Abstract
β-Catenin is an important regulator of dermal fibroblasts during cutaneous wound repair. However, the factors that modulate β-catenin activity in this process are not completely understood. We investigated the role of the extracellular matrix in regulating β-catenin and found an increase in β-catenin-mediated Tcf-dependent transcriptional activity in fibroblasts exposed to various extracellular matrix components. This occurs through an integrin-mediated GSK3β-dependent pathway. The physiologic role of this mechanism was demonstrated during wound repair in extra domain A-fibronectin-deficient mice, which exhibited decreased β-catenin-mediated signaling during the proliferative phase of healing. Extra domain A-fibronectin-deficient mice have wounds that fail at a lower tensile strength and contain fewer fibroblasts compared with wild type mice. This phenotype was rescued by genetic or pharmacologic activation of β-catenin signaling. Because fibronectin is a transcriptional target of β-catenin, this suggests the existence of a feedback loop between these two molecules that regulates dermal fibroblast cell behavior during wound repair.
Keywords: Beta-Catenin, Extracellular Matrix, Fibroblast, Fibronectin, Skin, Wound Repair
Introduction
The healing process in response to cutaneous injury is characterized by regeneration of the cellular and extracellular components of the skin. This regeneration progresses through overlapping phases of inflammation, proliferation, and remodeling (1). The proliferative phase of healing involves the re-epithelialization of the superficial surface layer and the reconstitution of the underlying dermis by fibroblasts. The secretion of extracellular matrix components, such as fibronectin and collagen, by dermal fibroblasts during the proliferative phase is critical for successful wound repair, because these molecules are needed to re-establish the mechanical strength of the wound (2).
β-Catenin is an important regulator of the wound phenotype during healing, where it modulates dermal fibroblast activity (3–5). Wound fibroblasts have elevated β-catenin protein levels and exhibit β-catenin-mediated T-cell factor (Tcf)-dependent3 transcriptional activity during the proliferative phase of healing. Furthermore, β-catenin levels correlate with the size of the scar that forms, and genetic stabilization of β-catenin leads to hyperplastic scars in mice (3, 6). β-Catenin acts as a transcriptional co-activator but also associates with cadherins at adherens junctions to maintain cell-cell contacts (7). As a transcriptional co-activator, it is a crucial component of the canonical Wingless (Wnt) signaling pathway. Stabilization of cytoplasmic β-catenin can occur via Wnt-mediated regulation of a multiprotein complex, which includes glycogen-synthase kinase (GSK) 3β (7). Elevation of the cytoplasmic β-catenin level enables its translocation to the nucleus where it associates with Tcf/Lef transcription co-factors to modulate gene expression. In the absence of an appropriate Wnt ligand, the multiprotein complex targets cytoplasmic β-catenin for proteasomal degradation via phosphorylation of N-terminal serine/threonine residues. The Wnt signaling inhibitor Dickkopf-1 (DKK-1) binds the Wnt co-receptor LRP6/Arrow to prevent Wnt-mediated signal transduction, thus inhibiting β-catenin-mediated Tcf-dependent transcription (8).
The factors that regulate β-catenin activity in fibroblasts during healing are not completely understood. The extracellular environment is known to influence intracellular signaling, and this interaction can have profound effects on cell behavior. As such, it is possible that the extracellular matrix plays a role regulating β-catenin during wound repair. Indeed, data from cell lines of epithelial origin demonstrate that β-catenin signaling can be modulated by extracellular matrix components, such as collagen I (9–12), Matrigel (10), and collagen XVIII fragments (13, 14). Collagen can also influence β-catenin levels in mesenchymal cells from the fibroproliferative disorder, Dupuytren disease (15).
A variety of extracellular matrix components, including fibronectin isoforms, are secreted by dermal fibroblasts during cutaneous wound repair (2). Interestingly, the genes encoding some of these proteins, notably fibronectin, are β-catenin transcriptional targets (16). Some of these factors are exclusive to the wound healing process. For instance, the embryonic EDA (EIIIA) splice variant of fibronectin is uniquely expressed during wound healing in post-natal mice (17–20). Such extracellular matrix factors could modulate β-catenin activity during the process of cutaneous healing. Here we investigate the role of extracellular matrix components in the regulation of β-catenin in fibroblasts and examine the role of EDA-fibronectin in relation to β-catenin during wound repair in vivo.
EXPERIMENTAL PROCEDURES
Genetically Modified Mice
B6.129-Fn1tm2Feb mice (21) that lack the EDA splice variant of fibronectin (referred to as Eda Fn−/−) and their wild type littermates (referred to as Eda Fn+/+) were used to study the effect of EDA-fibronectin on β-catenin in vivo. The Eda Fn−/− mice are viable, although they have a shorter lifespan than their wild type littermates and exhibit a less compact cellular organization of the dermis during the proliferative phase of repair (21). Mice with conditional, loxP-flanked alleles of β-catenin were used to determine whether β-catenin activation could rescue the wound phenotype in Eda Fn−/− mice. The Catnbtm1Tak mouse possesses loxP sites flanking exon 3 of the Catnb (β-catenin) gene, which results in the conditional stabilization of β-catenin in response to Cre-recombinase (22). Double-mutant Eda Fn−/−;Catnbtm1Tak mice were generated by crossing Eda Fn−/− and Catnbtm1Tak mice and then breeding the heterozygous littermates. Catnbtm2Kem mice used in wound healing studies possess loxP sites located in introns 1 and 6 of the Catnb gene, which when subjected to Cre-recombinase, results in a null allele (23). Tcf reporter mice express LacZ under the control of a c-fos minimal promoter and three Tcf-binding sites; β-catenin/Tcf-mediated transcriptional activity thus leads to the production of β-galactosidase (3).
Primary Cells and Cell Lines
Primary dermal fibroblasts from Tcf reporter mice were established by enzymatic digestion of dorsal dermal skin (24), using collagenase I. Tcf reporter fibroblasts and primary murine dermal fibroblasts containing a loxP-flanked β1-integrin gene (25) were used between passages 3 and 10 and were maintained in DMEM containing 10% FBS and 1% of an antibiotic/antimycotic mixture in a humidified incubator at 37 °C and 5% CO2. The murine embryonic fibroblast NIH3T3 cell line (American Type Culture Collection, Manassas, VA) and the β1-integrin-deficient GD25 embryonic fibroblastic cell line (26) were maintained in culture conditions similar to those described above. Although GD25 cells lack expression of the β1-integrin, they can adhere to substrates, such as fibronectin, via integrin αVβ3 (26). The GSK3β +/+ and GSK3β−/− murine embryonic fibroblasts (27) are immortalized cell lines originally established from wild type and GSK3β-null embryos. These cell lines were maintained in DMEM containing 3.7 g/liter of sodium bicarbonate, 10% FBS, and 1% antibiotic/antimycotic mixture.
Treatment with Extracellular Matrix Substrates and Peptides
For comparison of the effects of different extracellular matrix substrates on β-catenin levels and activity, fibroblasts were seeded (35,000–50,000 cells/10 cm2) at equal densities on tissue culture plastic and thin layer preparations of the following extracellular matrix molecules: collagen I (5 μg/cm2) from rat tail (BD Biosciences, San Jose, CA); collagen IV (4 μg/cm2) from mouse (BD Biosciences); Matrigel (10 μg/cm2) (BD Biosciences); and human fibronectin (2 μg/cm2) (Sigma-Aldrich) and harvested after 48 h. For peptide treatments, Tcf reporter fibroblasts were treated with 1 mm RGDS peptide (Sigma-Aldrich) or vehicle control and harvested 48 h later. Fibroblasts containing loxP sites flanking the β1 integrin gene (25) were cultured overnight prior to treatment with 200 multiplicity of infection of a Cre-expressing, GFP-tagged, adenoviral vector (Vector Biolabs, Philadelphia, PA), or a GFP-expressing control virus. The cells were then cultured for an additional 4 days prior to lysis. Successful infection was verified by fluorescence microscopy, and recombination was assessed by Western blotting.
