Abstract
Cellular receptors for collagens belong to the family of β1 integrins. In the epidermis, integrin α2β1 is the only collagen-binding integrin present. Its expression is restricted to basal keratinocytes with uniform distribution on the cell surface of those cells. Although α2β1 receptors localized at the basal surface interact with basement membrane proteins collagen IV and laminin 111 and 332, no interaction partners have been reported for these integrin molecules at the lateral and apical membranes of basal keratinocytes. Solid phase binding and surface plasmon resonance spectroscopy demonstrate that collagen XXIII, a member of the transmembrane collagens, directly interacts with integrin α2β1 in an ion- and conformation-dependent manner. The two proteins co-localize on the surface of basal keratinocytes. Furthermore, collagen XXIII is sufficient to induce adhesion and spreading of keratinocytes, a process that is significantly reduced in the absence of functional integrin α2β1.
Keywords: Cell-Cell Interaction, Collagen, Extracellular Matrix, Membrane Proteins, Skin
Introduction
Collagen XXIII belongs to the class of transmembrane collagens in type II orientation, which comprises the collagens XIII, XVII, XXIII, and XXV and other related proteins such as ectodysplasin A, class A macrophage scavenger receptors, and the colmedins (1, 2). Collagen XXIII forms homotrimers consisting of a short intracellular domain, a single-pass transmembrane domain and three extracellular collagen domains interrupted by short noncollagenous sequences (3, 4). It exists either as a full-length, membrane-anchored protein or as a soluble ectodomain. The proteolytic processing releasing the ectodomain is mediated by furin and is regulated by the plasma membrane microenvironment (5). Collagen XXIII is expressed in the epidermis and other epithelia such as those in the tongue, gut, and lung but also in the brain and kidney (4). In prostate, collagen XXIII expression was shown to correlate with tumor progression (6).
Four integrins, α1β1, α2β1, α10β1, and α11β1 bind native collagens (7). This β1 subunit-containing subclass of integrins is characterized by the presence of an inserted domain (I domain) with homology to von Willebrand factor A domains in their α-subunit, which harbors the ligand-binding site (8, 9). The integrins α10β1 and α11β1 have a restricted expression pattern on differentiated chondrocytes and developing muscle cells (10, 11), whereas α1β1 and α2β1 show a broader distribution, with integrin α1β1 being predominantly expressed by cells of mesenchymal origin and integrin α2β1 by epithelial cells and platelets. In vitro studies suggested an implication of integrin α2β1 in cell attachment and migration (12, 13), generation of mechanical forces and contraction of collagen matrices (14), induction of collagenase activity and matrix remodeling (15, 16), as well as angiogenesis (17) and epithelial branching morphogenesis (18). Mice lacking the integrin α2 subunit exhibit defects in mammary gland branching morphogenesis (19), delayed platelet aggregation and formation of unstable thrombi (20, 21), and enhanced angiogenesis in wounds (22) and tumors (23).
In the skin, integrin α2β1 is expressed by endothelial and some immune cells, fibroblasts and most prominently by keratinocytes of the basal layer. Expression is abrogated during terminal differentiation of keratinocytes subsequent to loss of contact with the extracellular matrix of the dermo-epidermal basement membrane (24, 25). Integrin α2β1 efficiently interacts with collagen I and has a lower affinity for basement membrane collagen IV and laminins 111 and 332 (12, 26–28).
Because collagen I is absent from epidermis, we raised the question of whether integrin α2β1 was engaged in cell-extracellular matrix interactions in this tissue and if so, which would be the matrix ligand. Here we present evidence that collagen XXIII directly interacts with integrin α2β1 and is sufficient to induce integrin α2β1-dependent attachment and spreading of keratinocytes. We postulate that the interaction of collagen XXIII with integrin α2β1 may contribute to cell-cell binding in the basal epidermis.
EXPERIMENTAL PROCEDURES
Recombinant Proteins and Antibodies
Recombinant production and purification of collagen XXIII ectodomain (4), integrin αI-domains2 (29), and integrin α2β1 ectodomain (30) were done as described previously. The polyclonal anti-collagen XXIII antibody was described previously (4). Rat monoclonal antibody against integrin α2 was purchased from Emfret, and mouse monoclonal antibody against E-cadherin was purchased from BD Transduction Laboratories. Rabbit polyclonal antibody against laminin 332 was a gift from Robert E. Burgeson. For blocking experiments, mouse monoclonal antibodies were employed: clone P1E6 to integrin α2, clone P1B5 to integrin α3 (Chemicon), and clone AIIB2 to integrin β1 (Developmental Studies Hybridoma Bank). The GST detection module (GE Healthcare) was applied to detect GST fusion proteins, polyclonal anti-vinculin antibody was purchased from Sigma, and desmoglein 1 was detected with clone DG3.10 (Roche Applied Science).
Immunohistochemistry
Immunohistochemistry was performed on frozen embedded sections of fetal (embryonic day 18.5) and adult (postnatal day 60) mice and on frozen sections of wounds as described previously (22, 31).
Solid Phase Binding Assay
Purified proteins were diluted in TBS (20 mm Tris, 150 mm NaCl, 2 mm MgCl2, 1 mm MnCl2, pH 7.4), and 10 μg/ml (500 ng/well) were coated onto 96-well plates (Nunc Maxisorb) at 4 °C overnight. To determine whether the interaction between collagen XXIII and integrin α2β1 depends on native folding of the collagen, the collagen XXIII ectodomain was heat-denatured for 10 min at 80 °C prior to immobilization. After washing with TBS, unspecific binding sites were blocked with 1% BSA in TBS for 2 h at room temperature. Ligands, diluted to concentrations between 0.3 and 3000 nm in blocking buffer, were incubated for 1.5 h. Excess ligand was removed by washing twice with HEPES buffer (20 mm HEPES, 150 mm NaCl, 2 mm MgCl2, 1 mm MnCl2), bound ligands were fixed with 2.5% (v/v) glutaraldehyde for 10 min. The amounts of bound ligand were detected with primary antibodies against collagen XXIII, GST or the integrin β1 subunit, followed by incubation with secondary horseradish peroxidase-coupled antibodies. For enzymatic reaction, the wells were incubated with 50 μl of 0.25 mm tetramethylbenzidine and 0.005% (v/v) H2O2 in 0.1 m sodium acetate, pH 6.0, for 10 min. The reaction was stopped with 50 μl/well 2.5 m H2SO4, and absorbance was read at 450 nm using a microplate reader (Labsystems Multiscan MS). Apparent KD constants were calculated assuming a 1:1 interaction. All of the buffers contained 2 mm MgCl2 and 1 mm MnCl2 unless otherwise noted.
