Abstract
Epithelial Na+ channels (ENaCs) play an essential role in the regulation of body fluid homeostasis. Certain transition metals activate or inhibit the activity of ENaCs. In this study, we examined the effect of extracellular Cu2+ on human ENaC expressed in Xenopus oocytes and investigated the structural basis for its effects. External Cu2+ inhibited human αβγ ENaC with an estimated IC50 of 0.3 μm. The slow time course and a lack of change in the current-voltage relationship were consistent with an allosteric (non pore-plugging) inhibition of human ENaC by Cu2+. Experiments with mixed human and mouse ENaC subunits suggested that both the α and β subunits were primarily responsible for the inhibitory effect of Cu2+ on human ENaC. Lowering bath solution pH diminished the inhibition by Cu2+. Mutations of two α, two β, and two γ His residues within extracellular domains significantly reduced the inhibition of human ENaC by Cu2+. We identified a pair of residues as potential Cu2+-binding sites at the subunit interface between thumb subdomain of αhENaC and palm subdomain of βhENaC, suggesting a counterclockwise arrangement of α, β, and γ ENaC subunits in a trimeric channel complex when viewed from above. We conclude that extracellular Cu2+ is a potent inhibitor of human ENaC and binds to multiple sites within the extracellular domains including a subunit interface.
Keywords: Copper, Epithelial Cell, Ion Channels, Kidney, Lung, Protein Domains, ASIC, Amiloride, ENaC, Xenopus Oocytes
Introduction
The epithelial Na+ channel (ENaC)2 mediates Na+ transport across apical membranes of high resistance epithelia in kidney, colon, and lung. ENaC has important roles in the maintenance of extracellular fluid volume and the regulation of airway surface liquid volume (1). Alterations in ENaC activity have been associated with several human diseases. For example, enhanced ENaC activity is responsible for the hypertension seen in Liddle's syndrome, contributes to the mucociliary dysfunction seen in cystic fibrosis, and is believed to contribute to hypervolemia associated with nephrotic syndrome (2, 3).
A variety of intracellular and extracellular factors regulate ENaC activity by distinct mechanisms (4). External amiloride analogs, cations, anions, nucleotides, serine proteases, and laminar shear stress inhibit or stimulate endogenous or exogenous ENaCs (5–12). All of these extracellular regulators appear to directly alter the activity of ENaCs in plasma membranes rather than affect channel subunit trafficking (1). Their primary targets likely reside within the characteristically large extracellular domains (ECDs) of ENaC subunits. This notion is in line with the well defined subdomains within the ECDs of the chicken acid-sensing ion channel 1 (cASIC1), a member of the ENaC/degenerin family, revealed in a crystal structure and the identification of proton binding sites within the ECDS (13).
We have previously examined the effects of the transition metals, Ni2+ and Zn2+, on ENaC activity. External Ni2+ inhibits and Zn2+ activates mouse ENaCs in Xenopus oocytes by directly interacting with the channels and altering channel gating (6, 7). Some of these metal effects are thought to be related to Na+ self-inhibition, a down-regulation of open probability (Po) by extracellular Na+ (7, 14). Yu et al. (14) have also examined the effects of several transition metals on the single channel activity of native Xenopus ENaCs in A6 cells. These metals differentially affect Xenopus ENaC Po and channel number in membrane patches without changing the single channel conductance. However, the exact binding sites and detailed mechanisms for the metal effects on ENaCs remain largely unknown.
Copper is the third most abundant trace metal in humans and has a variety of important biological functions. Excessive Cu2+ is highly toxic to cells, and its content in cells is carefully maintained at low levels. Indeed, Cu2+ is implicated in several human diseases such as Wilson disease, Menkes disease, neurodegenerative disorders, and cancers (15, 16). The therapeutic potential of copper chelators and copper complexes is being intensively investigated (16). In addition, particulate matters contain high amounts of transitional metals including copper. Soluble metals in airborne particles contribute to pulmonary and cardiovascular toxicity (17, 18). Recent studies suggest that copper nanoparticles are highly toxic (19). The underlying mechanisms for the harmful effects of Cu2+ are not fully understood. Many studies have suggested that certain metals exert their toxic effects in part by altering functions of ion channels or transporters (20, 21). Clearly, a better understanding of the interactions between copper and biological molecules is crucial to an elucidation of its physiological, pathological, and toxicological roles in human health.
In this report, we examined the effects of external Cu2+ on amiloride-sensitive Na+ currents in oocytes expressing αβγ human ENaC (hENaC) and probed the structural basis by site-directed mutagenesis. We found that external Cu2+ is a potent inhibitor of hENaC. The inhibitory effect of Cu2+ on hENaC depends on the α and β subunits. The most important site for Cu2+ inhibition was identified at the α/β subunit interface.
EXPERIMENTAL PROCEDURES
cDNA Constructs and Site-directed Mutagenesis
Wild-type α, β, and γ hENaC cDNAs (22, 23) were in pSPORT, pBluescript KS+, and pCDNA3 vectors, respectively. Point mutations were introduced into hENaC cDNAs using the QuikChange II XL site-directed mutagenesis kit (Stratagene). The presence of intended mutations and the absence of unwanted mutations were verified by direct DNA sequencing. Mouse α, β, and γ ENaC (mENaC) cDNAs were in pBluescript SK− vector. Wild-type and mutant hENaC cRNAs were made using SP6 (αhENaC) or T7 (β and γ hENaC) RNA polymerase (Ambion, Inc.). All of the mENaC cRNAs were made using T3 RNA polymerase (Ambion, Inc.). The synthesized cRNAs were purified with an RNA purification kit (Qiagen) and quantified by spectrophotometry.
ENaC Expression and Two-electrode Voltage Clamp
ENaC expression in Xenopus oocytes and current measurements by two-electrode voltage clamp were performed as previously reported (6). Stage V and VI oocytes with the follicle cell layer removed were injected with 50 nL/cell of mixed cRNAs composed of 2 ng of each hENaC subunit or 1 ng of each mENaC subunit. Injected oocytes were incubated at 18 °C in modified Barth's solution (88 mm NaCl, 1 mm KCl, 2.4 mm NaHCO3, 15 mm HEPES, 0.3 mm Ca(NO3)2, 0.41 mm CaCl2, 0.82 mm MgSO4, 10 μg/ml of sodium penicillin and streptomycin sulfate, 100 μg/ml gentamycin sulfate, pH 7.4). All of the experiments were performed at room temperature (20–24 °C) 20–30 h following injection. The oocytes were placed in a recording chamber from Warner Instruments (Hamden, CT) and perfused with bath solutions at a constant flow rate of 12–15 ml/min. Voltage clamp was performed using Axoclamp 900A amplifier and DigiData 1440A interface controlled by pClamp 10 (Molecular Devices Corporation, Sunnyvale, CA). The oocytes were either continuously clamped to −100 mV to monitor current change over a period of time or stepwise clamped to a series of voltages (−140 to 60 mV) to determine the current-voltage relationship.