Immunoblotting
Whole cell lysates were harvested from fibroblasts in reporter gene lysis buffer containing protease and phosphatase inhibitor cocktails (Roche Applied Science). Protein concentrations were determined using the BCA assay (28). Proteins were resolved by SDS-polyacrylamide gel electrophoresis. The antibody that recognizes activated (unphosphorylated at Ser-37 and Thr-41) β-catenin (clone 8E7) was from Millipore (Billerica, MA) (29, 30), whereas total β-catenin was detected using an antibody from BD Biosciences. The antibodies for phospho-GSK3β (serine 9) and phospho-STAT1 (Tyr-701) were obtained from Cell Signaling (Danvers, MA), whereas the total GSK3β (31) and Smad2/3 antibodies were from BD Biosciences. Western blots for phospho-GSK3β (serine 9) were stripped and reprobed with the antibody for the “total” form of the protein. Ad-DKK-1 was detected using an anti-His antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (32), and antibodies for β1 integrin and GFP were also obtained from Santa Cruz. Protein expression levels were compared relative to the housekeeping proteins actin (Calbiochem, San Diego, CA) or GAPDH (Abcam, Cambridge, MA). Densitometric analysis was performed using AlphaEaseFC 4.0 (Alpha Innotech Corp., San Leandro, CA) software. The immunoblots shown are representative of a minimum of three independent experiments.
KinexTM Antibody Microarray
Cell pellets from primary dermal Tcf reporter fibroblasts grown on fibronectin and plastic were shipped to Kinexus Bioinformatics Corporation (Vancouver, Canada) where they were lysed and analyzed using the KinexTM antibody microarray (KAM 1.2), which contains 800 (500-pan-specific and 300-phosphorylation site-specific) antibodies. Ingenuity pathways analysis (Ingenuity Systems, Redwood City, CA) was used to identify the most highly up-regulated candidates, and the signaling pathways activated, from the log2(treated average/control average) expression data.
β-Galactosidase Assay
Whole cell lysates were obtained from primary dermal fibroblasts derived from Tcf reporter mice (3). Samples were assayed for β-galactosidase activity by measuring the optical density at 570 nm, following hydrolysis of the enzyme substrate, chlorophenol red β-d-galactopyranoside (Sigma-Aldrich) at 37 °C.
Topflash/Fopflash Assays
The cells were seeded on 6-well 35-mm-diameter plates and transfected 24 h later with 0.7 μg of the Tcf-responsive pTOPFLASH reporter construct, which contains three Tcf-binding sites, or 0.7 μg of the corresponding pFOPFLASH construct, which contains three mutated Tcf sites (33). A β-galactosidase expression vector (0.3 μg) served as a control for transfection efficiency. For each experiment, transfections were performed in triplicate using FuGENE 6 (Roche Applied Science) transfection reagent as per the manufacturer's instructions. Lysates were assayed for luciferase activity on a luminometer using luciferase assay substrate and buffer (Promega, Madison, WI). TOPFLASH or FOPFLASH luciferase activity was normalized to β-galactosidase activity.
Wound Healing Experiments
Eda Fn+/+ and Eda Fn−/− mice used in experiments were between 8 and 12 weeks of age, and each study group contained five mice of the same genotype. For the studies involving the double-mutant Eda Fn−/−;Catnbtm1Tak and Eda Fn+/+;Catnbtm1Tak mice, each treatment group (Ad-GFP or Ad-CRE, with Ad-CRE conferring β-catenin protein stabilization) contained at least three mice between 8 and 16 weeks of age. These mice were homozygous for Eda Fn−/− or Eda Fn+/+ and contained at least one Catnbtm1Tak allele. Mice in the different groups were littermate controls. All of the experiments using mice were reviewed and approved by the Animal Care Committee of the Hospital for Sick Children (Toronto, Canada). Sterile skin biopsy punches (Miltex Inc., York, PA) with a diameter of 4 mm were used to create four full-thickness skin wounds on the dorsal surface (back) of the mouse. Circular wounds were harvested during the proliferative phase of healing at days 5–8 post-wounding or during the remodeling phase at days 12–14 postinjury. For mechanical testing of wound strength, 4-mm-long full thickness linear wounds were made on the dorsal surface of the mouse, using a scalpel. Seven days postwounding, 4-mm-wide linear sections were prepared as strips and tested for tensile mechanical properties on an Instron 4301 machine as previously described. A stress strain curve was generated, and loads were normalized to the cross-sectional area of the wound. The ultimate tensile strength and the work to failure were calculated (34, 35).
Treatment of Mice with Lithium Chloride, Ad-DKK-1, or Ad-CRE
Treatment of mice with LiCl or NaCl as a control, was initiated 24 h prior to wounding and terminated at the time of wound harvest. NaCl or LiCl was added to the drinking water to a final concentration of 0.014 m. This dosage was previously shown to elevate β-catenin in mesenchymal cells in mice (6, 36). An adenovirus that expresses DKK-1 (32), CRE-recombinase, or GFP as a control (6), was administered to wild type or genetically modified mice 4 days prior to wounding. The mice were injected subcutaneously with the adenovirus at a titer of 1 × 108 plaque-forming units at the future wound site, and also intraperitoneally, as previously reported (6, 37, 38); Eda Fn−/−;Catnbtm1Tak mice were injected with a dose of 5 × 107 plaque-forming units/site of an adenovirus driving expression of CRE-recombinase, or GFP, as previously reported (6). Wounds were harvested at either 5, 7, 12, or 14 days after injury, and the wounds were examined for size, cellularity, and β-catenin protein levels. Successful infection was determined by detecting the virus in the wound biopsy samples, and successful recombination was determined using DNA and/or protein analysis as previously reported (6, 38). Because DKK-1 is a secreted protein, only small numbers of cells need to be infected for it to have an inhibitory effect on Wnt signaling (32).
Protein and RNA Extraction from Wounds
Cutaneous wounds were dissected from the surrounding unwounded skin. Protein was isolated from tissue in lysis buffer, as described above, whereas mRNA was extracted from tissue using the Qiagen RNeasy mini kit (Qiagen) as per the manufacturer's instructions. Lysates from fracture repair were taken from those harvested from previously reported studies (38).
Real Time PCR Assays
mRNA was reverse-transcribed to cDNA using Superscript reverse transcriptase (Invitrogen) and oligod(T)15 primers (Invitrogen). Real time PCR analysis of the relative expression of collagen I and fibronectin in relation to GAPDH in mouse whole wounds, 7–8 days postinjury, was performed using TaqMan® gene expression assays (Applied Biosystems Inc., Foster City, CA). All of the reactions were run on an Applied Biosystems 7900 HT Fast real time PCR machine.
