Abstract
IFNγ and TNFα are potent inhibitors of hematopoiesis and have been implicated in the pathophysiology of bone marrow failure and myelodysplastic syndromes (MDS). We examined the role of protein kinase R (PKR) in the generation of the inhibitory effects of these myelosuppressive cytokines on hematopoiesis. Our data demonstrate that PKR is rapidly phosphorylated/activated in response to engagement of IFNγ or TNFα receptors in normal human hematopoietic progenitors. Such engagement of PKR is important for the suppressive effects of these cytokines on normal hematopoiesis. Pharmacological targeting of PKR using a specific inhibitor or siRNA-mediated PKR knockdown results in partial reversal of the suppressive effects of IFNγ and TNFα on normal human CD34+-derived myeloid (colony-forming unit-granulocyte-monocytic) and erythroid (burst-forming unit-erythroid) progenitors. Importantly, inhibition of PKR activity or expression increases hematopoietic colony formation from human MDS progenitors, suggesting that drugs that target PKR may provide a novel approach for the treatment of MDS and marrow failure syndromes. Altogether, our data establish that beyond its key role in the induction of IFN-antiviral responses, PKR plays important roles in signaling for IFNγ and other myelosuppressive cytokine receptors as a common mediator of signals for hematopoietic suppression.
Keywords: Antiviral Agents, Cytokine, Hematopoiesis, Interferon, Signal Transduction, Protein Kinase R (PKR)
Introduction
IFNγ is a cytokine with major roles in the regulation of innate immunity against infections and in the immune surveillance against cancer (1–3). Over the years, the important properties of IFNγ have led to extensive investigative efforts to understand and define the signaling events that are elicited upon binding of this cytokine to its receptor and to define the mechanisms by which it generates its biological effects. It is now well established that IFNγ-dependent transcriptional regulation is controlled by engagement of JAKs and STATs by the Type II IFN receptor and formation of STAT1-STAT1 homodimers that regulate gene transcription by binding to gamma interferon activation site elements in the promoters of IFN-stimulated genes (1–4). There is also accumulating evidence that auxillary pathways, including the PI3K/mammalian target of rapamycin signaling cascade, play important roles in the generation of IFNγ signals and IFNγ-dependent mRNA translation for genes that mediate antiviral and growth inhibitory responses (5, 6).
Beyond its antiviral and antitumor properties, IFNγ also exhibits potent suppressive effects on normal hematopoiesis and has been implicated in the pathogenesis of bone marrow failure syndromes and MDS2 (7–10). Similarly, another proinflammatory cytokine, TNFα, also plays a key role in the pathophysiology of bone marrow suppression and MDS (10, 11). Interestingly, although IFNγ and TNFα and other myelosuppressive cytokines transduce signals via distinct pathways (1–6, 12, 13), there is evidence that they activate common signaling cascades in hematopoietic progenitor cells to mediate suppressive responses on primitive hematopoietic precursors. This has been shown by studies that have established that the p38 MAPK pathway is a common element in the signaling pathways of these cytokines in hematopoietic cells (14, 15) and functions as a mediator of myelosuppressive signals in the bone marrow of patients suffering from marrow failure or MDS (15–17).
Protein kinase R (PKR) is an IFN-inducible double-stranded RNA-activated serine-threonine protein kinase. This kinase is a major mediator of the antiviral and antiproliferative activities of IFNs (18–24), but its role in the regulation of normal and malignant hematopoiesis is not known. In the present study, we examined the engagement and functional role of PKR in the generation of the suppressive effects of IFNγ and TNFα on primitive hematopoietic progenitors. Our data provide evidence that IFNγ or TNFα treatment of expanded human hematopoietic progenitors from normal bone marrow results in phosphorylation/activation of PKR and its downstream target eIF2α. We also demonstrate that PKR regulates downstream cytokine-dependent activation of MAPKs, including ERK1/2 and the JNK kinase pathway in hematopoietic cells. In studies using siRNA-mediated knockdown of PKR, we show that the function of this signaling cascade is essential for generation of myelosuppressive responses and operates in parallel, but independently of the p38 MAPK, to promote induction of such responses.
MATERIALS AND METHODS
Cells and Reagents
U937 cells were grown in RPMI 1060 supplemented with 10% (v/v) fetal bovine serum and antibiotics. Recombinant human IFNγ was obtained from PBL Interferon Source (Piscataway, NJ). Recombinant human TNFα was obtained from Invitrogen (Carlsbad, CA). An antibody against the phosphorylated form of PKR (Thr-446) was purchased from Abcam. Antibodies against PKR, p38, ERK1/2, JNK, and eIF2α and the phosphorylated forms of p38 (Thr-180/Tyr-182), ERK1/2 (Thr-202/Tyr-204), JNK (Thr-183/Tyr-185), and eIF2α (Ser-51) were obtained from Cell Signaling Technology (Danvers, MA). An antibody against GAPDH was obtained from Chemicon (Billerica, MA). The PKR inhibitor and the corresponding inactive inhibitor were purchased from EMD Biosciences (Darmstadt, Germany). Primary normal CD34+ progenitor cells were either obtained from Stem Cell Technologies (Vancouver, Canada) or Lonza (Basel, Switzerland). Differentiation of human CD34+ cells to colony-forming unit-erythroid (CFU-E) was achieved by culture in a cytokine mixture, as previously described (14). Two distinct sets of siRNAs targeting human PKR as well as nontargeting siRNAs were obtained either from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) which was a pool of three siRNAs: sequence 1, GAAGGUGAAGGUAGAUCAA; sequence 2, CACACUCGCUUCUGAAUCA; and sequence 3, CGACCUAACACAUCUGAAA. The second set of siRNA targeting human PKR as well as nontargeting siRNAs were obtained from Dharmacon (Thermo Scientific), which was a single targeting sequence: GCGAGAAACUAGACAAAGU.