Examination of the Effects of Cu2+ on ENaCs in Oocytes
The oocytes were perfused with normal bath solution (NaCl-110, containing 110 mm NaCl, 2 mm KCl, 2 mm CaCl2, pH 7.4) while clamped to −100 mV. Bath solution was buffered with either 10 mm HEPES or 5 mm MES and 5 mm MOPS. Although HEPES reportedly forms complex with Cu2+ (24), we observed similar responses to Cu2+ using bath solution buffered with either HEPES or MES and MOPS that do not complex Cu2+. Inward currents were continuously recorded, whereas bath solutions supplemented with or without Cu2+ were exchanged. Following Cu2+ washout, bath solution was switched to NaCl-110 with 10 μm amiloride to determine the amiloride-insensitive current. The effects of Cu2+ on ENaCs were analyzed by comparison of the amiloride-sensitive currents prior to and after Cu2+ application. A Cu2+ stock solution of 1 m was prepared by dissolving CuSO4·5H2O (purity of 99.999%; Sigma-Aldrich) in deionized water and was diluted to its final concentration in NaCl-110. The highest concentration of Cu2+ solution that could be made in this bath solution and remain relatively stable was 100 μm, and precipitates appeared at higher concentration. Bath solutions containing Cu2+ were prepared fresh prior to experiments to minimize the reduction of Cu2+ concentration because of the slow formation of insoluble Cu(OH)2 at neutral pH. Nominal concentrations of Cu2+ were used. Oocytes with unstable currents were not used in these experiments.
Examination of Na+ Self-inhibition
The Na+ self-inhibition responses were examined as previously reported (25, 26). Briefly, Na+ self-inhibition was examined by rapidly replacing a low [Na+] bath solution (NaCl-1; containing 1 mm NaCl, 109 mm N-methyl-d-glutamine (NMDG), 2 mm KCl, 2 mm CaCl2, 10 mm HEPES, pH 7.4) with a high [Na+] bath solution (NaCl-110), whereas the oocytes were continuously clamped to −100 mV. Bath solution exchange was done with a Teflon valve perfusion system controlled by computer (AutoMate Scientific Inc, Berkeley, CA). Upon completion of the experiment, 10 μm amiloride was added to the bath solution so as to determine the amiloride-insensitive portion of the whole cell current. To avoid complications from the observable variability in the Na+ self-inhibition response of WT ENaCs among different batches of oocytes (27), the response of WT channels was always tested in an alternating manner with mutants in the same batch of oocytes. Steady state current (Iss) was measured at 40 s after Ipeak. The amiloride-insensitive currents were subtracted from Iss and Ipeak currents to determine the amiloride-sensitive current ratio of Iss/Ipeak, the index for the magnitude of Na+ self-inhibition.
Examination of the Effect of Cu2+ on hENaCs in Human Airway Epithelial Monolayer
Primary human airway epithelial cells were cultured from excess pathological tissue following lung transplantation and organ donation under a protocol approved by the University of Pittsburgh Investigational Review Board. Human airway epithelial cells were cultured on human placental collagen-coated Costar Transwell filters (0.33 cm2) as described previously (28) and used for experimentation following 4–6 weeks of culture at an air-liquid interface. Short circuit currents (Isc) were measured as previously described (28). In brief, cells cultured on filter supports were mounted in modified Ussing chambers, and the cultures were continuously short circuited with an automatic voltage clamp (Physiologic Instruments). The bathing Ringer's solution was composed of 120 mm NaCl, 10 mm HEPES, 3.3 mm KH2PO4, 0.8 mm K2HPO4, 0.6 mm MgCl2, 0.6 mm CaCl2, and 10 mm glucose (pH, 7.4). Chambers were constantly gassed with a mixture of 95% O2 and 5% CO2 at 37 °C, which maintained a pH of 7.35. Simultaneous transepithelial resistance was recorded by applying a 10-mV pulse/s via an automated pulse generator. Acquire and Analyze 2.3 (Physiological Instruments) was used to control the voltage clamp and analyze the Isc data. Isc recordings included a 30-min equilibration period, followed by the addition of Cu2+ from a 1 m Cu2+ stock solution in water, followed by the addition of 20 μm amiloride.
Statistical Analysis
The data are presented as the means ± S.E. Significance comparisons between groups were performed using Student's t test. Curve fittings were performed with Origin Pro 8.0 (OriginLab Corporation, Northampton, MA).
RESULTS
External Cu2+ Inhibits Human αβγ ENaC
We examined the effect of extracellular Cu2+ on whole cell currents in Xenopus oocytes expressing αβγ hENaC. Oocytes were continuously clamped at −100 mV to monitor inward current changes before, during (10 min), and after Cu2+ application in bath solution (NaCl-110 buffered with 5 mm MES and 5 mm MOPS). Because ENaC activity in oocytes typically experiences a rundown (i.e. a spontaneous decline of current over time) (29, 30), control experiments were performed the same way except with no Cu2+ addition. The rundown was nearly linear but best fit with an exponential equation. The estimated time constant was 736.6 ± 67.2 s (n = 13). In response to external application of 0.1 μm Cu2+, the current increased slightly at first and then declined in a similar way to the control recording (Fig. 1A), indicating a lack of inhibitory effect. At higher concentrations, Cu2+ reduced the currents in a dose-dependent manner. The time course of the current changes in the absence and presence of Cu2+ are shown in Fig. 1B, and the estimated time constants are listed in Table 1.
FIGURE 1.
External Cu2+ inhibits human ENaC. A, representative recordings of whole cell currents from oocytes expressing αβγ hENaCs before, during, and after Cu2+ applications. Each trace represents at least five independent observations. The oocytes were clamped at −100 mV. Cu2+ was added to the bath solution (NaCl-110 with 110 mm Na+, buffered with 5 mm MES and 5 mm MOPS) at 0.1, 0.3, 1, 3, 10, and 30 μm. Control oocytes were clamped in the same way except no Cu2+ application. Inward currents were shown in negative values by convention. The durations of Cu2+ and amiloride (10 μm) applications are indicated by black and open bars, respectively. Black arrows point to the transient increases in currents after initiation of Cu2+ applications. B, time courses of the current changes in the absence (control) or presence of Cu2+. The relative currents were the ratios of the amiloride-sensitive currents measured every minute after the beginning of the Cu2+ applications and the amiloride-sensitive currents prior to Cu2+ additions (at time 0). The data were from 17 oocytes for control and 9 or 10 oocytes for Cu2+ applications. The black arrowheads indicate the times when datum points were chosen for dose-response analysis (2 min for 3 and 10 μm Cu2+, 5 min for 1 μm Cu2+, and 10 min for 0.1 and 0.3 μm Cu2+). C, dose response of Cu2+ on αβγ hENaCs. The relative currents from B were adjusted to deduct rundown contribution from the observed current decreases at the corresponding time with the formula: IR (corrected) = IR + (1 − IR control). IR and IR control were the relative currents with and without Cu2+, respectively. The line was from a best fit with the Hill equation. The parameters were: IC50, 0.31 μm; n, 1.28, B, 0.23; and R2, 0.99.
TABLE 1.
Time constants for current decreases in the absence (control) and presence of various concentrations of Cu2+ in oocytes expressing αβγ hENaC
Time constants were obtained from best fit of the current changes within 10 min using built-in exponential function in Clampfit 10. The data were from Fig. 1. The time constant for 0.1 μm Cu2+ could not be obtained.
| [Cu2+] | Tau | Oocytes |
|---|---|---|
| s | ||
| 0 μm (control) | 736.6 ± 67.2 | 13 |
| 0.1 μm | ND | |
| 0.3 μm | 199.9 ± 8.5 | 7 |
| 1 μm | 92.1 ± 4.4 | 8 |
| 3 μm | 35.7 ± 2.3 | 10 |
| 10 μm | 31.8 ± 2.7 | 9 |
ND, not determined.