Histology and Immunohistochemistry
Formalin-fixed, paraffin-embedded wounds were used for histological and immunohistochemical analysis. Sectioning of wounds was performed as previously described, with serial sections through the circular wound examined to identify the center (longest diameter) section of the wound (3, 6, 31, 39). Wound size was examined using trichrome stained sections, whereas the general histologic appearance was examined in hematoxylin- and eosin-stained sections. Heat-mediated antigen retrieval in sodium citrate buffer was performed for immunohistochemical analysis, with the exception of tissue sections stained with F4/80 and GFP, where antigen retrieval was performed using proteinase K. β-Catenin localization in wound sections was assessed using a polyclonal anti-rabbit β-catenin antibody (Millipore, Billerica, MA) and a biotinylated anti-rabbit secondary antibody (Vector Labs Inc., Burlingame, CA). Fibroblasts (40, 41) in the wound sections were identified using an antibody that recognizes fibroblast activation protein (Abcam), myofibroblasts were detected using an antibody to α-smooth muscle actin (Abcam), macrophages were labeled using an antibody to F4/80 (42) (AbD Serotec, Kidlington, UK), and keratinocytes were localized using a keratin-14 (43) antibody (Covance Inc., Princeton, NJ). GFP was detected using an antibody from Abcam. The appropriate biotinylated secondary antibody was used in combination with each primary antibody (Vector Labs Inc., Burlingame, CA). Strepdavidin-Dylight Conjugate (Thermo Fisher Scientific Ltd., Rockford, IL) was used for fluorescent imaging of GFP.
Statistical Analysis
The mean, standard deviation, and 95% confidence interval were calculated for each data set using Microsoft Excel (Redmond, WA). The data are presented as the means and 95% confidence intervals. Differences between groups of data were determined using the two-tailed Student's t test. Statistical significance is defined as p ≤ 0.05.
RESULTS
Wnt Ligand Inhibition Does Not Completely Abrogate the Increase in β-Catenin Levels during Wound Repair
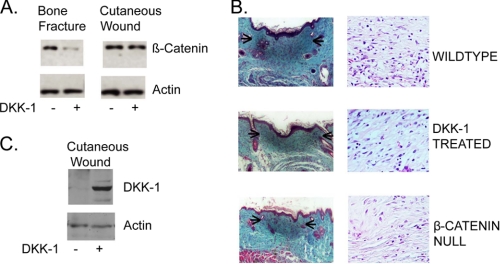
During the proliferative phase of bone fracture repair, inhibition of Wnt signaling by overexpression of DKK-1 resulted in a complete abrogation of the elevation of the β-catenin protein level (38). To examine whether DKK-1 would inhibit the increase in β-catenin protein levels during cutaneous wound healing, we used an approach similar to that utilized in the studies of fracture repair. Mice subjected to a full thickness cutaneous wound were treated with either a DKK-1-expressing adenovirus or a control adenovirus (32, 38). Intriguingly, we found only a modest decline in the level of β-catenin in wounds from DKK1-treated mice during the proliferative phase of cutaneous healing and no significant effect on the ultimate size or cellularity of the scar that developed (Fig. 1, A and B). Effective infection was verified by observation of viral protein production using Western analysis (Fig. 1C) and by observation of green fluorescent protein (GFP). The lack of a change in wound size or cellularity (with an average of 62 cells/high powered field in wild type mice and 58 cells in DKK-1-treated mice) contrasts starkly with data from mice expressing a conditional null allele of β-catenin activated using an identical adenovirus but that expresses Cre-recombinase instead of DKK-1 (average of 47 cells/high powered wound field), as previously reported (6). This suggests that an alternative pathway other than Wnt ligand activation plays a role in maintaining the β-catenin protein level during the proliferative phase of cutaneous wound repair.
FIGURE 1.
Wnt ligand inhibition does not completely abrogate the increase in β-catenin level during the proliferative phase of cutaneous wound repair. A, mice subjected to a full thickness cutaneous wound were treated with either a DKK-1-expressing adenovirus or a control adenovirus. The level of total β-catenin protein, relative to actin, does not change significantly in wounds treated with the Wnt-inhibitor DKK-1 1 week following wounding. This is in contrast to the situation in fracture repair, where DKK-1 treatment substantially reduced the β-catenin protein level. B, DKK-1 treatment had no effect on the size of the scar that developed following wounding. This is in contrast to treatment of mice expressing a conditional null allele of β-catenin, in which the wound was smaller and less cellular (arrows represent the maximal diameter of the wound). The lengths of the scale bars are 200 and 50 μm in the low and high powered fields, respectively. Representative immunoblots and histologic sections are shown. C, effective infection is shown by Western analysis of DKK-1 expression, using an antibody to the His tag.
Extracellular Matrix Proteins Stimulate β-Catenin Activity in Dermal Fibroblasts
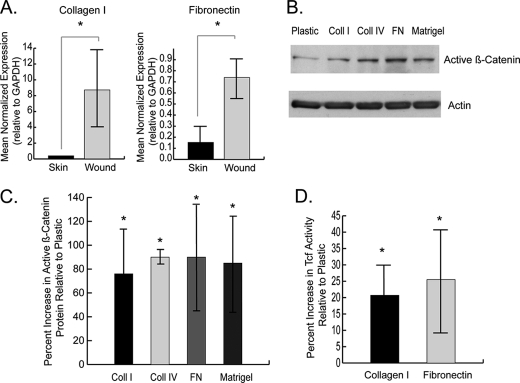
The expression levels of extracellular matrix components that are expressed during wound repair (2, 44, 45) were examined in murine cutaneous wounds using real time PCR. We found that the expression of fibronectin and collagen type I transcripts was elevated during the proliferative phase of healing (Fig. 2A).
FIGURE 2.
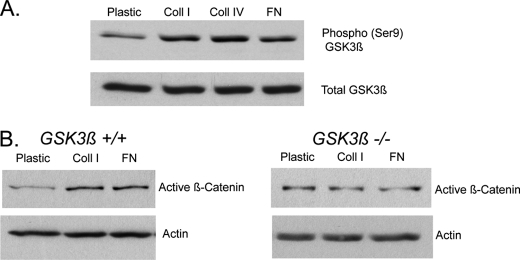
β-Catenin protein is regulated by extracellular matrix components in primary dermal fibroblasts. A, real time RT-PCR shows that the expression of collagen I and fibronectin, relative to GAPDH, is higher during the proliferative phase of wound healing compared with unwounded skin. The data are presented as the means and 95% confidence intervals. A significant difference is indicated by an asterisk. This confirms that the expression of new extracellular matrix components is increased during wound healing. B and C, immunoblot analysis shows that the protein level of activated β-catenin is increased in primary dermal fibroblasts cultured on extracellular matrix proteins compared with tissue culture plastic (B). These data are represented as a graph in C. Expression of active β-catenin was quantified by densitometry relative to the housekeeping protein. D, β-catenin-mediated Tcf-dependent transcriptional activity, represented by β-galactosidase activity in primary dermal Tcf reporter fibroblasts, is elevated in fibroblasts cultured on extracellular matrix components compared with plastic. The data are given as the mean percentages of increase in Active β-catenin protein OR Tcf reporter activity, compared with cells grown on plastic, ± 95% confidence intervals. A statistically significant difference for a given substrate compared with plastic is indicated by an asterisk. Coll, collagen; FN, fibronectin.
Primary dermal fibroblasts from Tcf reporter mice were cultured on tissue culture plastic and thin layer preparations of collagen I, collagen IV, fibronectin, and Matrigel. Immunoblot analysis of cell lysates showed that levels of activated (unphosphorylated at Ser-37 and Thr-41) β-catenin protein were elevated in fibroblasts grown on the various extracellular matrix components compared with tissue culture plastic (Fig. 2, B and C). An increase in Tcf transcriptional activation was also observed in the fibroblasts grown on extracellular matrix components (Fig. 2D). Thus, fibroblasts increase their level of β-catenin-mediated transcriptional activity when exposed to select extracellular matrices.
