Abstract
The exquisite specificity of the adenine-responsive riboswitch toward its cognate metabolite has been shown to arise from the formation of a Watson-Crick interaction between the adenine ligand and residue U65. A recent crystal structure of a U65C adenine aptamer variant has provided a rationale for the phylogenetic conservation observed at position 39 for purine aptamers. The G39-C65 variant adopts a compact ligand-free structure in which G39 is accommodated by the ligand binding site and is base-paired to the cytosine at position 65. Here, we demonstrate using a combination of biochemical and biophysical techniques that the G39-C65 base pair not only severely impairs ligand binding but also disrupts the functioning of the riboswitch in vivo by constitutively activating gene expression. Folding studies using single-molecule FRET revealed that the G39-C65 variant displays a low level of dynamic heterogeneity, a feature reminiscent of ligand-bound wild-type complexes. A restricted conformational freedom together with an ability to significantly fold in monovalent ions are exclusive to the G39-C65 variant. This work provides a mechanistic framework to rationalize the evolutionary exclusion of certain nucleotide combinations in favor of sequences that preserve ligand binding and gene regulation functionalities.
Keywords: Crystal Structure, Gene Regulation, RNA Folding, RNA Structure, Transcription Regulation, Riboswitch, Evolution, Single-molecule FRET
Introduction
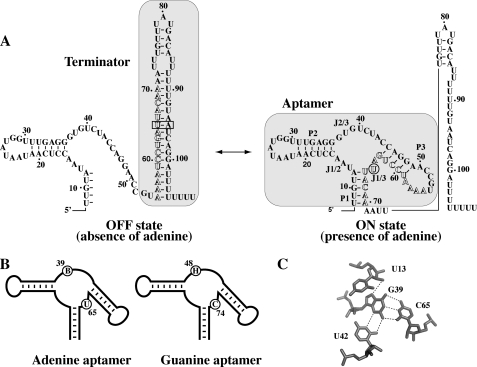
Riboswitches are naturally occurring genetic elements that regulate gene expression usually by binding to specific cellular metabolites (1–3). These genetic switches are highly structured domains that are mostly found in untranslated regions of prokaryotic and eukaryotic messenger RNA (4). Riboswitches are involved in the control of genes associated with the biosynthesis or transport of metabolites by using various cellular factors (5). They are composed of two modular domains corresponding to the aptamer and the expression platform. The aptamer domain is the most conserved region of the riboswitch and is involved in the specific recognition of the ligand. The expression platform can vary to a high degree in structure given that it regulates at various biological levels, such as transcription, translation, and splicing (1). Bioinformatics has recently revealed that more non-coding RNAs, some of them being riboswitches, are still very likely to be discovered (6, 7). Adenine and guanine riboswitches are members of the purine riboswitch class and are involved in the transport and regulation of purines (8, 9). The adenine riboswitch is an activating switch that positively modulates transcription elongation in response to adenine binding by preventing the formation of a Rho-independent transcription terminator (Fig. 1A). The pbuE gene codes for a purine efflux pump that presumably reduces the intracellular concentration of purines upon activation of the riboswitch. However, the guanine riboswitch negatively regulates transcription of the xpt-pbuX operon that is involved in the purine metabolism by promoting the formation of the transcription terminator in the presence of guanine (8, 10). Although adenine and guanine aptamers are very similar in their tertiary structure (11, 12), they exhibit exquisite selectivity toward their cognate metabolite (8, 9). The main difference between both aptamers is the residue at position 65 (position 74 according to the guanine riboswitch nomenclature (8) (Fig. 1B)), which dictates binding specificity through the formation of a Watson-Crick base pair with the ligand (9). According to previous studies, the identity of residue 65 does not have a significant effect on the structural organization of aptamers (11–13), limiting its role to the riboswitch specificity.
FIGURE 1.
A, schematic representing the ligand-induced pbuE adenine riboswitch transcription activation. Shaded regions represent the transcription terminator and the aptamer structure. Outlined letters represent nucleotides involved in the formation of both the terminator and the aptamer structures. The nucleotide U65 and the base pair U65-A95 are identified by a circle in the on state and by a rectangle in the off state, respectively. B, graphics representing the adenine and guanine aptamers. The sequence consensus of equivalent positions is shown for both the adenine and the guanine aptamers. The B consensus represents nucleotides C, G, and U, and the H consensus represents nucleotides A, C, and U. Consensus sequences were obtained here and from previous studies (14, 15). C, hydrogen bonding of G39 within the ligand binding site in the G39-C65 aptamer variant (16). Note that the variant adopts a very similar architecture when compared with the bound form of wild-type purine aptamers (11, 12).
Based on limited sequence alignment analysis, it was previously determined that both adenine and guanine riboswitches exhibit an unsuspected sequence restriction at the level of the core region, at position 39 (or 48 using the guanine riboswitch nomenclature; Fig. 1B). The adenine riboswitch shows a B consensus (C, G, and U), whereas the guanine riboswitch exhibits an H consensus (A, C, and U) at the corresponding position (Fig. 1B) (14, 15). Therefore, sequence combinations consisting of A39-U65 or G39-C65 do not naturally occur for the adenine and guanine riboswitches, respectively. It was experimentally shown that the presence of an A39-U65 combination perturbed ligand binding activity by factors of ∼5- and ∼14-fold in two naturally occurring adenine aptamers (15). However, a much more drastic effect was observed for the G39-C65 combination, which strongly inhibited ligand binding (14). Because the G39-C65 combination induced such a strong inhibitory effect, it was further studied by obtaining a crystal structure of a purine aptamer mutant exhibiting a G39-C65 combination (16). It was found that the presence of the base pair induces a ligand-free compact architecture resembling that of a purine-bound aptamer structure (Fig. 1C).
Here, we studied the impact of the evolutionarily excluded G39-C65 base pair on riboswitch folding and activity using a combination of biochemical and biophysical assays. Our data show that a riboswitch carrying a G39-C65 base pair exhibits reduced ligand binding affinity and perturbed gene regulation. The presence of the base pair induces a sufficient network of aptamer-core interactions to mimic those present in the wild-type aptamer-ligand complex, thus resulting in the riboswitch being constitutively activated. Interestingly, it was also found that the G39-C65 base pair constrains the conformational landscape of the aptamer sensor, bypassing intermediate states that are obligatory on the multistep folding pathway of the wild-type aptamer (17). Remarkably, the low level of dynamic heterogeneity found here for the folding and unfolding rates of the base pair variant under all experimental conditions resembles that seen for the wild-type adenine aptamer at saturating ligand concentrations. These findings are consistent with the idea that purine riboswitch aptamers have evolved to adopt ligand-free structures that preclude non-productive aptamer conformations.
