Abstract
We examined intracellular pH (pHi) of ten cancer cell lines derived from different organs and two normal cell lines including human embryonic lung fibroblast cells (HEL) and human umbilical vein endothelial cells (HUVEC) in vitro, and found that pHi of most of these cancer cells was evidently higher (pH 7.5 to 7.7) than that of normal cells (7.32 and 7.44 for HEL and HUVEC, respectively) and that of primary leukemic cells and erythrocytes hitherto reported (≤7.2). Higher pHi in these cancer cells could be related to the Warburg effect in cancer cells with enhanced glycolytic metabolism. Since reversal of the Warburg effect may perturb intracellular homeostasis in cancer cells, we looked for compounds that cause extensive reduction of pHi, a major regulator of the glycolytic pathway and its associated metabolic pathway. We found that phenoxazine compounds, 2-aminophenoxazine-3-one (Phx-3) and 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one (Phx-1) caused a rapid and drastic dose-dependent decrease of pHi in ten different cancer cells within 30 min, though the extent of the decrease of pHi was significantly larger for Phx-3 (ΔpHi = 0.6 pH units or more for 100 µM Phx-3) than for Phx-1 (ΔpHi = 0.1 pH units or more for 100 µM Phx-1). This rapid and drastic decrease of pHi in a variety of cancer cells caused by Phx-3 and Phx-1 possibly perturbed their intracellular homeostasis, and extensively affected the subsequent cell death, because these phenoxazines exerted dose-dependent proapoptotic and cytotoxic effects on these cells during 72 h incubation, confirming a causal relationship between ΔpHi and cytotoxic effects due to Phx-3 and Phx-1. Phx-3 and Phx-1 also reduced pHi of normal cells including HEL and HUVEC, although they exerted less proapoptotic and cytotoxic effects on these cells than on cancer cells. Drugs such as Phx-3 and Phx-1 that reduce pHi and thereby induce cellular apoptosis might serve as benevolent anticancer drugs.
Keywords: phenoxazines, cancer cells, intracellular pH, apoptosis
Introduction
Since cancer cells actively proliferate, it is reasonable that aerobic metabolism including tricarbonic acid cycle producing much amounts of ATP, may be enhanced. Despite this expectation, cancer cells incorporate more glucose, and prefer glycolysis, an anaerobic metabolic pathway, even in the presence of ample oxygen. This paradoxical behavior of cancer cells with enhanced glycolysis is known as the Warburg effect.1) However, the actual cause of the Warburg effect of cancer cells is still unknown,2) though this characteristic behavior of cancer cells has been practically applied for detecting tumors by fluorodeoxyglucose positron emission tomography (FDG-PET),2) and more recently has attracted much attention in terms of cancer therapy targeting the inhibition of this fragile energy metabolism.3,4)
Another feature of tumors is that the extracellular pH (pHe) is extremely acidified and intracellular pH (pHi) is relatively high in the regions of the solid tumors.5,6) According to the classical Donnan’s membrane equilibrium, pHi in cancer cells may decrease as extracellular pH of these cells decreases. Actually, the pHi of human erythrocytes decreases according to the acidification of extracellular medium.7,8) However, in cancer cells, pHi is not decreased to the expected level, in spite of the acidification of extracellular pH,9,10) suggesting that the existence of a regulating system that maintains a higher pH in the cells. Later, Na+/H+ exchanger isoform 1 (NHE1) in the plasma membrane was found to be responsible for regulating pHi in cancer cells and for keeping pHi higher.11,12) Some reports indicated that pHi increased in several species of cancer cells,13–16) though general agreements on the elevation of pHi in cancer cells has not been achieved. Recently, Che et al.17) demonstrated that pHi in KB-3-1 cells (human epidermoid carcinoma cell line: pHi = 7.65) and K562 cells (human chronic myeloid leukemia cell line: pHi = 7.8) was much higher than that in intact normal cells (generally, pHi ≤ 7.2). This alkalinization of pHi in cancer cells may be beneficial for maintaining homeostasis in these cells, which depends primarily on the energy metabolism delivered from glycolysis, as compared with normal cells.
Therefore, the drugs used to reduce pHi in cancer cells may possibly be useful in cancer therapy because intracellular acidification is associated not only with inhibition of the glycolytic pathways, but also with activation of the apoptotic events in cancer cells,15,16,18) while a higher pHi enhances the glycolytic metabolism and proliferation of these cells.19) In this context, phenoxazines such as 2-aminophenoxazine-3-one (Phx-3) and 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one (Phx-1), which are prepared by reacting bovine hemoglobin with o-aminophenol or 2-amino-5-methylphenol20,21) may be most likely candidates for treating cancer because they are known to both decrease the pHi of a few cancer cell lines17) and cause apoptosis of these cells.22–27) However, there has been no systematic investigation of the causal relationship between pHi decrease and apoptotic events in cancer cells induced by Phx-3 and Phx-1.
In this manuscript, we first studied the pHi of ten human cancer cell lines derived from different organs and two normal cell lines including human embryonic lung fibroblast cells (HEL) and human umbilical vein endothelial cells (HUVEC) in vitro, in order to see whether pHi, a critical factor in regulating intracellular metabolism including glycolysis, might be elevated in cancer cells, since the Warburg effects could be explained by the elevation of pHi in the cells. Second we examined the effects of the oxidative phenoxazines such as Phx-3 and Phx-1 on pHi change, apoptosis induction, and the viability of these cells, seeking for a plausible mechanism for the anticancer effects of these phenoxazines, and for benevolent anticancer drugs in general.
Materials and methods
Phx-3 and Phx-1.
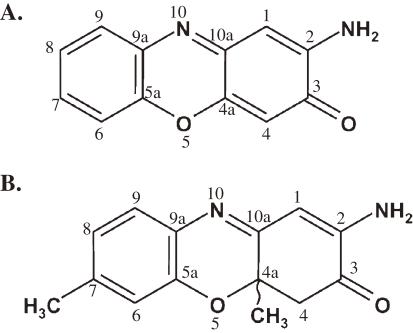
For this study, Phx-3 and Phx-1 were prepared by the reaction of bovine hemoglobin with o-aminophenol and 2-amino-5-methylphenol, respectively, as described by Shimizu et al.20) and Tomoda et al.21) The chemical structure of Phx-3 and Phx-1 is depicted in Fig. 1. Phx-3 and Phx-1 were dissolved in a mixture of dimethylsulfoxide (DMSO) and ethyl alcohol (3:1) as a vehicle to make 20 mM solution.
Figure 1.
Chemical structure of Phx-3 (A) and Phx-1 (B).
Cell line and culture condition.
Human breast cancer cell line MCF-7, human epidermoid carcinoma cell line A431, cisplatin-resistant cell line KCP-4 derived from KB-3-1 human epidermoid cancer cells, human lung adenocarcinoma cell line A549, human pancreatic cancer cell line KLM-1 and MIA PaCa-2, human renal carcinoma cell line ACHN, human colon adenocarcinoma cell line LoVo-1 and human glioblastoma cell line U251MG were cultured in DMEM (Nissui Seiyaku Co., Tokyo) supplemented with 10% fetal calf serum (Equitech-Bio, Kerrville, TX), 2 mM glutamine, and 100 units/ml of penicillin. Human retinoblastoma cell line Y-79 was cultured in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM glutamine, and 100 units/ml of penicillin. Human lung embryonic fibroblast cell line HEL was cultured in FBM supplemented with hFGF-β, insulin, 2% FBS. Human umbilical vein endothelial cells (HUVEC) were cultured in EBM-2 supplemented with hydrocortisone, hFGF-β, VEGF, IGF-1, ascorbic acid, hEGF, heparin and 2% FBS. All the cells were cultured at 37 ℃ in a 5% CO2 humidified atmosphere.
Influence of Phx-1 and Phx-3 on the pHi in a variety of cancer cells and normal cells (HEL and HUVEC).
For a variety of cancer cells (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79) and normal cells (HEL and HUVEC), pHi was determined according to the method described by Litman et al.14) Briefly, the cells (4 × 107/ml) were loaded with a pH-sensitive fluorescent probe BCECF-AM (3 µM) (Dojin Chemical, Kumamoto, Japan) in HEPES buffer (153 mM NaCl, 5 mM KCl, 5 mM glucose, 20 mM HEPES, pH 7.4) at 37 ℃ for 30 min. After being washed once with HEPES buffer, the cells were resuspended in HEPES buffer. The cells (3 × 106) were treated with 0, 5, 10, 20, 50 and 100 µM Phx-3 for 20 min. Fluorescence was measured at an excitation wavelength of 500 nm and an emission wavelength of 530 nm, using a FP750 microplate fluorescence reader (Jasco Co. Ltd., Tokyo). To calibrate fluorescence, BCECF-AM-loaded cells (3 × 106) were suspended in pH 6.6, 7.0, 7.4, 7.8 and 8.2 calibration buffer (130 mM KCl, 10 mM NaCl, 1 mM MgSO4, 10 mM Na-MOPS) and 10 µg/ml nigericin was added to equilibrate the external and internal pH. The relative fluorescence ratio values were plotted against corresponding pHi values, in order to determine the unknown pHi. A linear calibration curve for pHi was obtained (data not shown): the optical density of the solution including BCECF-AM increased linearly with an increase of the pH. Therefore, it was possible to estimate the pHi of cells loaded with BCECF-AM.