Lentivirus particles targeted against human PKR were purchased from Santa Cruz Biotechnology, Inc., which was a pool of three targeting sequences. Puromycin was purchased from Sigma. Bone marrow or peripheral blood from MDS patients was collected after obtaining consent approved by the institutional review board of Northwestern University.
Cell Lysis and Immunoblotting
Cells were treated with 1 × 104 IU/ml of IFNγ or 20 ng/ml of TNFα for the indicated times and then subsequently lysed in phosphorylation buffer as described previously (26). In the experiments using siRNAs targeted against human PKR, the cells were nucleofected following the manufacturer's protocol (Amaxa AG, Cologne, Germany). 24 h later the cells were treated with IFNγ or TNFα, as indicated. The cells were then collected and lysed in phosphorylation lysis buffer. Immunoblotting was performed using an ECL method as in previous studies (27).
Cell Proliferation Assays
The cells were seeded in 96-well plates and incubated in the presence or absence of human TNFα and IFNγ at the indicated concentrations at 37 °C for 7 days. Cell proliferation was assessed using a methyl-thiazolyl-tetrazolium assay system as in our previous studies (28, 29).
RNA Isolation and Quantitative RT-PCR
Cellular mRNA was reverse transcribed into cDNA using the Omniscript RT kit and oligo(dT) primer (Qiagen) as previously described. Quantitative real time RT-PCR was carried out as in our previous studies (30, 31). Commercially available FAM-labeled probes and primers (Applied Biosystem) were used to determine PKR mRNA expression. GAPDH was used for normalization.
Hematopoietic Progenitor Cell Assays
Formation of burst-forming unit-erythroid (BFU-E) or colony-forming unit-granulocyte-monocytic (CFU-GM) was assessed in clonogenic assays in methylcellulose, as in our previous studies (32–34).
RESULTS
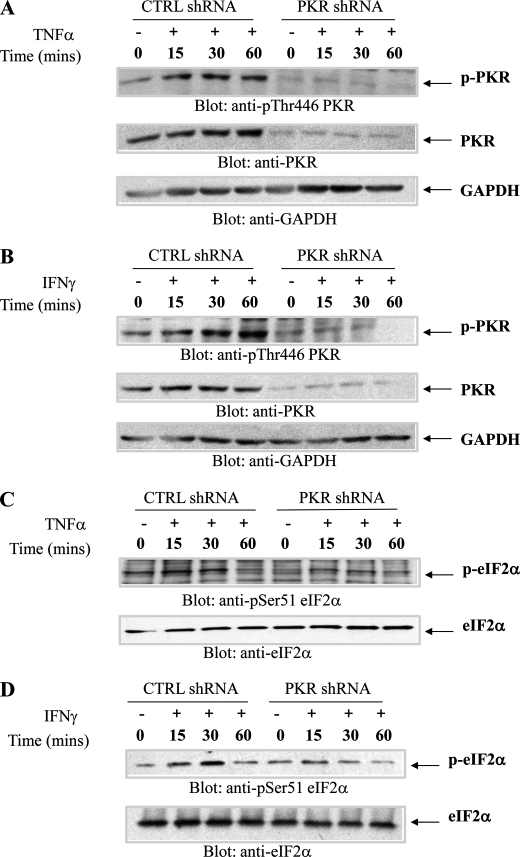
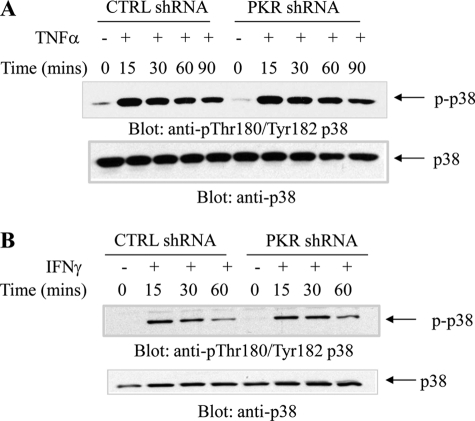
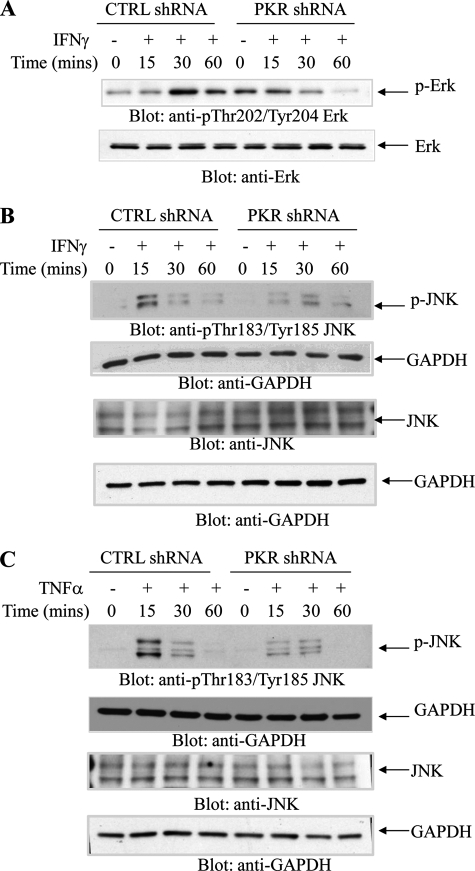
In initial studies, we examined whether TNFα and IFNγ induce phosphorylation/activation of PKR in hematopoietic cells. U937 cells expressing PKR or U937 cells in which PKR was stably knocked down were treated with TNFα (Fig. 1A) or IFNγ (Fig. 1B), and cell lysates were immunoblotted with an antibody against phosphorylated PKR on Thr-446. Treatment with either TNFα or IFNγ resulted in rapid phosphorylation of PKR that was noticeable within 15–30 min of the cells, whereas no signal was detectable in cells in which PKR was knocked down (Fig. 1, A and B). Moreover, both TNFα (Fig. 1C) and IFNγ (Fig. 1D) induced phosphorylation of e-IF2α on Ser-51, and such phosphorylation was decreased by knockdown of PKR (Fig. 1, C and D). On the other hand, phosphorylation of p38 MAPK in U937 cells by either TNFα or IFNγ was found to be PKR-independent (Fig. 2, A and B). In other studies in which the IFNγ-inducible activation of ERK and JNK was examined, we found that engagement of both ERK and JNK were PKR-dependent, although some degree of phosphorylation/activation was still detectable in cells in which PKR was knocked down (Fig. 3, A and B). Similarly, TNFα-mediated phosphorylation/activation of JNK was also PKR-dependent (Fig. 3C).