We generated a dose-response curve by plotting the relative currents against Cu2+ concentrations (Fig. 1C). Measurements were taken at times equivalent to approximately three time constants after the addition of Cu2+ (Table 1 and black arrowheads in Fig. 1B). Time constant for 0.1 μm Cu2+ could not be determined; values at 10 min were used. The data for 30 μm Cu2+ were taken at 2 min from experiments with Cu2+ applied for 3 min (Fig. 1A). The relative currents were adjusted to deduct rundown contribution from the observed current decreases at the corresponding time as described in details in the figure legend. Fitting the data with the Hill equation (ICu/I = IC50n/(Cn + IC50n) + B, n for Hill coefficient, C for Cu2+ concentration, and B for bottom plateau) yielded the following parameters: IC50, 0.31 μm; n, 1.28; and B, 0.23 with the coefficient of determination (R2) of 0.98.
Prior to the inhibitory effect of Cu2+ on hENaC, a small transient increase in current was typically seen (arrows in Fig. 1A). The transient change in current was not an artifact, although its magnitude varied among different batches of oocytes. It likely resulted from a rapid activation of the channel prior to the inhibitory effect of Cu2+.
The inhibitory effect of Cu2+ on hENaCs was not readily reversible. Washout of Cu2+ for 1 min only moderately restored the inhibited currents (Figs. 1 and 2A). This slow reversal could result from either tight binding of Cu2+ to the channel complex or permanent inactivation of channels. To distinguish these two possibilities, we utilized a high affinity Cu2+ chelator, tetrathiomolybdate (TTM), to facilitate Cu2+ removal from its binding site(s). Inhibition of hENaC by 10 μm Cu2+ was nearly completely reversed following the addition of 10 μm TTM (Fig. 2, B and D). TTM alone did not change the current (Fig. 2C). The results suggested that the slow reversibility of Cu2+ inhibition reflects tight binding of Cu2+ to hENaC.
FIGURE 2.
Time course and reversibility of Cu2+ inhibition of hENaC. Seven oocytes expressing αβγ hENaCs were used for each experiment in A–C. A, a typical recording showing the current changes following 1 min of application and 1 min of washout of 10 μm Cu2+. B, representative recording showing the current changes following 1 min of application of 10 μm Cu2+ and 1 min of washout with 10 μm TTM. C, representative recording showing effects of 10 μm TTM and its washout on the current. Relative currents (amiloride-sensitive currents after 1 min of application of 10 μm TTM normalized to those prior to TTM treatment) were 0.98 ± 0.02 (n = 7, p > 0.05 from paired Student's t test). D, summary data for experiments shown in A and B. The asterisk indicates that the relative current after washout with TTM was significantly higher than that after washout with NaCl-110 (p < 0.001). To minimize rundown of ENaC currents typically seen under continuous hyperpolarization, 10 μm Cu2+ was applied for only 1 min and washed out for 1 min. The current was approaching a plateau at 1 min of Cu2+ application, and the ICu/I was slightly greater than that with 3 min of application. Bath solution was buffered with 5 mm MES and 5 mm MOPS.
External Cu2+ Inhibits hENaC by Acting on Sites outside of the Pore
Because Cu2+ was applied in bath solution in our study, its action site is likely within either the ECD or the transmembrane domain. We carried out experiments to examine these two possibilities. First, we examined the effect of Cu2+ on the current-voltage (I-V) relationship of hENaC. As shown in Fig. 3A, the I-V curve remained linear following a 3-min application of 10 μm Cu2+. The voltage independence of Cu2+ inhibition is inconsistent with a pore blocking effect. A positively charged pore blocker such as amiloride preferentially blocks the inward current causing an outward rectification in the I-V curve (Fig. 3A, curve for amiloride). Second, we tested whether pore blocker amiloride could protect the channels from inhibition by Cu2+. ENaC currents completely recovered after washout of 10 μm amiloride (Fig. 3, B and D). However, when oocytes were pretreated with 10 μm amiloride followed by 10 μm Cu2+, currents only partially recovered after washout of both Cu2+ and amiloride (Fig. 3, C and D). Apparently, Cu2+ inhibited hENaC current after the pore had been occupied by amiloride. These results suggest that Cu2+ acts at a site that is external to the amiloride-binding site within the pore. Based on the above observations, we conclude that external Cu2+ inhibits human ENaCs likely by binding to sites outside of the pore.
FIGURE 3.
Cu2+ binding site is likely not located within hENaC pore. A, I-V curves before and after 10 μm Cu2+. Oocytes expressing αβγ hENaCs (n = 5) were clamped to a series of voltages (−140 to 60 mV) for 500 ms, and the currents were measured at 400 ms. B, recording trace showing current changes before, during, and after washout of 10 μm amiloride (80 s). Oocytes were clamped at −100 mV. The current decay from a peak current following amiloride washout reflects Na+ self-inhibition response (25). C, recording from a similar experiment to the one in B except that 10 μm Cu2+ was co-applied with 10 μm amiloride for 60 s. Cu2+ was added 10 s after amiloride and withdrawn 10 s before amiloride to ensure that Cu2+ was only applied in the presence of amiloride. Bath solution was buffered with 5 mm MES and 5 mm MOPS. D, current recovery after amiloride washout with (open bar) or without (black bar) Cu2+. The values were the ratios of the amiloride-sensitive currents measured after amiloride washout for 60 s and before amiloride application. The current recovery following co-application of amiloride and Cu2+ was significantly lower than that following amiloride application alone (p < 0.001, n = 10 for amiloride and 7 for amiloride + Cu2+).
Human α and β Subunits Are Necessary to Confer the Response of hENaC to Cu2+
We tested whether Cu2+ had a similar effect on mouse ENaC and observed that 10 μm Cu2+ did not significantly inhibit the current in oocytes expressing αβγ mENaC (Fig. 4E). To determine the hENaC subunits (α, β, and/or γ) that are necessary for Cu2+ inhibition, we expressed hybrid channels containing mixed human and mouse subunits in Xenopus oocytes. Replacing human α or β subunits with their mouse counterpart significantly reduced the inhibitory effect of 10 μm Cu2+, whereas replacing the γ subunit did not alter the effect of 10 μm Cu2+ (Fig. 4). These results suggest that both the α and β subunits are required for the inhibitory effect of Cu2+ on hENaC activity.
FIGURE 4.
Both α and β subunits contribute to the distinct responses of hENaC and mENaC to external Cu2+. The effects of 10 μm Cu2+ on whole cell currents were examined in oocytes expressing αβγ hENaC (A, HαHβHγ), α mENaC with β and γ hENaC (B, MαHβHγ), α and γ hENaC with β mENaC (C, HαMβHγ), α and β hENaC and γ mENaC (D, HαHβMγ), or αβγ mENaC (E, MαMβMγ). Each recording represents five observations. The gray bars show the presence of 10 μm amiloride. F, relative current (ICu/I, n = 5). Bath solution was buffered with 10 mm HEPES. The ICu/I values were the ratios of amiloride-sensitive currents measured after and before 10 μm Cu2+ applications. Rundown was not deducted from the observed current decreases in this and all subsequent figures. The solid bars indicate that the values are significantly different from that of αβγhENaC obtained in the same batches of oocytes (p < 0.05 for MαHβHγ, p < 0.001 for HαMβHγ and MαMβMγ).