Detection of the loss of mitochondrial membrane potential in a variety of cancer cells and normal cells (HEL and HUVEC).
Reduced mitochondrial membrane potential is considered as initial and irreversible step towards apoptosis. Therefore, the loss of mitochondrial membrane potential of ten cancer cells (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79) and two normal cells (HEL and HUVEC) treated with Phx-3 was examined by 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) in order to detect the apoptosis of these cells. The cells (2 × 105) were seeded in 12-well plates and treated with 0, 7 and 14 µM Phx-3 for 3, 6 and 9 h. The cells were then rinsed twice with PBS, then stained with 1 ml RPMI-1640 or DMEM medium containing 5 µmol/L JC-1 (Molecular Probes, USA) for 30 min at 37 ℃. Cells were then rinsed twice with ice-cold PBS, resuspended in 1 ml ice-cooled PBS, and instantly assessed for red and green fluorescence using a Coulter FACSCAN (Becton Dickinson, San Jose, CA). A 488 nm filter was used for the excitation of JC-1. An emission filter of 535 nm was used to quantify the population of mitochondria with green (JC-1 monomers) fluorescence and an emission filter of 595 nm was used to quantify the population of mitochondria with red (JC-1 aggregates) fluorescence. The decrease of orange fluorescence (FL-2) was analyzed to estimate the population of the early apoptotic cells which lost the mitochondrial membrane potentials.
Estimation of viability of a variety of cancer cells and normal cells (HEL and HUVEC) treated with different concentrations of Phx-3 or Phx-1.
Ten different cancer cells (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79) and two normal cells (HEL and HUVEC) (3,000/ml) were incubated with or without various concentrations of Phx-3 or Phx-1 for 72 h in 96-well plates. Next, 3-(4,5-dimethyl-thiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml) was added to each well, and the cells were incubated for an additional 4 h. The MTT formazan precipitate was dissolved in 100 µl of DMSO after removal of the culture medium by aspiration. The plates were shaken for 5 min and read immediately at 570 nm using a model 550 Micro Plate Reader (Bio-Rad, Hercules, CA).
Data analysis.
Student’s t-test was used to compare the values for the population of apoptotic cells. The p-value of <0.01 and <0.001 was considered to be statistically significant (Figs. 3A and 3B). Statistical analysis for Fig. 5 was performed by regression analysis.
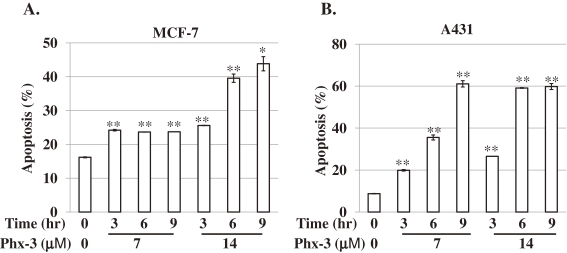
Figure 3.
Proapoptotic effects of Phx-3 on MCF-7 and A431 cells. The effects of different concentrations of Phx-3 (0, 7 and 14 µM) on the apoptosis induction of MCF-7 and A431 cells were examined after 0, 3, 6 and 9 h. The population of apoptotic cells (%) was detected as the loss of mitochondrial membrane potential as described in Materials and methods. *p < 0.01, **p < 0.001.
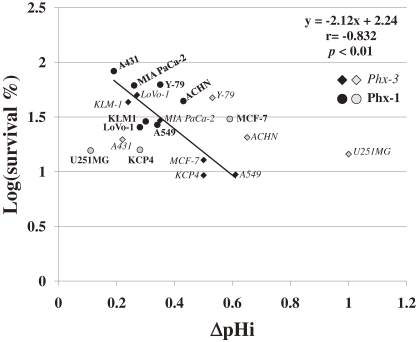
Figure 5.
Logarithmic plot of the survival rate (%) against ΔpHi in ten cancer cells treated with 20 µM Phx-3 or 100 µM Phx-1. The survival rate (%) of ten cancer cell lines (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79) in the presence of 20 µM Phx-3 or 100 µM Phx-1 (data from Figs. 4A and 4B) (expressed logarithmically) was plotted as a function of ΔpHi (data from Tables 1 and 2). The cells treated with Phx-3 were indicated by the italic letters (closed and gray rhombuses). The cells treated with Phx-1 were indicated by the bold letters (closed and gray circles). Closed rhombuses and circles show the values which are close to the equation (y = −2.12x + 2.24, r = −0.832, p < 0.01), and gray rhombuses and circles show those which deviated from the equation.
Results
Effects of Phx-3 or Phx-1 on pHi in human breast cancer cell line MCF-7 and human skin cancer cell line A431.
We found that the pHi in human breast cancer cell line, MCF-7 was as high as 7.62. During 30 min incubation of MCF-7 cells, no changes in pHi were indicated in the cells without treatment with Phx-3 or Phx-1. However, the pHi in the cells decreased from 7.62 to 6.57, within several minutes after the addition of 100 µM Phx-3. When 100 µM Phx-1 was added to MCF-7 cells, pHi (initial pHi = 7.53) was gradually decreased to 6.94 within 30 min (the data not shown).
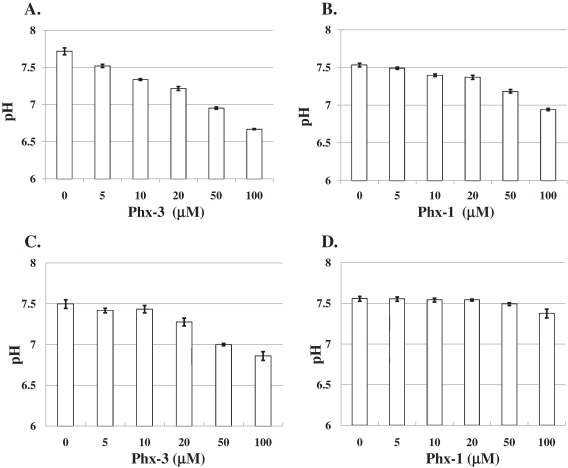
Thus, we studied the changes in the pHi in MCF-7 cells 30 min after the addition of different concentrations (0, 5, 10 20, 50 and 100 µM) of Phx-3 or Phx-1 (Figs. 2A and 2B). Treatment with Phx-3 decreased the pHi in MCF-7 cells in a dose-dependent manner (Fig. 2A). Though the effects of Phx-1 on the decrease of pHi in MCF-7 cells were weaker than those of Phx-3, the decrease of pHi was obviously indicated in the cells with 20 µM or more Phx-1 (Fig. 2B).
Figure 2.
Changes in pHi in MCF-7 and A431 cells treated with different concentrations of Phx-3 or Phx-1. Human breast cancer cell line MCF-7 and human epidermoid carcinoma cell line A431 were incubated with or without different concentrations of Phx-3 or Phx-1 (5, 10, 20, 50, and 100 µM) at 37 ℃ for 30 min, in order to examine changes in pHi in the cells. A. Effects of Phx-3 on pHi in MCF-7 cells; B. Effects of Phx-1 on pHi in MCF-7 cells; C. Effects of Phx-3 on pHi in A431 cells; D. Effects of Phx-1 on pHi in A431 cells.
Furthermore, we examined the effects of different concentrations of Phx-3 or Phx-1 on human skin cancer cell line, A431 (Figs. 2C and 2D). During 30 min incubation of A431 cells with Phx-3, pHi in the cells was dose-dependently decreased by Phx-3 (Fig. 2C). The pHi in A431 cells whose initial pHi was 7.5 remained at 7.5 during 30 min in the absence of Phx-3, but it decreased to 6.86, 30 min after the addition of 100 µM Phx-3. With regard to Phx-1, pHi (initial pHi = 7.56) in A431 cells was not affected by 50 µM Phx-1, but was reduced to 7.37 by adding 100 µM Phx-1 (Fig. 2D), demonstrating that Phx-1 has less capacity of reducing pHi in A431 cells than Phx-3.
