possibility.
Naturally occurring additional disulfide bonds are found in camel single-domain antibody fragments (VHH fragments) (between CDR1 and CDR3) and in the shark IgNAR (Ig new antigen receptor) V domain (between CDR3 and frameworks) (18, 19). The thermostability of a single isolated antibody domain is typically increased by 10 °C after introduction of an additional disulfide bond (20). The strategy is similar to that used in the design of m01. It would be interesting to determine whether shortening such domains with a naturally occurring second disulfide bond and also in general other antibody domains could result in an increase in their stability.
We also found that shortening m01 (m01s) does not perturb, to any significant degree, some other properties, including binding to shFcRn. Because of the lack of CH3, the binding of m01s to shFcRn was relatively weak compared with whole Fc. However, interestingly, there was an increase in binding compared with CH2. We are currently further improving binding of m01s to shFcRn, which may lead to an extended half-life of m01s in vivo. However, the removal of N-terminal residues from CH2 may affect binding to Fcγ receptors and related effector functions. Further studies are in progress to elucidate this and other possible effects due to the N-terminal shortening, including binding to complement.
These findings could have implications for exploration of the unfolding mechanisms of antibody domains and for the development of candidate therapeutic proteins with increased stability and extended half-lives. Whether the observed increase in stability against temperature and chemical agents and binding to shFcRn in vitro will result in increased stability and long half-lives in vivo remains to be seen.
Supplementary Material
Acknowledgments
We thank Dr. Sergey G. Tarasov for helpful discussions, Marzena A. Dyba for technical support, and Dr. K. Dane Wittrup for providing plasmid pCTCON2.
This work was supported by the National Institutes of Health Intramural AIDS Targeted Antiviral Program (IATAP), the National Institutes of Health NIAID Intramural Biodefense Program, and the National Institutes of Health NCI Center for Cancer Research Intramural Research Program. This work was supported, in whole or in part, by federal funds from National Institutes of Health NCI Contract N01-CO-12400.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Fig. S1.
- shFcRn
- soluble human neonatal Fc receptor.
REFERENCES
- 1. Dimitrov D. S. (2010) mAbs 2, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kolmar H., Skerra A. (2008) FEBS J. 275, 2667. [DOI] [PubMed] [Google Scholar]
- 3. Skerra A. (2007) Curr. Opin. Biotechnol. 18, 295–304 [DOI] [PubMed] [Google Scholar]
- 4. Nygren P. A., Skerra A. (2004) J. Immunol. Methods 290, 3–28 [DOI] [PubMed] [Google Scholar]
- 5. Feige M. J., Walter S., Buchner J. (2004) J. Mol. Biol. 344, 107–118 [DOI] [PubMed] [Google Scholar]
- 6. Gong R., Vu B. K., Feng Y., Prieto D. A., Dyba M. A., Walsh J. D., Prabakaran P., Veenstra T. D., Tarasov S. G., Ishima R., Dimitrov D. S. (2009) J. Biol. Chem. 284, 14203–14210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dimitrov D. S. (2009) mAbs 1, 26–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yasmeen D., Ellerson J. R., Dorrington K. J., Painter R. H. (1976) J. Immunol. 116, 518–526 [PubMed] [Google Scholar]
- 9. Ellerson J. R., Yasmeen D., Painter R. H., Dorrington K. J. (1972) FEBS Lett. 24, 318–322 [DOI] [PubMed] [Google Scholar]
- 10. Hackel B. J., Kapila A., Wittrup K. D. (2008) J. Mol. Biol. 381, 1238–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prabakaran P., Vu B. K., Gan J., Feng Y., Dimitrov D. S., Ji X. (2008) Acta Crystallogr. D Biol. Crystallogr. 64, 1062–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng Y., Gong R., Dimitrov D. S. (2011) Protein Expr. Purif., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu Z., Dimitrov D. S. (2009) Methods Mol. Biol. 525, 129–142, xv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chao G., Lau W. L., Hackel B. J., Sazinsky S. L., Lippow S. M., Wittrup K. D. (2006) Nat. Protoc. 1, 755–768 [DOI] [PubMed] [Google Scholar]
- 15. Greene R. F., Jr., Pace C. N. (1974) J. Biol. Chem. 249, 5388–5393 [PubMed] [Google Scholar]
- 16. Suzuki T., Ishii-Watabe A., Tada M., Kobayashi T., Kanayasu-Toyoda T., Kawanishi T., Yamaguchi T. (2010) J. Immunol. 184, 1968–1976 [DOI] [PubMed] [Google Scholar]
- 17. De Sancho D., Doshi U., Muñoz V. (2009) J. Am. Chem. Soc. 131, 2074–2075 [DOI] [PubMed] [Google Scholar]
- 18. Saerens D., Pellis M., Loris R., Pardon E., Dumoulin M., Matagne A., Wyns L., Muyldermans S., Conrath K. (2005) J. Mol. Biol. 352, 597–607 [DOI] [PubMed] [Google Scholar]
- 19. Stanfield R. L., Dooley H., Flajnik M. F., Wilson I. A. (2004) Science 305, 1770–1773 [DOI] [PubMed] [Google Scholar]
- 20. Hagihara Y., Mine S., Uegaki K. (2007) J. Biol. Chem. 282, 36489–36495 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.