EXPERIMENTAL PROCEDURES
Synthesis of RNA Molecules
Templates for transcriptions were made by PCR from synthetic DNA oligonucleotides (Sigma Genosys Canada). All transcribed RNA molecules begin with a 5′-GCG sequence to minimize the 5′-heterogeneity of the RNA population (18). RNA molecules containing fluorophores were purchased from Integrated DNA Technologies. Reconstituted dual-labeled fluorescent aptamers were prepared and purified as described previously (17).
Single-round in Vitro Transcription Assays
DNA templates for single-round in vitro transcriptions were prepared as described previously (19). Briefly, transcription reactions were performed using the Escherichia coli RNA polymerase from Epicenter Biotechnologies and used 300 fmol of DNA templates. Reactions were performed essentially as reported previously (19). Experiments have been conducted at least three times, and all exhibited very similar uncertainties (<10%). Mutations introduced are indicated in the text.
SHAPE Analysis
Wild-type and mutant riboswitch aptamers were prepared as described previously (19). The wild-type aptamer sequence used is the following (5′ to 3′ direction): GCGTTGTATAACCTCAATAATATGGTTTGAGGGTGTCTACCAGGAACCGTAAAATCCTGATTACAACGCAAAGCACTGCCACCTAGATGGTAG. Underlined letters correspond to the sequence added at the 3′-end to allow binding of a complementary DNA primer. The selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE)5 reaction was executed as described previously (19). Briefly, samples were allowed to fold in a solution of 0.33× Tris-EDTA, 100 mm K-HEPES, pH 8.0, 100 mm NaCl, 10 mm MgCl2, and the required concentration of ligand. SHAPE reactions were initiated by adding N-methylisatoic anhydride (Invitrogen) dissolved in dimethyl sulfoxide (DMSO), and the solution was allowed to react for 80 min at 37 °C. Reverse transcriptions were performed and analyzed as described previously (20).
Transcriptional β-Galactosidase Assays
Constructs were engineered from the wild-type pbuE sequence previously described (17). Transformed Bacillus subtilis cells were grown to mid-exponential phase with shaking at 37 °C in a minimal medium (17), and indicated ligands were added to a final concentration of 0.5 mg/ml. Cells at mid-exponential stage were resuspended in minimal medium in the absence or presence of ligands and were grown for an additional 3 h, at which time 200 μl of culture was assayed for β-galactosidase activity (17). Experiments have been performed at least three times, and the average as well as the S.D. are shown.
2-Aminopurine Fluorescence Spectroscopy
Fluorescence spectroscopy was performed as described previously (21). The fluorescence data were fitted to a simple two-state model that assumes an all-or-none conformational transition induced by the binding of magnesium ions, with a Hill coefficient n and an apparent association constant KA. The proportion of folded aptamer (α) is given by
As the parameters KA and n co-vary, we present the [Mg2+]½ = (1/KA)1/n, which gives a robust estimate for the affinity of magnesium ions (17, 22). The experiments have been performed at least three times, and all exhibited very similar uncertainties (<5%).
FRET Analysis
Fluorescence spectroscopy was performed on a Quanta Master fluorometer, and spectra were corrected for lamp fluctuations and instrument variations as described (17). Data were obtained at 4 °C in 1× Tris borate and the required concentration of magnesium ions. Fluorescein and Cy3 fluorophores were excited at 490 and 547 nm, respectively. Values of EFRET were determined using the acceptor normalization method (23, 24). Experiments were performed at least three times, and all exhibited very similar uncertainties (<5%).
Single-molecule FRET Analysis
Single-molecule FRET experiments were carried out as described previously (17). Briefly, Cy3 donor and Cy5 acceptor intensities were recorded from single immobilized aptamers using a total internal reflection setup equipped with a 532 nm laser and a back-illuminated EMCCD camera (Andor Technology, Belfast, Northern Ireland, UK). FRET trajectories were acquired with 50 ms unless stated otherwise. The buffer used for imaging was 50 mm Tris-HCl (pH 8.1), 6% (w/w) glucose, 1% 2-mercaptoethanol, 0.1 mg/ml glucose oxidase (Sigma), 0.02 mg/ml glucose catalase (Sigma). Specified concentrations of MgCl2 were included in the imaging buffer. Measurements were performed at room temperature (22 °C). Single-molecule FRET efficiency after background correction was approximated by IA/(IA+ID), where IA and ID are the fluorescence intensities of the acceptor and donor, respectively. Single-molecule FRET histograms were obtained by averaging the first 10 frames of each FRET trace for every individual molecule after manually filtering photobleaching and blinking effects. Rapidly fluctuating molecules undergo more transitions than slowly fluctuating ones, and thus to avoid bias toward fast rates, dwell time histograms were obtained by using a weighting factor inversely proportional to the number of transitions observed for each molecule. These dwell time histograms were then fitted to a single-exponential function to obtain the lifetimes of each.
NMR Spectroscopy
Preparation of 15N-labeled G39-C65 variant RNA for nuclear magnetic resonance (NMR) studies has been described previously (16). The purified RNA was first prepared in NMR buffer (10 mm sodium cacodylate (pH 6.5), 50 mm KCl, 0.05 mm NaN3, in 90% H2O, 10% D2O) supplemented with 5 mm MgCl2 and analyzed using a two-dimensional 1H-15N HSQC spectrum to verify proper folding (not shown). Subsequently, the RNA sample (0.34 mm) was exchanged in NMR buffer supplemented with 500 mm EDTA to remove MgCl2 before exchanging in NMR buffer with ∼5 μm EDTA using Amicon Ultra4 (3K) centrifugal filter devices. NMR experiments were collected on this sample at 288 K on a Varian UnityINOVA 600-MHz NMR spectrometer equipped with pulsed field gradient units and actively shielded z-gradient HCN triple resonance probes. The following NMR experiments were conducted: one-dimensional 15N-decoupled WATERGATE (25), two-dimensional 1H-15N HSQC (26); and three-dimensional 15N-edited NOESY-HSQC (τm = 120 ms) (27). 1H and 15N chemical shifts were referenced to an external standard of 2,2-dimethyl-2-silapentane-5-sulfonic acid at 0.00 ppm. NMR data were processed using the NMRPipe/NMRDraw package (28) and analyzed with NMRView (29).
RESULTS
Naturally Occurring Purine Aptamers Do Not Exhibit a G39-C65 Base Pair
The adenine and guanine aptamers fold into nearly identical tertiary structures, where each relies on similar nucleotides to coordinate ligand binding (11, 12). We previously determined the consensus sequence and secondary structures of adenine and guanine aptamers (14, 15) and concluded that both aptamers exhibit phylogenetic restrictions at position 39. Both NMR and crystallography data showed that a guanine at position 39 can base-pair with nucleotide C65, which is predicted to be highly detrimental for riboswitch gene regulation. Using the RNAMotif program (30) to expand the number of riboswitch representatives, we searched for sequence patterns matching that of the purine aptamer exhibiting a cytosine at position 65. A total of 789 sequences were obtained, from which a sequence alignment was produced (supplemental Fig. S1). As expected, among all sequences retrieved, no guanine was observed at position 39, strongly suggesting that the presence of a guanine at position 39 is not allowed in naturally occurring guanine riboswitches. Our findings are also supported by the absence of the G39-C65 combination in the 1,244 purine riboswitch sequences of the Rfam database (release 9.1).
