Abstract
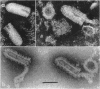
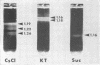
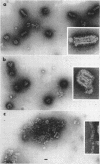
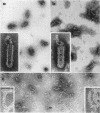
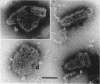
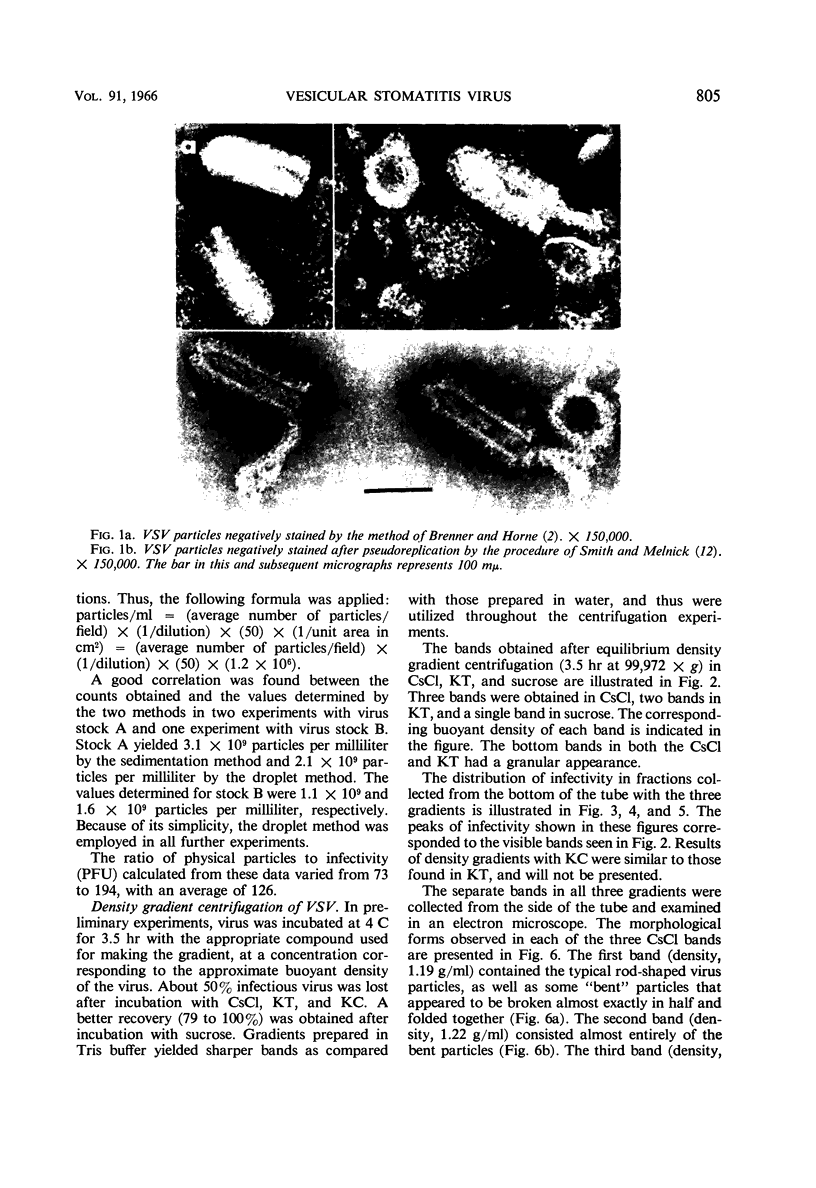
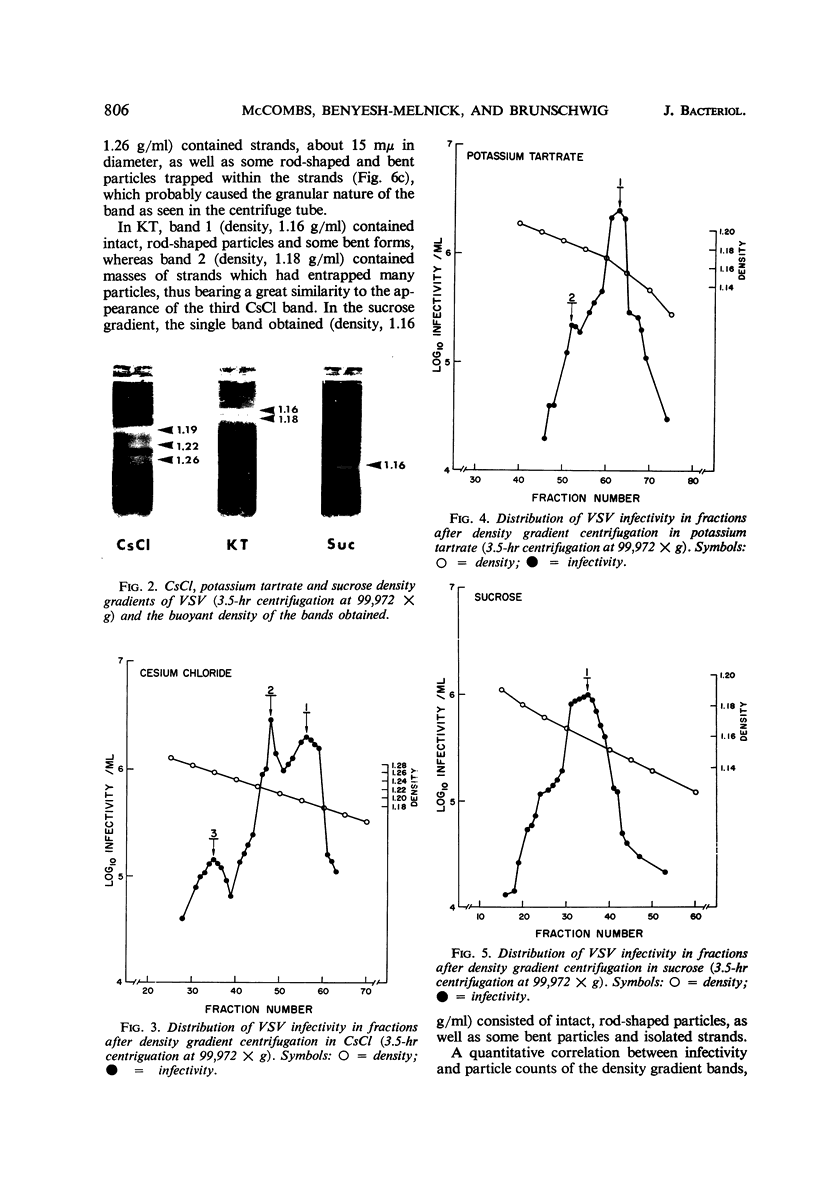
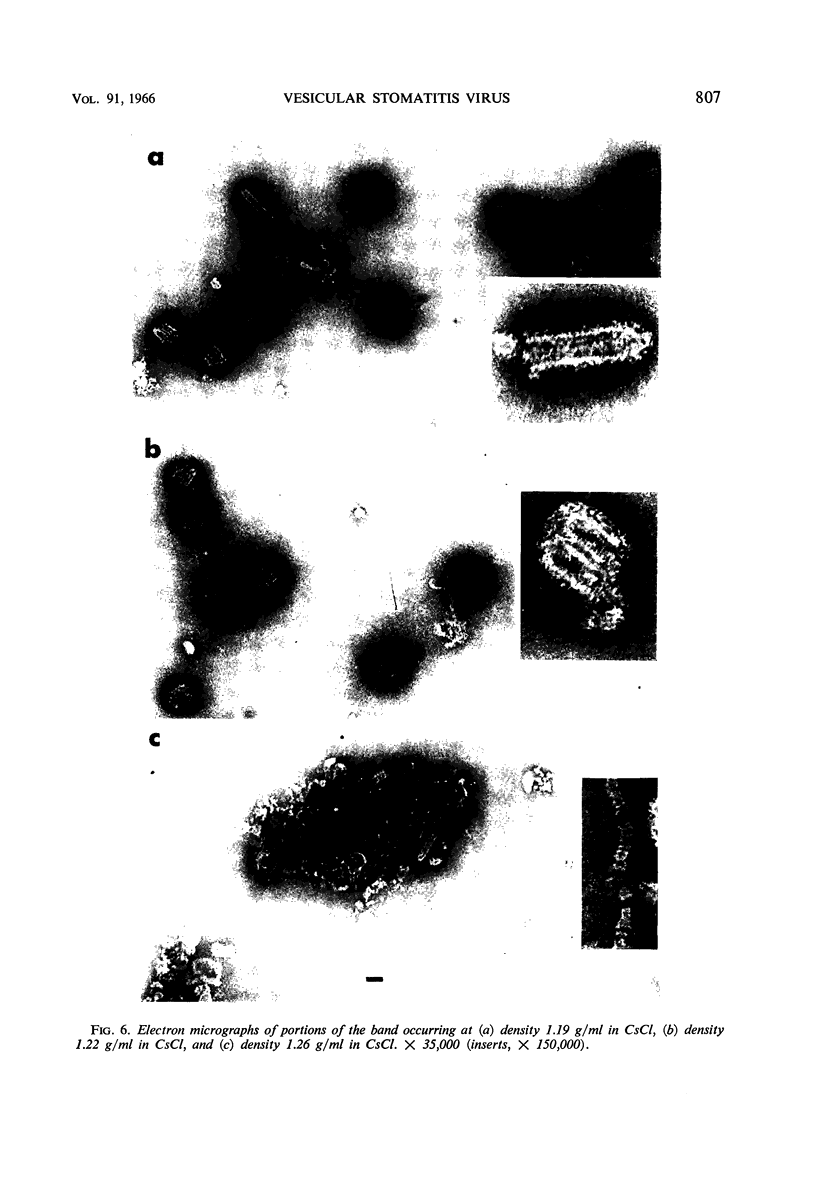
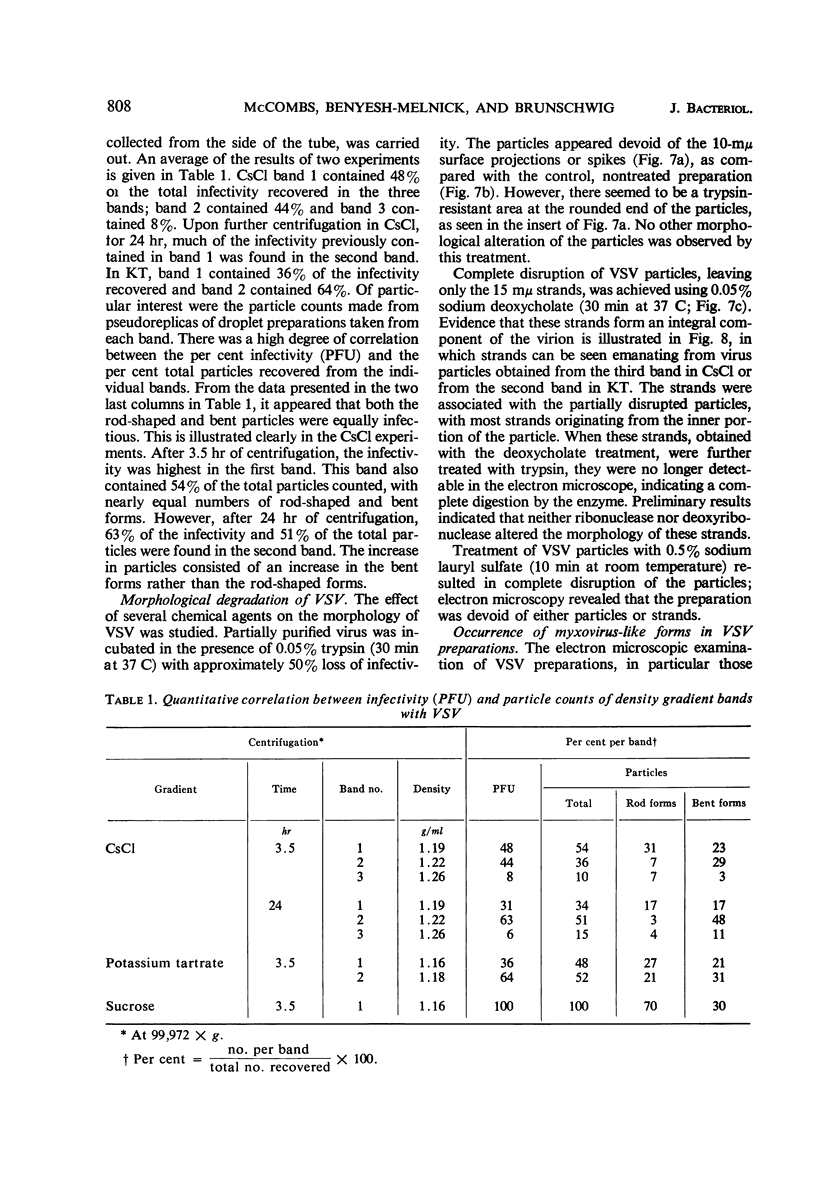
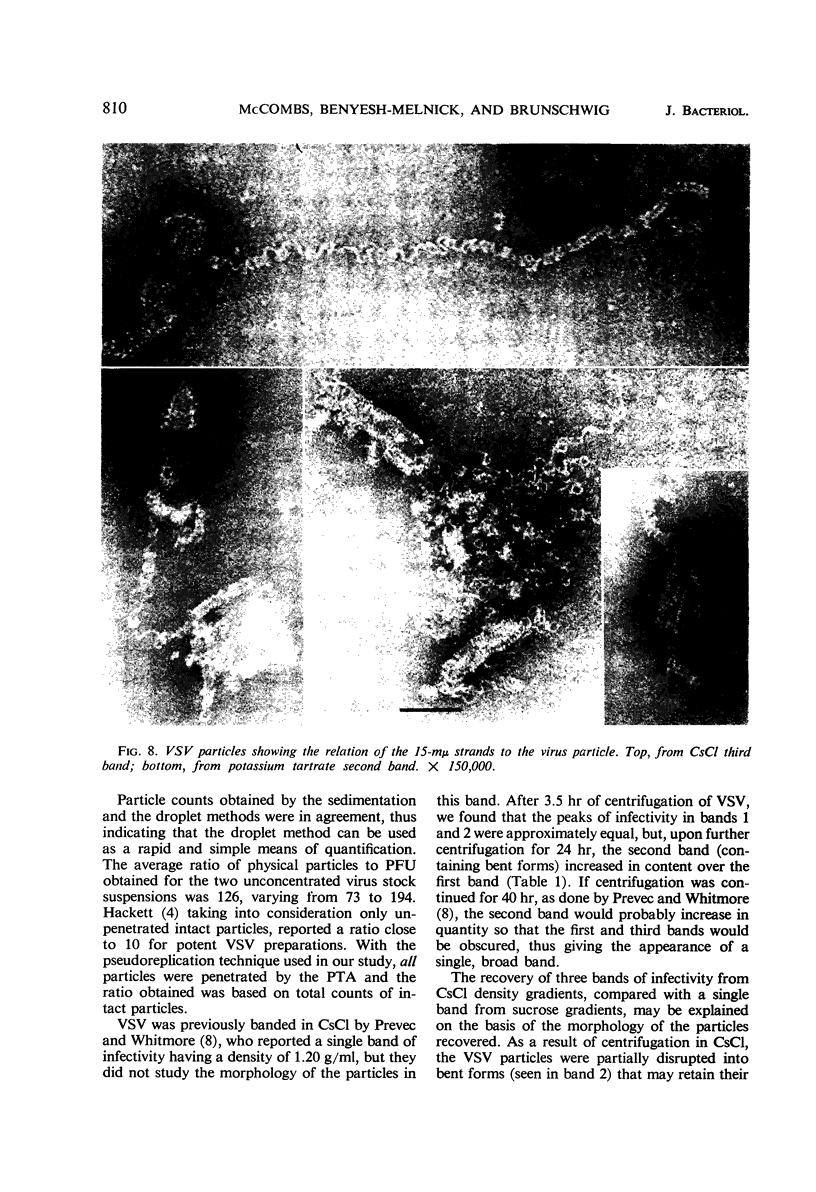
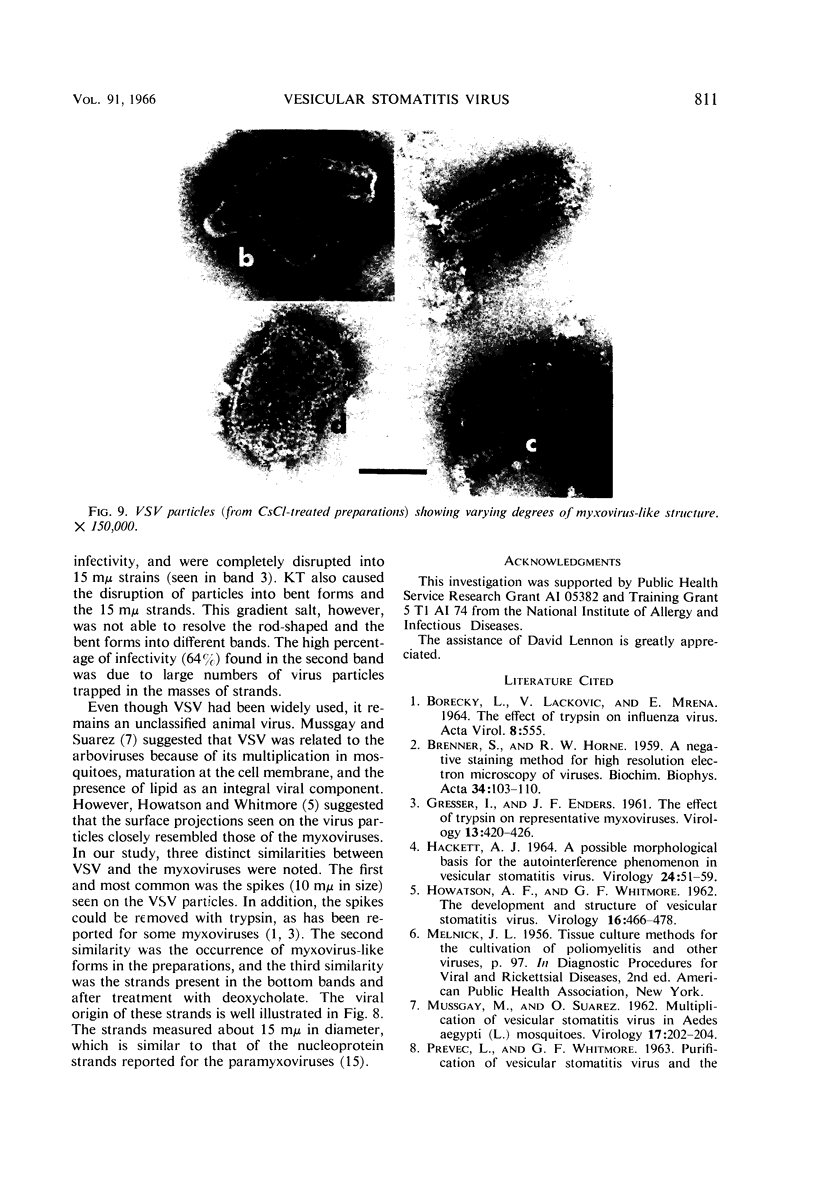
McCombs, Robert M. (Baylor University College of Medicine, Houston, Tex.), Matilda Benyesh-Melnick, and Jean P. Brunschwig. Biophysical studies of vesicular stomatitis virus: J. Bacteriol. 91:803–812. 1966.