Surface Plasmon Resonance Spectroscopy
Surface plasmon resonance spectroscopy was performed using a Biacore 2000 (BIAcore AB) system. For measurement of protein-protein interactions, the integrin α2I-domains carrying the activating mutation E318W or the integrin α2β1 ectodomain were coupled in 25 mm sodium acetate, pH 4.5, with a flow rate of 5 μl/min to a CM5 chip. The chip was previously activated with N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride. After coupling the required amount of protein (∼1000 RU), unbound reactive groups were saturated with 1 m ethanolamine hydrochloride, pH 8.5. The experiments were carried out using serial dilutions of the collagen XXIII ectodomain in running buffer (20 mm HEPES, 150 mm NaCl, 2 mm MgCl2, 1 mm MnCl2, 0.005% P20). The analyte was passed over the sensor chip with a constant flow rate of 30 μl/min for 120–180 s, and dissociation was measured over 250–300 s. Fittings of the data, overlay plots, and calculation of KD values were done with BIAevaluation software 3.2 assuming a 1:1 model.
Tissue Culture and Cell Adhesion Assays
HaCaT cells were grown in DMEM/nutrient mixture F-12 with GlutaMAXTM (Invitrogen) containing 10% fetal calf serum (Biochrom AG). Human keratinocytes were obtained from Robert E. Burgeson, and mouse keratinocytes were isolated from newborn skin of wild-type and integrin α2-deficient mice and placed in culture according to established procedures (14). For determination of cell adhesion to collagen XXIII, the purified ectodomain was immobilized on 96-well tissue culture-treated plates (Costar) overnight at 4 °C. Unspecific binding sites were blocked with 1% BSA in TBS for 3 h at 4 °C. Single cell suspensions of HaCaT or primary keratinocytes were incubated in serum-free medium supplemented with 2 mm MgCl2 and 1 mm MnCl2 at densities of 1 × 106 or 5 × 105 cells/ml for 30 or 60 min at 37 °C and 5% CO2. For inhibition experiments, appropriate dilutions of integrin function-blocking antibodies were mixed with suspended cells before plating. For competition experiments, both the coated plates and the cells in suspension were presupplemented with the competitor. Nonadherent cells were removed by a single PBS wash, and the cells were fixed with 1% (v/v) glutaraldehyde in PBS followed by staining with 0.1% (w/v) crystal violet. To evaluate the shape and area, the adherent cells were photographed with a digital camera (PowerShot G5, Canon) mounted on a phase contrast microscope (Axiovert 100; Carl Zeiss) followed by evaluation with ImageJ software (National Institutes of Health). Adhesion was quantified by lysing the adherent cells with 0.2% Triton X-100 and determining the amount of released dye by absorption at 540 nm using a microplate reader (Labsystems Multiscan MS).
Cell Spreading and Formation of Focal Contacts
Focal contact formation analysis was performed as described previously (34). Briefly, glass chamber slides (Nunc) were coated with collagen XXIII ectodomain or bovine collagen I (both 40 μg/ml) overnight at 4 °C. After blocking of unspecific binding sites with 0.1% BSA in TBS for 1 h at room temperature, HaCaT cells were suspended in medium with or without 1 mm MnCl2, plated at a density of 2 × 105 cells/ml on substrate-coated wells, and incubated for 2 h at 37 °C and 5% CO2. The adherent cells were fixed with 2% paraformaldehyde followed by permeabilization with blocking buffer (0.5% saponin, 2.5% methanol, 2% horse serum in PBS) for 45 min at room temperature. Focal contacts were visualized by staining with anti-vinculin anybody diluted in blocking buffer followed by fluorescence labeled secondary antibody and subsequent analysis with an Axiophot microscope equipped with an AxioCam MRc camera (Carl Zeiss).
Wound Healing
Skin wounding and tissue collections were performed as described previously (22). In brief, adult C57BL/6 mice were anesthesized, and two full thickness excisional wounds of 6 mm each were created at each side of the scapular region by excising skin and the subcutaneous muscle panniculus carnosus. Wounds were left uncovered and harvested at 4 days after wounding (22).
RESULTS
Collagen XXIII Partially Co-localizes with Integrin α2β1 in the Epidermis and Is Present in the Nondesmosomal Keratinocyte Plasma Membrane
We recently described the expression pattern of collagen XXIII in mouse embryos and showed that it is present in a variety of different epithelia (4). To extend these observations, we compared expression of collagen XXIII with that of integrin α2β1 in the epidermis and in tongue epithelium of embryonic (E18.5) and adult (P60) mice (Fig. 1). Integrin α2β1 is the only collagen-binding integrin present in those tissues (22, 24). Confocal immunofluorescence microscopy indicated cell surface staining of collagen XXIII in all layers of the epidermis, whereas the expression of integrin α2β1 was restricted to the surface of keratinocytes in the stratum basale. Overlay analysis revealed co-localization of collagen XXIII with integrin α2β1 at the surface of basal keratinocytes.
FIGURE 1.
Distribution of collagen XXIII and integrin α2β1 and co-localization of the two proteins in murine tissues. Immunohistochemistry was performed on cryosections of wild-type (C57BL/6) embryonic (E18.5) (A) as well as adult (P 60) mouse skin (B) and tongue (C). Co-staining was accomplished using sequential incubation with polyclonal anti-collagen XXIII antibody (red) and a mixture of monoclonal antibodies against the α2 integrin subunit (green). Note that the two proteins co-localize (yellow) on the surface of the basal keratinocytes and in hair follicles. Bar, 40 μm.
To more precisely define the localization of collagen XXIII molecules on the membrane, immunoelectron microscopy was performed. Employing classical embedding and labeling techniques proved to be difficult because the antibodies recognizing collagen XXIII did not detect the epitopes, which were potentially masked. However, application of the recently described method cryo-ultramicrotomy (32, 33), which avoids harsh fixation before ultrasectioning, resulted in successful labeling of collagen XXIII in sections of keratinocyte membranes that were interspersed between electron dense structures (supplemental Fig. S1A), which stained positively for desmoglein-1 (supplemental Fig. S1B), thus identifying desmosomes. This indicates a cell surface localization of collagen XXIII at the nondesmosomal membrane of the keratinocytes.