Effects of Phx-3 or Phx-1 on pHi in a variety of cancer cell lines and normal cell lines (HEL and HUVEC).
We examined pHi in 10 cancer cell lines (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79) derived from the different organs and two normal cell lines (HEL and HUVEC), in order to see whether higher pHi in cancer cells and pHi decrease caused by Phx-3 and Phx-1 as seen in MCF-7 and A431 cells, might be commonly occurring, and to see the level of pHi in the cultured normal cells, HEL and HUVEC in the presence or absence of these phenoxazines. We therefore examined the initial pHi in these cells without addition of Phx-3 and Phx-1, and then the reduction of pHi in these cells during 30 min incubation with 20 µM or 100 µM Phx-3 or Phx-1 (Tables 1 and 2).
Table 1.
Changes in pHi of various species of cancer cells and normal cells (HEL and HUVEC) caused by 20 and 100 µM Phx-3. The pHi of various species of cancer cells and normal cells including human embryonic lung fibroblast (HEL) and human umbilical vein endothelial cells (HUVEC) was examined at 30 min without the addition of Phx-3, and 30 min after the addition of 20 or 100 µM Phx-3 (Fig. 2A). ΔpHi indicates the difference between pHi at 30 min with and without 20 or 100 µM Phx-3. Value in the table is the means of triplicate trials.
| Phx-3 | 0 µM | 20 µM | 100 µM | ||
|---|---|---|---|---|---|
| pHi | pHi | ΔpHi | pHi | ΔpHi | |
| Cancer cell lines | |||||
| MCF-7 | 7.62 | 7.12 | 0.50 | 6.57 | 1.05 |
| A431 | 7.50 | 7.28 | 0.22 | 6.86 | 0.64 |
| KCP-4 | 7.67 | 7.07 | 0.50 | 6.90 | 0.77 |
| A549 | 7.61 | 7.00 | 0.61 | 6.69 | 0.92 |
| KLM-1 | 7.64 | 7.40 | 0.24 | 6.78 | 0.88 |
| MIA PaCa-2 | 7.29 | 6.94 | 0.35 | 6.30 | 0.99 |
| ACHN | 7.52 | 6.87 | 0.65 | 6.32 | 1.20 |
| LoVo-1 | 7.61 | 7.34 | 0.27 | 6.63 | 0.98 |
| U251MG | 7.46 | 6.46 | 1.00 | 6.43 | 1.03 |
| Y-79 | 7.52 | 6.99 | 0.53 | 6.42 | 1.10 |
| Normal cell lines | |||||
| HEL | 7.32 | 7.03 | 0.29 | 6.46 | 0.86 |
| HUVEC | 7.44 | 7.07 | 0.37 | 6.34 | 1.10 |
Table 2.
Changes in pHi of various species of cancer cells and normal cells (HEL and HUVEC) caused by 20 and 100 µM Phx-1. The pHi of various species of cancer cells and normal cells (HEL and HUVEC) was examined at 30 min without the addition of Phx-1, and 30 min after the addition of 20 or 100 µM Phx-1 (Fig. 2B). ΔpHi indicates the difference between pHi at 30 min with and without 20 or 100 µM Phx-1. Value in the table is the means of triplicate trials.
| Phx-1 | 0 µM | 20 µM | 100 µM | ||
|---|---|---|---|---|---|
| pHi | pHi | ΔpHi | pHi | ΔpHi | |
| Cancer cell lines | |||||
| MCF-7 | 7.53 | 7.37 | 0.16 | 6.94 | 0.59 |
| A431 | 7.56 | 7.54 | 0.02 | 7.37 | 0.19 |
| KCP-4 | 7.72 | 7.64 | 0.08 | 7.44 | 0.28 |
| A549 | 7.61 | 7.52 | 0.09 | 7.33 | 0.28 |
| KLM-1 | 7.57 | 7.42 | 0.15 | 7.23 | 0.34 |
| MIA PaCa-2 | 7.31 | 7.26 | 0.05 | 7.05 | 0.26 |
| ACHN | 7.65 | 7.49 | 0.16 | 7.22 | 0.43 |
| LoVo-1 | 7.56 | 7.44 | 0.12 | 7.26 | 0.30 |
| U251MG | 7.55 | 7.54 | 0.01 | 7.44 | 0.11 |
| Y-79 | 7.46 | 7.34 | 0.12 | 7.11 | 0.35 |
| Normal cell lines | |||||
| HEL | 7.32 | 7.25 | 0.07 | 7.03 | 0.29 |
| HUVEC | 7.44 | 7.41 | 0.03 | 7.15 | 0.29 |
Table 1 summarizes the pHi of these cells before or 30 min after the addition of 20 or 100 µM Phx-3, and ΔpHi (the differences in pHi without and with Phx-3). Results indicate that pHi in all the cancer cells (MCF-7, A431, KCP-4, A549, KLM-1, ACHN, LoVo-1, U251MG and Y-79) except for human pancreatic cancer cell line MIA PaCa-2 (pHi = 7.29), was maintained between 7.46 and 7.67, being extensively higher than that in normal cell lines HEL and HUVEC (7.32 and 7.44) or the hitherto reported pHi for the hematopoietic cells such as peripheral blood mononuclear cells and bone marrow mononuclear cells (pHi ≤ 7.1)28) and erythrocytes8,29) (pHi ≤ 7.2).
Such higher pHi in cancer cells was reduced to the range below 7.0 within 30 min, when these cells were treated with 100 µM Phx-3 (Table 1). The pHi decrease (ΔpHi) ranged from 0.22 to 1.00 and from 0.64 to 1.20 pH units in every cancer cells with 20 µM and 100 µM Phx-3, respectively. When these cells were treated with 20 or 100 µM Phx-1 (Table 2), the extent of pHi decrease was much smaller compared with the case with Phx-3, and the ΔpHi ranged between 0.01 to 0.16 and 0.11 and 0.59 pH units for 20 µM and 100 µM Phx-1, respectively.
With regard to two normal cell lines HEL and HUVEC, Phx-3 and Phx-1 caused significant decrease of pHi, whose extents were comparable to those in cancer cells (Tables 1 and 2).
Proapoptotic effects of Phx-3 or Phx-1 on MCF-7 and A431 cells detected by the loss of mitochondrial membrane potentials.
In order to see whether rapid and drastic changes in pHi in MCF-7 and A431 cells caused by Phx-3 (Figs. 2A and 2C) might be associated with the onset of cellular apoptosis, we studied the effects of different concentrations of Phx-3 (0, 7 and 14 µM) on the mitochondrial membrane potential of these cells during 3, 6 and 9 h incubation at 37 ℃. The population of apoptotic cells detected by the decreased mitochondrial potential was increased as a function of time (3, 6, and 9 h), and dependent on the concentration of Phx-3 (7 and 14 µM) (Fig. 3A). Apoptosis induced by Phx-3 was evident in A431 cells as well (i.e., the population of apoptotic cells increased time- and dose-dependently, after the addition of 7 or 14 µM Phx-3) (Fig. 3B).
Since the initial pHi of MCF-7 (7.62) and that of A431 (7.50) was reduced to 7.12 and 7.28, respectively, during 30 min incubation of these cells with 20 µM Phx-3 (Figs. 2A and 2C), it is likely that the onset of apoptosis of MCF-7 and A431 cells might be preceded by early acidification in these cells that was caused by Phx-3.
Proapoptotic effects of Phx-3 or Phx-1 on a variety of cancer cells and normal cells (HEL and HUVEC) detected by the loss of mitochondrial membrane potentials.
Since we found that Phx-3 exerted significant proapoptotic effects on MCF-7 and A431 cells, we studied whether Phx-3 might cause the apoptosis of a variety of cancer cells (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79) and two normal cells (HEL and HUVEC). Table 3 summarizes the population of apoptotic cells detected by the loss of mitochondrial membrane potential, in these cancer cells and normal cells (HEL and HUVEC) treated with Phx-3 for 9 h. At 7 and 14 µM Phx-3, the population of apoptotic cells significantly increased with time, in cancer cells except for human pancreatic cancer cell lines, KLM-1 and MIA PaCa-2 and human retinoblastoma cell line, Y-79. Negligible increase in the population of apoptotic cells was seen in normal cells (HEL and HUVEC) treated with 7 µM or 14 µM Phx-3 (Table 3).
Table 3.