The Presence of a G39-C65 Base Pair Mimics a Ligand-bound Aptamer and Reduces Ligand Affinity
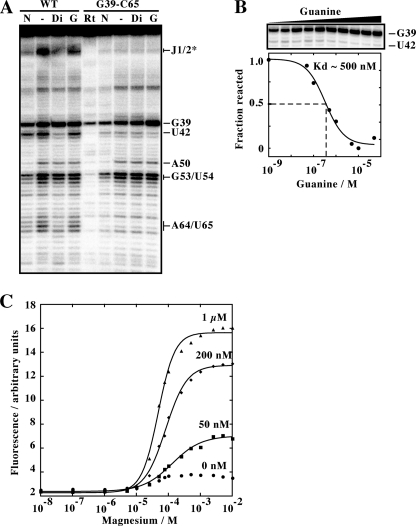
Using NMR spectroscopy and x-ray crystallography, we have previously determined that a purine aptamer having a G39-C65 mutation shows a compact structure mimicking the ligand-bound state of purine aptamers (16). To gain more insights into the effects of the G39-C65 base pair on the local architecture of the riboswitch aptamer and its influence on ligand binding, we performed SHAPE (31). This technique is useful to study local nucleotide flexibility, where 2′-OH groups located in flexible regions are prone to react with the electrophile N-methylisatoic anhydride (NMIA). SHAPE assays are an excellent way to study the ligand-induced structural rearrangements intrinsic to riboswitch function (32, 33). We first subjected the wild-type adenine aptamer to NMIA reaction as a function of ligand concentration. For this assay, we used 2,6-diaminopurine, which has been shown to exhibit very tight binding to the adenine aptamer (9). We observed clear differences in the core region of the aptamer (Fig. 2A). For example, strong NMIA protections were observed in the presence of diaminopurine in the J1/2 region and for nucleotides U42, A64, and U65, consistent with a ligand-induced reorganization of the aptamer, as observed previously (32). No SHAPE protection was observed in the presence of guanine (Fig. 2A). We next investigated an aptamer having a G39-C65 base pair using SHAPE assays. In the absence of ligand, a reactivity pattern very similar to the ligand-bound wild-type aptamer was obtained where the region J1/2 and nucleotides U42, A64, and U65 were protected from NMIA modification (Fig. 2A). No further differences in the reactivity pattern were observed upon the addition of diaminopurine. This absence of binding could be caused by the guanine specificity of the aptamer or by an inefficient competition of the ligand with the G39-C65 base pair or both. Nevertheless, our results obtained in the absence of ligand strongly indicate that the aptamer variant mimics the ligand-bound state of the wild-type molecule, which is in excellent agreement with our previously reported crystal structure of the G39-C65 aptamer (16).
FIGURE 2.
A, SHAPE modification of the adenine aptamer and the related G39-C65 variant. Reactions were performed in the absence of ligand (−) or in the presence of 10 μm of 2,6-diaminopurine (Di) or guanine (G). Positions where NMIA reaction was observed are indicated on the right. N represents a reaction in which NMIA was substituted by dimethyl sulforide. Nucleotide identification is based on sequencing reactions as reported previously (32). The asterisk indicates a region assigned based on a previous study (32). Rt represents a primer extension performed using unreacted RNA. B, SHAPE modification of the G39-C65 variant as a function of the guanine concentration. The concentrations used are 0 nm, 1 nm, 10 nm, 50 nm, 0.1 μm, 0.5 μm, 1 μm, 5 μm, 10 μm, and 50 μm. A plot depicting the normalized fraction of reacted RNA versus the guanine concentration reveals an apparent Kd value of ∼500 nm, which is 100-fold higher than reported for the xpt guanine aptamer (8). C, plot of normalized 2AP fluorescence as a function of Mg2+ concentration. Titrations were performed at the indicated concentrations of adenine. Note that the observed change in the absence of adenine is not significant enough to allow informative data to be extracted from curve fitting.
The addition of guanine resulted in SHAPE reactivity changes for positions G39 and U42 (Fig. 2A), suggesting the formation of a complex between guanine and the aptamer variant. Because the presence of the G39-C65 base pair is expected to reduce affinity for guanine, we monitored the reactivity of position G39 as a function of guanine concentration using SHAPE assays (Fig. 2B). By normalizing the reactivity of G39, we determined an apparent dissociation constant of ∼500 nm, which represents a decrease in affinity of 100-fold when compared with the wild-type guanine aptamer (8). Thus, although the G39-C65 base pair does not completely prevent ligand binding, its presence strongly perturbs the formation of a riboswitch-ligand complex, in agreement with our previous findings (15, 16).
Residue 39 Becomes Solvent-exposed upon Ligand Binding
In the ligand-bound state of the wild-type adenine aptamer, position 39 is completely exposed to the solvent (11), thus making the way nucleotides 39 and 65 can form a Watson-Crick base pair intriguing in the context of the variant. To gain further knowledge about the local environment of position 39, we introduced the purine analog 2-aminopurine (2AP) at position 39 and monitored the fluorescence of the wild-type aptamer as a function of magnesium ions (Fig. 2C). 2AP fluorescence is a widely used technique to investigate changes in the local environment of the 2AP probe when incorporated in a DNA or RNA sequence. Because its fluorescence strongly decreases upon interaction with surrounding bases, variation in fluorescence intensity can report local changes in the RNA structure (34).
In the absence of ligand, a slight increase in fluorescence was observed when titrating the Mg2+ concentration. However, when the titration was performed in the presence of 50 nm adenine, a significant increase of 2AP fluorescence was detected (Fig. 2C). Further increase of the ligand concentration resulted in higher fluorescence, consistent with 2AP becoming more exposed to the solvent upon adenine binding. The fluorescence data were fitted using a two-state model that allowed us to estimate values for apparent Mg2+ binding parameters. Using the model, apparent affinities for magnesium ions [Mg2+]½ were found to decrease as a function of adenine concentration, suggesting that adenine assists Mg2+ binding to the aptamer (supplemental Table S1). In addition, the Hill coefficient increased to a value of 1.7 ± 0.1 at 1 μm adenine. This suggests a cooperative role for adenine in the Mg2+-induced folding of the aptamer core region.