FIGURE 1.
TNFα and IFNγ induce phosphorylation/activation of PKR in leukemia cells. U937 cells stably expressing CTRL shRNA or PKR shRNA were treated with human TNFα (A and C) or IFNγ (B and D) for the indicated times. Total lysates were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. CTRL, control.
FIGURE 2.
TNFα- or IFNγ-dependent activation of p38 MAPK is PKR-independent phosphorylation. U937 cells stably expressing control (CTRL) shRNA or PKR shRNA were treated with human TNFα (A) or IFNγ (B) for the indicated times. Total lysates were resolved by SDS-PAGE and immunoblotted with the indicated antibodies.
FIGURE 3.
PKR engagement is required for activation of ERK1/2 or JNK in response to TNFα or IFNγ. A, U937 cells stably expressing control (CTRL) shRNA or PKR shRNA were treated with human IFNγ for the indicated times, and cell lysates were resolved by SDS-PAGE and immunoblotted with anti-phospho-Thr-202/Tyr-204-Erk or anti-Erk, as indicated. B, U937 cells stably expressing control shRNA or PKR shRNA were treated with human IFNγ for the indicated times, and cell lysates were resolved by SDS-PAGE and immunoblotted with antibodies against phosphor-JNK or GAPDH as indicated (top two panels). Equal amounts of cell lysates from the same experiment were resolved separately by SDS-PAGE and immunoblotted with antibodies against JNK or GAPDH, as indicated (bottom two panels). C, U937 cells stably expressing CTRL shRNA or PKR shRNA were treated with TNFα for the indicated times, and cell lysates were resolved by SDS-PAGE and immunoblotted with antibodies against phosphor-JNK or GAPDH as indicated (top two panels). Equal amounts of cell lysates from the same experiment were resolved separately by SDS-PAGE and immunoblotted with antibodies against JNK or GAPDH, as indicated (bottom two panels).
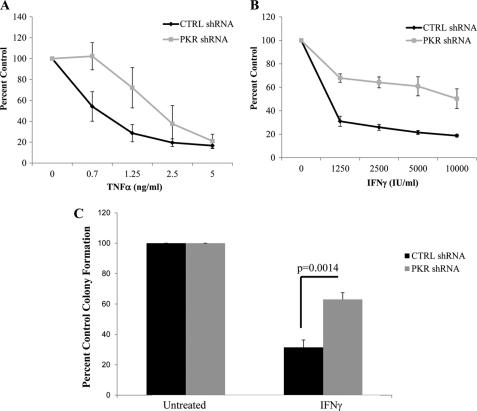
To define the functional relevance of engagement of PKR by IFNγ and TNFα in hematopoietic cells, we examined the ability of these cytokines to generate growth inhibitory responses in cells in which PKR was stably knocked down. Treatment with either IFNγ or TNFα resulted in decreased cell viability of U937 cells expressing PKR, whereas such responses were significantly impaired in cells in which PKR was knocked down (Fig. 4, A and B). Importantly, in experiments in which effects on leukemic progenitor colony formation were assessed by clonogenic assays in methylcellulose, we found that PKR knockdown led to partial reversal of IFNγ-induced suppressive responses on leukemic CFU-L progenitors (Fig. 4C).
FIGURE 4.
Requirement for PKR kinase mediates the antiproliferative effects of TNFα and IFNγ on the growth of U937 cells. A and B, U937 cells stably expressing control shRNA or PKR shRNA were incubated for 7 days in the presence or absence of the indicated concentrations of human TNFα (A) or human IFNγ (B), and cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays. The data are expressed as percentages of control of untreated cells and represent the means ± S.E. of five experiments. C, U937 cells stably expressing control shRNA or PKR shRNA were incubated in the presence or absence of IFNγ as indicated and leukemic progenitor (CFU-L) colonies were scored after 7 days in methylcellulose cultures. The data are expressed as percentages of control colony formation from untreated cells and represent the means ± S.E. of five experiments. Paired t test analysis showed p = 0.0014 for the effects of IFNγ on PKR shRNA versus control shRNA expressing cells. CTRL, control.