Effects of different concentrations of Phx-3 on the population (%) of apoptotic cells in various species of cancer cells and normal cells (HEL and HUVEC) evaluated by the decrease of mitochondrial membrane potential. The mitochondrial membrane potential in each cell was measured after 9 h incubation with or without 7 or 14 µM Phx-3.
| Cell lines | population (%) of apoptotic cells | ||
|---|---|---|---|
| Phx-3 (0 µM) | Phx-3 (7 µM) | Phx-3 (14 µM) | |
| Cancer cell lines | |||
| MCF-7 | 16.18 | 23.71 | 43.84 |
| A431 | 8.76 | 61.09 | 59.81 |
| KCP-4 | 16.11 | 65.42 | 69.3 |
| A549 | 13.37 | 90.82 | 96.67 |
| KLM-1 | 7.54 | 8.04 | 10.33 |
| MIA PaCa-2 | 41.13 | 46.57 | 46.09 |
| ACHN | 15.76 | 32.25 | 36.97 |
| LoVo-1 | 19.74 | 36.51 | 38.75 |
| U251MG | 7.38 | 28.92 | 36.10 |
| Y-79 | 4.64 | 4.48 | 5.14 |
| Normal cell lines | |||
| HEL | 8.18 | 7.99 | 8.40 |
| HUVEC | 4.03 | 5.44 | 6.98 |
Cytotoxic effects of Phx-3 and Phx-1 on cancer cells and normal cells in vitro.
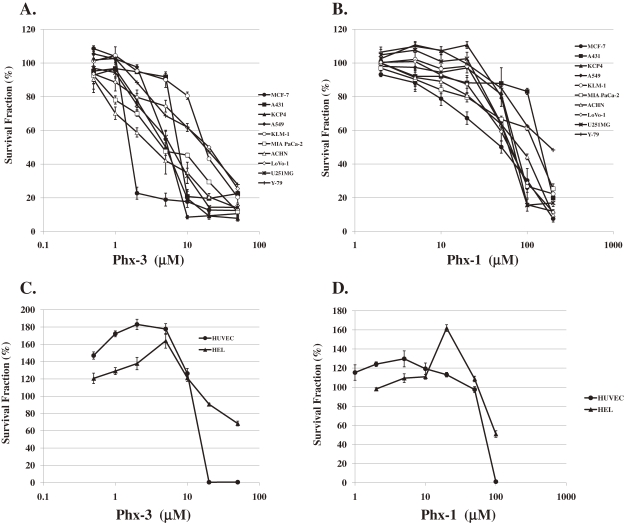
Figure 4A depicts the cytotoxic effects of Phx-3 on ten different cancer cells (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79). All these cancer cells except for KLM-1, LoVo-1 and Y-79 cells were vulnerable to Phx-3 at the concentrations less than 10 µM. The cytotoxic effects of Phx-1 on these cancer cells were much less than those of Phx-3 (Fig. 4B), being comparable to pHi change in these cells treated with Phx-1 and Phx-3 (Tables 1 and 2).
Figure 4.
Cytotoxic effects of Phx-3 and Phx-1 on a variety of cancer cells and normal cells. Ten species of cancer cell lines (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79) and two normal cell lines (HEL and HUVEC) were treated with different concentrations of Phx-3, at 37 ℃ for 24 h. The viability of these cells (%) was depicted as a function of different concentrations of Phx-3 or Phx-1. A: Cytotoxicity of Phx-3 to a variety of cancer cell lines; B: Cytotoxicity of Phx-1 to a variety of cancer cell lines; C: Cytotoxicity of Phx-3 to normal cells (HEL and HUVEC); D: Cytotoxicity of Phx-1 to normal cells (HEL and HUVEC).
With respect to normal cells including HEL and HUVEC, Phx-3 and Phx-1 exerted paradoxical effects on these cells, according to the dose of these phenoxazines (Figs. 4C and 4D). Namely, when these cells were treated with less than 10 µM Phx-3, the number of viable cells was increased to a great extent (maximally 180% for HUVEC, and 160% for HEL), while that of these cells was extensively decreased by 20 µM or more Phx-3 (Fig. 4C). Similar behavior of these cells was indicated when the cells were treated with Phx-1, dependent on the doses, though the cytotoxic effects of Phx-1 were much smaller than that of Phx-3 (Fig. 4D).
From these results, IC50 (the concentration to suppress the viability of the cells by 50%) of Phx-3 and Phx-1 against ten cancer cell lines (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79) and two normal cell lines (HEL and HUVEC) was obtained (Table 4). Though the sensitivity of cancer cells to Phx-3 changed according to cell species, seven cancer cell lines (except for KLM-1, LoVo-1 and Y-79 cells) were sensitive to this phenoxazine compound (IC50: ∼ less than 8 µM). The IC50 of Phx-3 for KLM-1, LoVo-1 and Y-79 cells was 21.5 µM, 20 µM and 18 µM, respectively. The cytotoxic effects of Phx-1 on these cancer cells were much less than those of Phx-3, because IC50 of Phx-1 was more than 48 µM for every cancer cell.
Table 4.
Cytotoxicity effects of Phx-3 and Phx-1 on various cell lines and on normal cells (HEL and HUVEC). Cell survival was determined with an MTT assay of these cells treated with Phx-3 or Phx-1 for 72 h. The IC50 values are the means ± S.D. of triplicates.
| Cell lines | IC50 (µM) | |
|---|---|---|
| Phx-3 | Phx-1 | |
| Cancer cell lines | ||
| MCF-7 | 1.67 ± 0.021 | 48.12 ± 4.14 |
| A431 | 7.94 ± 0.21 | 152.14 ± 5.89 |
| KCP-4 | 5.03 ± 0.21 | 73.06 ± 4.85 |
| A549 | 5.48 ± 0.38 | 78.29 ± 5.11 |
| KLM-1 | 21.50 ± 3.22 | 62.70 ± 3.82 |
| MIA PaCa-2 | 7.16 ± 1.54 | 131.75 ± 4.72 |
| ACHN | 3.58 ± 0.22 | 85.16 ± 4.28 |
| LoVo-1 | 20.03 ± 4.98 | 65.20 ± 0.29 |
| U251MG | 6.34 ± 1.05 | 64.03 ± 3.87 |
| Y-79 | 18.03 ± 1.11 | 187.34 ± 15.38 |
| Normal cell lines | ||
| HEL | >50 | >200 |
| HUVEC | 16.06 ± 0.19 | 74.06 ± 0.86 |
The IC50 of Phx-3 for the normal cells, HEL and HUVEC was >50 µM and 16 µM, respectively, indicating that these cells are less sensitive to Phx-3 than cancer cells. The IC50 of Phx-1 for HEL and HUVEC was >200 µM and 74 µM, respectively, showing less sensitivitiy of these cells to Phx-1.
Discussion
Agents that induce the apoptotic cell death of cancer cells but not of normal cells would be ideal chemotherapeutic drugs for patients with cancer. Though many agents for treating cancer have been developed, they also have extensive adverse effects on normal cells and the human body. Actinomycin D found in streptomyces is the oxidative form of phenoxazine and exhibits strong anticancer activity exclusively against Wilms’s tumor,30) but also has the adverse effects, including the bone marrow suppression. Most phenoxazines, which are chemically synthesized, conform to the reductive form, are hardly soluble in water, and exert little anticancer effect, except for some compounds.31) Phx-3 and Phx-1 are synthesized by the biological reactions of o-aminophenol or 2-amino-5-methylphenol with bovine hemoglobin solution or bovine erythrocytes, conform to the oxidative form, as Actinomycin D, and are relatively water-soluble.20,21,32,33) Phx-3 is identical to questiomycin A that was identified as an antibiotic against Mycobacterium tuberculosis in a streptomyces isolated from the soil in Tokyo in 195934) and has recently been characterized as exhibiting anticancer activity against several cancer cell lines,24,32,33) strong antimicrobial activity against Helicobacter pylori, in vitro,35) anti-angiogenic activity indicated by the inhibition of the expression of angiogenic factors in HUVEC treated with high concentrations of glucose,36) and anti-inflammatory activity.37) Phx-1 has been characterized as exerting antiviral effects against poliovirus and porcine parvovirus,38) and antimicrobial effects against Clamydia pneumoniae,39) along with anticancer activity against several cancer cell lines, with less efficacy than Phx-3,26) but with far higher efficacy when treated with tumor necrosis factor-related apoptosis inducing ligand (TRAIL).40) However, the general availability of Phx-3 and Phx-1 for treating cancer awaits clarification of the detailed mechanism for the anticancer activity of Phx-3 and Phx-1, which has remained unclear. In the present study, we aimed at investigating the pHi-reducing activity of these phenoxazine compounds against ten cancer cell lines derived from various organs, targeting the perturbation of cancer specific metabolism that is characterized by the Warburg effect.