β-Catenin-mediated Signaling Is Reduced during the Proliferative Phase of Wound Repair in Mice Lacking the EDA Variant of Fibronectin
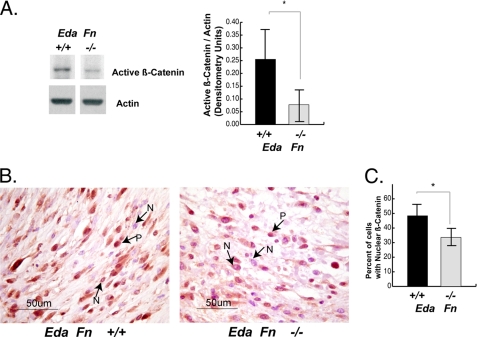
Because the embryonic EDA splice variant of fibronectin is uniquely expressed in the adult animal during wound repair (17), it is possible that this extracellular matrix component regulates β-catenin signaling during wound healing. To determine whether this is the case, we examined genetically modified Eda Fn−/− mice, which lack the EDA splice variant of fibronectin (21). Previous studies of these mice show that they have a wound phenotype characterized by a less compact cellular organization (21) that is reminiscent of that found in mice expressing a conditional null allele of β-catenin (6). We found that wounds from Eda Fn−/− mice have a reduced level of activated β-catenin protein (Fig. 3A) and exhibit a lower percentage of cells in which there is nuclear localization of the β-catenin protein (Fig. 3B) during the proliferative phase of repair than wild type littermate mice (Eda Fn+/+). This demonstrates that extracellular matrix components can regulate β-catenin in fibroblasts in vivo.
FIGURE 3.
β-Catenin-mediated signaling is decreased during wound healing in mice lacking the EDA splice variant of fibronectin. A, wounds from Eda Fn−/− mice have reduced levels of activated β-catenin protein compared with the wounds of their wild type (Eda Fn+/+) littermates, during the proliferative phase of healing, 5 days postinjury. The level of activated β-catenin was quantified relative to actin. B and C, immunohistochemistry shows that the percentage of cells exhibiting nuclear β-catenin staining is reduced in wounds from Eda Fn−/− mice compared with wounds from Eda Fn+/+ mice (B). P, positive stain; N, negative stain. This is represented as a graph in C. The data are expressed as the mean percentage per 40× microscope field ± 95% confidence intervals. A statistically significance difference is indicated by an asterisk over the line illustrating the comparison.
Integrin Receptors Mediate the Regulation of β-Catenin by the Extracellular Matrix
To determine how the extracellular matrix might affect cell behavior in a manner that would influence β-catenin, proteins regulated by exposure to fibronectin were identified using antibody array technology (KinexTM antibody microarray, KAM 1.2; Kinexus Bioinformatics Corporation). (GEO accession number GSE30135). Seven of the ten most highly up-regulated molecules in the array, detected using phosphorylation-site specific antibodies, are associated with integrin signaling (Table 1). We undertook a Western analysis to verify the direction of the observed expression change for Smad2/3 and phospho-STAT1 (Tyr-701), two proteins up-regulated in the array, in 5-day wound lysates from Eda Fn1+/+ and Eda Fn1−/− mice. Because integrins link the extracellular and intracellular environment, this raises the possibility that integrins mediate the regulation of β-catenin activation.
TABLE 1.
Seven of the top ten up-regulated proteins identified by antibody array have a known association with integrin signaling
Seven of the top ten up-regulated proteins identified by antibody array have a known association with integrin signaling. Candidate proteins regulated by exposure to fibronectin in primary dermal fibroblasts were identified using a KinexTM antibody array. Seven of the ten most highly up-regulated proteins in the array, detected using phosphorylation site-specific antibodies, have a known association with integrin signaling (indicated by +).
| Protein name | Gene | Log2 ratio up-regulated (fibronectin vs. plastic) | Known association with integrin signaling | Reference |
|---|---|---|---|---|
| Mitogen-activated protein kinase 14 | MAPK14 | 3.370 | + | 70 |
| Synapsin 1 | SYN1 | 3.054 | + | 71 |
| Mitogen-activated protein kinase kinase 6 | MAP2K6 | 2.443 | − | |
| Phospholipase Cγ1 | PLCG1 | 2.427 | + | 72 |
| Signal transducer and activator of transcription 5A | STAT5A | 2.366 | + | 73 |
| Mitogen-activated protein kinase kinase 1 | MAP2K1 | 2.322 | + | 74 |
| Ribosomal protein S6 kinase, 90 kDa, polypeptide 1 | RPS6KA1 | 2.264 | − | |
| Serine/threonine-protein kinase PAK 1/2/3 (p21-activated kinase 1/2/3) | PAK1 | 1.814 | + | 75, 76 |
| Retinoblastoma 1 | RB1 | 1.794 | − | |
| Myristoylated alanine-rich protein kinase C substrate | MARCKS | 1.776 | + | 77 |
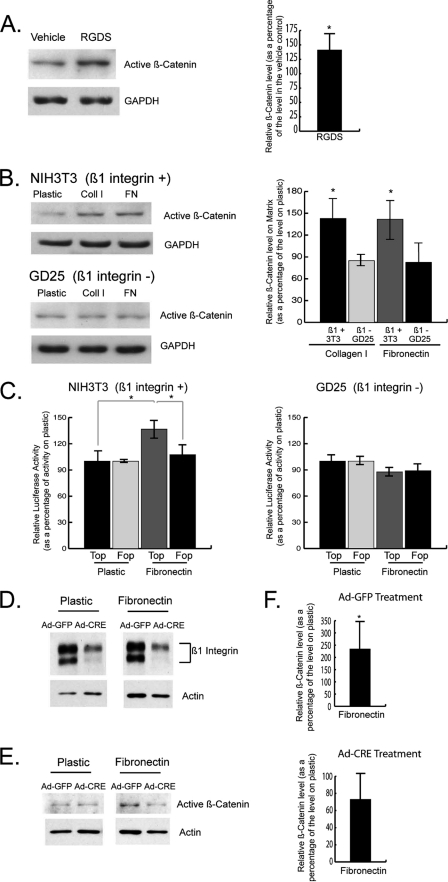
Primary dermal fibroblasts were exposed to a peptide that represents a major integrin-binding site on fibronectin (RGD, which mediates fibronectin binding to α5β1 and α3β1 integrins) (46–49) to determine whether integrin receptor activation modulates β-catenin. We found that the RGD peptide increased the level of activated β-catenin protein compared with the control (Fig. 4A). We then compared the β1-integrin-deficient embryonic fibroblastic GD25 cell line (26) to NIH3T3 embryonic fibroblasts, which express the β1-integrin. NIH3T3 fibroblasts grown on extracellular matrix components demonstrated increased activated β-catenin protein and transcriptional activity, similar to that observed in primary dermal fibroblast cultures, whereas this effect was absent in the β1-integrin null GD25 cells (Fig. 4, B and C).
FIGURE 4.
Integrins mediate the regulation of β-catenin activity by extracellular matrix proteins. A, immunoblot analysis shows that the peptide, RGDS, increases the level of activated β-catenin protein compared with vehicle treatment in primary murine dermal fibroblasts. β-Catenin expression was quantified relative to GAPDH. B, Western analysis shows that activated β-catenin protein is up-regulated in NIH3T3 embryonic fibroblasts grown on collagen I and fibronectin compared with tissue culture plastic. There is no effect by these extracellular matrix components on β-catenin in the β1-integrin-deficient GD25 embryonic fibroblastic cell line. β-Catenin levels were quantified relative to GAPDH. C, β-catenin-mediated Tcf-dependent transcriptional activity is elevated in NIH3T3 fibroblasts grown on fibronectin compared with tissue culture plastic. This effect is not observed in the β1-integrin-deficient GD25 fibroblastic cell line. D, Western analysis shows that Ad-CRE treatment of primary dermal fibroblasts containing a floxed version of the β1 integrin gene greatly reduces the β1 integrin expression level, compared with Ad-GFP treated fibroblasts. E, primary dermal fibroblasts with decreased β1 integrin expression (Ad-CRE) did not show an increase in activated β-catenin protein when grown on fibronectin, in contrast to Ad-GFP-treated fibroblasts. F, expression of active β-catenin, relative to actin, in fibroblasts grown on fibronectin versus plastic was quantified by densitometry. The data are presented as the means and 95% confidence intervals, relative to control. Statistical significance at the p ≤ 0.05 level is indicated by an asterisk. Top, Topflash reporter; Fop, Fopflash reporter; Coll, collagen; FN, fibronectin.