Collagen XXIII Mediates Integrin α2β1-dependent Cell Adhesion of HaCaT Keratinocytes and Triggers the Formation of Focal Adhesion Plaques
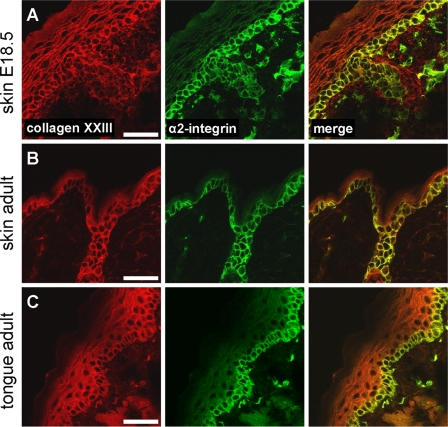
To determine whether collagen XXIII is capable of mediating cell attachment, its ectodomain (4) was utilized as attachment substratum for human keratinocytes and HaCaT keratinocytes. HaCaT cells express the integrins α2β1, α3β1 (35), α5β1, αVβ1, αVβ6 (36), and α6β4 (37) on their cell surface, whereof only integrin α2β1 belongs to the group of collagen-binding integrins. HaCaT keratinocytes attached to immobilized collagen XXIII in a concentration-dependent manner. Furthermore, the attachment of human keratinocytes to immobilized collagen XXIII was analyzed, and the binding was comparable with the one seen for HaCaT keratinocytes. (Fig. 2A). Adhesion was completely abrogated by EDTA and increased by the addition of Mg2+ and Mn2+ in a manner that is characteristic of an integrin-mediated process. Reduced attachment was observed to heat-denatured collagen XXIII, indicating the functional significance of the correct folding of collagen XXIII (Fig. 2, A and B). To assess whether adhesion of HaCaT keratinocytes to immobilized collagen XXIII was mediated by binding of integrin α2β1, the cellular interactions were challenged with function blocking antibodies directed against the integrin subunits β1 (AIIB2), α2 (P1E6), and α3 (P1B5). The presence of the antibodies against the β1 and α2 subunits during the experiments resulted in a concentration-dependent inhibition of HaCaT attachment (Fig. 2C). Similar results were found for the integrin α2-specific inhibitor rhodocetin (data not shown). In contrast, the antibody directed against the α3 integrin subunit showed no effect on HaCaT attachment. Taken together, this strongly suggests that HaCaT attachment to collagen XXIII is mediated by integrin α2β1. Moreover, the addition of soluble heparin in attachment experiments showed no effect, suggesting that glycosaminoglycan binding to collagen XXIII is not required for HaCaT attachment (Fig. 2D). Because collagen XXIII harbors an RGD sequence in its Col1 domain (3), which might confer binding to RGD-binding integrins, inhibition experiments were performed in the presence of RGD peptides. These did not show an effect on the attachment of HaCaT keratinocytes to collagen XXIII (Fig. 2D), thus ruling out the involvement of RGD-binding integrins.
FIGURE 2.
Adhesion of primary human keratinocytes and HaCaT keratinocytes to collagen XXIII depends on integrin α2β1 but is independent of RGD-binding integrins or glycosaminoglycans. A, ion- and folding-dependent attachment of primary human keratinocytes to collagen XXIII. Serial dilutions of native or heat-denatured collagen XXIII ectodomain (0.3–20 μg/ml) were immobilized on microtiter plates. Human keratinocytes were allowed to attach to the substratum for 45 min in the absence (untreated) or presence of either Mg2+/Mn2+ or EDTA, followed by staining with crystal violet. Relative adhesion is presented as ΔE (measured extinction minus nonspecific cell attachment to BSA). B, ion-dependent attachment of HaCaT cells to collagen XXIII. Serial dilutions of collagen XXIII ectodomain (0.3–20 μg/ml) were immobilized, and HaCaT keratinocytes were allowed to attach to the substratum for 30 min. Detection and calculation of the relative adhesion ΔE as described above. C, modulation of HaCaT adhesion to collagen XXIII by function blocking antibodies directed to the indicated integrin subunits. HaCaT keratinocytes were preincubated with monoclonal antibodies AIIB2, P1E6, or P1B5 at the indicated concentrations and subsequently allowed to attach to microtiter plates coated with collagen XXIII ectodomain (5 μg/ml). ab, antibody. D, the contribution of RGD-binding integrins and the influence of glycosaminoglycans to HaCaT attachment on collagen XXIII was analyzed by preincubating the cells and microtiter plates with the indicated concentrations of RGD peptide or heparin. HaCaT keratinocytes and microtiter plates coated with collagen XXIII ectodomain (5 μg/ml) were preincubated with the indicated concentrations of heparin. For the experiments shown in C and D, microtiter plates were coated with collagen XXIII ectodomain (5 μg/ml). Attachments were analyzed in the presence of 2 mm Mg2+ and 1 mm Mn2+, and the values are presented as percentages of inhibition in comparison with noninhibited controls. Nonspecific cell attachment to BSA was subtracted from all of the values. All of the data represent the mean values ± S.D. (n = 3).
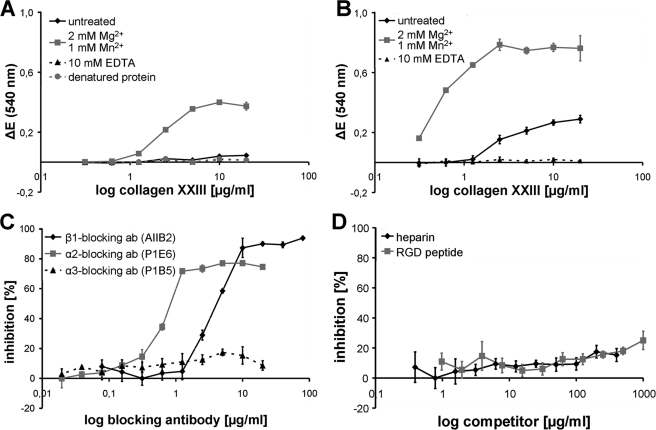
The first step in integrin-mediated signal transduction is the formation of focal contacts and focal adhesion plaques. To test whether cellular interactions with collagen XXIII are sufficient to induce the formation of these supramolecular structures, HaCaT cells attached to immobilized collagen XXIII were stained for the focal adhesion marker vinculin. Immunofluorescence analysis demonstrated the formation of distinct vinculin-positive focal adhesion plaques in HaCaT keratinocytes seeded on a collagen XXIII substrate, a process that was enhanced by the addition of Mn2+ and partially inhibited by the addition of an integrin β1 subunit blocking antibody (Fig. 3, A–C).
FIGURE 3.
Collagen XXIII promotes cell spreading and induces formation of focal contacts. HaCaT keratinocytes were seeded on collagen XXIII ectodomain (40 μg/ml; A–C), collagen I (40 μg/ml; D), or glass (E) and incubated for 2 h in the absence (A, D, and E) or presence of 1 mm Mn2+ (B) or 20 μg/ml of the monoclonal antibody AIIB2 (C). After fixation, the cells were stained for the focal contact marker vinculin. Bar, 15 μm.