The Warburg effect implies preferential utilization of glycolysis rather than the oxidative phosphorylation even under aerobic conditions, in cancer cells,1,2) and has been recognized as the metabolic fragility distinguishing cancer cells from normal cells, now being targets of cancer chemotherapy.3,4) It is reasonable to suggest that the cellular death of these cells may be induced by reversing the Warburg effect, i.e. by inhibiting of glycolysis. In this context, 2-deoxyglucose has recently attracted much attention. 2-Deoxyglucose inhibits the activity of hexokinase, an initial rate-limiting enzyme of the glycolytic pathway, and thereby reduces intracellular ATP in cancer cells, resulting in the activation of AMP-activated protein kinase, which is involved in the apoptotic mechanism in the cells.3) Recently, it has been shown that a single use of 2-deoxyglucose often stimulates the surviving mechanism including the serine/threonine kinase Akt in cancer cells,3,4) but that the combined application of 2-deoxyglucose with anticancer drugs drastically perturbs the intracellular energy metabolism and inhibits the Akt signaling in cancer cells, providing useful therapeutics for treating cancer in mice.3,4) However, it is possible to suppress glycolysis in cancer cells without use of 2-deoxyglucose by reducing pHi in cancer cells. The glycolytic pathway is extensively suppressed by lowering pHi in cancer cells, due to suppression of the activity of the rate-limiting enzymes of the glycolytic pathway, specifically hexokinase, pyruvate kinase, and exclusively phosphofructokinase,41) as the glycolytic activity at pHi 6.6 is suppressed by 75% to 80% from that at pHi 7.4 in human erythrocytes.29) Such a drastic decrease of glycolytic pathway in cancer cells may affect the pentose cycle, which is directly connected with the glycolytic pathway and is necessary for the producing nucleic acids in the cells. Therefore, lowering of pHi may reverse the Warburg effect and thereby induce apoptosis in cancer cells.
It is easy to decrease pHi in normal cells by lowering the pHe. However, it is very difficult to decrease pHi in cancer cells by such a change in pHe because of the action of a proton pump (Na+/H+ exchanger isoform 1: NHE1) in the plasma membranes of cancer cells.11,12) In order to overcome the difficulty of decreasing pHi in cancer cells, NHE1 specific inhibitors, amiloride and 5-(N,N-hexamethylene)-amiloride (HMA) have been experimentally used. Kim and Lee42) demonstrated that the addition of amiloride alone did not significantly decrease pHi in Hela cells, but that the combined use of amiloride and TRAIL caused a significant decrease of pHi (0.2 pH units) and apoptotic cell death in the cells. Furthermore, Rich et al.28) found that HMA, a derivative of amiloride with much stronger anti-NHE1 activity, induced apoptosis in primary leukemic cells obtained from patients with acute lymphocyte leukemia, causing a significant decrease of pHi in the cells after five hours. These results suggest that the agents used extensively to lower pHi may be effective in causing profound apoptotic cell death of cancer cells.
We found in the present study that pHi in nine of ten different species of cancer cell lines (MCF-7, A431, KCP-4, A549, KLM-1, ACHN, LoVo-1, U251MG and Y-79) (except for MIA PaCa-2 cells) was significantly elevated (Table 1) compared with the extracellular medium (pHe = 7.4), pHi in normal cells such as HEL (7.32) and HUVEC (7.44) (Table 1), human peripheral blood mononuclear cells (pHi ≤7.1),28) bone marrow mononuclear cells (pHi ≤7.1)28) and human erythrocytes (pH ≤7.2),8) being consistent with the results described in several reports.14,17) Such higher levels of pHi in cancer cells could be achieved by NHE1 of the plasma membrane of cancer cells, as suggested in many reports.11,12) Elevation of pHi in these cancer cells may be advantageous for the proliferative stimuli and oncogene transformation18,19) as well. The microenvironment of some regions of solid cancers is often 0.5 pH units lower than the normal tissues, while pHi in the solid tumors is maintained at or close to normal levels.5,6) The present results that pHi in a variety of cancer cell lines is significantly higher than the extracellular pH of 7.4, are very consistent with these in vivo results.5,6) Therefore, if we take account the fact that the activity of the glycolytic pathway is markedly accelerated at alkaline pH in both normal and cancer cells,29,43) due to the activation of phosphofructokinase, a key enzyme of the glycolytic pathway,44) the Warburg effect, though still considered enigmatic,2) may be explained simply by the elevation of pHi in cancer cells.
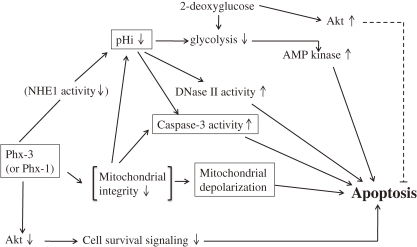
Then, it is conceivable that if the Warburg effect were reversed by lowering pHi of the cancer cells, these cells may be obliged to kill themselves through the apoptotic mechanism. Considering this view, we examined the effects of the oxidative phenoxazines, Phx-3 and Phx-1 on the reduction of pHi and apoptotic events in various cancer cells. We found that pHi in ten different species of cancer cell lines (MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79) decreased rapidly and dose-dependently, when these cells were treated with Phx-3 (Figs. 2A and 2C, Table 1) or Phx-1 (Figs. 2B and 2D, Table 2). In particular, 100 µM Phx-3 decreased pHi by 0.6 pH units or more in these cancer cells (Table 1), while 100 µM Phx-1 decreased it by 0.1 pHi units or more (Table 2). The drastic decrease in pHi occurred within several minutes and continued for more than several hours (data not shown), which would possibly lead to extensive suppression of glycolysis accompanying a reversal of the Warburg effect in cancer cells, effectively promoting apoptotic events that occur after a time lag of several hours, as seen in Table 3. Therefore, a plausible and comprehensive mechanism for the anticancer effects of Phx-3 and Phx-1 on cancer cells including the reversal of the Warburg effect was proposed in Scheme 1.
Scheme 1.
A plausible mechanism for inducing apoptosis in a variety of cancer cells caused with Phx-3 or Phx-1.
The pHi decrease in these cancer cells caused by Phx-3 or Phx-1 may be primarily attributed to the inhibition of NHE1 in the plasma membrane of these cells, as has been suggested for various anticancer drugs,45,46) and was indicated by Nagata et al.33) that Phx-3 inhibited NHE1 in human gastric cell lines, MNK 45 and MNK74 cells, inducing a rapid and drastic decrease of pHi in the cells. However, the possibility that the decrease of pHi was caused by the mitochondrial perturbation in these cancer cells with Phx-3 or Phx-1 cannot be ruled out, judging from the indication of Matsuyama et al.47,48) that the intracellular acidification occurs rapidly after the administration of mitochondria-dependent stimuli including staurosporine and ultraviolet irradiation to cancer cells, followed by release of cytochrome c, activation of caspases and mitochondrial swelling and depolarization, and the recent reports24,26) that Phx-3 and Phx-1 causes the apoptosis of cancer cells accompanying the activation of caspase-3.
The extensive decrease of pHi in cancer cells may induce various perturbation of intracellular homeostasis, in addition of suppression of the glycolytic metabolism, promoting proapoptotic signaling, in these cells. Matsuyama et al.47) and Lagadic-Gossmann et al.49) indicated that the decrease of pHi would cause the activation of caspase-3, a crucial executing enzyme of the apoptosis and finally induce the apoptotic cell death of cancer cells. On the other hand, Barry et al.16) indicated that DNase II, a pH-dependent endonuclease which is responsible for the DNA fragmentation, may act at lower pH and therefore may be associated with the apoptosis in the cells. Furthermore, Pérez-Sala et al.18) demonstrated that when HL-60 cells (a human leukemia cell line) were treated with either ionomycin or lovastatin, cellular apoptosis was induced due to intracellular acidification and activation of DNase II, although the extent of pHi decrease was 0.2 to 0.3 pH units. Therefore, the activation of caspase-3 and DNase II may be involved in the apoptotic cell death in the Phx-3- or Phx-1-treated cancer cells, in which pHi is extremely reduced. These views were supported by our finding that the apoptotic cell death was apparently caused in different species of cancer cells, 9 h after the addition of Phx-3, as detected by the loss of mitochondrial membrane potential (Table 3, Figs. 3A and 3B).
Depolarization of the mitochondria has been known as an eminent change at a final stage of apoptotic events in cancer cells.47,48) Our present findings in Table 3 indicated that the depolarization of the mitochondria was extensively caused in seven of ten different cancer cells (except for human pancreatic cancer cell lines, KLM-1 and MIA PaCa-2, and human retinoblastoma cell line, Y-79 cells), 9 h after the addition of 7 or 14 µM Phx-3. These results demonstrate that Phx-3 has a strong ability to depolarize the mitochondria in cancer cells, consistent with recent reports that Phx-3 is involved in depolarization of the mitochondria in multiple myeloma cell line U266 cells.50,51) Currently, it is unclear why Phx-3 had less effect on the depolarization of mitochondria in KLM-1, MIA PaCa-2 cells and Y-79 cells.