The G39-C65 Interaction Promotes Transcription Elongation of the pbuE Riboswitch
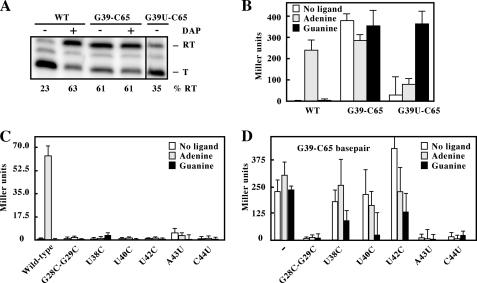
According to the pbuE riboswitch regulation mechanism, adenine binding to the riboswitch results in transcription elongation to allow gene expression (Fig. 1A). As a way to address the influence of the G39-C65 base pair on riboswitch activity, we developed an in vitro transcription assay where the proportion of elongated transcripts is analyzed as a function of experimental conditions. The assay was performed in single-round conditions in which reactions were done by using E. coli RNA polymerase as a function of ligand (19). As expected, when using the wild-type riboswitch, a very low fraction (23%) of transcription elongation was obtained in the absence of ligand (Fig. 3A). However, in the presence of 2,6-diaminopurine, a high percentage of transcription elongation products was obtained (63%), consistent with previous results showing that 2,6-diaminopurine efficiently binds to the aptamer and activates riboswitch transcription elongation (9, 19). In contrast to the wild-type riboswitch, the G39-C65 variant showed efficient transcription elongation (61%), both in the absence and in the presence of the ligand (Fig. 3A), suggesting that the G39-C65 base pair provides a constitutive activity to the riboswitch. Because the presence of the base pair introduces a mismatch in the terminator stem (Fig. 1A), the observed transcription elongation could result from the terminator stem being destabilized by the C65-A95 mismatch. To determine the relative importance of the mismatch, we introduced a G39U mutation as a way to disrupt the G39-C65 base pair while keeping the mismatch intact. When we performed the transcription assay using this variant, a low percentage (35%) of full-length transcripts was observed in the absence of ligand, suggesting that the presence of the terminator mismatch does not result in high levels of transcription elongation. These results confirm that the G39-C65 base pair is responsible for the constitutive transcription elongation activity of the riboswitch.
FIGURE 3.
A, single-round in vitro transcriptions performed for wild-type (WT), G39-C65 base pair, and G39U-C65 riboswitch variants. Reactions were carried out in the absence (−) of ligand and in the presence of 10 μm 2,6-diaminopurine (DAP; +). Two portions of the same gel, separated by a black line, were juxtaposed for better comparison. Readthrough (RT) and terminated (T) products are indicated on the right. Percentages of readthrough (% RT) products are indicated below the gel. B, β-galactosidase assays of the WT, G39-C65 base pair, and G39U-C65 constructs. Enzymatic activities were assayed in B. subtilis grown in minimal medium in the absence or presence of the indicated ligands (0.5 mg/ml) after an incubation of 3 h. Each experiment was performed three times, and the average as well as the S.D. are shown. C, β-galactosidase assays of the wild type and selected riboswitch mutants. The assays were performed in the absence or presence of the indicated ligands. D, β-galactosidase assays of the G39-C65 variant (−−) and selected mutants in the context of the G39-C65 riboswitch. The assays were performed in the absence or presence of the indicated ligands.
The G39-C65 Interaction Constitutively Activates the pbuE Riboswitch in Vivo
We and others have previously shown that the pbuE riboswitch activates gene expression in the presence of adenine (9, 17). By using transcriptional fusions in which the sequence of the pbuE riboswitch is fused to a β-galactosidase reporter gene in B. subtilis, we studied the impact of the G39-C65 base pair on the riboswitch activity in vivo (Fig. 3B). When growing cells in minimal medium in the absence of adenine, no significant β-galactosidase was observed for the wild-type riboswitch. However, the addition of adenine resulted in an increased enzymatic activity, consistent with the riboswitch positively modulating gene expression. No such effect was observed using guanine (Fig. 3B), as shown previously (9, 17). We next studied the G39-C65 riboswitch and observed a constitutive expression of the β-galactosidase regardless of the ligands used (Fig. 3B). These results are consistent with the idea that the G39-C65 base pair confers to the riboswitch a constitutive activity, as determined from single-round in vitro transcriptions (Fig. 3A). No constitutive expression was obtained when using a mutant not allowing the G39-C65 interaction (G39U-C65; Fig. 3B). Instead, an increase in enzymatic activity was only observed in the presence of guanine (Fig. 3B), consistent with the idea that the disruption of the G39-C65 base pair results in a guanine-responsive riboswitch. Together, these results suggest that the presence of the G39-C65 base pair makes the aptamer adopt a ligand-bound state in vivo, resulting in the constitutive activation of the riboswitch.
The G39-C65 Interaction Does Not Rely on Ligand Binding Determinants
The ligand binding site of purine aptamers is very conserved and exhibits a high degree of structural complexity, required to recognize the ligand with high affinity and specificity (11, 12). The bound ligand participates with residues U38 and U42 to constitute a base quadruple (Fig. 1C), which is stabilized on both sides by base stacking (11). The binding site also relies on additional tertiary interactions, such as the residue U40, which is involved with the U11-A67 base pair of the P1 stem.
To explore the influence of the G39-C65 base pair on riboswitch activity, we performed a mutational analysis on the riboswitch core. Given that no study had previously addressed the functional importance of riboswitch core nucleotides in vivo, we first introduced mutations in the context of the wild-type riboswitch with the goal of disrupting specific tertiary interactions. We first destabilized the loop-loop interaction by introducing G28C/G29C mutations. As expected, no ligand-dependent gene expression was observed when compared with the wild-type riboswitch (Fig. 3C and supplemental Fig. S2). We next performed transition mutations for nucleotides U38, U40, and U42, which are located in the aptamer core region and interact with either the ligand or the P1 stem (11, 12). None of the mutants showed a ligand-dependent β-galactosidase activation, reinforcing the importance of U38, U40, and U42 in the formation of an active riboswitch-ligand complex. We also assessed the importance of two Watson-Crick base pairs U13-A43 and G37-C44 taking place in the core region. The disruption of either base pair by mutations resulted in the complete loss of riboswitch activity, clearly indicating their importance for riboswitch function.
We next repeated the mutational analysis in the context of a G39-C65 riboswitch. As we have found for the wild type, the disruption of the loop-loop interaction was detrimental for the riboswitch activity (Fig. 3D). However, incorporation of single-point mutations U38C, U40C, or U42C resulted in a ligand-independent riboswitch activity (Fig. 3D and supplemental Fig. S2), consistent with the idea that the G39-C65 base pair does not rely on nucleotides involved in ligand binding. Guanine-induced gene repression was observed for mutations U38C and U40C, suggesting that these double mutants restored binding activity to some degree. No significant β-galactosidase expression was observed when disrupting the base pairs U13-A43 and G37-C44, suggesting their importance in the adoption of the active structure (Fig. 3D). Together, these results show that the effect of the G39-C65 base pair on riboswitch activity is not dependent on interactions occurring between the aptamer and the bound ligand in the wild-type context, but rather on interactions involved in the aptamer architecture (e.g. loop-loop interaction).