We further examined the role of the β1 integrin in primary dermal fibroblasts containing a floxed version of the β1 integrin gene (25). Treatment of the cells with a Cre-expressing adenovirus resulted in a substantial reduction of the protein level of β1 integrin compared with cells treated with a control GFP-expressing virus (Fig. 4D). As observed in Fig. 4 (E and F), Ad-Cre-treated fibroblasts grown on fibronectin did not display an increase in activated β-catenin, which is in contrast to the induction observed in Ad-GFP-treated fibroblasts. Collectively, these data suggest that the β1 integrin mediates the up-regulation of active β-catenin in response to extracellular matrix (ECM) stimulation.
GSK3β Is Required for the Regulation of β-Catenin by Extracellular Matrix Components
GSK3β is a crucial component of the multiprotein complex that targets β-catenin for degradation (50, 51). We found that there was a higher level of GSK3β phosphorylation on serine 9, which is an indirect indicator of inhibition of GSK3β activity, on fibroblasts grown on extracellular matrix compared with tissue culture plastic (Fig. 5A). To determine whether the presence of GSK3β is required for the activation of β-catenin, we examined the effect of extracellular matrix components on β-catenin in GSK3β null (−/−) and wild type (+/+) mouse embryonic fibroblasts (27). The elevation of activated β-catenin protein in response to collagen and fibronectin in wild type fibroblasts was absent in the GSK3β-null fibroblasts (Fig. 5B).
FIGURE 5.
GSK3β is required for the regulation of β-catenin by extracellular matrix components. A, an increased level of GSK3β phosphorylated on serine 9 (Phospho-GSK3β (Ser9)) was observed for fibroblasts grown on collagen I and fibronectin compared with plastic, as determined using Western analysis. B, immunoblot analysis of activated β-catenin expression shows that collagen I and fibronectin up-regulate activated β-catenin expression in wild type mouse embryonic fibroblasts compared with tissue culture plastic, but not in GSK3β-null mouse embryonic fibroblasts. β-Catenin levels are relative to actin. Coll, collagen; FN, fibronectin.
β-Catenin Activation Rescues the Wound Phenotype Observed in EDA-Fibronectin-deficient Mice
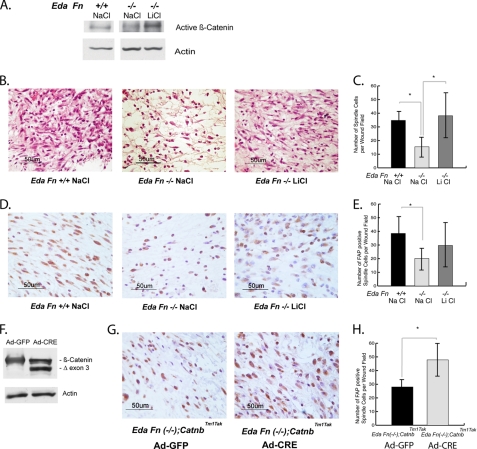
Mice lacking EDA-fibronectin (Eda Fn−/−) possess a wound phenotype characterized by a less compact cellular organization of the dermis (21). To determine whether the wound phenotype of these mice is due in part to the inability to maintain β-catenin protein activation, we activated β-catenin signaling through both genetic and pharmacologic means. To pharmacologically stimulate β-catenin, we treated Eda Fn−/− mice with LiCl, which stabilizes β-catenin through regulation of GSK3β (6, 38, 50) or NaCl as a control. Treatment with LiCl increased the level of activated β-catenin protein in wounds from Eda Fn−/− mice toward that found in wild type mice (Fig. 6A). We observed that wounds from Eda Fn−/− mice treated with NaCl possess fewer cells with the spindle cell morphology typical of fibroblasts (41) in hematoxylin- and eosin-stained wound sections than their NaCl-treated Eda Fn+/+ counterparts, 5 days postinjury. Treatment of Eda Fn−/− mice with LiCl rescued this aspect of the wound phenotype by increasing the number of spindle-shaped cells in dermal wound tissue to a level similar to that observed in Eda Fn+/+ mice (Fig. 6, B and C). We further characterized the identity of the spindle cells by using an antibody against fibroblast activation protein (40, 41, 52, 53) and found that the above pattern was reflected in the number of fibroblast activation protein-expressing spindle cells in the healing wounds (Fig. 6, D and E). The finding was similar when β-catenin was stabilized by genetic means. Eda Fn−/− mice were bred with mice expressing a conditionally stabilized form of β-catenin (Catnbtm1Tak) to generate a Eda Fn−/−;Catnbtm1Tak cross. Eda Fn−/−;Catnbtm1Tak mice treated with a Cre-expressing adenovirus to stabilize β-catenin (Fig. 6F) showed more spindle-shaped cells expressing fibroblast activation protein as a fibroblast marker, in the central area of their healing wounds, 5 days postinjury, than did mice treated with a control (GFP)-expressing adenovirus (Fig. 6, G and H). Together, these data suggest that β-catenin stabilization rescues the depleted fibroblast population in wounds from Eda Fn−/− mice.
FIGURE 6.
The number of fibroblasts during wound healing in EDA-fibronectin-deficient mice is regulated by β-catenin. A, treatment of Eda Fn−/− mice with LiCl increases the level of activated β-catenin protein in whole cutaneous wounds toward that observed in wild type (Eda Fn+/+) wounds. β-Catenin levels were assessed relative to actin. B, Eda Fn−/− mice treated with NaCl had fewer cells with the spindle-shaped morphology typical of fibroblasts per field of dermal wound proliferative phase tissue than wild type (Eda Fn+/+) NaCl-treated mice. Treatment with LiCl increased the number of spindle cells in Eda Fn−/− mice to a level similar to that in Eda Fn+/+ mice. Wound sections are stained with hematoxylin and eosin. C, graphical representation of the number of spindle cells per field in the different mice and treatment groups. D, immunohistochemical analysis using the fibroblast marker, fibroblast activation protein (FAP), confirmed the identity of the spindle cells as fibroblasts. FAP-expressing spindle cells were reduced in the central wound area of NaCl-treated Eda−/− mice. E, quantitation of fibroblast-activation-protein expressing cells in the different mice and treatment groups. F, genetic stabilization of β-catenin in Eda Fn−/−;Catnbtm1Tak mice was achieved using a GFP-tagged CRE-expressing adenovirus, whereas control mice received a GFP-expressing adenovirus. Stabilization of the β-catenin level is demonstrated by Western blot, which shows a higher level of the stabilized form of β-catenin (exon 3 deletion) in the wounds of Ad-CRE-treated mice. G, immunohistochemical analysis using the fibroblast marker, FAP. Eda Fn−/−; Catnbtm1Tak mice expressing stabilized β-catenin (Ad-Cre) contained more FAP-expressing spindle cells in the central area of their wounds than wounds from the control mice (Ad-GFP). H, quantification of the FAP-expressing cells. The data are presented as the mean numbers of cells per 40× microscope field of dermal proliferative phase wound tissue ± 95% confidence intervals. A significant difference at the p ≤ 0.05 level is represented by an asterisk over the line illustrating the comparison.