Mutations of Multiple His Residues within the ECDs Reduce Cu2+ Inhibition
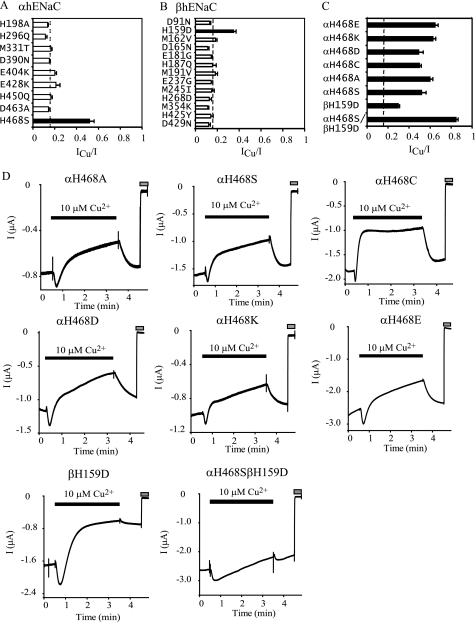
We hypothesized that the ECDs of the α and β hENaCs contain residues that are required to confer the inhibitory response to Cu2+. Because Cu2+ is often coordinated by side chains of His, Cys, Met, Glu, and Asp (31), we replaced His, Met, Glu, and Asp residues of α or β hENaC to their counterparts seen in mENaC at equivalent sites. All 16 Cys residues within α ECDs and 18 Cys residues within β ECDs are conserved between human and mouse ENaCs and therefore were not included in this analysis. Among nine α mutants, αH468S significantly reduced Cu2+ inhibition (Fig. 5A). Substitution of αHis468 with Cys, Asp, Glu, Lys, or Ala similarly attenuated Cu2+ inhibition (Fig. 5, C and D). Moreover, these mutations also rendered the Cu2+ inhibition reversible. Certain mutations at αHis468 also decreased the rates of Cu2+ inhibition or increased the rates of current recovery following washout (Table 2). Interestingly, αH468C dramatically increased both rates, suggesting that the introduced Cys side chain might retain certain capability of this residue interacting with Cu2+. After His, Cys is the second most frequent Cu2+ coordinating residue (31). One of the 13 β mutants, βH159D, showed a significantly reduced response to 10 μm Cu2+ but did not alter its reversibility or rate (Fig. 5, B and D). Double mutant αH468S-βH159D showed a minimal inhibitory response to 10 μm Cu2+ (ICu/I = 0.85 ± 0.01, n = 6, p < 0.0001 versus WT; Fig. 5).
FIGURE 5.
Identification of αHis468 and βHis159 as potential sites mediating Cu2+ interaction with hENaCs. Point or double mutations were introduced in one hENaC subunit, which was co-expressed with other WT hENaC subunits. Mutational sites were selected to substitute nonconserved residues capable of binding Cu2+ in α or β hENaC subunits with their corresponding residues in α or β mENaC-based on sequence alignments. A, ICu/I at 10 μm Cu2+ from oocytes expressing mutant α and WT β and γ hENaCs (n = 5). The ICu/I values in this and all other subsequent figures were obtained in the same way as in Fig. 4F. B, ICu/I from oocytes expressing mutant β and WT α and γ hENaCs (n = 5 except n = 4 for D91N and D429N). C, ICu/I from oocytes expressing αHis468 mutants and αH468S/βH159D (n = 5–7). The black bars in A–C indicate that the values were significantly different from that of WT αβγ hENaCs obtained in the same batches of oocytes (p < 0.01). The dashed line shows the average ICu/I values from all oocytes expressing WT αβγ hENaCs used in this experiment (0.15 ± 0.00, n = 164) for reference only but not for statistical significance analyses. D, representative recordings showing the effect of 10 μm Cu2+ on mutant hENaCs. The gray bars indicate the times when 10 μm amiloride was present. Bath solution was buffered with 10 mm HEPES.
TABLE 2.
Time constants for current changes during (application, 3 min) and after (washout, 1 min) 10 μm Cu2+ applications in oocytes expressing WT or mutant hENaC
Time constants were obtained from best fit of the current changes using built-in exponential function in Clampfit 10. The values for WT hENaC were pooled from all oocytes of different batches for reference only. Statistical tests were performed by comparing the values from WT and mutants obtained in the same batches of similar numbers of oocytes. ND, not determined.
| hENaC | Application | Washout | Oocytes |
|---|---|---|---|
| s | s | ||
| WT | 27.1 ± 0.8 | 50.0 ± 7.3 | 67 |
| αH255A | 21.3 ± 1.7 | 36.0 ± 6.0 | 5 |
| αH255C | 33.9 ± 3.4 | 35.9 ± 6.0 | 5 |
| αH255D | 29.4 ± 1.5 | 21.5 ± 4.7 | 6 |
| αH255R | 27.0 ± 2.1 | 44.3 ± 7.7 | 5 |
| αH468A | 32.4 ± 3.3 | 14.7 ± 1.4a | 5 |
| αH468C | 10.2 ± 1.5a | 15.6 ± 1.3a | 5 |
| αH468D | 46.4 ± 4.6a | 31.0 ± 3.4 | 5 |
| αH468E | 64.6 ± 6.7a | 17.3 ± 3.2 | 4 |
| αH468K | 49.6 ± 3.2a | 32.4 ± 3.7 | 7 |
| αH468S | 23.7 ± 3.8 | 27.6 ± 2.5a | 5 |
| βH159A | 29.7 ± 2.5 | 18.3 ± 2.6 | 6 |
| βH159D | 34.1 ± 2.0 | 17.7 ± 3.2 | 5 |
| βH160A | 28.9 ± 1.3 | 64.1 ± 5.9 | 5 |
| βE254A | 29.5 ± 2.3 | 24.8 ± 3.9 | 5 |
| βE254C | 38.1 ± 1.9 | 22.7 ± 2.5 | 5 |
| βE254D | 33.7 ± 3.8 | 22.0 ± 3.3 | 5 |
| βE254H | 33.5 ± 2.0 | 22.0 ± 1.7 | 5 |
| βE254Q | 28.5 ± 2.1 | 20.6 ± 2.6 | 5 |
| βE254R | 32.2 ± 2.9 | 16.9 ± 2.6 | 5 |
| γH88A | 38.5 ± 3.1 | 44.0 ± 4.6 | 5 |
| γH233A | 18.3 ± 2.1 | ND | 5 |
| γH233C | 21.6 ± 2.2 | ND | 5 |
| γH233D | 13.0 ± 1.8a | ND | 5 |
| γH233R | 22.5 ± 2.4a | ND | 5 |
| γH277A | 35.9 ± 1.1 | 18.3 ± 1.7a | 5 |
| γH332A | 17.1 ± 0.4a | ND | 5 |
a The values were significantly different from that of WT in the same batch of oocytes (p < 0.01).
Histidine has an ionizable imidazole ring with an average pKa of 6.6 ± 1.0 in folded proteins (32). If His residues are indeed involved in Cu2+ inhibition of hENaC, lowering the pH of the bath solution containing Cu2+ should reduce Cu2+ inhibition because of increased protonation of imidazole nitrogens. We therefore examined the effect of 10 μm Cu2+ in pH 6.0 bath solution on hENaC currents. Changing the pH of the bath solution from 7.4 to 6.0 moderately increased currents in oocytes expressing αβγ hENaCs (relative current = 1.19 ± 0.01, n = 7, p < 0.001; Fig. 6A) as reported by Collier and Snyder (8). At pH 6.0, we observed a minimal inhibitory effect of 10 μm Cu2+ (ICu/I = 0.86 ± 0.01, n = 7, p < 0.001), in contrast to the large inhibitory effect at pH 7.4 (p < 0.001; Fig. 6B). These results demonstrate that Cu2+ inhibition of hENaC is pH-dependent and are consistent with His side chains mediating the interaction of Cu2+ with hENaC.