Integrin α2-deficient Keratinocytes Exhibit Reduced Cell Attachment and Cell Spreading on a Collagen XXIII Matrix
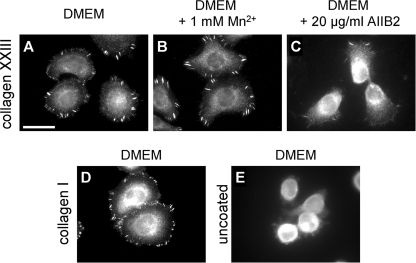
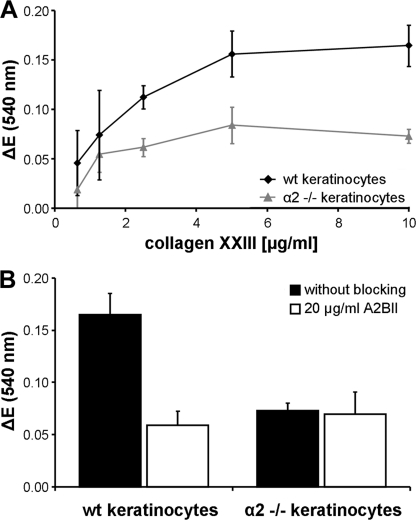
Integrin α2β1 is the only collagen-binding integrin expressed by keratinocytes. Cells deficient for this integrin are virtually incapable of adhering to collagen I and IV matrices (14). In the epidermis, integrin α2β1 is uniformly distributed along the basolateral surfaces of basal keratinocytes (this work and Refs. 25, 34), being available for interactions with basement membrane proteins at the basal cell surface. Thus far, no interaction partners for this receptor have been identified at the lateral surfaces. To determine whether collagen XXIII might be such an elusive ligand, we investigated the adhesion of keratinocytes isolated from wild-type or integrin α2-deficient mice to immobilized collagen XXIII. The wild-type cells showed a concentration-dependent attachment to collagen XXIII, whereas that of α2-deficient keratinocytes was reduced by more than 50% (Fig. 4A).
FIGURE 4.
Integrin α2 chain-deficient primary keratinocytes show reduced attachment to collagen XXIII. A, serial dilutions of collagen XXIII ectodomain (0.63–10 μg/ml) were immobilized on microtiter plates. Primary keratinocytes from wild-type (wt) or α2 integrin-deficient (α2 −/−) mice were allowed to attach to the substratum for 60 min in the presence of 2 mm Mg2+ and 1 mm Mn2+ followed by staining with crystal violet. B, attachment of primary keratinocytes from wild-type (wt) or α2 integrin-deficient (α2 −/−) mice to collagen XXIII in the presence or absence of the β1 integrin blocking antibody AIIB2 (20 μg/ml). Note that residual attachment of α2 integrin-deficient keratinocytes is not affected by β1 integrin inhibition. All of the data represent the mean values ± S.D. (n = 3). ΔE, measured extinction minus nonspecific cell attachment to BSA.
The addition of the integrin β1 subunit blocking antibody AIIB2 during the experiments attenuated binding of the wild-type keratinocytes to a level comparable with that of noninhibited α2-deficient keratinocytes, whereas addition of the antibody had no effect on the α2-deficient cells (Fig. 4B). Taken together, this identifies collagen XXIII also as a substrate for attachment of primary mouse keratinocytes and that the binding is mediated by integrin α2β1. However, in contrast to the human cells, residual binding was still observed.
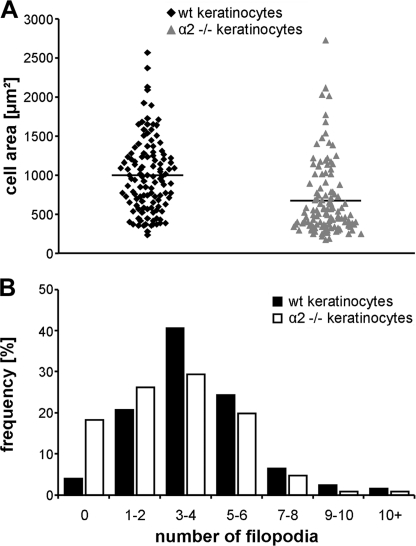
To further investigate the cell binding and to discriminate between fully attached, spread cells and only slightly attached, rounded cells, we quantified the area covered by the attached cells. The average area of cell spreading on a collagen XXIII matrix was significantly higher for wild type than for α2-deficient keratinocytes (Fig. 5A). In addition, the average number of filopodia was significantly reduced in α2-deficient keratinocytes compared with wild-type controls (Fig. 5B). Especially the number of cells that did not form filopodia upon attachment was four times higher for the α2-deficient cells. Those results show that proper attachment and spreading of primary keratinocytes on immobilized collagen XXIII depends on integrin α2β1.
FIGURE 5.
The integrin α2 chain is vital for proper spreading of primary keratinocytes on a collagen XXIII substrate. Primary keratinocytes from wild-type (wt) or α2 integrin-deficient (α2 −/−) mice were allowed to attach to collagen XXIII ectodomain (10 μg/ml) coated onto microtiter plates for 60 min. For analysis, the cells were fixed, and phase contrast pictures were taken. A, each dot indicates the area of one analyzed cell (data points; n = 240). The average area of wild-type cells was 998 ± 470 μm2, and for α2 integrin-deficient keratinocytes it was 675 ± 460 μm2 (p < 0.0001 by unpaired t test). B, histogram showing the percentage of cells grouped according to the number of filopodia/cell. The average number of filopodia on the α2 integrin-deficient keratinocytes (3.09 ± 2.28) was reduced in comparison with wild-type keratinocytes (4.03 ± 2.27). Note the high number of integrin α2-deficient keratinocytes that did not form any filopodia and the low number of them that formed many filopodia (n = 383; p < 0.0002 by unpaired t test).
Collagen XXIII Directly Interacts with Integrin α2β1
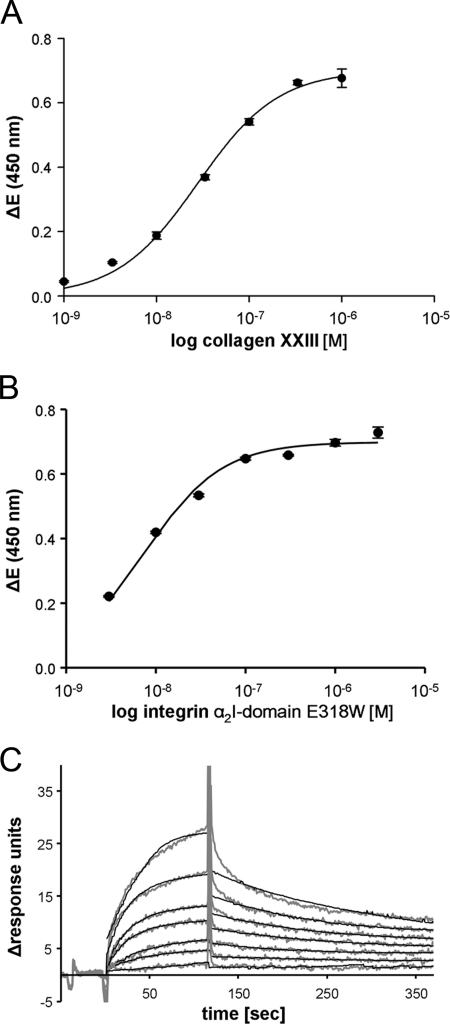
Direct interaction between collagen XXIII and integrin α2β1 was further investigated employing the recombinant collagen XXIII ectodomain and recombinant αI-GST (marked as αI) fusion protein containing the activation mutation E318W (38). Titration experiments clearly demonstrated dose-dependent saturable binding using either the collagen XXIII ectodomain or the α2I domain as soluble ligand. From the titration curves an apparent KD value of 2.8 ± 0.16 × 10−8 m was determined for the interaction of soluble collagen XXIII with the immobilized α2I domain (Fig. 6A). Conversely, the apparent KD value was 7.2 ± 0.78 × 10−9 m using collagen XXIII as immobilized and the α2I domain as soluble interaction partner, i.e. binding was stronger using immobilized versus soluble collagen XXIII (Fig. 6B). This discrepancy might be caused by partial dimerization of the α2I domains by way of their GST tag. These binding results were validated by surface plasmon resonance spectroscopy. The associations and dissociations of the obtained binding curves were analyzed in a Langmuir 1:1 binding model (Fig. 6C). Here, a KD value of 3.0 × 10−8 m was calculated using collagen XXIII as soluble interaction partner, which is in good agreement with the solid phase binding data.