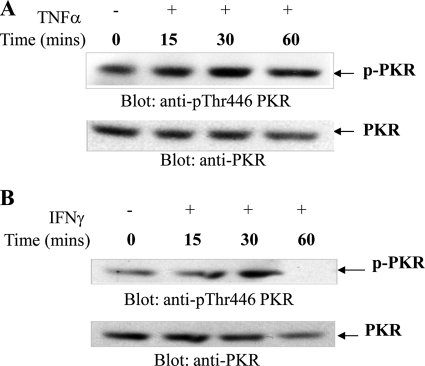
To define the role of PKR in the regulation of hematopoiesis by myelosuppressive cytokines in a more physiologically relevant system, experiments were performed using primary human hematopoietic progenitors from normal bone marrow. In initial experiments, we used a previously described methodology to expand normal bone marrow-derived human erythroid (CFU-E) progenitors (35, 36). As shown in Fig. 5, both myelosuppressive cytokines induced phosphorylation of PKR on Thr-446, indicating that the pathway is engaged by myelosuppressive cytokines in primitive hematopoietic precursors.
FIGURE 5.
Cytokine-mediated engagement of PKR in expanded human progenitors. CFU-E progenitor cells were treated with human TNFα (A) or human IFNγ (B) for the indicated times. Total lysates were separated by SDS-PAGE and immunoblotted with an antibody against phosphorylated PKR (Thr-446). The same blots were subsequently stripped and reprobed with an antibody against PKR.
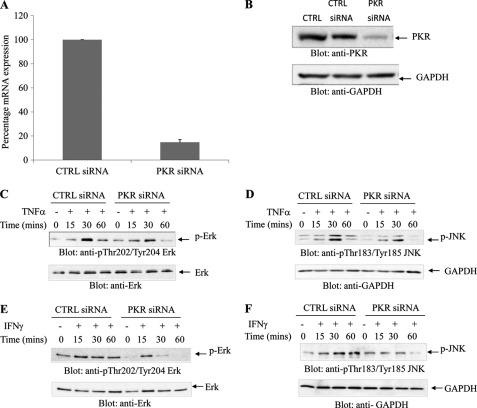
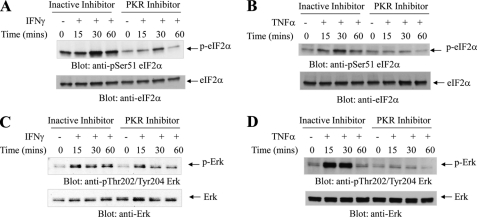
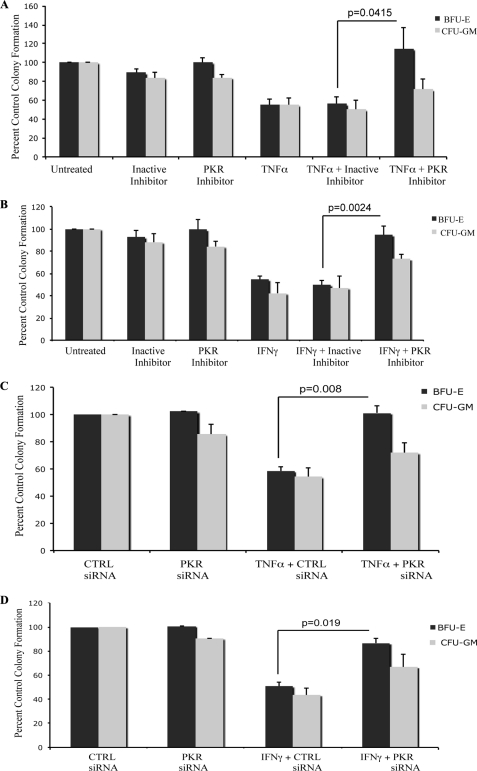
PKR-mediated signaling has been previously shown to engage a number of different stress signals downstream of the activated kinase (18), but its requirement in normal human cells of hematopoietic origin has not been known. Because our data in the leukemia cell line U937 suggested a role for PKR in the regulation of Erk and JNK by myelosuppressive cytokines, we examined the phosphorylation/activation of these MAPKs in response in expanded normal erythroid human bone marrow progenitors (CFU-E), in which PKR expression was knocked down (Fig. 6, A and B). When CFU-E progenitors were treated with TNFα, there was induction of phosphorylation/activation of ERK and JNK, but such phosphorylation was defective in cells in which PKR was knocked down (Fig. 6, C and D). Similarly, IFNγ-dependent phosphorylation of ERK and JNK was defective in cells in which PKR was knocked down (Fig. 6, E and F). We also examined the effects of pharmacological inhibition of PKR on downstream effectors in human hematopoietic progenitors. As shown in Fig. 7, the IFNγ or TNFα-induced phosphorylation of eIF2α and ERK were inhibited in the presence of PKR inhibitor. Altogether, these studies established that PKR is a common effector in the signaling pathways of both TNFα and IFNγ in human hematopoietic precursors. To examine the functional relevance of PKR in the generation of the suppressive effects of these cytokines on hematopoiesis, human CD34+ bone marrow cells were treated with TNFα or IFNγ in the presence or absence of a pharmacological inhibitor of PKR, and normal myeloid (CFU-GM) or erythroid (BFU-E) colony formation was assessed in clonogenic assays in methylcellulose. As shown in Fig. 8, treatment with either TNFα (Fig. 8A) or IFNγ (Fig. 8B) resulted in suppression of hematopoietic progenitor colony formation, but in both cases the effects were reversed by a PKR inhibitor. Importantly, siRNA-mediated knockdown of PKR resulted in reversal of the suppressive effects of TNFα (Fig. 8C) or IFNγ (Fig. 8D), establishing an important role for PKR as a mediator of myelosuppressive responses in normal hematopoiesis. Similar results were obtained using a different PKR siRNA (Fig. 9).
FIGURE 6.