FIGURE 6.
Lowering pH of bath solution diminishes the inhibitory effect of Cu2+. Oocytes expressing WT αβγ hENaCs were clamped at −100 mV. A, a typical recording showing the effects of lowering bath solution pH from 7.4 (buffered with 5 mm MES and 5 mm MOPS) to 6.0 (buffered with 10 mm MES) on the base-line current and Cu2+ inhibition at 10 μm. The dark and light gray bars indicate the time periods in the presence of 10 μm amiloride in pH 6.0 and 7.4, respectively. There was no difference between the currents with amiloride at pH of 6.0 and 7.4. B, ICu/I at pH 7.4 and 6.0 (n = 7, p < 0.001). A control experiment at pH 7.4 was performed in the same way as in Fig. 2A. In experiments with both pH 6.0 and 7.4, 10 μm Cu2+ was applied for only 1 min to minimize potential complication from intracellular acidification, which inhibits ENaCs (47). The ICu/I obtained with 1 min of application of 10 μm Cu2+ at pH 7.4 was slightly greater than that with 3 min of application.
There are 10, 13, and 14 His residues in the ECDs of α, β, and γ hENaC, respectively. We individually mutated each His residue to identify other sites for Cu2+ interaction with the channel. In addition to the αHis468 mutants (Fig. 5), substitution of αHis255 with Ala, Cys, Asp, or Arg significantly reduced channel inhibition by 10 μm Cu2+ (Fig. 7A). For the β subunit, two mutations (βH159A and βH160A) significantly reduced Cu2+ inhibition (Fig. 7B). The change in Cu2+ inhibition by βH159A was similar to that of βH159D (Fig. 5B). Two γ His mutants (γH88A and γH277A) showed a significantly reduced inhibitory effect of 10 μm Cu2+ (Fig. 7C). We also noted that substitutions at two γECD sites (γHis233 and γHis332) increased the inhibitory effect of 10 μm Cu2+ (p < 0.01).
FIGURE 7.
Mutations of multiple His residues within the ECDs reduce the inhibitory effect of Cu2+ on hENaCs. The ICu/I values reflecting the magnitudes of the inhibitory effect of 10 μm Cu2+ were obtained from oocytes expressing mutant α together with WT β and γ hENaCs (A), mutant β with WT α and γ hENaCs (B), or mutant γ with WT α and β hENaCs (C). Black bars in A–C indicate that the values were significantly greater than that of WT αβγ hENaCs obtained in the same batches of oocytes (p < 0.01, n = 5 for both mutants and WT). The values in γH233R, γH233A, γH233D, and γH332A (gray bars) were significantly less than that of WT (p < 0.01). The dashed line shows the average ICu/I value from all oocytes expressing WT αβγ hENaCs used in this experiment (0.15 ± 0.00, n = 164) for reference only but not for statistical significance analyses. ICu/I for αH468A was from Fig. 5C and is shown for comparison. D, representative recordings of currents with 3-min applications of 10 μm Cu2+. The gray bars in D indicate the presence of 10 μm amiloride. Bath solution was buffered with 10 mm HEPES.
We examined whether mutations of His residues in multiple subunits would enhance the loss of the inhibitory effect of 10 μm Cu2+, as we observed with the αH468SβH159D mutant (Fig. 5). Double mutations such as αH468A/βH160A (ICu/I = 1.01 ± 0.02, n = 5, p < 0.0001 versus WT) and αH468A/γH277A (ICu/I = 0.97 ± 0.03, n = 4, p < 0.0001 versus WT) eliminated inhibitory effect of 10 μm Cu2+. In contrast, βH159AγH277A greatly reduced but did not eliminate 10 μm Cu2+ inhibition (ICu/I = 0.48 ± 0.03, n = 5, p < 0.0001 versus WT; Fig. 8). These data suggest that αHis468 is a key site for conferring the inhibitory effect of external Cu2+.
FIGURE 8.
Double mutations of αHis468 and βHis160 or γHis277 eliminate the inhibitory effect of 10 μm Cu2+ on hENaCs. Oocytes expressing two mutant hENaC subunits and one WT hENaC subunit were clamped to −100 mV. The effects of 10 μm Cu2+ were examined together with WT αβγ hENaCs in the same way as in previous figures. A–C, representative recordings showing the responses of the double mutants to 10 μm Cu2+. Gray bars show the presence of 10 μm amiloride. D, ICu/I obtained from four or five oocytes expressing the double mutants. Black bars show that the values were significantly different from that of WT obtained in the same batches of oocytes (p < 0.001). The dashed line shows the average ICu/I from all WT expressing oocytes used in this particular experiment (0.16 ± 0.01, n = 19) for the purpose of reference. Bath solution was buffered with 10 mm HEPES.
A Cu2+ Binding Site Is Located at a Subunit Interface
In certain ligand-gated channels, transition metal-binding sites are located at subunit interfaces (33, 34). We reasoned that external Cu2+ might bind at a contact site between two subunits. Residue αHis468 aligned with Lys355 of cASIC1. The cASIC1 structure shows that the side chain of Lys355, located at the carboxyl end of an α helix (α5) in the thumb domain interacts with side chain of Glu178 within the β3-β4 loop of the palm domain of an adjacent subunit (Fig. 9A) (13). The distance between NZ of Lys355 and OE2 of Glu178 is 3.2 Å, well within the range of distance for ion pairs in proteins (35). Lys355 of cASIC1 aligns with hENaC αHis468, βArg437, and γGlu446, whereas Glu178 of cASIC1 aligns with hENaC αVal287, βGlu254, and γVal265 (Fig. 9C). Of these six hENaC residues, only αHis468 and βGlu254 could form an ion pair. If a His residue resides at the position of Lys355 in subunit A of cASIC1, the distances between an imidazole nitrogen of Lys355 and a carboxyl oxygen of Glu178 in subunit C of cASIC1 would be 3.4–5.5 Å, close to the sum of average distances for Cu2+-N-His (2.0∼2.1 Å) and Cu2+-O-Glu (2.2 Å) in proteins (36). We therefore hypothesized that αHis468 and βGlu254 coordinate the same Cu2+ at the α and β subunit interface. To test the hypothesis, we mutated βGlu254 to Ala, Arg, Gln, His, Asp, and Cys. All of the substitutions except βE254D led to a modest but significant reduction of inhibition by 10 μm Cu2+ (Fig. 9D). The double mutant αH468A/βE254A nearly eliminated the inhibitory effect of 10 μm Cu2+ (ICu/I = 0.83 ± 0.01, n = 5, p < 0.0001 versus WT, αH468A or βE254A; Fig. 9E). Our observations are consistent with the above hypothesis that αHis468 and βGlu254 contribute to coordination of the same Cu2+ ion. The close proximity of αHis468 and βGlu254 requires an obligate counterclockwise subunit arrangement of α-β-γ when viewed from above (Fig. 9F). A clockwise arrangement of α, β, and γ subunits would place γGlu446 and βGlu254 in close proximity (Fig. 9G). Charge reversal mutation γE446R did not alter the inhibitory effect of 10 μm Cu2+ (ICu/I = 0.08 ± 0.01, n = 4, p > 0.7 versus WT). The result was not consistent with an involvement of γGlu446 in Cu2+ binding and a pairing between γGlu446 and βGlu254 that would otherwise require a clockwise organization of α, β, and γ subunits.
FIGURE 9.