—The infectivity and morphology of vesicular stomatitis virus (VSV) were studied after density gradient centrifugation in cesium chloride (CsCI), potassium tartrate (KT), and sucrose. Centrifugation in CsCl revealed two equally infectious bands corresponding to densities of 1.19 and 1.22 g/ml, and a third (density, 1.26 g/ml) band of low infectivity. Two bands (densities of 1.16 and 1.18 g/ml) were observed in the KT gradient, in which the lighter band contained most of the infectivity. Centrifugation in sucrose resulted in a single broad infectious band (density, 1.16 g/ml). The typical rod-shaped VSV particles were found mainly in the lighter bands obtained in CsCl (1.19 g/ml) and KT (1.16 g/ml) and in the single sucrose gradient band (1.16 g/ml). Bent particles equally as infectious as the rod-shaped particles were a constant finding in the CsCl preparations, and were observed mainly in the second band (density, 1.19 g). Numerous strands 15mμ wide were found in the third CsCl (density, 1.26 g/ml) and the second KT (1.18 g/ml) bands. Similar strands could be liberated from VSV particles after treatment with deoxycholate. Internal transverse striations were found to be a regular feature of VSV particles examined with the pseudoreplication negative-staining technique. For crude virus stocks, the physical particle-to-infectivity ratio ranged from 73 to 194. Several morphological similarities between VSV and myxoviruses were observed, including 10 mμ surface projections, pleomorphic morphological forms, and 15 mμ seemingly nucleoprotein strands.