Requirement of PKR activity for activation of MAPK pathways in primitive hematopoietic precursors. A, expanded human CFU-E cells were nucleofected with control siRNA or PKR siRNA, as described under “Materials and Methods.” Expression of PKR mRNA was assessed by real time RT-PCR and is shown as a percentage of the levels of control siRNA-treated cells. The data shown represent the means ± S.E. of two experiments. B, CFU-E cells were nucleofected with control siRNA or PKR siRNA. Equal amounts of protein were separated by SDS-PAGE and then immunoblotted with antibodies against PKR or GAPDH. C–F, CFU-E cells were nucleofected with either control siRNA or PKR siRNA for 24 h and were then treated with human TNFα (C and D) or IFNγ (E and F) for the indicated times. Equal amounts of protein were separated by SDS-PAGE and then immunoblotted with the indicated antibodies. CTRL, control.
FIGURE 7.
Effects of pharmacological inhibition of PKR activity on eIF2a and Erk phosphorylation in normal hematopoietic progenitors. Expanded human CFU-E cells were pretreated with inactive inhibitor or PKR inhibitor (1 μm) for 1 h and were then treated with human IFNγ (A and C) or TNFα (B and D) for the indicated times. Equal amounts of protein were separated by SDS-PAGE and then immunoblotted with the indicated antibodies.
FIGURE 8.
PKR kinase mediates the antiproliferative effects of TNFα and IFNγ on normal hematopoiesis. A and B, normal CD34+ bone marrow-derived cells were incubated in the presence or absence of TNFα (A) or IFNγ (B), in the presence of a PKR inhibitor or a corresponding control-inactive inhibitor, in clonogenic assays in methylcellulose, as indicated. CFU-GM and BFU-E progenitor colonies were scored after 14 days in culture. The data are expressed as percentages of control colony formation from untreated cells and represent the means ± S.E. of five independent experiments for A and four independent experiments for B. p values for the indicated paired t test analyses are shown. C and D, normal human bone marrow-derived CD34+ cells were transfected with control siRNA or PKR siRNA, as indicated and incubated in clonogenic assays in methylcellulose in the presence or absence of TNFα (C) or IFNγ (D), as indicated. CFU-GM and BFU-E progenitor colonies were scored after 14 days in culture. The data are expressed as percentages of colony formation from control siRNA transfected cells and represent the means ± S.E. of five experiments for C and four experiments for D. p values for the indicated paired t test analyses are shown. CTRL, control.
FIGURE 9.
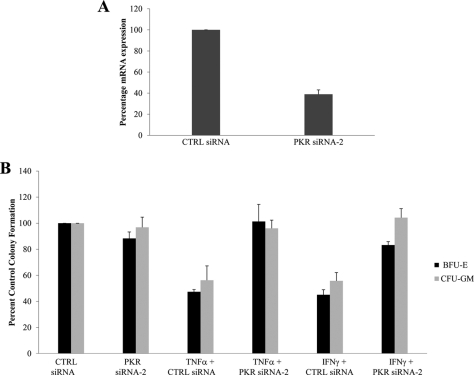
Effects of PKR knockdown on the generation of the antiproliferative effects of TNFα and IFNγ on normal hematopoiesis. A, U937 cells nucleofected with control siRNA or PKR siRNA2, as described under “Materials and Methods.” Expression of PKR mRNA was assessed by real time RT-PCR. The data are expressed as percentages of mRNA expression for control siRNA-treated cells and represent the means ± S.E. of two experiments. B, normal human bone marrow-derived CD34+ cells were transfected with control siRNA or PKR siRNA2, as indicated and incubated in clonogenic assays in methylcellulose in the presence or absence of TNFα or IFNγ, as indicated. CFU-GM and BFU-E progenitor colonies were scored after 14 days in culture. The data are expressed as percentages of colony formation from control siRNA transfected cells and represent the means ± S.E. of four experiments. CTRL, control.
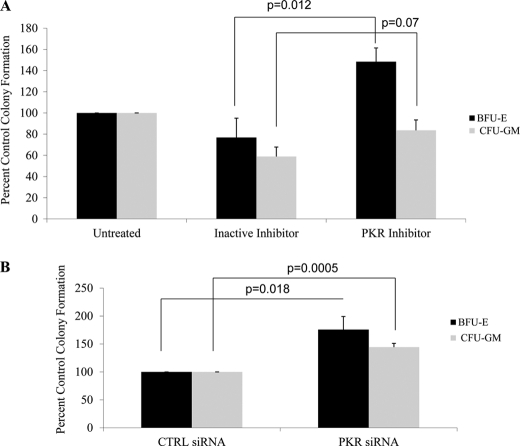
Because our studies established that PKR is involved in the generation of the suppressive effects of TNFα or IFNγ on normal hematopoiesis and overproduction of these cytokines has been implicated in the pathophysiology of bone marrow failure and myelodysplastic syndromes (MDS) (7–10), we performed studies to determine whether inhibition of PKR activity reverses myelosuppression in MDS. Bone marrow or peripheral blood mononuclear cells from MDS patients were treated with PKR inhibitor or an inactive control inhibitor, and myeloid (CFU-GM) or erythroid (BFU-E) colony formation was assessed. Treatment with the PKR inhibitor enhanced both erythroid (BFU-E) and myeloid (CFU-GM) progenitor colony formation from MDS patient samples (Fig. 10A). Consistent with this, in experiments involving siRNA-mediated knockdown of PKR, there was a significant increase in hematopoietic progenitor colony formation from MDS patients marrow or blood samples (Fig. 10B), consistent with an important role for PKR as a mediator of the effects of myelosuppressive cytokines in MDS.
FIGURE 10.