The G39-C65 Base Pair Modifies the Folding Pathway of the Riboswitch Aptamer
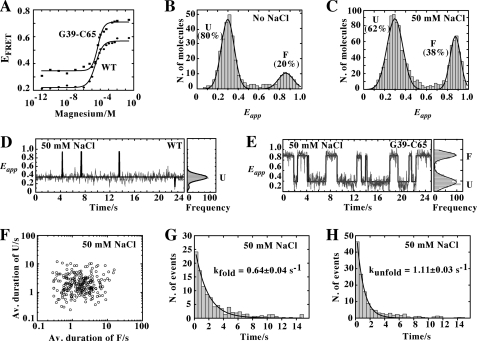
Using a combination of ensemble and single-molecule FRET techniques, we have previously studied the formation of the loop-loop interaction for the adenine aptamer (17). We found that this interaction is promoted by the presence of adenine, suggesting that ligand binding stabilizes the folded state. Here, using a similar approach, we have investigated the influence of the G39-C65 base pair on the dynamics of the loop-loop interaction. In our FRET assays, we introduced donor and acceptor fluorophores in the P2 and P3 loops, respectively, and monitored the loop-loop formation as a function of magnesium concentration. The FRET efficiency was first measured in steady state as a function of Mg2+ concentration. When compared with the wild-type aptamer, the G39-C65 variant exhibited a similar Mg2+-induced increase in FRET efficiency (Fig. 4A), consistent with the formation of the loop-loop interaction. However, the G39-C65 aptamer showed higher FRET efficiency at all Mg2+ concentrations, suggesting more efficient folding when compared with the wild-type molecule. These results are reminiscent of what was observed for the adenine riboswitch where ligand binding was found to increase folding efficiency (17).
FIGURE 4.
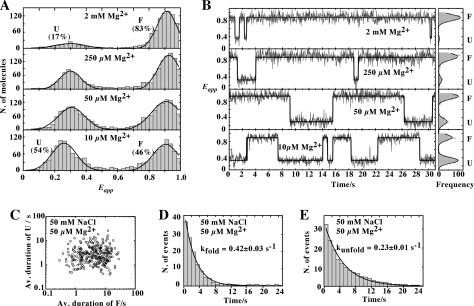
A, variation in FRET efficiency as a function of Mg2+ concentration for the wild-type (circles) and the G39-C65 (squares) aptamers. The experimental data were fitted (lines) to a two-state model where the binding of ions to the aptamer induces loop-loop formation. B, single-molecule FRET histogram of the G39-C65 aptamer in the absence of NaCl showing two populations centered at Eapp ∼0.3 and Eapp ∼0.85 corresponding to the U and F states, respectively. The single-molecule histogram was fitted using a two-Gaussian model to extract the relative contribution of each population. C, single-molecule FRET histogram in the presence of 50 mm NaCl showing two populations with mean FRET values centered at Eapp ∼0.3 (U) and ∼0.85 (F). D, single-molecule FRET trajectories for the wild-type aptamer (33 ms of integration time) obtained at 50 mm NaCl showing that the aptamer remains most of the time in the U state with occasional short-lived fluctuations to the folded state F. Corresponding FRET histograms are shown on the right. E, single-molecule FRET trajectories for the G39-C65 aptamer obtained at 50 mm NaCl showing the interconversion between U and F states. F, scatter plot showing the average duration (Av. duration) of U and F states from 250 molecules at 50 mm NaCl. G and H, dwell time histograms of the unfolded (G) and folded state (H) in the presence of 50 mm NaCl. The data have been fitted to single-exponential decay functions to give the rates of folding (G) and unfolding (H).
The observed increase in average FRET efficiency obtained at low Mg2+ concentration for the aptamer variant (EFRET ∼0.35), when compared with the wild-type structure (EFRET ∼0.21) (Fig. 4A), could result either from a different structural organization in the unfolded state or from an increased population of the folded state. To evaluate this, we investigated at the single-molecule level whether the presence of Na+ in the reaction buffer could promote a significant folding of the aptamer variant. 5′-Biotinylated molecules were immobilized on a quartz slide via biotin-streptavidin interactions and mounted on a wide-field total internal reflection microscope (see “Experimental Procedures”). We first tested experimental conditions in the absence of added Na+ and obtained a FRET histogram that showed a main peak centered at Eapp ∼0.3 (80%) and another one of minor contribution centered at Eapp ∼0.85 (20%; Fig. 4B). However, when the concentration of Na+ was increased to 50 mm, the contributions of the two peaks were significantly changed (Fig. 4C), with peaks centered at Eapp ∼0.3 (68%) and Eapp ∼0.85 (38%). Given that very similar FRET values were obtained for the wild-type aptamer (17), we assigned the FRET populations centered at Eapp ∼0.3 and Eapp ∼0.85 to the unfolded (U) and folded (F) states. These values indicate that global conformations of the U and F states are similar in both the wild-type and the G39-C65 aptamers. Contrary to the wild-type molecule (Fig. 4D), single-molecule time trajectories of the G39-C65 variant showed frequent transitions between the U and F states in the presence of only monovalent ions (Fig. 4E and supplemental Fig. S3), clearly showing its ability to fold in the absence of Mg2+. Moreover, the G39-C65 variant showed direct interconversion between the U and F states with no evidence for an intermediate state, in contrast to the wild-type aptamer (17). The absence of this intermediate state was tested at all ionic conditions and confirmed even at the highest time resolution of our single-molecule total internal reflection microscope (8 ms).
We next evaluated the average dwell time of the U and F states for 250 aptamer molecules in a background of 50 mm Na+ (Fig. 4F). We have previously used a similar approach to quantify the folding heterogeneity of the ligand-free and ligand-bound states of the adenine aptamer (17). In these experiments, we monitor the formation of the docking and undocking processes of the loop-loop interaction, which correspond to the folding and unfolding transitions of the interaction, respectively. The two-dimensional scatter plots of the mean dwell times at 50 mm Na+ revealed an ∼50-fold heterogeneity in the average folding and unfolding rates (Fig. 4F). Dwell time histograms were calculated for the unfolded and folded aptamer states (Fig. 4, G and H, respectively). The histograms were fitted to single-exponential decay functions, giving rate constants of kfold = 0.64 ± 0.04 s−1 and kunfold = 1.11 ± 0.03 s−1 for the docking and undocking processes, respectively. Using these rate constants, we obtained an equilibrium constant of 1.8 and a value of free energy of 0.3 kcal mol−1, similar to that previously observed for the wild-type aptamer at saturating adenine and Mg2+ concentrations (17). These results suggest that the presence of the G39-C65 base pair confers to the aptamer variant dynamic properties that are similar to those obtained for the wild-type structure bound to the adenine ligand (17).