FIGURE 6.
Concentration-dependent interaction of collagen XXIII with the α2I-domain. Binding was determined using the triple helical collagen XXIII ectodomain and an integrin α2I-domain GST fusion protein carrying the activating mutation E318W in solid phase binding assays and by surface plasmon resonance spectroscopy. Solid phase binding assays were performed with immobilized α2I-domain E318W and soluble collagen XXIII (A) or immobilized collagen XXIII and soluble α2I-domain E318W (B). Binding was detected in an ELISA style manner with a monoclonal anti-GST antibody or polyclonal antibody against collagen XXIII. The resulting saturation curves were used to calculate apparent KD values assuming a 1:1 interaction. ΔE equals the measured extinction minus blank value. The values represent the means ± S.D. (n = 3). C, surface plasmon resonance spectroscopy was performed with collagen XXIII as the soluble analyte. The amount of interacting analyte was monitored by measuring the variation in the plasmon resonance angle as a function of time and expressed in response units. The background signal has been subtracted from each curve. Fittings and overlay plots were done with the Biaevaluation software version 3.2. The continuous black lines represent the fitted curves.
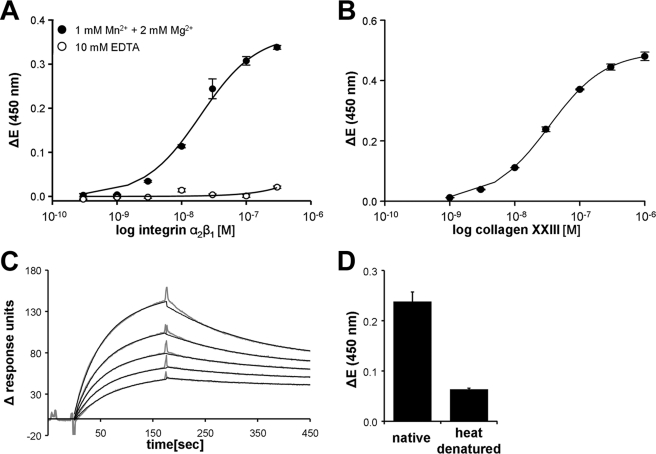
Although the αI-domains of collagen-binding integrins have been successfully employed in various interaction studies (28, 39), the binding properties of integrin molecules strongly depend on intramolecular domain-domain interactions (8). Therefore, to apply a model system that more closely resembles the in vivo situation, we used the recombinant heterodimeric ectodomain of integrin α2β1 for further binding experiments. Utilizing the integrin α2β1 ectodomain (30) as a soluble or immobilized interaction partner for collagen XXIII in solid phase binding assays, a dose-dependent saturable interaction was observed. The apparent KD values were calculated to 1.9 ± 0.23 × 10−8 m for immobilized collagen XXIII and soluble integrin α2β1 and 3.3 ± 0.7 × 10−8 m for the reverse experiment (Fig. 7, A and B). The absence of binding after addition of EDTA revealed the dependence on bivalent cations commonly seen in integrin-collagen interactions and furthermore indicated the specificity of the observed interaction (Fig. 7A). The measurements were confirmed by surface plasmon resonance spectroscopy, from which a KD value of 4.0 × 10−8 m was determined using collagen XXIII as a soluble interaction partner (Fig. 7C).
FIGURE 7.
Concentration-dependent interaction of collagen XXIII with the heterodimeric integrin α2β1 ectodomain. A and B, biochemical interaction was determined in solid phase binding assays and by surface plasmon resonance spectroscopy using the triple helical collagen XXIII ectodomain and the heterodimeric integrin α2β1 ectodomain. Solid phase binding assays were performed with immobilized collagen XXIII and soluble integrin α2β1 (A) or immobilized integrin α2β1 and soluble collagen XXIII (B). Binding was detected in an ELISA style manner with a polyclonal antibody against the integrin β1 subunit or a polyclonal anti-collagen XXIII antibody. The resulting saturation curves were used to calculate apparent KD values assuming an 1:1 interaction. C, surface plasmon resonance spectroscopy was performed with collagen XXIII as a soluble analyte. The amount of interacting analyte was monitored by measuring the variation in the plasmon resonance angle as a function of time and expressed in response units. The background signal has been subtracted from each curve. Fittings and overlay plots were done with the Biaevaluation software version 3.2. The continuous black lines represent the fitted curves. D, The interaction of collagen XXIII with integrin α2β1 requires native triple helical conformation. Native or heat-denatured collagen XXIII ectodomain (10 min, 80 °C) was immobilized on a microtiter plate, and the interaction with 100 nm soluble integrin α2β1 ectodomain was determined by solid phase assay. ΔE, measured extinction minus blank value. The values represent the means ± S.D. (n = 3).
Another prerequisite for the interaction of collagen to collagen-binding integrins is the presence of an intact collagen triple helix folding (40). The native collagen XXIII ectodomain contains three triple helical domains separated and flanked by four nonhelical segments with 75% of the ectodomain folded into a triple helix. To test whether the native conformation is required for the collagen XXIII-integrin α2β1 interaction, collagen XXIII was denatured by heating and applied as an immobilized partner to solid phase binding assays. Disruption of the native folding strongly reduced binding to soluble integrin α2β1 (Fig. 7D), demonstrating the functional importance of the native triple helical folding of the collagen XXIII ectodomain.