Identification of αHis468 and βGlu254 pair at subunit interface. A, a structural model of chicken ASIC1. The trimeric cASIC1 structure (45) was rendered as three colored ribbons (subunit A, B, and C in red, green, and blue, respectively) with PyMol (version 1.3) using coordinates from Protein Data Bank (identification code 3HGC). The ECDs on the top part of the structure are linked to the transmembrane (TM) domain via six short coiled segments termed the wrist (13). The boxed area is enlarged on the right to show the contact between the thumb domain Lys355 of subunit A and the palm domain Glu178 of subunit C in the cASIC1 structure. For clarity the area is shown with semitransparent surface and ribbon rendering. The side chains of Lys355 and Glu178 are shown as sticks with carbon, oxygen, and nitrogen atoms colored in cyan, red, and blue, respectively. B, nontransparent surface rendering of the same region as in A with subunit A in red and subunit C in blue to highlight the subunit interface. Lys355 was mutated to a His. Homologous hENaC residues to K355H and Glu178 are shown in parentheses. The surface near the two residues are omitted. C, sequence alignments of human, mouse ENaCs, and cASIC1 in regions surrounding βGlu254 (−) and αHis468 (+). Secondary structures are shown according to the cASIC1 structure (13). D, ICu/I from oocytes expressing β mutant and WT α and γ hENaCs. Black bars indicate that the values were significantly different from that of WT in the same batches of oocytes (p < 0.01, n = 5). The dashed line shows the average value from pooled WT-expressing oocytes in this experiment (0.16 ± 0.01, n = 10). Bath solution was buffered with 10 mm HEPES. E, superimposed traces showing the responses of WT (black), βE254A (blue), αH468A (red), and αH468A/βE254A (purple) and ICu/I values in corresponding colors. All of the mutant values were significantly greater than that of WT (p < 0.001), and the asterisk shows that the value of the double mutant was significantly greater than that of the either single mutant (p < 0.001, n = 5). F, an illustration showing a counterclockwise arrangement of three ENaC subunits in a top view. Three rectangles represent the approximate spaces occupied by α (red), β (blue), and γ (green) subunits. Overlapped regions highlight intersubunit contacts including three-way interaction at the center and two-way interactions at more distal regions. Subunit-subunit interactions happen primarily along the two inner laterals. We designated the long inner sides as + and the short inner side as − by convention used in other ligand-gated channels. G, an alternative (clockwise) arrangement of α, β, and γ ENaC subunits.
Cu2+ Inhibition and Na+ Self-inhibition
We studied the effect of Cu2+ on hENaC in a bath solution containing 110 mm Na+, which is typically used by investigators so as to produce easily measurable currents. At this concentration, extracellular Na+ reduces the Po of ENaC via a process of Na+ self-inhibition (25, 26, 37, 38). The degree of Na+ self-inhibition dramatically affects the effects of other extracellular regulators on ENaCs such as Zn2+, H+, and Cl− (7–9). We suspected that some of the six His mutations might alter the Na+ self-inhibition response, and as a secondary effect Cu2+ inhibition appeared to be reduced. We therefore examined the Na+ self-inhibition responses of these mutant ENaCs.
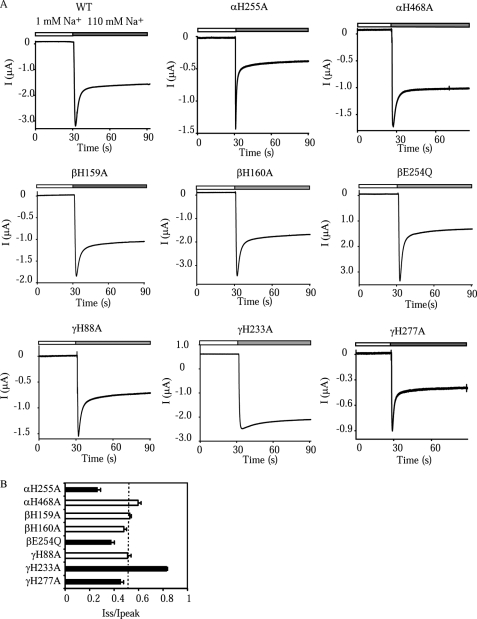
As shown in Fig. 10, αH255A greatly enhanced Na+ self-inhibition, whereas βE254Q and γH277A moderately increased the magnitude of Na+ self-inhibition. In contrast, αH468A, βH159A, βH160A, and γH88A did not significantly change the Na+ self-inhibition response. Na+ self-inhibition was largely eliminated in the γH233A mutant, as previously reported (8). These observations suggest that αHis468, βHis159, βHis160, and γHis88 have specific roles in Cu2+ inhibition of hENaC.
FIGURE 10.
Na+ self-inhibition responses of mutant hENaCs. A, representative traces showing Na+ self-inhibition responses of WT and αH255A, αH468A, βH159A, βH160A, βE254Q, γH88A, γH233A, and γH277A mutants. The oocytes were clamped at −100 mV, and the whole cell currents were continuously recorded, whereas bath [Na+] was rapidly increased from 1 mm (open bar) to 110 mm (gray bar). Both bath solutions were buffered with 10 mm HEPES. The traces are representative of at least five independent observations. The current decay following an increase in [Na+] represents Na+ self-inhibition. B, Iss/Ipeak of mutant hENaCs. Ipeak was the peak current, and Iss was the current 40 s after the peak current. The ratio is inversely proportional to the magnitude of Na+ self-inhibition. The black bars indicate that values were significantly different from that of WT obtained in the same batches of oocytes (p < 0.01, n = 5). The dashed line indicates the average value of WT hENaCs pooled from all oocytes in this experiment (0.50 ± 0.01, n = 84) for reference only.
Mutations that altered the Na+ self-inhibition response also changed the magnitude of the transient activation of current that appeared before the inhibitory effect of Cu2+ (Figs. 1A and 7D). The transient activation was absent in mutants with Na+ self-inhibition eliminated (for example, γH233D; Fig. 7D). It suggests that the transient activation by Cu2+ was caused by a relief of Na+ self-inhibition, in a manner similar to that of ENaC activation by external Zn2+ and H+ (7, 8). Accordingly, αH255D and γH277A that enhanced the Na+ self-inhibition response increased the magnitude of the transient Cu2+ activation by 5.3- and 3.1-fold, respectively, compared with WT (p < 0.001). These observations suggest that the reduced Cu2+ inhibition in αHis255 and γHis277 mutants (i.e. greater ICu/I; Fig. 7) was related to the augmented stimulatory effect of Cu2+, which resulted from enhanced Na+ self-inhibition. Other mutations including αHis468 mutations (Fig. 5D), βH159A (Fig. 7D), βH160A (Fig. 7D), βGlu254 mutations (Fig. 9E), and γH88A (Fig. 7D) did not significantly change the transient Cu2+ stimulation, consistent with their specific role in the inhibitory effect of Cu2+.
Cu2+ Inhibits Short Circuit Current in Human Airway Epithelial Monolayers
We studied whether external Cu2+ had an inhibitory effect on natively expressed human ENaCs similar to that on cloned ENaCs expressed in oocytes by examining the effect of apically applied Cu2+ on the short circuit currents in human airway epithelial monolayers. The portion of Isc that was blocked by 10 μm amiloride was considered ENaC-mediated current. As shown in Fig. 11, Cu2+ at doses above 10 μm reduced the Isc in a dose-dependent manner.
FIGURE 11.