We next studied the influence of magnesium ions on the folding of the loop-loop interaction in the presence of the G39-C65 base pair. As the concentration of Mg2+ was increased, the proportion of the U state decreased concomitantly with an increase in the proportion of the F state (Fig. 5A). The single-molecule FRET trajectories showed a significant increase in the lifetime of the F state as Mg2+ concentration was raised (Fig. 5B). We also examined the folding heterogeneity of the G39-C65 aptamer in the presence of 50 μm magnesium ions. As similarly obtained in the absence of Mg2+, the two-dimensional scatter plots of the mean dwell times revealed an ∼50-fold heterogeneity in the average folding and unfolding rates (Fig. 5C). The histograms were fitted to single-exponential decay functions, giving rate constants of kfold = 0.42 ± 0.03 s−1 and kunfold = 0.23 ± 0.01 s−1 (Fig. 5, D and E, respectively), showing that Mg2+ stabilizes the F state mostly by decreasing the unfolding rate by a factor of 5-fold.
FIGURE 5.
A, single-molecule FRET histograms of the G39-C65 aptamer as a function of Mg2+. The two-Gaussian fits are shown for each histogram. Individual time traces were manually filtered to remove blinking and photobleaching events. B, representative single-molecule FRET trajectories (33 ms of time resolution) at the specified Mg2+ concentrations. Corresponding FRET histograms for each trajectory are shown on the right. C, scatter plot showing the average duration (Av. duration) of U and F states from 300 molecules at 50 μm Mg2+. D and E, dwell time histograms of the unfolded (D) and folded state (E) in the presence of 50 μm Mg2+. The data have been fitted to single-exponential decay functions to give the rates of folding (kfold) and unfolding (kunfold).
Imino NMR Spectroscopy of the G39-C65 Aptamer in the Presence of Na+
According to our FRET data, the formation of the loop-loop interaction occurs very efficiently in the context of the G39-C65 aptamer, which is particularly striking in the absence of Mg2+. To further characterize this aptamer variant in the absence of Mg2+, we employed the same imino NMR methods that we previously used to obtain high-resolution structural information of this RNA (16). The two-dimensional 1H-15N HSQC spectrum of the G39-C65 variant recorded in the absence of Mg2+ (supplemental Fig. S4) is very similar to that previously collected in the presence of Mg2+ (16), and all signals could be easily assigned based on the similarity of imino NMR data (chemical shifts and NOE; supplemental Fig. S4 and supplemental Tables S2 and S3). Overall, these data indicate that in the absence of Mg2+, the global conformation of the G39-C65 aptamer is identical to that observed in the presence of magnesium ions, including the stems, the loop-loop interaction, and the core.
DISCUSSION
Similarly to the ligand-bound form, the riboswitch ligand-free conformation is crucial for genetic regulation because it has to remain accessible for ligand binding by adopting structures compatible with metabolite recognition. Several studies focused on the characterization of these ligand-free states have revealed that nucleotide conservation may be important to prevent the aptamer from adopting non-permissive ligand binding states (16, 35, 36). In this regard, the presence of the G39-C65 base pair makes the unbound purine aptamer adopt a stable conformation that is highly detrimental for riboswitch function, providing a rationale for its absence across naturally occurring purine riboswitches. It is also interesting to note that despite its smaller but significant effect on ligand binding (15), the A39-U65 base pair is not found across purine aptamers according to the Rfam database (release 9.1), suggesting an evolutionary pressure to select against purine aptamers exhibiting a base pair involving positions 39 and 65.
Upon close inspection of crystal structures of purine aptamers complexed to their cognate metabolite (11, 12), it can be observed that a high proportion of the metabolite-bound surface is inaccessible to the bulk solvent. This suggests that the apo-form of riboswitch aptamers needs to be in an “open” conformation compatible with ligand binding to enable the subsequent ligand-induced rearrangement required for genetic modulation. Because G39 is completely exposed to the solvent in crystallized complexes (11, 12), it is intriguing how it can come in close proximity to position 65 to form a Watson-Crick base pair. Interestingly, it was previously proposed that the ligand recognition mechanism of the guanine riboswitch relies on the reorganization of the J2/3 single-strand junction, in which position 39 is located (13). In agreement with this, we have observed that a 2AP introduced at position 39 exhibits an increase in fluorescence upon adenine binding (Fig. 2C). This finding is consistent with the idea that 2AP can initially sample an alternative location, where the fluorescence is quenched (13). Together, these results are consistent with a model in which nucleotide 39 samples stacked conformations in the unbound form, which could help the formation of the G39-C65 base pair.
In addition to conferring a conformation similar to the ligand-bound aptamer (16), the formation of the G39-C65 base pair results in a constitutive transcription elongation regardless of ligand (Fig. 3, A and B). This constitutive modulation was previously attributed to the mismatch created in the terminator stem caused by the introduction of the U65C mutation (9). However, based on our in vitro transcription and gene reporter assays, constitutive expression is strongly dependent on the G39-C65 base pair but not on the mismatch (Fig. 3, A and B). Instead, the terminator mismatch was found to have a minimal effect on lacZ expression in the absence of ligand (Fig. 3B). Also, the constitutive modulation caused by the G39-C65 base pair has different requirements from those of the wild-type purine aptamer. Indeed, none of the nucleotides involved in ligand recognition are important for the riboswitch constitutive activity (Fig. 3D), most probably because G39 is held in place via the RNA backbone and because it does not require tight interaction with residues involved in ligand binding other than C65. However, the constitutive activity relies on structural elements such as the loop-loop interaction and Watson-Crick base pairs located in the aptamer core (Fig. 3D).
Our mutagenesis data showed that the presence of a U42C mutation increased by almost 2-fold the activity of the G39-C65 riboswitch variant in the absence of ligand (Fig. 3D). This can be rationalized from a previous mutagenesis study performed on the 2′-deoxyguanosine (dG) riboswitch (37), where it was shown that the U42C mutation favors a stable interaction with the sugar edge of the dG ligand. In a similar way, the U42C mutation could stabilize the G39-C65 base pair and thereby promote riboswitch activation. In addition, our results show an inhibitory effect from guanine binding for U40C and U42C that could be attributed to the fact that the bound guanine could form a less favorable interaction with the riboswitch core, resulting in lower genetic expression (Fig. 3D).