Inhibition of PKR enhances colony formation from MDS bone marrow or peripheral blood. A, mononuclear cells derived from peripheral blood or bone marrow of patients with MDS were incubated in clonogenic assays in methylcellulose in the presence of PKR inhibitor or inactive inhibitor, as indicated. CFU-GM and BFU-E progenitor colonies were scored after 14 days in culture. p values for the indicated paired t test analyses are shown. B, mononuclear cells derived from peripheral blood or bone marrow of patients with MDS. The cells were nucleofected with control siRNA or PKR siRNA, as incubated, and CFU-GM and BFU-E progenitor colony formation was assessed in clonogenic assays in methylcellulose. The data are expressed as percentages of colony formation from control siRNA transfected cells and represent the means ± S.E. of four independent experiments. p values for the indicated paired t test analyses are shown. CTRL, control.
DISCUSSION
For optimal production and maturation of cells of different hematopoietic lineages, a fine balance must exist between the actions of hematopoietic growth factors and myelosuppressive cytokines (39–43). Deregulation in the production of myelosuppressive cytokines promotes development of various pathophysiological states, including marrow failure and myelodysplastic syndromes (MDS) (7, 45). MDS includes a heterogeneous group of diseases characterized by dysplastic hematopoiesis (46, 47), in which immune deregulation appears to play important pathobiologic roles. Recent work has provided evidence for expansion of abnormal auto reactive CD8+ T lymphocytes in the bone marrow of patient with low to intermediate risk MDS, suggesting a mechanism for the overproduction of myelosuppressive cytokines that target hematopoietic precursors in MDS (47–50). In previous work, we have identified signaling elements that may act as common effectors for signals generated by receptors of different myelosuppressive cytokines to mediate bone marrow suppression and inhibition of hematopoiesis. Among them, the p38 MAPK pathway appears to play a critical and essential role in the regulation of hematopoiesis in normal or abnormal states (14–17), whereas certain isoforms of the PKC family may also participate in a similar regulation (reviewed in Ref. 51), although their involvement requires further investigation.
The double stranded RNA-dependent PKR is a ubiquitously expressed kinase that integrates signals in response to Toll-like receptor activation, growth factor receptors, and diverse cellular stress responses (18–24, 37, 38). PKR was originally identified as a mediator of the innate immune and antiviral responses, but a number of studies over the years subsequently linked this kinase to cell growth and differentiation, inflammation, and cytokine signaling (18–24, 37). PKR is present as a monomer in resting cells and upon its engagement in response to various stimuli, it homodimerizes and undergoes autophosphorylation and activation of its kinase domain (38). Beyond its well established role as a kinase for eIF-2α, recent studies have demonstrated that PKR regulates phosphorylation of other molecules, such as IκBα, and plays important roles in transcriptional regulation and as a mediator of growth inhibitory and proapoptotic responses (19, 22).
Although PKR plays a central role as mediator of the biological functions of IFNs (20), to the best of our knowledge, there has been no previous work to define its role in the growth inhibitory effects of IFNs or other myelosuppressive cytokines on normal hematopoietic progenitors. However, a recent study (52) demonstrated that PKR is activated in the bone marrow of MDS patients and that its subcellular localization directly correlates with disease severity. The authors of that study found that the levels of phosphorylated PKR were increased in all MDS samples compared with normal bone marrow, and in the case of high risk MDS the subcellular localization was different (52).
In the present study, we sought to determine the involvement of PKR in the generation of the suppressive effects of IFNγ and TNFα on leukemic cells and normal hematopoietic precursors. Our data demonstrated that both of these cytokines, which have been previously implicated in the pathophysiology of marrow failure syndromes (7, 45), induce phosphorylation of PKR and its downstream effector eIF2a. Importantly, our studies established that molecular targeting of PKR results in partial reversal of their inhibitory effects on normal human marrow-derived erythroid (BFU-E) or myeloid (CFU-GM) hematopoietic progenitors. Although the precise mechanisms of such effects occur remain to be defined, in experiments involving siRNA-mediated knockdown of human PKR in expanded primary hematopoietic progenitors, we found a requirement for the kinase in the activation of ERK1/2 and JNK. These findings suggest a potential mechanism by which PKR may control regulation and differentiation of hematopoietic cells, involving MAPK pathways. ERK1/2 has been previously shown to be involved in controlling survival and differentiation of hematopoietic progenitors (53), whereas JNK kinase has been implicated in the regulation of erythropoiesis (54) and myeloid differentiation (55). Such regulatory effects of PKR on hematopoiesis appear to be particularly relevant in MDS and, possibly, marrow failure syndromes, because our studies demonstrate that pharmacological or molecular targeting of PKR results in enhanced colony formation from bone marrow or peripheral blood of patients with MDS.
The lack of effects of PKR targeting on p38 MAPK activation suggests the existence of parallel cascades that control normal hematopoiesis and can be deregulated in pathophysiological states. p38 MAPK is known to play critical roles in the pathophysiology of MDS and bone marrow failure (16, 17, 56). There has been previous evidence indicating a requirement for PKR for p38 MAPK activity in nonhematopoietic cells (44). Studies with PKR null MEFs have previously shown that PKR is required for p38 MAPK activation in response to double-stranded RNA, LPS, and other proinflammatory cytokines (44). The lack of regulatory effects on p38 MAPK in hematopoietic cells indicates cell type-dependent selectivity of MAPK targets for PKR activity and suggests that such differential targeting may account for the versatility of responses and cellular processes regulated by this kinase. Independently of the precise mechanisms involved, our findings raise the possibility that PKR may be an appropriate therapeutic target for the ultimate development of new therapeutic approaches for the treatment of MDS, and studies in that direction are warranted.
This work was supported, in whole or in part, by National Institutes of Health Grants CA77816, CA121192, and AG029138. This work was also supported by a merit review grant from the Department of Veterans Affairs.