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORECKY L., LACKOVIC V., MIRENA E. THE EFFECT OF TRYPSIN ON INFLUENZA VIRUS. Acta Virol. 1964 Nov;8:555–555. [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- GRESSER I., ENDERS J. F. The effect of trypsin on representative myxoviruses. Virology. 1961 Apr;13:420–426. doi: 10.1016/0042-6822(61)90273-2. [DOI] [PubMed] [Google Scholar]
- HACKETT A. J. A POSSIBLE MORPHOLOGIC BASIS FOR THE AUTOINTERFERENCE PHENOMENON IN VESICULAR STOMATITIS VIRUS. Virology. 1964 Sep;24:51–59. doi: 10.1016/0042-6822(64)90147-3. [DOI] [PubMed] [Google Scholar]
- HOWATSON A. F., WHITMORE G. F. The development and structure of vesicular stomatitis virus. Virology. 1962 Apr;16:466–478. doi: 10.1016/0042-6822(62)90228-3. [DOI] [PubMed] [Google Scholar]
- MUSSGAY M., SUAREZ O. Multiplication of vesicular stomatitis virus in Aedes aegypti (L.) mosquitoes. Virology. 1962 May;17:202–204. doi: 10.1016/0042-6822(62)90100-9. [DOI] [PubMed] [Google Scholar]
- RECZKO E. [Electron microscopic research on the virus of vesicular stomatitis]. Arch Gesamte Virusforsch. 1961;10:588–605. [PubMed] [Google Scholar]
- SMITH K. O., BENYESH-MELNICK M., FERNBACH D. J. STUDIES ON HUMAN LEUKEMIA. II. STRUCTURE AND QUANTITATION OF MYXOVIRUS-LIKE PARTICLES ASSOCIATED WITH HUMAN LEUKEMIA. J Natl Cancer Inst. 1964 Sep;33:557–570. [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. A method for staining virus particles and identifying their nucleic acid type in the electron microscope. Virology. 1962 Jul;17:480–490. doi: 10.1016/0042-6822(62)90143-5. [DOI] [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. Electron microscopic counting of virus particles by sedimentation on aluminized grids. J Immunol. 1962 Aug;89:279–284. [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L., BIANCHI M. Factors influencing enterovirus and reovirus growth and plaque formation. Tex Rep Biol Med. 1962;20:693–702. [PubMed] [Google Scholar]
- WATERSON A. P., CRUICKSHANK J. G. FORM OF THE NUCLEOPROTEIN COMPONENT AS A TAXONOMIC CRITERION FOR THE MYXOVIRUSES. Nature. 1964 Feb 8;201:640–641. doi: 10.1038/201640a0. [DOI] [PubMed] [Google Scholar]