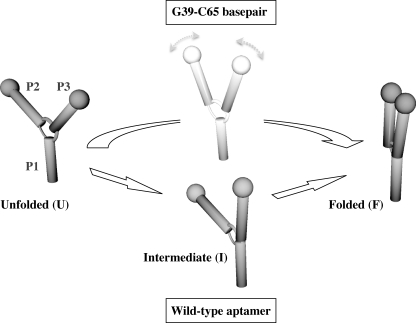
Our previous studies of the wild-type adenine aptamer revealed that the formation of the loop-loop interaction requires Mg2+ and proceeds via a discrete intermediate state (17). Although the exact nature of the transient intermediate remains to be determined, it has been suggested that it may correlate with the formation of the P1-P3 coaxial stacking (38). The ability of the G39-C65 aptamer to fold without Mg2+ and the absence of the intermediate at all experimental conditions provide strong evidences for a different folding mechanism. A plausible explanation for this finding arises from a detailed analysis of the two main structural differences observed in the G39-C65 aptamer when compared with the wild-type aptamer (11, 12, 16). First, G39 is found to occupy the ligand binding site of the aptamer, as opposed to being extruded in the wild-type molecule. Secondly, G39-flanking bases U38 and U40 adopt a different conformation in which they stack one to another without interacting with nucleotides of the core region. Both of these structural characteristics, which are absent from the ligand-bound wild-type structure, may stabilize a collapsed form of the G39-C65 aptamer that brings loops L2 and L3 in close proximity to form the loop-loop interaction in a single folding step (Fig. 6). Stabilization of the loop-loop interaction by ligand binding was previously observed for both purine aptamers (17, 39). Thus, it is very likely that the G39-C65 base pair promotes loop-loop formation as ligand binding does in the wild-type molecule.
FIGURE 6.
Folding mechanisms of the wild-type and the G39-C65 base pair aptamers. The wild-type adenine aptamer folds from the unfolded (U) to an intermediate state (I) by the formation of the P1-P3 coaxial stacking interaction. The folded state (F) is then adopted by the formation of the loop-loop interaction. The G39-C65 aptamer most likely folds directly from the U to the F state without going through the I state. The absence of the I structure most likely results from the formation of the G39-C65 base pair. Folding schemes only represent global structures and not structural differences that occur at the local level in core domains.
In contrast to the natural aptamer, the G39-C65 variant is substantially folded in monovalent ions (Fig. 4C). This is in agreement with the imino NMR data obtained in the absence of Mg2+ (supplemental Fig. S4), which supports a very similar structure to that previously characterized at 5 mm Mg2+ (16). The NMR data not only confirm formation of the U42·G39-C65 base triple, but also stacking on both sides of this base triple. Such preorganization of the aptamer core in a locked conformation likely assists the formation of the observed L2-L3 loop-loop contacts. As a result, the formation of both the base triple and the stacking interactions leads to a highly stabilized aptamer core that can fold following a concerted mechanism that bypasses the conformational flexibility and intermediate steps observed for the wild-type aptamer.
Further insights into the dynamic behavior of the G39-C65 variant can be obtained by comparing it with the natural sequence. We found that the folding and unfolding rates of G39-C65 aptamer molecules in the presence of monovalent (Fig. 4F) and divalent metal ions (Fig. 5C) span over approximately a 50-fold range, a value very similar to that reported for the natural aptamer at saturating adenine concentrations (17). This excellent agreement suggests that the global scaffold of the G39-C65 variant inherits the dynamic features of the wild-type RNA-ligand complex and makes the G39-C65 base pair not only a perfect structural but also dynamic match of the ligand-bound form. A recent single-molecule FRET study of the guanine aptamer has shown only a very slight decrease in the folding heterogeneity between the ligand-free and ligand-bound states (39), thus suggesting that the reduced heterogeneity could be aptamer-specific. Although this clearly needs further investigation, the observation that the global structure of guanine riboswitches is less dynamic than for adenine riboswitches has been proposed on the basis of NMR data (40). In addition, the degree of heterogeneity observed in this work for the G39-C65 variant not only confirms the previously reported collapse of heterogeneity on the wild-type aptamer upon the addition of adenine ligand but also reinforces that the high level of heterogeneity observed in the ligand-free form of the wild-type structure is an intrinsic feature of the RNA structure and it is not caused by surface-immobilization artifacts.
Collectively, our findings suggest a mechanism where the G39-C65 variant fluctuates between globally identical U and F states to those observed for the natural aptamer, but with folding taking place in a more restricted conformational landscape with no access to structural intermediates (Fig. 6). A comparison of the two folding models suggests that although the aptamer core is mostly stabilized by the formation of the loop-loop interaction with the aptamer core remaining locally flexible and able to adapt to ligand binding, folding of the G39-C65 variant is mostly driven by stabilizing interactions within its core that are concomitant with the formation of the loop-loop interaction in a single folding step.
In the light of our results, it is worth considering to which extent evolutionarily excluded nucleotides preventing ligand binding could be present in other regulatory mRNAs, either using a similar mimicking strategy as that found for the G39-C65 base pair or through alternative routes inhibiting ligand recognition. Here, because of the more elaborate and diverse chemical structure exhibited by other riboswitch ligands (i.e. S-adenosylmethionine, thiamine pyrophosphate, and flavin mononucleotide), it seems unlikely that a single-point mutation, or even a combination of mutations within the aptamer domain, could replicate the network of interactions necessary to mimic the bond-like structure of these riboswitch classes. Obviously, additional riboswitch-activating mutations could be obtained through the stabilization of the P1 stem, as shown previously for riboswitches sensing adenine, lysine, and S-adenosylmethionine (19, 20, 41). Evidences in favor of alternative mechanisms involving the inhibition of ligand binding arise from the large body of existing data regarding riboswitch mutations induced by various antibiotics (42–45). In most cases, mutations within the aptamer domain have been found to inhibit antibiotic binding. Nevertheless, as observed in this work, the concept of evolutionary exclusion of certain residue combinations that favor folded but inactive conformations may extend not only to other riboswitches but to functional RNAs in general (20, 32, 41, 46–48). Well known examples include tRNA mutations that induce pronounced changes in the tRNA structure, most of which are related to diseases such as diabetes, myopathies, and encephalopathies (49).
In summary, our study provides the molecular basis to understand how sequence composition within the purine riboswitch aptamer core has evolved not only to maximize affinity and specificity toward the ligand, but also to minimize the possibility of forming rigid ligand-free stable structures that cannot adaptively respond to ligand concentrations.
Supplementary Material
Acknowledgments
We thank members of the Lafontaine laboratory for helpful discussions, Patricia Bouchard for the NMR data analysis, and Alain Lavigueur for critical reading of the manuscript.
This work was supported by a grant from the National Sciences and Engineering Research Council of Canada (NSERC) (to E. B.), and by Canadian Institutes for Health Research (CIHR) Grants MOP-86502 (to P. L.), and MOP-166290 (to D. A. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and Tables S1–S3.
- SHAPE
- selective 2′-hydroxyl acylation analyzed by primer extension
- NMIA
- N-methylisatoic anhydride
- 2AP
- 2-aminopurine
- HSQC
- heteronuclear single quantum correlation.