We did not observe any differences in macrophage, myofibroblast (54), and keratinocyte populations among the different types of mice using F4/80, α-smooth muscle actin, and keratin 14 staining, respectively. Furthermore, although EDA-fibronectin deficiency did not have a statistically significant effect on the wound surface diameter of the scar formed from circular wounds at 12 days postwounding, there was a trend toward a mean surface width of wounds from Eda−/− mice of ∼20% smaller than wild type.
Scar Strength Is Reduced in EDA-fibronectin-deficient Mice, and This Defect Is Rescued by Stabilization of β-Catenin
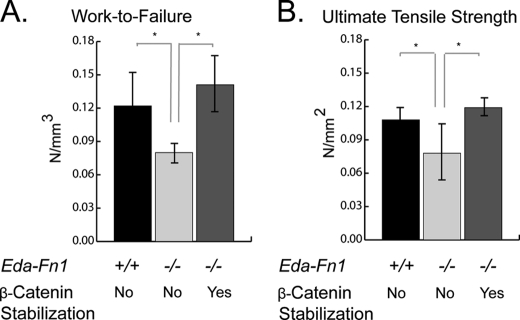
We reasoned that the phenotype of the healing wounds from EDA-fibronectin-deficient mice, including their reduced fibroblast numbers, might influence the mechanical properties of the wound. To investigate this, we conducted mechanical testing of wound strength in Eda Fn+/+;Catnbtm1Tak and Eda Fn−/−;Catnbtm1Tak mice with and without the activation of the conditionally stabilized β-catenin allele. Mechanical testing of strips prepared from linear wounds, 7 days postinjury, showed a decrease in work to failure (Fig. 7A) and ultimate tensile strength (Fig. 7B) in healing wounds lacking EDA fibronectin. This defect was rescued in mice that also expressed the stabilized form of β-catenin. Thus, EDA-fibronectin deficiency, and the consequent decrease in β-catenin activation and fibroblast infiltration, has a functional effect on wound scar strength. Although this may in part be caused by deficiency in EDA-fibronectin, the rescue by β-catenin stabilization suggests that fibroblast behavior independent of EDA-fibronectin plays a major role in wound strength.
FIGURE 7.
Mechanical strength of wounds. Linear wounds from Eda Fn−/− mice displayed decreased work-to-failure (A) and ultimate tensile strength (B) compared with wounds from wild type mice. The decreased tensile mechanical properties of the wounds were rescued by stabilization of β-catenin (Eda Fn−/−;Catnbtm1Tak) using Ad-CRE. Control mice received Ad-GFP. The data are presented as the means ± 95% confidence intervals. A significant difference at the p ≤ 0.05 level is represented by an asterisk over the line illustrating the comparison.
DISCUSSION
Here we show that fibronectin and other extracellular matrix components regulate β-catenin activity in fibroblasts through an integrin-mediated, GSK3β-dependent pathway. Furthermore, we found that this mechanism plays a physiologic role in vivo during wound repair. Indeed, the smaller number of dermal fibroblasts in Eda Fn−/− mice is most likely caused by a lower level of β-catenin during the proliferative phase of wound repair. This results in a significant difference in wound strength.
β-Catenin is activated during the initial phase of wound repair, and its activity is maintained during the proliferative phase (3). There are several factors that might initiate the activation of β-catenin during wound healing. Growth factors released by early response cells and liberated from the matrix, such as TGF-β, are known to activate β-catenin-mediated signaling during wound repair (31). Wnt ligands expressed during the early phases of wound healing (55) may also play a role. Our data show that once β-catenin is activated by such factors, its expression and activity are maintained during the proliferative phase of repair not by continued Wnt ligand activation, but by cellular interactions with the immature extracellular matrix deposited during the early phases of healing.
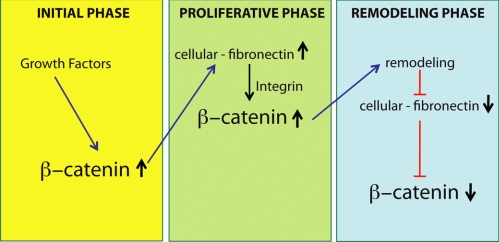
The EDA splice variant of fibronectin is synthesized early in the repair process (2, 17, 18), and its expression peaks during the proliferative phase (18, 19). EDA fibronectin contains additional integrin-binding sites compared with other splice variants (49), suggesting that it may be able to activate β-catenin more strongly than other forms of fibronectin. Intriguingly, fibronectin is also a β-catenin-regulated gene (16), raising the possibility of a “feedback” loop between β-catenin and fibronectin during wound repair (Fig. 8). In this regulatory loop, β-catenin is activated in the early phases of healing by growth factors, causing the expression of extracellular matrix components such as fibronectin. Fibronectin and other extracellular matrix components then maintain activation of β-catenin throughout the proliferative phase of repair. In their immature state, these extracellular matrix proteins contain larger numbers of integrin-binding sites. During wound remodeling, EDA fibronectin, which contains the EDGIHEL and RGD motifs that bind integrins α4β1 (56) and α5β1/α3β1 (46–49), respectively, becomes replaced with bundles of collagen I, a structure in which the RGD motif is exposed only upon denaturation (57, 58). Therefore, as the wound matures and the composition of the extracellular matrix changes, EDA fibronection and other immature extracellular components are no longer present to activate integrin receptors, and β-catenin levels would return to baseline.
FIGURE 8.
Schematic illustrating the proposed reciprocal regulatory loop between β-catenin and the extracellular matrix in dermal fibroblasts during wound healing. During the early phases of wound repair, growth factors activate β-catenin-mediated signaling, which stimulates the expression of extracellular matrix components, such as fibronectin. Such extracellular matrix components in turn give feedback to maintain activation of β-catenin throughout the proliferative phase of healing. As the wound matures, the extracellular matrix remodels, removing the immature extracellular matrix components that stimulate β-catenin signaling. This results in a decline in β-catenin levels back to preinjury levels.
We found that EDA-fibronectin deficiency, and the consequent reduction in activated β-catenin levels and the dermal fibroblast population, had an effect on the mechanical properties of the wound. Because this phenotype was rescued by stabilization of β-catenin, it is likely that the increased fragility of EDA-fibronectin-deficient wounds may be caused both by the deficiency of this molecule, as well as changes in fibroblast behavior linked to β-catenin.
We found a different mechanism of regulation of β-catenin by extracellular matrix components in dermal fibroblasts than that found in cells of epithelial origin. The mechanism of activation of β-catenin by extracellular matrix in fibroblasts involves an integrin-mediated, GSK3β-dependent pathway that stimulates Tcf-dependent transcription. This suggests involvement of the β1-integrin-associated integrin-linked kinase (59–61). In cells of epithelial origin (9, 11, 62, 63) and in natural killer cells (64), β-catenin is modulated in response to extracellular matrix and/or integrin stimulation by tyrosine phosphorylation and consequent liberation from cadherin binding. The activation of β-catenin released from E-cadherin by integrin signaling is not associated with GSK3β (11, 63). Furthermore, Kim et al. (63) showed that β-catenin freed from adherens junctions by integrin signaling in cells of epithelial origin does not activate Tcf-lef transcription but rather forms a transcriptional complex with phospho-Smad2 (62, 63). Thus, disparate mechanisms of integrin-mediated stimulation of β-catenin exist that activate different downstream signaling pathways and likely reflect separate functions and means of regulation.