In order to quantitatively estimate the effects of a pHi decrease caused by Phx-3 or Phx-1 on the viability of ten cancer cell lines, we compared the cause and effect relationship between these factors based on the results in Table 1, Table 2, Fig. 4A and Fig. 4B. Figure 5 shows a logarithmic plot of the survival rate (%) as a function of ΔpHi in ten cancer cell lines with 20 µM Phx-3 or 100 µM Phx-1. It was found that suppression of the survival rate was closely related with the magnitude of ΔpHi with regard to the effects of 20 µM Phx-3 and 100 µM Phx-1 on four of ten cancer cell lines (A549, KLM-1, MIA PaCa-2, and LoVo-1 cells), with regard to the effects of 20 µM Phx-3 on two cancer cell lines (MCF-7 and KCP-4 cells), and with regard to the effect of Phx-1 on three cancer cell lines (A431, Y-79 and ACHN cells) (y = −2.12x + 2.24, r = −0.832, p < 0.01). Only U251MG cells deviated from this equation. Therefore it may be possible to say that the cytotoxic effects of Phx-3 may primarily be attributed to the magnitude of ΔpHi in cancer cells with these phenoxazines.
Serine/threonine kinase Akt has been recognized to mediate a variety of survival signaling, participating in growth factor maintenance of cell survival and preventing cancer cells from becoming apoptotic.52,53) Therefore, it will be important to suppress the Akt signaling to prevent the survival of cancer cells. Enoki et al.54) initially reported that Phx-1, a phenoxazine compound, has significant activity in suppressing the phosphorylation of Akt in rat basophilic leukemia RBL-2H3 cells. Hara et al.40) demonstrated that Phx-1 has the effect of inhibiting the proliferation and serum-induced phosphorylation of Akt in Jurkat cells, a human T cell leukemic cell line. Thimmaiah et al.55) examined in detail the effect of various kind of synthetic phenoxazines and demonstrated that N10-substituted phenoxazines such as 10-[4′-(N-diethylamino)butyl]-2-chlorophenoxazine and 10-[4′-[(β-hydroxyethyl)piperazino]butyl]-2-chlorophenoxazine, strongly inhibited Akt phosphorylation. Thus, we examined whether or not Phx-3 inhibits Akt in human lung adenocarcinoma cell line A549 cells. It was found that Phx-3 inhibited the phosphorylation of Akt with time in the cells (data not shown), suggesting that Phx-3 strongly suppresses Akt signaling in cancer cells. We are now preparing to investigate whether or not the inhibition of Akt signaling caused by Phx-3 commonly occurs in many cancer cells. Taken together, it is quite possible that Phx-3 and Phx-1 are involved in inhibiting Akt signaling in cancer cells, and contribute to inducing apoptosis in cancer cells, as summarized in Scheme 1. These phenoxazines seem to exert anti-cancer activity against cancer cells, manifesting a decrease of pHi, reversal of the Warburg effect (= inhibition of glycolysis), activation of DNase II (pH-dependent endonuclease) and caspase-3, promotion of mitochondrial depolarization, and inhibition of Akt phosphorylation associated with the cell survival.
According to Hendrich et al.,56) phenoxazine molecules are located close to the polar/apolar interface of lipid bilayers and weakly interact with lipid bilayers, altering the lipid phase properties of the cellular and mitochondrial membranes. Thus, it is probable that NHE1, which is located in the cellular membrane and regulates the discharge of hydrogen ion from the cytozol to the outside,11,12) may be affected by Phx-3 or Phx-1 penetrating into the lipid bilayers of the cellular membrane of cancer cells, possibly causing the inhibition of NHE1 activity. The integrity of the mitochondria may be also disturbed by the penetration of phenoxazine molecules, resulting in the depolarization of the mitochondria. The present results in Table 3 are in good accordance with this view. Recently, Zheng et al.27) demonstrated that Phx-3 has higher affinity to the mitochondria in human lung adenocarcinoma cell line A549 cells and depolarizes the mitochondria in cells, consistent with the above-stated report of Hendrich et al.56)
Differences between Phx-3 and Phx-1 in the effectiveness to cancer cells may be related with differences in the chemical structures. For example, 2,7-diamino-3,8-dimethylphenazine, a derivative of phenazine with a tricyclic structure similar to phenoxazine (Fig. 1), exerts mutagenic effect.57) However, vitamin B2, a derivative of phenazine which carries methyl group at position 7 and 8 of the tricyclic structure is not toxic to the human body. This suggests that methyl group at the position 7 and 8 of the tricyclic structure may to some degree buffer the original toxic action of phenazine. This may explain why Phx-1, which has one methyl group at the position 7 of the tricyclic strucuture (Fig. 1), exerts far milder effect than Phx-3 in terms of cytotoxic effects against cancer cells (IC50 in Table 4), though the present view is still speculative. Cinnabarinic acid, another oxidative phenoxazine produced by the reaction of 3-hydroxyanthranilic acid with human hemoglobin,58) has carboxyl group at the position 1 and 9 of the tricyclic structure, and has been shown to be carcinogenic.59) Actinomycin D contains the phenoxazine structure, in which methyl group is present at the position 4 and 6, and has strong adverse effects to the human body.60) We found that a derivative of Phx-3 with methyl group at the position 4 and 6 of the tricyclic structure, caused fetal death when administered to mice (our unpublished data). Therefore, the relationship between chemical structure and bioactivity of these tricyclic chromophores may become an interesting theme in the future.
It will be noteworthy that despite the significant decrease of pHi in a human embryonic lung fibroblast HEL and human umbilical vein endothelial cells (HUVEC) in the presence of 20 or 100 µM Phx-3 (Table 1), the depolarization of the mitochondria was not seen in these normal cells, 9 h after the addition of this phenoxazine (Table 3). This result suggests that the decrease in pHi is fatal to cancer cells, but not to normal cells, and some protective mechanism may be operating in normal cells such as HEL and HUVEC. Our present results are consistent with the indication of Rich et al.28) that HMA, a strong inhibitor of NHE1, is more effective to leukemic cells with higher pHi, but less effective to normal hematopoietic cells with relatively lower pHi, in terms of cytotoxicity. These findings may be related to the least adverse effects of Phx-3 to mice. Namely, Miyano-Kurosaki et al.24) reported that the bone marrow suppression and bodyweight loss were not indicated, when Phx-3 (0.5 mg/kg), which exerted strong anticancer effects on mouse melanoma cells, was administered to mice, while Mori et al.61) reported that 5-fluorouracil, a potential anticancer drug, exerts severe adverse effects including decrease of leukocyte counts and bodyweight loss at the dose of 5 mg/kg to mice. Kohno et al.37) recently reported that no bodyweight loss and gastro-intestinal injury were observed, when 500 mg to 1500 mg/kg Phx-3 was orally administered to mice. In addition, at lower concentrations of Phx-3 less than 10 µM, the number of viable cells increased to a great extent in normal cell lines HEL (160% increase) and HUVEC (180% increase) (Fig. 4C), suggesting that Phx-3 may activate normal cells at lower concentrations. The explanation for this paradoxical effects of Phx-3 is a topic for further investigation. Concerning the toxicity of Phx-1, it has been reported by Mori et al.61) and Shimamoto et al.22) that Phx-1 does not exert significant adverse effects including the bodyweight loss, piloerection, or decrease of leukocyte counts in mice.
In conclusion, the drugs that cause drastic acidification of cancer cells, reverse the Warburg effect, depolarize the mitochondria, suppress Akt signaling and thereby induce apoptosis of these cells are anticipated to be benevolent therapeutics for cancer.3,10,62) Phx-3 and Phx-1 are suitable for this purpose because they can cause extensively decrease pHi (more than 0.6 pH units in Phx-3 and more than 0.1 pH units in Phx-1), induce apoptosis and exert cytotoxic effects in the cancer cells, exerting least adverse effects.
Acknowledgments
The present study was supported by funds from the High-Tech Research Project for Private Universities and a matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology, Japan (2008–2013), and by Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (2009–2010) (No. 21790324).