REFERENCES
- 1. Serganov A., Patel D. J. (2007) Nat. Rev. Genet 8, 776–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roth A., Breaker R. R. (2009) Annu. Rev. Biochem. 78, 305–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montange R. K., Batey R. T. (2008) Annu. Rev. Biophys. 37, 117–133 [DOI] [PubMed] [Google Scholar]
- 4. Nudler E. (2006) Cell 126, 19–22 [DOI] [PubMed] [Google Scholar]
- 5. Serganov A. (2009) Curr. Opin. Struct. Biol. 19, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weinberg Z., Barrick J. E., Yao Z., Roth A., Kim J. N., Gore J., Wang J. X., Lee E. R., Block K. F., Sudarsan N., Neph S., Tompa M., Ruzzo W. L., Breaker R. R. (2007) Nucleic Acids Res. 35, 4809–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irnov I., Sharma C. M., Vogel J., Winkler W. C. (2010) Nucleic Acids Res. 38, 6637–6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mandal M., Boese B., Barrick J. E., Winkler W. C., Breaker R. R. (2003) Cell 113, 577–586 [DOI] [PubMed] [Google Scholar]
- 9. Mandal M., Breaker R. R. (2004) Nat. Struct. Mol. Biol. 11, 29–35 [DOI] [PubMed] [Google Scholar]
- 10. Christiansen L. C., Schou S., Nygaard P., Saxild H. H. (1997) J. Bacteriol. 179, 2540–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serganov A., Yuan Y. R., Pikovskaya O., Polonskaia A., Malinina L., Phan A. T., Hobartner C., Micura R., Breaker R. R., Patel D. J. (2004) Chem. Biol. 11, 1729–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batey R. T., Gilbert S. D., Montange R. K. (2004) Nature 432, 411–415 [DOI] [PubMed] [Google Scholar]
- 13. Gilbert S. D., Stoddard C. D., Wise S. J., Batey R. T. (2006) J. Mol. Biol. 359, 754–768 [DOI] [PubMed] [Google Scholar]
- 14. Mulhbacher J., Lafontaine D. A. (2007) Nucleic Acids Res. 35, 5568–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lemay J. F., Lafontaine D. A. (2007) RNA 13, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delfosse V., Bouchard P., Bonneau E., Dagenais P., Lemay J. F., Lafontaine D. A., Legault P. (2010) Nucleic Acids Res. 38, 2057–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lemay J. F., Penedo J. C., Tremblay R., Lilley D. M., Lafontaine D. A. (2006) Chem. Biol. 13, 857–868 [DOI] [PubMed] [Google Scholar]
- 18. Pleiss J. A., Derrick M. L., Uhlenbeck O. C. (1998) RNA 4, 1313–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lemay J. F., Desnoyers G., Blouin S., Heppell B., Bastet L., St-Pierre P., Massé E., Lafontaine D. A. (2011) PLoS Genet 7, e1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blouin S., Chinnappan R., Lafontaine D. A. (2011) Nucleic Acids Res. 39, 3373–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blouin S., Lafontaine D. A. (2007) RNA 13, 1256–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lafontaine D. A., Wilson T. J., Zhao Z. Y., Lilley D. M. (2002) J. Mol. Biol. 323, 23–34 [DOI] [PubMed] [Google Scholar]
- 23. Clegg R. M., Murchie A. I., Zechel A., Carlberg C., Diekmann S., Lilley D. M. (1992) Biochemistry 31, 4846–4856 [DOI] [PubMed] [Google Scholar]
- 24. Murchie A. I., Clegg R. M., von Kitzing E., Duckett D. R., Diekmann S., Lilley D. M. (1989) Nature 341, 763–766 [DOI] [PubMed] [Google Scholar]
- 25. Piotto M., Saudek V., Sklenár V. (1992) J. Biomol. NMR 2, 661–665 [DOI] [PubMed] [Google Scholar]
- 26. Kay L. E., Keifer P., Saarinen T. (1992) J. Am. Chem. Soc. 114, 10663–10665 [Google Scholar]
- 27. Zhang O., Kay L. E., Olivier J. P., Forman-Kay J. D. (1994) J. Biomol. NMR 4, 845–858 [DOI] [PubMed] [Google Scholar]
- 28. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 29. Johnson B. A., Blevins R. A. (1994) J. Biomol. NMR 4, 603–614 [DOI] [PubMed] [Google Scholar]
- 30. Macke T. J., Ecker D. J., Gutell R. R., Gautheret D., Case D. A., Sampath R. (2001) Nucleic Acids Res. 29, 4724–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merino E. J., Wilkinson K. A., Coughlan J. L., Weeks K. M. (2005) J. Am. Chem. Soc. 127, 4223–4231 [DOI] [PubMed] [Google Scholar]
- 32. Stoddard C. D., Gilbert S. D., Batey R. T. (2008) RNA 14, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garst A. D., Héroux A., Rambo R. P., Batey R. T. (2008) J. Biol. Chem. 283, 22347–22351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ward D. C., Reich E., Stryer L. (1969) J. Biol. Chem. 244, 1228–1237 [PubMed] [Google Scholar]
- 35. Gilbert S. D., Love C. E., Edwards A. L., Batey R. T. (2007) Biochemistry 46, 13297–13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stoddard C. D., Montange R. K., Hennelly S. P., Rambo R. P., Sanbonmatsu K. Y., Batey R. T. (2010) Structure 18, 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edwards A. L., Batey R. T. (2009) J. Mol. Biol. 385, 938–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gilbert S. D., Batey R. T. (2006) Chem. Biol. 13, 805–807 [DOI] [PubMed] [Google Scholar]
- 39. Brenner M. D., Scanlan M. S., Nahas M. K., Ha T., Silverman S. K. (2010) Biochemistry 49, 1596–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noeske J., Schwalbe H., Wöhnert J. (2007) Nucleic Acids Res. 35, 5262–5273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heppell B., Blouin S., Dussault A. M., Mulhbacher J., Ennifar E., Penedo J. C., Lafontaine D. A. (2011) Nat. Chem. Biol. 7, 384–392 [DOI] [PubMed] [Google Scholar]
- 42. Kim J. N., Blount K. F., Puskarz I., Lim J., Link K. H., Breaker R. R. (2009) ACS Chem. Biol. 4, 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blount K. F., Wang J. X., Lim J., Sudarsan N., Breaker R. R. (2007) Nat. Chem. Biol. 3, 44–49 [DOI] [PubMed] [Google Scholar]
- 44. Blount K. F., Breaker R. R. (2006) Nat. Biotechnol. 24, 1558–1564 [DOI] [PubMed] [Google Scholar]
- 45. Mulhbacher J., Brouillette E., Allard M., Fortier L. C., Malouin F., Lafontaine D. A. (2010) PLoS Pathog. 6, e1000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lafontaine D. A., Norman D. G., Lilley D. M. (2001) EMBO J. 20, 1415–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lafontaine D. A., Norman D. G., Lilley D. M. (2002) EMBO J. 21, 2461–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bassi G. S., Murchie A. I., Walter F., Clegg R. M., Lilley D. M. (1997) EMBO J. 16, 7481–7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wittenhagen L. M., Kelley S. O. (2003) Trends Biochem. Sci. 28, 605–611 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.