Fibronectin may have an important role in regulating β-catenin in other mesenchymal repair processes, in addition to its function in cutaneous wound healing. EDA fibronectin is expressed during bone healing (65, 66) where it might modulate β-catenin in select local cell populations. In chondrocytes, fragments of fibronectin that contain the EDA region stimulate matrix metalloproteinase expression (67), and because β-catenin can also regulate matrix metalloproteinase expression (68), it raises the prospect that this effect may be mediated by β-catenin activity. The finding that EDA fibronectin is expressed in tumor metastasis (69) suggests that it could potentially regulate β-catenin in this process. Thus, it is possible that the mechanism we identified plays a role in a variety of biologic processes. In contrast to its role in repair, the observation that mice lacking EDA-fibronectin do not demonstrate developmental effects (21) suggests that other pathways can compensate for any effect on β-catenin during normal fetal development.
Here we found a central role for the extracellular matrix as a regulator of β-catenin activation during the cutaneous repair process. Our findings suggest that changes in the extracellular matrix could alter normal wound healing by modulating cell signaling. Indeed, the manipulation of β-catenin activity during wound healing by the use of EDA fibronectin or related bioactive peptides may present a clinical application to accelerate wound repair in patients who suffer from delayed healing.
Acknowledgments
We thank Dr. Jim Woodgett (Mt. Sinai Hospital Samuel Lunenfeld Research Institute and University of Toronto, Toronto, Canada) for providing us with the GSK3β knock-out and wild type mouse embryonic fibroblasts; Dr. Andrew Leask (University of Western Ontario, London, Canada) for the primary murine fibroblasts containing a floxed version of the β1 integrin gene; Dr. Arsenio Armagno (EMMA, Italy) for the Eda Fn−/− mouse genotyping information; and Dr. Calvin J. Kuo (Stanford University School of Medicine) for the adenovirus vector expressing DKK-1. We also thank Puviindran Nadesan, Chunying Yu, Karan Abraham, and Winston Yu for technical assistance.
This work was supported by Canadian Institutes of Health Research Grant RFN 15136.
- Tcf
- T-cell factor
- GSK
- glycogen-synthase kinase
- GFP
- green fluorescent protein
- FAP
- fibroblast activation protein
- EDA
- extra domain A
- DKK-1
- Dickkopf-1
- ECM
- extracellular matrix.
REFERENCES
- 1. Singer A. J., Clark R. A. (1999) N. Engl. J. Med. 341, 738–746 [DOI] [PubMed] [Google Scholar]
- 2. Clark R. A. (1990) J. Invest. Dermatol. 94, 128S–134S [DOI] [PubMed] [Google Scholar]
- 3. Cheon S. S., Cheah A. Y., Turley S., Nadesan P., Poon R., Clevers H., Alman B. A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 6973–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowley E., O'Gorman D. B., Gan B. S. (2007) J. Surg. Res. 138, 141–150 [DOI] [PubMed] [Google Scholar]
- 5. Zhang D. L., Gu L. J., Liu L., Wang C. Y., Sun B. S., Li Z., Sung C. K. (2009) Biochem. Biophys. Res. Commun. 378, 149–151 [DOI] [PubMed] [Google Scholar]
- 6. Cheon S. S., Wei Q., Gurung A., Youn A., Bright T., Poon R., Whetstone H., Guha A., Alman B. A. (2006) FASEB J. 20, 692–701 [DOI] [PubMed] [Google Scholar]
- 7. Cadigan K. M., Peifer M. (2009) Cold Spring Harb. Perspect. Biol. 1, a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bafico A., Liu G., Yaniv A., Gazit A., Aaronson S. A. (2001) Nat. Cell Biol. 3, 683–686 [DOI] [PubMed] [Google Scholar]
- 9. Li A., Zhou T., Guo L., Si J. (2010) Oncol. Rep. 23, 1247–1255 [DOI] [PubMed] [Google Scholar]
- 10. Larsen M. C., Brake P. B., Pollenz R. S., Jefcoate C. R. (2004) Toxicol. Sci. 82, 46–61 [DOI] [PubMed] [Google Scholar]
- 11. Koenig A., Mueller C., Hasel C., Adler G., Menke A. (2006) Cancer Res. 66, 4662–4671 [DOI] [PubMed] [Google Scholar]
- 12. Alman B. A., Li C., Pajerski M. E., Diaz-Cano S., Wolfe H. J. (1997) Am. J. Pathol. 151, 329–334 [PMC free article] [PubMed] [Google Scholar]
- 13. Hanai J., Gloy J., Karumanchi S. A., Kale S., Tang J., Hu G., Chan B., Ramchandran R., Jha V., Sukhatme V. P., Sokol S. (2002) J. Cell Biol. 158, 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quélard D., Lavergne E., Hendaoui I., Elamaa H., Tiirola U., Heljasvaara R., Pihlajaniemi T., Clément B., Musso O. (2008) PLoS One 3, e1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vi L., Njarlangattil A., Wu Y., Gan B. S., O'Gorman D. B. (2009) BMC Musculoskelet. Disord. 10, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gradl D., Kühl M., Wedlich D. (1999) Mol. Cell Biol. 19, 5576–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. (1989) J. Cell Biol. 109, 903–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown L. F., Dubin D., Lavigne L., Logan B., Dvorak H. F., Van de Water L. (1993) Am. J. Pathol. 142, 793–801 [PMC free article] [PubMed] [Google Scholar]
- 19. Singh P., Reimer C. L., Peters J. H., Stepp M. A., Hynes R. O., Van De Water L. (2004) J. Invest. Dermatol. 123, 1176–1181 [DOI] [PubMed] [Google Scholar]
- 20. White E. S., Baralle F. E., Muro A. F. (2008) J. Pathol. 216, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muro A. F., Chauhan A. K., Gajovic S., Iaconcig A., Porro F., Stanta G., Baralle F. E. (2003) J. Cell Biol. 162, 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., Taketo M. M. (1999) EMBO J. 18, 5931–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O., Kemler R. (2001) Development 128, 1253–1264 [DOI] [PubMed] [Google Scholar]
- 24. Wu C., Wei Q., Utomo V., Nadesan P., Whetstone H., Kandel R., Wunder J. S., Alman B. A. (2007) Cancer Res. 67, 8216–8222 [DOI] [PubMed] [Google Scholar]
- 25. Liu S., Xu S. W., Blumbach K., Eastwood M., Denton C. P., Eckes B., Krieg T., Abraham D. J., Leask A. (2010) J. Cell Sci. 123, 3674–3682 [DOI] [PubMed] [Google Scholar]
- 26. Wennerberg K., Lohikangas L., Gullberg D., Pfaff M., Johansson S., Fässler R. (1996) J. Cell Biol. 132, 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoeflich K. P., Luo J., Rubie E. A., Tsao M. S., Jin O., Woodgett J. R. (2000) Nature 406, 86–90 [DOI] [PubMed] [Google Scholar]
- 28. Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985) Anal. Biochem. 150, 76–85 [DOI] [PubMed] [Google Scholar]
- 29. van Noort M., Meeldijk J., van der Zee R., Destree O., Clevers H. (2002) J. Biol. Chem. 277, 17901–17905 [DOI] [PubMed] [Google Scholar]