- MDS
- myelodysplastic syndrome(s)
- PKR
- protein kinase R
- CFU-E
- colony-forming unit-erythroid
- BFU-E
- burst-forming unit-erythroid
- CFU-GM
- colony-forming unit-granulocyte-monocytic.
REFERENCES
- 1. Platanias L. C. (2005) Nat. Rev. Immunol. 5, 375–386 [DOI] [PubMed] [Google Scholar]
- 2. Borden E. C., Sen G. C., Uze G., Silverman R. H., Ransohoff R. M., Foster G. R., Stark G. R. (2007) Nat. Rev. Drug Discov. 6, 975–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schoenborn J. R., Wilson C. B. (2007) Adv. Immunol. 96, 41–101 [DOI] [PubMed] [Google Scholar]
- 4. Joshi S., Kaur S., Kroczynska B., Platanias L. C. (2010) Cytokine 52, 123–127 [DOI] [PubMed] [Google Scholar]
- 5. Stark G. R. (2007) Cytokine Growth Factor Rev. 18, 419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Platanias L. C., Fish E. N. (1999) Exp. Hematol. 27, 1583–1592 [DOI] [PubMed] [Google Scholar]
- 7. Young N. S., Scheinberg P., Calado R. T. (2008) Curr. Opin. Hematol. 15, 162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hofmann W. K., Koeffler H. P. (2005) Annu. Rev. Med. 56, 1–16 [DOI] [PubMed] [Google Scholar]
- 9. Young N. S. (2002) Ann. Intern. Med. 136, 534–546 [DOI] [PubMed] [Google Scholar]
- 10. Kitagawa M., Saito I., Kuwata T., Yoshida S., Yamaguchi S., Takahashi M., Tanizawa T., Kamiyama R., Hirokawa K. (1997) Leukemia 11, 2049–2054 [DOI] [PubMed] [Google Scholar]
- 11. Gersuk G. M., Beckham C., Loken M. R., Kiener P., Anderson J. E., Farrand A., Troutt A. B., Ledbetter J. A., Deeg H. J. (1998) Br. J. Haematol. 103, 176–188 [DOI] [PubMed] [Google Scholar]
- 12. Naude P. J., den Boer J. A., Luiten P. G., Eisel U. L. (2001) FEBS J. 278, 888–898 [DOI] [PubMed] [Google Scholar]
- 13. Parameswaran N., Patial S. (2010) Crit. Rev. Eukaryot. Gene Expr. 20, 87–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verma A., Deb D. K., Sassano A., Uddin S., Varga J., Wickrema A., Platanias L. C. (2002) J. Biol. Chem. 277, 7726–7735 [DOI] [PubMed] [Google Scholar]
- 15. Verma A., Deb D. K., Sassano A., Kambhampati S., Wickrema A., Uddin S., Mohindru M., Van Besien K., Platanias L. C. (2002) J. Immunol. 168, 5984–5988 [DOI] [PubMed] [Google Scholar]
- 16. Katsoulidis E., Li Y., Yoon P., Sassano A., Altman J., Kannan-Thulasiraman P., Balasubramanian L., Parmar S., Varga J., Tallman M. S., Verma A., Platanias L. C. (2005) Cancer Res. 65, 9029–9037 [DOI] [PubMed] [Google Scholar]
- 17. Navas T. A., Mohindru M., Estes M., Ma J. Y., Sokol L., Pahanish P., Parmar S., Haghnazari E., Zhou L., Collins R., Kerr I., Nguyen A. N., Xu Y., Platanias L. C., List A. A., Higgins L. S., Verma A. (2006) Blood 108, 4170–4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. García M. A., Gil J., Ventoso I., Guerra S., Domingo E., Rivas C., Esteban M. (2006) Microbiol. Mol. Biol. Rev. 70, 1032–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clemens M. J. (1997) Int. J. Biochem. Cell Biol. 29, 945–949 [DOI] [PubMed] [Google Scholar]
- 20. Pindel A., Sadler A. (2011) J. Interferon Cytokine Res. 31, 59–70 [DOI] [PubMed] [Google Scholar]
- 21. George C. X., Li Z., Okonski K. M., Toth A. M., Wang Y., Samuel C. E. (2009) J. Interferon Cytokine Res. 29, 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sadler A. J., Williams B. R. (2007) Curr. Top Microbiol. Immunol. 316, 253–292 [DOI] [PubMed] [Google Scholar]
- 23. McAllister C. S., Toth A. M., Zhang P., Devaux P., Cattaneo R., Samuel C. E. (2010) J. Virol. 84, 380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toth A. M., Zhang P., Das S., George C. X., Samuel C. E. (2006) Prog. Nucleic Acids Res. Mol. Biol. 81, 369–434 [DOI] [PubMed] [Google Scholar]
- 25. Zhang P., Samuel C. E. (2007) J. Virol. 81, 8192–8200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uddin S., Lekmine F., Sharma N., Majchrzak B., Mayer I., Young P. R., Bokoch G. M., Fish E. N., Platanias L. C. (2000) J. Biol. Chem. 275, 27634–27640 [DOI] [PubMed] [Google Scholar]
- 27. Uddin S., Yenush L., Sun X. J., Sweet M. E., White M. F., Platanias L. C. (1995) J. Biol. Chem. 270, 15938–15941 [DOI] [PubMed] [Google Scholar]
- 28. Giafis N., Katsoulidis E., Sassano A., Tallman M. S., Higgins L. S., Nebreda A. R., Davis R. J., Platanias L. C. (2006) Cancer Res. 66, 6763–6771 [DOI] [PubMed] [Google Scholar]
- 29. Kambhampati S., Li Y., Verma A., Sassano A., Majchrzak B., Deb D. K., Parmar S., Giafis N., Kalvakolanu D. V., Rahman A., Uddin S., Minucci S., Tallman M. S., Fish E. N., Platanias L. C. (2003) J. Biol. Chem. 278, 32544–32551 [DOI] [PubMed] [Google Scholar]