GXXGER Motives in Collagen XXIII Do Not Mediate Integrin α2β1 Binding
Structural analysis of the integrin α2β1 binding site in collagens has revealed that the amino acid sequence GFOGER (O being 4-hydroxyproline) confers high affinity binding to the integrin extracellular part (41, 42). The sequence of mouse collagen XXIII does not contain this motif, but two similar motives containing GER triplets, 434GTSGER439 and 488GEKGER493, are present. The latter is conserved in collagen XXIII of all species so far sequenced (4). To analyze whether those motives function as integrin α2β1 binding sites, we inserted them into model fusion proteins containing a bacteriophage foldon and flanking GPP5 linkers (43). Both sequences were expressed as fusion proteins in bacteria, and folding into triple helical conformation of purified peptides was confirmed by CD spectroscopy (supplemental Fig. S2, A and B). Cell attachment assays showed that the control peptide (GPP)5GFPGER(GPP)5 supported HaCaT attachment to a similar extent as the collagen XXIII ectodomain. By contrast, the peptides (GPP)5GTSGER(GPP)5 and (GPP)5GEKGER(GPP)5 failed to support cell attachment, indicating that those motives in collagen XXIII are not used for binding to integrin α2β1 (supplemental Fig. S2C).
Expression Levels and Distribution of Collagen XXIII Are Not Significantly Altered in the Skin of Integrin α2-deficient Mice
To verify the functional significance of the collagen XXIII-integrin α2β1 interaction in vivo, we investigated the level of collagen XXIII expression and distribution in the skin of wild-type and integrin α2-deficient mice. Western blot and immunofluorescence analysis did not show significant differences in either expression or distribution of collagen XXIII in integrin α2-deficient compared with wild-type mice (supplemental Fig. S3). This result might suggest that in addition to integrin α2β1, collagen XXIII is held in place also by other interaction partners, e.g. intracellular adapter proteins or glycosaminoglycan side chain-containing proteins.
Collagen XXIII Is Mostly Absent in Migrating Keratinocytes at the Wound Edge in Skin
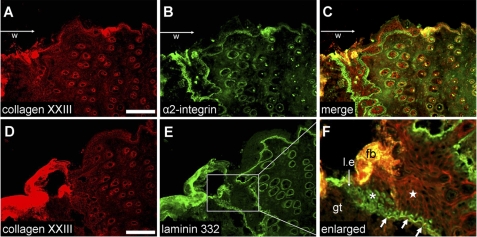
To investigate the role of collagen XXIII during wound healing, the localization of collagen XXIII was studied in full-thickness wounds (extending beyond the panniculus carnosus, i.e. the subcutaneous muscle layer of murine skin). Wound sections 4 days after wounding were analyzed. Interestingly, collagen XXIII was not present at the cell-cell junction of migrating keratinocytes at the leading wound edge (Fig. 8F). Migrating keratinocytes synthesize large amounts of laminin 332 as seen by the intracellular staining (Fig. 8, E and F, asterisk). Collagen XXIII can be again detected at cell-cell junctions of keratinocytes (star) where a basement membrane is newly formed, visualized by a positive basement membrane staining for laminin 332 (Fig. 8F, thicker arrows). Integrin α2 expression of basal keratinocytes is also detectable at this time point, and the strongest staining signals can be detected at the wound edge (Fig. 8B).
FIGURE 8.
Distribution of collagen XXIII in skin wounds: collagen XXIII red (A and D), integrin α2β1 green (B), and laminin 332 green (E). Immunohistochemisty was performed on cryosections of wounds of 6-week-old mice (collected 4 days after wounding). The wound areas (w) are located at the left sides of the images, and the keratinocytes in the leading edges (l.e.) are moving from the right to the left. Co-staining was accomplished using sequential incubation with a polyclonal guinea pig anti-collagen XXIII antibody (red) followed by incubation with either a monoclonal antibody against the α2 integrin subunit (green) or a polyclonal rabbit anti-laminin 332 antibody (green). B and C, strong α2 integrin subunit staining can be detected at the wound edge (left sides of the pictures). As previously shown, the staining is most prominent at the wound edge. In the nonwounded areas and in the keratinocytes of the hair follicles, the signals are much weaker. D and F, collagen XXIII staining can be detected in all keratinocyte cell layers at cell-cell junctions. Near the wound edge, collagen XXIII was not present at cell-cell junctions (white asterisk); however, intracellular staining for laminin 332 can be seen. In the area (E, F), where a newly formed basement membrane was detected by immunofluorescence staining (arrows), intracellular staining for laminin 332 disappears, whereas cell-cell staining for collagen XXIII becomes apparent (white star). l.e., leading edge; w, wound area; gt, granulation tissue; fb, fibrin clot. Bar, 100 μm.
DISCUSSION
Collagen XXIII belongs to the group of transmembrane collagens that exist in two forms, either as full-length cell surface anchored molecules or as shed soluble ectodomains (1). We showed previously that in skin collagen XXIII is predominantly present as full-length molecule, and it localizes to the cell surface of keratinocytes in all layers of the epidermis (5). In the epidermis, expression of integrin α2β1 is restricted to basal keratinocytes with uniform distribution on the cell surface of those cells (this work and Refs. 25 and 34). Although α2β1 receptors localized at the basal surface interact with basement membrane collagen IV, laminin 111 and 332, no interaction partners have been reported for these integrin molecules at the lateral and apical membranes of basal keratinocytes. This work identifies the interaction collagen XXIII and integrin α2β1 by solid phase and surface plasmon resonance spectroscopy and describes their co-localization on the surface of basal keratinocytes in mouse skin at the microscopic level. In addition, we show at the ultrastructural level that collagen XXIII is localized in nondesmosomal areas of the keratinocyte membrane, a localization also suggested for integrin α2β1 (44).
Several binding assays based on different principles revealed strong interactions between collagen XXIII and the heterodimeric integrin α2β1 ectodomain and the integrin α2I domain (containing the activation mutation E318W). In the latter case, the domain shifts into an open conformation, which resembles the ligand-bound state (38). The I domain of integrin α2β1 harbors the collagen-binding site, and structural studies revealed that coordination of a metal ion between a glutamate residue of the collagen and the MIDAS motif of the I domain is crucial for the interaction. Binding is limited by steric reasons to only one integrin molecule at any given site within the collagen molecule, despite the presence of three binding sites in a homotrimeric collagen helix (40). Therefore, the estimation of a 1:1 interaction between collagen XXIII and integrin α2β1 is feasible, but the presence of more than one integrin-binding motif in collagen XXIII cannot be excluded. Based on this assumption, the KD value for the collagen XXIII-integrin α2β1 interaction lies in the range of 20–40 nm, which is comparable with the interaction of collagen I with integrin α2β1 (28, 45). Consistent with the general model for collagen-integrin α2β1 interactions (46, 47), binding of the receptor to collagen XXIII requires the native trimeric collagen conformation and bivalent cations, i.e. Mn2+ and Mg2+, and it is completely abolished by bivalent ion removal with EDTA. For integrin α2β1, only few binding motives have been identified, all of which are variations of the GFOGER motif that was first identified and can be summarized as GXXGER (48). Of the 23 glutamate residues present in the collagenous domains of collagen XXIII, only two motives follow the GXXGER rule. Interestingly, those two motives do not support binding to integrin α2β1. Therefore, the actual binding motif is still elusive and may represent a novel group of integrin α2β1 binding motives other than GXXGER.