References
- 1).Warburg O. (1956) On the origin of cancer cells. Science 24, 309–314 [DOI] [PubMed] [Google Scholar]
- 2).Hsu P.P., Sabatini D.M. (2008) Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707 [DOI] [PubMed] [Google Scholar]
- 3).Zhon D., Xiong L., Liu T., Liu X., Liu X., Chen J., Sun S.-Y., Khuri F.R., Zong Y., Zhou Q., Zhou W. (2009) The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGFlR. J. Biol. Chem. 284, 23225–23233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Shara I.B., Tanti J.-F., Bust F. (2010) The combination of metformin and 2-deoxyglucose inhibits autophagy and induces AMPK-dependent apoptosis in prostate cancer cells. Autophagy 6, 670–671 [DOI] [PubMed] [Google Scholar]
- 5).Izumi H., Torigoe T., Ishiguchi H., Uramoto H., Yoshida Y., Tanabe M., Ise T., Murakami T., Yoshida T., Nomoto M., Kohno K. (2003) Cellular pH regulators: potentially promising molecular target for cancer chemotherapy. Cancer Treat. Rev. 29, 541–549 [DOI] [PubMed] [Google Scholar]
- 6).Vaupel P., Kallinowski F., Okunieff P. (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 49, 6449–6465 [PubMed] [Google Scholar]
- 7).Duhm J. (1971) Effects of 2,3-diphosphoglycerate and other organic phosphate compounds on oxygen affinity and intracellular pH of human erythrocytes. Pflugers Arch. 326, 341–356 [DOI] [PubMed] [Google Scholar]
- 8).Minakami, S., Tomoda, A. and Tsuda, S. (1975) Effect of intracellular pH (pHi) change on red cell glycolysis. In Erythrocyte Structure and Function (ed. Brewers, G.). Alan R. Liss Inc., New York, pp. 149–162. [Google Scholar]
- 9).Yamagata M., Tannock I.F. (1996) The chronic administration of drugs that inhibit the regulation of intracellular pH: in vitro and anti-tumor effects. Br. J. Cancer 73, 1328–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Wahl M.L., Owen J.A., Burd R., Herlands R.A., Nogami S.S., Rodeck U., Berd D., Leeper D.B., Owen C.S. (2002) Regulation of intracellular pH in human melanoma: potential therapeutic implications. Mol. Cancer Ther. 1, 617–628 [PubMed] [Google Scholar]
- 11).Tse C.M., Levine S.A., Yun C.H.C., Nath S., Pouyssegur J., Donowitz M. (1994) Molecular properties, kinetics and regulation of mammalian Na+/H+ exchangers. Cell. Physiol. Biochem. 4, 2282–2300 [Google Scholar]
- 12).Putney L.K., Denker S.P., Barber B.L. (2002) The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu. Rev. Pharmacol. Toxicol. 42, 527–552 [DOI] [PubMed] [Google Scholar]
- 13).Shrode L.D., Tapper H., Grinstein S. (1997) Role of intracellular pH in proliferation, transformation and apoptosis. J. Bioenerg. Biomembr. 29, 393–399 [DOI] [PubMed] [Google Scholar]
- 14).Litman T., Pedersen S.F., Karamhoft B., Skovsgaad T., Hoffmann E.K. (1998) pH regulation in sensitive multidrug resistant Ehrlich ascites tumor cells. Cell. Physiol. Biochem. 8, 137–150 [DOI] [PubMed] [Google Scholar]
- 15).Goossens J.F., Henichrt J.P., Dassonneville L., Facompre M., Bailly C. (2000) Relation between intracellular acidification and camptothecin-induced apoptosis in leukemia cells. Eur. J. Pharm. Sci. 10, 125–131 [DOI] [PubMed] [Google Scholar]
- 16).Barry M.A., Reynolds J.E., Eastman A. (1993) Etoposide-induced apoptosis in human HL-60 cells is associated with intracellular acidification. Cancer Res. 53, 2349–2357 [PubMed] [Google Scholar]
- 17).Che X.F., Akiyama S., Tomoda A. (2008) Suppression of the proliferation of cancer cell lines, KB-3-1 and K562 cells preceded by a decrease in intracellular pH caused by phenoxazine derivatives. Oncol. Rep. 19, 1253–1258 [PubMed] [Google Scholar]
- 18).Pérez-Sala D., Collado-Escobar D., Mollinedo F. (1995) Intracellular alkalinization suppresses lovastatin-induced apoptosis in HL-60 cells through the inactivation of a pH-dependent endonuclease. J. Biol. Chem. 270, 6235–6242 [DOI] [PubMed] [Google Scholar]
- 19).Gillies, R.J. (1981) Intracellular pH and growth control in eukaryotic cells. In The Transformed Cell (ed. Cameron, I.L.). Academic Press, New York, pp. 348–382. [Google Scholar]
- 20).Shimizu S., Suzuki M., Tomoda A., Arai S., Taguchi H., Hanawa T., Kamiya S. (2004) Phenoxazine compounds produced by the reactions with bovine hemoglobin show antimicrobial activity against non-tuberculosis mycobacteria. Tohoku J. Exp. Med. 203, 47–52 [DOI] [PubMed] [Google Scholar]
- 21).Tomoda A., Arai S., Ishida R., Shimamoto T., Ohyashiki K. (2001) An improved method for the rapid preparation of 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one, a novel antitumor agent. Bioorg. Med. Chem. Lett. 11, 1057–1058 [DOI] [PubMed] [Google Scholar]
- 22).Shimamoto T., Tomoda A., Ishida R., Ohyashiki K. (2001) Antitumor effects of a novel phenoxazine derivative on human leukemia cell lines in vitro and in vivo. Clin. Cancer Res. 704, 704–708 [PubMed] [Google Scholar]
- 23).Koshibu-Koizumi J., Akazawa M., Iwamoto T., Takasaki M., Mizuno F., Kobayashi R., Abe A., Tomoda A., Hamatake M., Ishida R. (2002) Antitumor activity of a phenoxazine compound, 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one against human B cell and T cell lymphoblastoid cell lines: induction of mixed types of cell death, apoptosis and necrosis. J. Cancer Res. Clin. Oncol. 128, 363–368 [DOI] [PubMed] [Google Scholar]
- 24).Miyano-Kurosaki N., Kurosaki K., Hayashi M., Takaku H., Hayafune M., Shirato K., Kasuga T., Endo T., Tomoda A. (2006) 2-Aminophenoxazine-3-one suppresses the growth of mouse malignant melanoma B16 cells transplanted into C57BL/6Cr Slc mice. Biol. Pharm. Bull. 29, 2197–2201 [DOI] [PubMed] [Google Scholar]
- 25).Shirato K., Imaizumi K., Abe A., Tomoda A. (2007) Phenoxazine derivatives induce caspase-independent cell death in human glioblastoma cell lines, A-172 and U-231 MG. Oncol. Rep. 17, 201–208 [PubMed] [Google Scholar]
- 26).Miyano-Kurosaki N., Ikegami K., Kurosaki K., Endo T., Aoyagi H., Hanami M., Yasumoto J., Tomoda A. (2009) Anticancer effects of phenoxazine derivatives revealed by inhibition of cell growth and viability, disregulation of cell cycle, and apoptosis induction in HTLV-1-positive leukemia cells. J. Pharmacol. Sci. 110, 87–97 [DOI] [PubMed] [Google Scholar]
- 27).Zheng C.L., Che X.F., Akiyama S., Miyazawa K., Tomoda A. (2010) 2-Aminophenoxazine-3-one induces cellular apoptosis by causing rapid intracellular acidification and generating reactive oxygen species in human lung adenocarcinoma cells. Int. J. Oncol. 36, 641–650 [DOI] [PubMed] [Google Scholar]
- 28).Rich I.N., Worthington-White D., Garden O.A., Musk P. (2000) Apoptosis of leukemic cells accompanies reduction in intracellular pH after targeted inhibition of the Na+/H+ exchanger. Blood 95, 1427–1434 [PubMed] [Google Scholar]
- 29).Tomoda A., Tsuda-Hirota S., Minakami S. (1977) Glycolysis of red cell suspended in solutions of impermeable solutes: Intracellular pH and glycolysis. J. Biochem. 81, 697–701 [DOI] [PubMed] [Google Scholar]
- 30).Ward H.W.C. (1973) Actinomycin D for Wilms’s tumor. Br. Med. J. 2, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Motohashi N., Mitscher L.A., Meyer R. (1991) Potential antitumor phenoxazines. Med. Res. Rev. 11, 239–294 [DOI] [PubMed] [Google Scholar]
- 32).Nakachi T., Tabuchi T., Takasaki A., Arai S., Miyazawa K., Tomoda A. (2010) Anticancer activity of phenoxazines produced by bovine erythrocytes on colon cancer cells. Oncol. Rep. 23, 1517–1522 [DOI] [PubMed] [Google Scholar]