- 30. Deb D. K., Sassano A., Lekmine F., Majchrzak B., Verma A., Kambhampati S., Uddin S., Rahman A., Fish E. N., Platanias L. C. (2003) J. Immunol. 171, 267–273 [DOI] [PubMed] [Google Scholar]
- 31. Kaur S., Lal L., Sassano A., Majchrzak-Kita B., Srikanth M., Baker D. P., Petroulakis E., Hay N., Sonenberg N., Fish E. N., Platanias L. C. (2007) J. Biol. Chem. 282, 1757–1768 [DOI] [PubMed] [Google Scholar]
- 32. Kaur S., Sassano A., Dolniak B., Joshi S., Majchrzak-Kita B., Baker D. P., Hay N., Fish E. N., Platanias L. C. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 4808–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joshi S., Kaur S., Redig A. J., Goldsborough K., David K., Ueda T., Watanabe-Fukunaga R., Baker D. P., Fish E. N., Fukunaga R., Platanias L. C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kroczynska B., Kaur S., Katsoulidis E., Majchrzak-Kita B., Sassano A., Kozma S. C., Fish E. N., Platanias L. C. (2009) Mol. Cell Biol. 29, 2865–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wickrema A., Uddin S., Sharma A., Chen F., Alsayed Y., Ahmad S., Sawyer S. T., Krystal G., Yi T., Nishada K., Hibi M., Hirano T., Platanias L. C. (1999) J. Biol. Chem. 274, 24469–24474 [DOI] [PubMed] [Google Scholar]
- 36. Redig A. J., Sassano A., Majchrzak-Kita B., Katsoulidis E., Liu H., Altman J. K., Fish E. N., Wickrema A., Platanias L. C. (2009) J. Biol. Chem. 284, 10301–10314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Samuel C. E., Kuhen K. L., George C. X., Ortega L. G., Rende-Fournier R., Tanaka H. (1997) Int. J. Hematol. 65, 227–237 [DOI] [PubMed] [Google Scholar]
- 38. Li S., Peters G. A., Ding K., Zhang X., Qin J., Sen G. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10005–10010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lotem J., Shabo Y., Sachs L. (1991) Cell Growth Differ. 2, 421–427 [PubMed] [Google Scholar]
- 40. Bruserud Ø., Kittang A. O. (2010) Curr. Top Microbiol. Immunol. 341, 3–12 [DOI] [PubMed] [Google Scholar]
- 41. Bodo M., Baroni T., Tabilio A. (2009) J. Stem Cells 4, 57–69 [PubMed] [Google Scholar]
- 42. Kaushansky K. (2009) J. Thromb. Haemost. 1, 235–238 [DOI] [PubMed] [Google Scholar]
- 43. Baker S. J., Rane S. G., Reddy E. P. (2007) Oncogene 26, 6724–6737 [DOI] [PubMed] [Google Scholar]
- 44. Goh K. C., deVeer M. J., Williams B. R. (2000) EMBO J. 19, 4292–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Young N. S. (1996) Eur. J. Haematol. Suppl. 60, 55–59 [DOI] [PubMed] [Google Scholar]
- 46. Garcia-Manero G. (2010) Hematology Am. Soc. Hematol. Educ. Program 2010, 330–337 [DOI] [PubMed] [Google Scholar]
- 47. Olnes M. J., Sloand E. M. (2011) J. Am. Med. Assoc. 305, 814–819 [DOI] [PubMed] [Google Scholar]
- 48. Sloand E. M., Mainwaring L., Fuhrer M., Ramkissoon S., Risitano A. M., Keyvanafar K., Lu J., Basu A., Barrett A. J., Young N. S. (2005) Blood 106, 841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kochenderfer J. N., Kobayashi S., Wieder E. D., Su C., Molldrem J. J. (2002) Blood 100, 3639–3645 [DOI] [PubMed] [Google Scholar]
- 50. Chamuleau M. E., Westers T. M., van Dreunen L., Groenland J., Zevenbergen A., Eeltink C. M., Ossenkoppele G. J., van de Loosdrecht A. A. (2009) Haematologica 94, 496–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Redig A. J., Platanias L. C. (2007) J. Interferon Cytokine Res. 27, 623–636 [DOI] [PubMed] [Google Scholar]
- 52. Follo M. Y., Finelli C., Mongiorgi S., Clissa C., Bosi C., Martinelli G., Blalock W. L., Cocco L., Martelli A. M. (2008) Leukemia 22, 2267–2269 [DOI] [PubMed] [Google Scholar]
- 53. Geest C. R., Coffer P. J. (2009) J. Leukocyte Biol. 86, 237–250 [DOI] [PubMed] [Google Scholar]
- 54. Trivedi A. K., Bararia D., Christopeit M., Peerzada A. A., Singh S. M., Kieser A., Hiddemann W., Behre H. M., Behre G. (2007) Oncogene 26, 1789–1801 [DOI] [PubMed] [Google Scholar]
- 55. Arnold R., Frey C. R., Müller W., Brenner D., Krammer P. H., Kiefer F. (2007) Cell Death Differ. 14, 568–575 [DOI] [PubMed] [Google Scholar]
- 56. Navas T., Zhou L., Estes M., Haghnazari E., Nguyen A. N., Mo Y., Pahanish P., Mohindru M., Cao T., Higgins L. S., Platanias L. C., List A., Verma A., Bhagat T., Gajavelli S., Kambhampati S. (2008) Leuk. Lymphoma 49, 1963–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]