In vitro cell culture experiments provided clear evidence for an integrin-dependent attachment of keratinocytes to collagen XXIII. The use of HaCaT keratinocytes with a well defined integrin profile in combination with integrin subunit function blocking antibodies clearly identified integrin α2β1 as the major cellular receptor for cell adhesion to collagen XXIII. This initial attachment is independent of glycosaminoglycan binding to collagen XXIII. Furthermore, cell attachment is independent of RGD-binding integrins, most likely because the RGD sequence in the Col1 domain of collagen XXIII is not accessible in the folded state of the molecule.
In addition to attachment, collagen XXIII also supports cell spreading and the formation of focal adhesion plaques, processes that are significantly reduced in the absence of functional integrin α2β1. Because the formation of focal adhesion plaques is considered to be the first step in integrin-mediated signaling (8), those results suggest that collagen XXIII acts as a physiological agonist for integrin α2β1, triggering integrin-mediated cell responses such as spreading and alterations in cell morphology.
Collagen XXIII is localized at the cell surface of basal keratinocytes, and release of its ectodomain by proteolytic cleavage is tightly regulated (5). Thus three different spatial configurations are potentially feasible for the collagen XXIII-integrin α2β1 interaction: interaction of the two molecules on the same cell or between neighboring cells or, if the collagen XXIII ectodomain is cleaved, between cells and the extracellular matrix. In normal skin, where collagen XXIII is predominantly present in a noncleaved, membrane-anchored form (4, 5), its combined collagenous domains consist of 370 amino acids, resulting in a collagen fold of ∼110 nm in length, which is well capable of bridging the gap between adjacent cells. Taking this together with the localization of collagen XXIII at sites of cell-cell contacts (5), we would like to postulate a role for the collagen XXIII-integrin α2β1 interaction in cell-cell adhesion. Unfortunately, in cultured keratinocytes collagen XXIII is down-regulated, and almost no full-length form of collagen XXIII was detectable on the cell surface (not shown). Even overexpression of collagen XXIII in HaCaT cells did not solve the problem, because the full-length protein was internally cleaved by furin (not shown). Interestingly, in the in vivo situation, we also observed that collagen XXIII is not present on the cell surface of migrating keratinocytes at the wound edge. This would explain our findings that cultured keratinocytes do not display collagen XXIII on their cell surface. Hence, switching between the full-length and shed forms might enable the keratinocytes to dynamically adapt to a changing environment, for example, following injury, which induces keratinocyte migration and redistribution of integrin α2β1 to the basal surfaces and leading edge of the migrating keratinocytes (49). In such challenging situations, up-regulation of collagen XXIII proteolytic processing would release any collagen XXIII-mediated cell-cell adhesion restraints and could positively influence cell migration. Animal models to further substantiate this hypothesis are underway.
Supplementary Material
This work was supported by Deutsche Forschungsgemeinschaft Grants EC140/5-1 (to B. E. and T. K.), SFB/TR 23 and SFB815 (to J. A. E.), and SFB829 (to M. K., T. K., and B. E.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental text and Figs. S1–S3.
- I-domain
- inserted domain.
REFERENCES
- 1. Franzke C. W., Bruckner P., Bruckner-Tuderman L. (2005) J. Biol. Chem. 280, 4005–4008 [DOI] [PubMed] [Google Scholar]
- 2. Myllyharju J., Kivirikko K. I. (2004) Trends Genet. 20, 33–43 [DOI] [PubMed] [Google Scholar]
- 3. Banyard J., Bao L., Zetter B. R. (2003) J. Biol. Chem. 278, 20989–20994 [DOI] [PubMed] [Google Scholar]
- 4. Koch M., Veit G., Stricker S., Bhatt P., Kutsch S., Zhou P., Reinders E., Hahn R. A., Song R., Burgeson R. E., Gerecke D. R., Mundlos S., Gordon M. K. (2006) J. Biol. Chem. 281, 21546–21557 [DOI] [PubMed] [Google Scholar]
- 5. Veit G., Zimina E. P., Franzke C. W., Kutsch S., Siebolds U., Gordon M. K., Bruckner-Tuderman L., Koch M. (2007) J. Biol. Chem. 282, 27424–27435 [DOI] [PubMed] [Google Scholar]
- 6. Banyard J., Bao L., Hofer M. D., Zurakowski D., Spivey K. A., Feldman A. S., Hutchinson L. M., Kuefer R., Rubin M. A., Zetter B. R. (2007) Clin. Cancer Res. 13, 2634–2642 [DOI] [PubMed] [Google Scholar]
- 7. Leitinger B., Hohenester E. (2007) Matrix Biol. 26, 146–155 [DOI] [PubMed] [Google Scholar]
- 8. Hynes R. O. (2002) Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 9. Whittaker C. A., Hynes R. O. (2002) Mol. Biol. Cell 13, 3369–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Camper L., Hellman U., Lundgren-Akerlund E. (1998) J. Biol. Chem. 273, 20383–20389 [DOI] [PubMed] [Google Scholar]
- 11. Velling T., Kusche-Gullberg M., Sejersen T., Gullberg D. (1999) J. Biol. Chem. 274, 25735–25742 [DOI] [PubMed] [Google Scholar]
- 12. Decline F., Rousselle P. (2001) J. Cell Sci. 114, 811–823 [DOI] [PubMed] [Google Scholar]
- 13. Werr J., Johansson J., Eriksson E. E., Hedqvist P., Ruoslahti E., Lindbom L. (2000) Blood 95, 1804–1809 [PubMed] [Google Scholar]
- 14. Zhang Z. G., Bothe I., Hirche F., Zweers M., Gullberg D., Pfitzer G., Krieg T., Eckes B., Aumailley M. (2006) J. Cell Sci. 119, 1886–1895 [DOI] [PubMed] [Google Scholar]