Cu2+ inhibits native human ENaCs in human airway epithelial cells. A, superimposed short circuit recordings showing the responses of the currents to 1, 10, 30, and 100 μm Cu2+ applied in apical chamber. B, ICu/I, ratio of amiloride-sensitive current in the presence of Cu2+ and amiloride-sensitive current prior to Cu2+ application. The asterisks indicate that the values in the presence of 30 and 100 μm Cu2+ were significantly less than that of base-line currents (p < 0.001, n = 6).
DISCUSSION
In this study, we found that external Cu2+ inhibited human αβγ ENaC in both Xenopus oocytes and human airway epithelia. External Cu2+ at 10 μm does not inhibit mouse (Fig. 4) or rat ENaC.3 Native Xenopus ENaCs in A6 cells are activated by extracellular Cu2+ (14). Therefore, in the context of previous reports and our current observations, external Cu2+ appears to be a specific inhibitor of human ENaC among cloned ENaCs. The inhibitory effect of Cu2+ on the hENaC current in the human airway epithelia appeared to be smaller and weaker than that in oocytes (Fig. 11). We do not know the exact cause for the different responses to Cu2+. They could be related to certain experimental conditions utilized in the two systems, such as temperatures (i.e. 20–24 °C in oocytes and 37 °C in epithelia) and oxygen tensions, both of which regulate ENaC activity (25, 39, 40). The differences in the response to Cu2+ could also reflect the inherent differences between native and heterologously expressed channels. It has been reported that ENaCs in oocytes and epithelial monolayers display different sensitivities to the peptide inhibitors derived from the inhibitory domains of α and γ mouse ENaCs (41, 42).
The estimated IC50 of 0.31 μm for Cu2+ inhibition suggests that external Cu2+ is a potent hENaC inhibitor. A high affinity ENaC inhibitor may be useful in treating diseases associated with elevated ENaC-mediated Na+ absorption such as Liddle syndrome and cystic fibrosis (43, 44).
Recent studies have established a critical role for ENaCs in the regulation of airway surface liquid volume and excessive activity of ENaCs in airways contributes to the pathogenesis of cystic fibrosis (44). Impaired Na+ transport in alveoli leads to pulmonary edema (40). Airborne particles contain a considerate amount of transition metals including copper. Upon contact with biological fluids, free metal ions can be released from the particles and cause local and even remote toxic effects (17, 18). We speculate that the inhibitory effect of Cu2+ on human ENaCs in lung epithelia may contribute to the toxicological symptoms caused by inhaled particulate matters. Cu2+, released from particulate matters, may worsen particle-induced pulmonary edema by inhibition of Na+ absorption in airways and alveoli.
The main goal of this study was to probe the structural basis for hENaC inhibition by external Cu2+. Experiments with mixed human and mouse ENaC subunits demonstrate that α and β hENaC subunits are necessary for the specific response of hENaCs to Cu2+ (Fig. 4). Initial mutational screening of the hENaC-specific residues within α and β ECDs identified αHis468 and βHis159 as residues involved in Cu2+ inhibition (Fig. 5). The double mutant (αH468S-βH159D) converted the response to Cu2+ of the human channel to that of the mouse channel (Figs. 4 and 5), suggesting that these two hENaC-specific residues are primarily responsible for the distinct response to 10 μm Cu2+ of human versus mouse ENaC. Subsequently, systematic screening of His residues within ECDs of α, β, and γ subunits identified additional four sites (αHis255, βHis160, γHis88, and γHis277) where mutations significantly reduced the inhibitory effect of 10 μm Cu2+ (Fig. 7). However, further analyses showed that αHis255 and γHis277 mutations significantly increased the magnitudes of Na+ self-inhibition and Cu2+-induced transient activation preceding the inhibitory effect (Figs. 7D and 10), suggesting indirect roles for both His residues in the Cu2+ inhibition. Another residue, γHis88, does not appear to have an essential role in Cu2+ inhibition, given the small effect of its mutation on Cu2+ inhibition (Fig. 7). On the contrary, αHis468, βHis159, and βHis160 mutations specifically reduced Cu2+ inhibition, without affecting the Na+ self-inhibition response and the transient activation by Cu2+. We conclude that these three His residues are involved in Cu2+ inhibition.
Our data suggest that αHis468 has a key role in mediating Cu2+ inhibition. Taking advantage of the structural information for cASIC1 (13, 45), we predicted that αHis468 and βGlu254 could contribute to a Cu2+-binding site at the α/β subunit interface. Mutational analyses confirmed the prediction (Fig. 9). The identification of αHis468/βGlu254 pair suggests a counterclockwise configuration of α, β, and γ subunits when viewed from above the channel (Fig. 9F). This subunit arrangement is in agreement with a recent report by Collier and Snyder (46). However, we cannot rule out the presence of both the counterclockwise and clockwise subunit arrangements. Another limitation of the notion is that it is based on the assumption that ENaC, like ASIC1, has a trimeric architecture, which remains to be established experimentally.
ENaC Po is regulated by a variety of extracellular factors that may share common pathways in their regulation of ENaC gating. Indeed, the effects of external Zn2+, H+, and Cl− on ENaC activity rely on the existence of Na+ self-inhibition (7–9). However, Cu2+ inhibition of hENaC does not depend on the existence of Na+ self-inhibition. In fact, the magnitude of Cu2+ inhibition was increased by Na+ self-inhibition eliminating mutation (γH233A) and reduced by Na+ self-inhibition enhancing mutation (αH255A). Therefore, Cu2+ likely inhibits the hENaC via a pathway distinct from that of Na+ self-inhibition. In contrast, the transient activation of hENaC by Cu2+ appears to result from a relief of Na+ self-inhibition, because Na+ self-inhibition eliminating (γH233A) or enhancing (αH255A) mutations diminished or enhanced its magnitude accordingly.
In summary, we found that external Cu2+ is a high affinity inhibitor of human ENaC and identified a Cu2+-binding site at subunit interface within the extracellular domains. Structure-assisted mutational analyses suggest that a thumb domain His residue of α subunit and a palm domain Glu residue of the β subunit interact with a Cu2+ ion. This pairing (αHis468/βGlu254) requires a counterclockwise arrangement of α, β, and γ ENaC subunits when viewed from above.
Acknowledgments
We thank Dr. Thomas R. Kleyman for critical reading and comments on this manuscript, Dr. Joseph Pilewski at the Airway Epithelial Cell Culture Core at the University of Pittsburgh for providing human bronchial epithelial cultures, and Brandon M. Blobner for oocyte preparation.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 ES014701, K08 HL087932, P30 DK072506, and P30 DK079307. This work was also supported by a research grant from Dialysis Clinic, Inc. and the Cystic Fibrosis Foundation RDP to the University of Pittsburgh.
J. Chen and S. Sheng, unpublished observations.
- ENaC
- epithelial Na+ channel
- hENaC
- human ENaC
- mENaC
- mouse ENaC
- ASIC
- acid sensing ion channel
- ECD
- extracellular domain
- MES
- 2-(N-morpholino) ethanesulfonic acid
- MOPS
- 3-(N-morpholino) propanesulfonic acid
- TTM
- tetrathiomolybdate.