- 30. Staal F. J., Noort Mv M., Strous G. J., Clevers H. C. (2002) EMBO Rep. 3, 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheon S. S., Nadesan P., Poon R., Alman B. A. (2004) Exp. Cell Res. 293, 267–274 [DOI] [PubMed] [Google Scholar]
- 32. Kuhnert F., Davis C. R., Wang H. T., Chu P., Lee M., Yuan J., Nusse R., Kuo C. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. (1997) Science 275, 1787–1790 [DOI] [PubMed] [Google Scholar]
- 34. Gurung A., Uddin F., Hill R. P., Ferguson P. C., Alman B. A. (2009) Am. J. Pathol. 174, 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olaso E., Lin H. C., Wang L. H., Friedman S. L. (2011) Fibrogenesis Tissue Repair 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dehpour A. R., Sadr S. S., Azizi M. R., Namiranian K., Farahani M., Javidan A. N. (2002) Pharmacol. Toxicol. 90, 89–93 [DOI] [PubMed] [Google Scholar]
- 37. Chen Y., Whetstone H. C., Youn A., Nadesan P., Chow E. C., Lin A. C., Alman B. A. (2007) J. Biol. Chem. 282, 526–533 [DOI] [PubMed] [Google Scholar]
- 38. Chen Y., Whetstone H. C., Lin A. C., Nadesan P., Wei Q., Poon R., Alman B. A. (2007) PLoS Med. 4, e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheon S., Poon R., Yu C., Khoury M., Shenker R., Fish J., Alman B. A. (2005) Lab. Invest. 85, 416–425 [DOI] [PubMed] [Google Scholar]
- 40. Pilling D., Fan T., Huang D., Kaul B., Gomer R. H. (2009) PLoS One 4, e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kalluri R., Zeisberg M. (2006) Nat. Rev. Cancer 6, 392–401 [DOI] [PubMed] [Google Scholar]
- 42. Austyn J. M., Gordon S. (1981) Eur. J. Immunol. 11, 805–815 [DOI] [PubMed] [Google Scholar]
- 43. Purkis P. E., Steel J. B., Mackenzie I. C., Nathrath W. B., Leigh I. M., Lane E. B. (1990) J. Cell Sci. 97, 39–50 [DOI] [PubMed] [Google Scholar]
- 44. Whitby D. J., Ferguson M. W. (1991) Development 112, 651–668 [DOI] [PubMed] [Google Scholar]
- 45. Eckes E., Aumailley M., Krieg T. (1996) in The Molecular and Cellular Biology of Wound Repair (Clark R. A. F. ed) pp. 493–512, Plenum Press, New York [Google Scholar]
- 46. Hautanen A., Gailit J., Mann D. M., Ruoslahti E. (1989) J. Biol. Chem. 264, 1437–1442 [PubMed] [Google Scholar]
- 47. Bauer J. S., Schreiner C. L., Giancotti F. G., Ruoslahti E., Juliano R. L. (1992) J. Cell Biol. 116, 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Z., Vuori K., Reed J. C., Ruoslahti E. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 6161–6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pankov R., Yamada K. M. (2002) J. Cell Sci. 115, 3861–3863 [DOI] [PubMed] [Google Scholar]
- 50. Stambolic V., Ruel L., Woodgett J. R. (1996) Curr. Biol. 6, 1664–1668 [DOI] [PubMed] [Google Scholar]
- 51. Doble B. W., Woodgett J. R. (2007) Cells Tissues Organs 185, 73–84 [DOI] [PubMed] [Google Scholar]
- 52. Garin-Chesa P., Old L. J., Rettig W. J. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 7235–7239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Niedermeyer J., Scanlan M. J., Garin-Chesa P., Daiber C., Fiebig H. H., Old L. J., Rettig W. J., Schnapp A. (1997) Int. J. Cancer 71, 383–389 [DOI] [PubMed] [Google Scholar]
- 54. Darby I., Skalli O., Gabbiani G. (1990) Lab. Invest. 63, 21–29 [PubMed] [Google Scholar]
- 55. Okuse T., Chiba T., Katsuumi I., Imai K. (2005) Wound Repair Regen. 13, 491–497 [DOI] [PubMed] [Google Scholar]
- 56. Liao Y. F., Gotwals P. J., Koteliansky V. E., Sheppard D., Van De Water L. (2002) J. Biol. Chem. 277, 14467–14474 [DOI] [PubMed] [Google Scholar]
- 57. Yamamoto M., Yamato M., Aoyagi M., Yamamoto K. (1995) Exp. Cell Res. 219, 249–256 [DOI] [PubMed] [Google Scholar]
- 58. Leitinger B., Hohenester E. (2007) Matrix Biol. 26, 146–155 [DOI] [PubMed] [Google Scholar]
- 59. Hannigan G. E., Leung-Hagesteijn C., Fitz-Gibbon L., Coppolino M. G., Radeva G., Filmus J., Bell J. C., Dedhar S. (1996) Nature 379, 91–96 [DOI] [PubMed] [Google Scholar]
- 60. Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oloumi A., Syam S., Dedhar S. (2006) Oncogene 25, 7747–7757 [DOI] [PubMed] [Google Scholar]
- 62. Kim K. K., Wei Y., Szekeres C., Kugler M. C., Wolters P. J., Hill M. L., Frank J. A., Brumwell A. N., Wheeler S. E., Kreidberg J. A., Chapman H. A. (2009) J. Clin. Invest. 119, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim Y., Kugler M. C., Wei Y., Kim K. K., Li X., Brumwell A. N., Chapman H. A. (2009) J. Cell Biol. 184, 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang T., Liu S., Yang P., Han C., Wang J., Liu J., Han Y., Yu Y., Cao X. (2009) Blood 114, 4081–4088 [DOI] [PubMed] [Google Scholar]
- 65. Kilian O., Dahse R., Alt V., Zardi L., Hentschel J., Schnettler R., Kosmehl H. (2008) Calcif. Tissue Int. 83, 101–111 [DOI] [PubMed] [Google Scholar]
- 66. Kilian O., Dahse R., Alt V., Zardi L., Rosenhahn J., Exner U., Battmann A., Schnettler R., Kosmehl H. (2004) Bone 35, 1334–1345 [DOI] [PubMed] [Google Scholar]
- 67. Saito S., Yamaji N., Yasunaga K., Saito T., Matsumoto S., Katoh M., Kobayashi S., Masuho Y. (1999) J. Biol. Chem. 274, 30756–30763 [DOI] [PubMed] [Google Scholar]
- 68. Crawford H. C., Fingleton B. M., Rudolph-Owen L. A., Goss K. J., Rubinfeld B., Polakis P., Matrisian L. M. (1999) Oncogene 18, 2883–2891 [DOI] [PubMed] [Google Scholar]
- 69. Rybak J. N., Roesli C., Kaspar M., Villa A., Neri D. (2007) Cancer Res. 67, 10948–10957 [DOI] [PubMed] [Google Scholar]
- 70. Kim J., Erikson D. W., Burghardt R. C., Spencer T. E., Wu G., Bayless K. J., Johnson G. A., Bazer F. W. (2010) Matrix Biol. 29, 369–382 [DOI] [PubMed] [Google Scholar]
- 71. Chen Q., Zhu X., Zhang Y., Wetsel W. C., Lee T. H., Zhang X. (2010) J. Mol. Neurosci. 40, 284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tvorogov D., Wang X. J., Zent R., Carpenter G. (2005) J. Cell Sci. 118, 601–610 [DOI] [PubMed] [Google Scholar]
- 73. Defilippi P., Rosso A., Dentelli P., Calvi C., Garbarino G., Tarone G., Pegoraro L., Brizzi M. F. (2005) J. Cell Biol. 168, 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bao W., Strömblad S. (2004) J. Cell Biol. 167, 745–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Acconcia F., Barnes C. J., Singh R. R., Talukder A. H., Kumar R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6782–6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hunter M. P., Zegers M. M. (2010) Am. J. Physiol. Cell Physiol. 299, C21–C32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Disatnik M. H., Boutet S. C., Lee C. H., Mochly-Rosen D., Rando T. A. (2002) J. Cell Sci. 115, 2151–2163 [DOI] [PubMed] [Google Scholar]