- 15. Langholz O., Röckel D., Mauch C., Kozlowska E., Bank I., Krieg T., Eckes B. (1995) J. Cell Biol. 131, 1903–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zigrino P., Drescher C., Mauch C. (2001) Eur. J. Cell Biol. 80, 68–77 [DOI] [PubMed] [Google Scholar]
- 17. Senger D. R., Perruzzi C. A., Streit M., Koteliansky V. E., de Fougerolles A. R., Detmar M. (2002) Am. J. Pathol. 160, 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saelman E. U., Keely P. J., Santoro S. A. (1995) J. Cell Sci. 108, 3531–3540 [DOI] [PubMed] [Google Scholar]
- 19. Chen J., Diacovo T. G., Grenache D. G., Santoro S. A., Zutter M. M. (2002) Am. J. Pathol. 161, 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holtkötter O., Nieswandt B., Smyth N., Müller W., Hafner M., Schulte V., Krieg T., Eckes B. (2002) J. Biol. Chem. 277, 10789–10794 [DOI] [PubMed] [Google Scholar]
- 21. Kuijpers M. J., Pozgajova M., Cosemans J. M., Munnix I. C., Eckes B., Nieswandt B., Heemskerk J. W. (2007) Thromb. Haemost. 98, 1072–1080 [PubMed] [Google Scholar]
- 22. Zweers M. C., Davidson J. M., Pozzi A., Hallinger R., Janz K., Quondamatteo F., Leutgeb B., Krieg T., Eckes B. (2007) J. Invest. Dermatol. 127, 467–478 [DOI] [PubMed] [Google Scholar]
- 23. Woodall B. P., Nyström A., Iozzo R. A., Eble J. A., Niland S., Krieg T., Eckes B., Pozzi A., Iozzo R. V. (2008) J. Biol. Chem. 283, 2335–2343 [DOI] [PubMed] [Google Scholar]
- 24. Watt F. M. (2002) EMBO J. 21, 3919–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu J. E., Santoro S. A. (1994) Dev. Dyn. 199, 292–314 [DOI] [PubMed] [Google Scholar]
- 26. Käpylä J., Ivaska J., Riikonen R., Nykvist P., Pentikäinen O., Johnson M., Heino J. (2000) J. Biol. Chem. 275, 3348–3354 [DOI] [PubMed] [Google Scholar]
- 27. Pfaff M., Göhring W., Brown J. C., Timpl R. (1994) Eur. J. Biochem. 225, 975–984 [DOI] [PubMed] [Google Scholar]
- 28. Tulla M., Pentikäinen O. T., Viitasalo T., Käpylä J., Impola U., Nykvist P., Nissinen L., Johnson M. S., Heino J. (2001) J. Biol. Chem. 276, 48206–48212 [DOI] [PubMed] [Google Scholar]
- 29. Käpylä J., Pentikäinen O. T., Nyrönen T., Nissinen L., Lassander S., Jokinen J., Lahti M., Marjamäki A., Johnson M. S., Heino J. (2007) J. Med. Chem. 50, 2742–2746 [DOI] [PubMed] [Google Scholar]
- 30. Eble J. A., Beermann B., Hinz H. J., Schmidt-Hederich A. (2001) J. Biol. Chem. 276, 12274–12284 [DOI] [PubMed] [Google Scholar]
- 31. Veit G., Kobbe B., Keene D. R., Paulsson M., Koch M., Wagener R. (2006) J. Biol. Chem. 281, 3494–3504 [DOI] [PubMed] [Google Scholar]
- 32. Tokuyasu K. T. (1989) Histochem. J. 21, 163–171 [DOI] [PubMed] [Google Scholar]
- 33. Ishida-Yamamoto A., Simon M., Kishibe M., Miyauchi Y., Takahashi H., Yoshida S., O'Brien T. J., Serre G., Iizuka H. (2004) J. Invest. Dermatol. 122, 1137–1144 [DOI] [PubMed] [Google Scholar]
- 34. Eble J. A., Kassner A., Niland S., Mörgelin M., Grifka J., Grässel S. (2006) J. Biol. Chem. 281, 25745–25756 [DOI] [PubMed] [Google Scholar]
- 35. Scharffetter-Kochanek K., Klein C. E., Heinen G., Mauch C., Schaefer T., Adelmann-Grill B. C., Goerz G., Fusenig N. E., Krieg T. M., Plewig G. (1992) J. Invest. Dermatol. 98, 3–11 [DOI] [PubMed] [Google Scholar]
- 36. Koivisto L., Larjava K., Häkkinen L., Uitto V. J., Heino J., Larjava H. (1999) Cell Adhes. Commun. 7, 245–257 [DOI] [PubMed] [Google Scholar]
- 37. Hintermann E., Bilban M., Sharabi A., Quaranta V. (2001) J. Cell Biol. 153, 465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aquilina A., Korda M., Bergelson J. M., Humphries M. J., Farndale R. W., Tuckwell D. (2002) Eur. J. Biochem. 269, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 39. Käpylä J., Jäälinoja J., Tulla M., Ylöstalo J., Nissinen L., Viitasalo T., Vehviläinen P., Marjomäki V., Nykvist P., Säämänen A. M., Farndale R. W., Birk D. E., Ala-Kokko L., Heino J. (2004) J. Biol. Chem. 279, 51677–51687 [DOI] [PubMed] [Google Scholar]
- 40. Emsley J., Knight C. G., Farndale R. W., Barnes M. J., Liddington R. C. (2000) Cell 101, 47–56 [DOI] [PubMed] [Google Scholar]
- 41. Knight C. G., Morton L. F., Peachey A. R., Tuckwell D. S., Farndale R. W., Barnes M. J. (2000) J. Biol. Chem. 275, 35–40 [DOI] [PubMed] [Google Scholar]
- 42. Xu Y., Gurusiddappa S., Rich R. L., Owens R. T., Keene D. R., Mayne R., Höök A., Höök M. (2000) J. Biol. Chem. 275, 38981–38989 [DOI] [PubMed] [Google Scholar]
- 43. Frank S., Kammerer R. A., Mechling D., Schulthess T., Landwehr R., Bann J., Guo Y., Lustig A., Bächinger H. P., Engel J. (2001) J. Mol. Biol. 308, 1081–1089 [DOI] [PubMed] [Google Scholar]
- 44. Galle J., Reibiger I., Westermann M., Richter W., Löffler S. (2002) Cell Commun. Adhes. 9, 161–172 [DOI] [PubMed] [Google Scholar]
- 45. Calderwood D. A., Tuckwell D. S., Eble J., Kühn K., Humphries M. J. (1997) J. Biol. Chem. 272, 12311–12317 [DOI] [PubMed] [Google Scholar]
- 46. Kern A., Eble J., Golbik R., Kühn K. (1993) Eur. J. Biochem. 215, 151–159 [DOI] [PubMed] [Google Scholar]
- 47. Tuckwell D., Calderwood D. A., Green L. J., Humphries M. J. (1995) J. Cell Sci. 108, 1629–1637 [DOI] [PubMed] [Google Scholar]
- 48. Farndale R. W., Lisman T., Bihan D., Hamaia S., Smerling C. S., Pugh N., Konitsiotis A., Leitinger B., de Groot P. G., Jarvis G. E., Raynal N. (2008) Biochem. Soc. Trans. 36, 241–250 [DOI] [PubMed] [Google Scholar]
- 49. Martin P. (1997) Science 276, 75–81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.