- 33).Nagata H., Che X.-F., Miyazawa K., Tomoda A., Konishi M., Ubukata H., Tabuchi T. (2011) Rapid decrease of intracellular pH associated with inhibition of Na+/H+ exchanger precedes apoptotic events in the MNK and MNK74 gastric cancer cell lines treated with 2-aminophenoxazine-3-one. Oncol. Rep. 25, 341–346 [DOI] [PubMed] [Google Scholar]
- 34).Anzai K., Isono K., Okuma K., Suzuki S. (1959) The new antibiotics, questiomycins A and B. J. Antibiotics Ser. A 13, 125–132 [PubMed] [Google Scholar]
- 35).Hanawa T., Osaki T., Manzoku T., Fukuda M., Kawakami H., Tomoda A., Kamiya S. (2010) In vitro antibacterial activity of Phx-3 against Helicobacter pylori. Biol. Pharm. Bull. 33, 188–191 [DOI] [PubMed] [Google Scholar]
- 36).Fukuda G., Yoshitake N., Khan Z.A., Kanazawa M., Notoya Y., Che X.-F., Akiyama S., Tomoda A., Chakrabarti S., Odawara M. (2005) 2-Aminophenoxazine-3-one attenuates glucose-induced augmentation of embryonic form of myosin heavy chain, endothelin-1 and plasminogen activator inhibitor-1 in human umbilical vein endothelial cells. Biol. Pharm. Bull. 28, 797–801 [DOI] [PubMed] [Google Scholar]
- 37).Kohno K., Miyake M., Sano O., Tanaka-Kataoka M., Yamamoto S., Koya-Miyata S., Arai N., Fujii M., Watanabe H., Ushio S., Iwaki K., Fukuda S. (2008) Anti-inflammatory and immunomodulatory properties of 2-amino-3H-phenoxazin-3-one. Biol. Pharm. Bull. 31, 1938–1945 [DOI] [PubMed] [Google Scholar]
- 38).Iwata A., Yamaguchi T., Sato K., Yoshitake N., Tomoda A. (2005) Suppression of proliferation of poliovirus and porcine parvovirus by novel phenoxazines, 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one and 3-amino-1,4α-dihydro-4α,8-dimethyl-2H-phenoxazine-2-one. Biol. Pharm. Bull. 28, 9905–9907 [DOI] [PubMed] [Google Scholar]
- 39).Uruma T., Yamaguchi H., Fukuda M., Kawakami H., Goto H., Kishimoto T., Yamamoto Y., Tomoda A., Kamiya S. (2005) Clamydia pneumoniae growth inhibition in human monocytic THP-1 cells and human epithelial HEp-2 cells by a novel phenoxazine derivative. J. Med. Microbiol. 54, 1143–1149 [DOI] [PubMed] [Google Scholar]
- 40).Hara K., Okamoto M., Aki T., Yagita H., Tanaka H., Mizukami Y., Nakamura H., Tomoda A., Hamasaki N., Kang D. (2005) Synergistic enhancement of TRAIL- and tumor necrosis factor α-induced cell death by a phenoxazine derivative. Mol. Cancer Ther. 4, 11121–11127 [DOI] [PubMed] [Google Scholar]
- 41).Minakami S., Yoshikawa H. (1966) The effect of active cation transport, pH and inorganic phosphate concentration on erythrocyte glycolysis. J. Biochem. 59, 145–150 [DOI] [PubMed] [Google Scholar]
- 42).Kim K.M., Lee Y.J. (2005) Role of HER-2/neu signaling in sensitivity to tumor necrosis factor-related apoptosis-inducing ligand: Enhancement of TRAIL-mediated apoptosis by amiloride. J. Cell. Biochem. 96, 376–389 [DOI] [PubMed] [Google Scholar]
- 43).Wenner, C. (1975) Regulation of energy metabolism in normal and tumor tissues. In Cancer: A Comprehensive Treatise (ed. Becker, F.F.). Plenum, New York, pp. 389–403. [Google Scholar]
- 44).Ui M. (1966) A role of phosphofructokinase in pH dependent regulation of glycolysis. Biochim. Biophys. Acta 124, 310–322 [DOI] [PubMed] [Google Scholar]
- 45).Maidorn R.P., Cragoe E.J., Tannock I.F. (1993) Therapeutic intracellular pH as a possible mechanism of tumor selective therapy. Br. J. Cancer 67, 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Reshkin S.J., Bellizi A., Cardone R.A., Tommashino M., Casavola V., Paradiso A. (2003) Paclitaxel induces apoptosis via protein kinase A- and p38 mitogen-activated protein-dependent inhibition of the Na+/H+ exchanger (NHE) NHE isoform 1 in human breast cancer cells. Clin. Cancer Res. 9, 2366–2373 [PubMed] [Google Scholar]
- 47).Matsuyama S., Reed J.C. (2000) Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 7, 1155–1165 [DOI] [PubMed] [Google Scholar]
- 48).Matsuyama S., Liopis J., Deveraux Q.L., Tsien R.Y., Reed J.C. (2000) Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2, 318–325 [DOI] [PubMed] [Google Scholar]
- 49).Lagadic-Gossmann D., Hue L., Lecureur V. (2004) Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 11, 953–961 [DOI] [PubMed] [Google Scholar]
- 50).Shirato K., Imaizumi K., Miyazawa K., Takasaki A., Mizuguchi J., Che X.-F., Akiyama S., Tomoda A. (2008) Apoptosis induction preceded by mitochondrial depolarization in multiple myeloma cell line U266 by 2-aminophenoxazine-3-one. Biol. Pharm. Bull. 31, 62–67 [DOI] [PubMed] [Google Scholar]
- 51).Takasaki A., Hanyu H., Iwamoto T., Shirato K., Izumi R., Toyota H., Mizuguchi J., Miyazawa K., Tomoda A. (2009) Mitochondrial depolarization and apoptosis associated with sustained activation of c-jun-N-terminal kinase in the human multiple myeloma cell line U266 induced by 2-aminophenoxazine-3-one. Mol. Med. Report. 2, 199–203 [DOI] [PubMed] [Google Scholar]
- 52).Kennedy S.G., Wagner A.J., Conzen S.D., Jordan J., Bellacusa A., Tsichlis P.N., Hay N. (1997) The PI3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 11, 7701–7713 [DOI] [PubMed] [Google Scholar]
- 53).Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. (1999) Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96, 857–868 [DOI] [PubMed] [Google Scholar]
- 54).Enoki E., Sada K., Qu X., Kyo S., Miah S.M.S., Hanani T., Tomoda A., Yamamura H. (2004) The phenoxazine derivative Phx-1 suppresses IgE-mediated degranulation in rat basophilic leukemia RBL-2H3 cells. J. Pharmacol. Sci. 94, 329–333 [DOI] [PubMed] [Google Scholar]
- 55).Thimmaiah K.N., Easton J.B., Germain G.S., Morton C.L., Kamath S., Buolamwinini J.K., Houghton P.J. (2005) Identification of N10-substituted phenoxazines as potten and specific inhibitors of Akt signaling. J. Biol. Chem. 280, 31924–31935 [DOI] [PubMed] [Google Scholar]
- 56).Hendrich A.B., Stanczak K., Komorowska M., Motohashi N., Kawase M., Michalak K. (2006) A study on the perturbation of model lipid membranes by phenoxazines. Bioorg. Med. Chem. 14, 5948–5954 [DOI] [PubMed] [Google Scholar]
- 57).Watanabe T., Ono M., Hirayama T., Fukui S. (1987) 2,7-Diamino-3,8-dimethylphenazine as the major mutagenic product from the reaction of 2,4-diaminotoluene with hydrogen peroxide. Mutat. Res. 190, 113–117 [DOI] [PubMed] [Google Scholar]
- 58).Tomoda A., Shirasawa E., Nagano S., Minami M., Yoneyama Y. (1984) Involvement of oxidoreactive reactions of intracellular haemoglobin in the metabolism of 3-hydroxyanthranilic acid in human erythrocytes. Biochem. J. 222, 755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Dykens J., Sullivan S.G., Stern A. (1987) Oxidative reactivity of the tryptophan metabolites 3-hydroxyanthranilate, cinnabarinate, quinolinate and picolinate. Biochem. Pharmacol. 36, 211–217 [DOI] [PubMed] [Google Scholar]
- 60).Hollstein U. (1974) Actinomycin. Chemistry and mechanism of action. Chem. Rev. 74, 625–652 [Google Scholar]
- 61).Mori H., Honda K., Ishida R., Nohira T., Tomoda A. (2000) Antitumor activity of 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one against Meth A tumor transplanted into BALB/c mice. Anticancer Drugs 11, 653–657 [DOI] [PubMed] [Google Scholar]
- 62).Tannock I.F., Rotin D. (1989) Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 49, 4373–4384 [PubMed] [Google Scholar]