REFERENCES
- 1. Sheng S., Johnson J. P., Kleyman T. R. (2007) in Seldin and Giebisch's The Kidney: Physiology & Pathophysiology (Alpern R. J., Hebert S. C. eds.) 4th Ed., pp. 743–768, Academic Press, New York [Google Scholar]
- 2. Bhalla V., Hallows K. R. (2008) J. Am. Soc. Nephrol. 19, 1845–1854 [DOI] [PubMed] [Google Scholar]
- 3. Passero C. J., Hughey R. P., Kleyman T. R. (2010) Curr. Opin. Nephrol. Hypertens 19, 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garty H., Palmer L. G. (1997) Physiol. Rev. 77, 359–396 [DOI] [PubMed] [Google Scholar]
- 5. Van Driessche W., Zeiske W. (1985) Physiol. Rev. 65, 833–903 [DOI] [PubMed] [Google Scholar]
- 6. Sheng S., Perry C. J., Kleyman T. R. (2002) J. Biol. Chem. 277, 50098–50111 [DOI] [PubMed] [Google Scholar]
- 7. Sheng S., Perry C. J., Kleyman T. R. (2004) J. Biol. Chem. 279, 31687–31696 [DOI] [PubMed] [Google Scholar]
- 8. Collier D. M., Snyder P. M. (2009) J. Biol. Chem. 284, 792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collier D. M., Snyder P. M. (2009) J. Biol. Chem. 284, 29320–29325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nie H. G., Zhang W., Han D. Y., Li Q. N., Li J., Zhao R. Z., Su X. F., Peng J. B., Ji H. L. (2010) Am. J. Physiol. Renal Physiol. 298, F323–F334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kleyman T. R., Carattino M. D., Hughey R. P. (2009) J. Biol. Chem. 284, 20447–20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carattino M. D., Sheng S., Kleyman T. R. (2004) J. Biol. Chem. 279, 4120–4126 [DOI] [PubMed] [Google Scholar]
- 13. Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Nature 449, 316–323 [DOI] [PubMed] [Google Scholar]
- 14. Yu L., Eaton D. C., Helms M. N. (2007) Am. J. Physiol. Renal Physiol. 293, F236–F244 [DOI] [PubMed] [Google Scholar]
- 15. Ellingsen D. G., Horn N., Aaseth J. (2007) in Handbook on the Toxicology of Metals (Nordberg G. F., Fowler B. A., Norgberg M., Friberg L. T. eds.) 3rd Ed., pp. 529–547, Academic Press, Burlington, MA [Google Scholar]
- 16. Tisato F., Marzano C., Porchia M., Pellei M., Santini C. (2010) Med. Res. Rev. 30, 708–749 [DOI] [PubMed] [Google Scholar]
- 17. Adamson I. Y., Prieditis H., Vincent R. (1999) Toxicol. Appl. Pharmacol. 157, 43–50 [DOI] [PubMed] [Google Scholar]
- 18. Prieditis H., Adamson I. Y. (2002) Exp. Lung Res. 28, 563–576 [DOI] [PubMed] [Google Scholar]
- 19. Karlsson H. L., Cronholm P., Gustafsson J., Möller L. (2008) Chem. Res. Toxicol. 21, 1726–1732 [DOI] [PubMed] [Google Scholar]
- 20. Kiss T., Osipenko O. N. (1994) Pharmacol. Rev. 46, 245–267 [PubMed] [Google Scholar]
- 21. Restrepo-Angulo I., De Vizcaya-Ruiz A., Camacho J. (2010) J. Appl. Toxicol. 30, 497–512 [DOI] [PubMed] [Google Scholar]
- 22. McDonald F. J., Price M. P., Snyder P. M., Welsh M. J. (1995) Am. J. Physiol. 268, C1157–C1163 [DOI] [PubMed] [Google Scholar]
- 23. McDonald F. J., Snyder P. M., McCray P. B., Jr., Welsh M. J. (1994) Am. J. Physiol. 266, L728–L734 [DOI] [PubMed] [Google Scholar]
- 24. Hegetschweiler K., Saltman P. (1986) Inorg. Chem. 25, 107–109 [Google Scholar]
- 25. Chraïbi A., Horisberger J. D. (2002) J. Gen. Physiol. 120, 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheng S., Bruns J. B., Kleyman T. R. (2004) J. Biol. Chem. 279, 9743–9749 [DOI] [PubMed] [Google Scholar]
- 27. Sheng S., Maarouf A. B., Bruns J. B., Hughey R. P., Kleyman T. R. (2007) J. Biol. Chem. 282, 20180–20190 [DOI] [PubMed] [Google Scholar]
- 28. Myerburg M. M., Harvey P. R., Heidrich E. M., Pilewski J. M., Butterworth M. B. (2010) Am. J. Respir. Cell Mol. Biol. 43, 712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kellenberger S., Gautschi I., Rossier B. C., Schild L. (1998) J. Clin. Invest. 101, 2741–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Volk T., Konstas A. A., Bassalaý P., Ehmke H., Korbmacher C. (2004) Pflugers Arch. 447, 884–894 [DOI] [PubMed] [Google Scholar]
- 31. Dokmanić I., Sikić M., Tomić S. (2008) Acta Crystallogr. D Biol. Crystallogr. 64, 257–263 [DOI] [PubMed] [Google Scholar]
- 32. Pace C. N., Grimsley G. R., Scholtz J. M. (2009) J. Biol. Chem. 284, 13285–13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nevin S. T., Cromer B. A., Haddrill J. L., Morton C. J., Parker M. W., Lynch J. W. (2003) J. Biol. Chem. 278, 28985–28992 [DOI] [PubMed] [Google Scholar]
- 34. Nagaya N., Tittle R. K., Saar N., Dellal S. S., Hume R. I. (2005) J. Biol. Chem. 280, 25982–25993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barlow D. J., Thornton J. M. (1983) J. Mol. Biol. 168, 867–885 [DOI] [PubMed] [Google Scholar]
- 36. Rulísek L., Vondrásek J. (1998) J. Inorg. Biochem. 71, 115–127 [DOI] [PubMed] [Google Scholar]
- 37. Sheng S., Carattino M. D., Bruns J. B., Hughey R. P., Kleyman T. R. (2006) Am. J. Physiol. Renal Physiol. 290, F1488–F1496 [DOI] [PubMed] [Google Scholar]
- 38. Maarouf A. B., Sheng N., Chen J., Winarski K. L., Okumura S., Carattino M. D., Boyd C. R., Kleyman T. R., Sheng S. (2009) J. Biol. Chem. 284, 7756–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Askwith C. C., Benson C. J., Welsh M. J., Snyder P. M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6459–6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis I. C., Matalon S. (2007) Adv. Exp. Med. Biol. 618, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carattino M. D., Sheng S., Bruns J. B., Pilewski J. M., Hughey R. P., Kleyman T. R. (2006) J. Biol. Chem. 281, 18901–18907 [DOI] [PubMed] [Google Scholar]
- 42. Bruns J. B., Carattino M. D., Sheng S., Maarouf A. B., Weisz O. A., Pilewski J. M., Hughey R. P., Kleyman T. R. (2007) J. Biol. Chem. 282, 6153–6160 [DOI] [PubMed] [Google Scholar]
- 43. Rossier B. C., Pradervand S., Schild L., Hummler E. (2002) Annu. Rev. Physiol. 64, 877–897 [DOI] [PubMed] [Google Scholar]
- 44. Donaldson S. H., Boucher R. C. (2007) Chest 132, 1631–1636 [DOI] [PubMed] [Google Scholar]
- 45. Gonzales E. B., Kawate T., Gouaux E. (2009) Nature 460, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Collier D. M., Snyder P. M. (2011) J. Biol. Chem. 286, 6027–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chalfant M. L., Denton J. S., Berdiev B. K., Ismailov II, Benos D. J., Stanton B. A. (1999) Am. J. Physiol. 276, C477–C486 [DOI] [PubMed] [Google Scholar]