Abstract
Since I was involved in the molecular cloning of GM3 synthase (SAT-I), which is the primary enzyme for the biosynthesis of gangliosides in 1998, my research group has been concentrating on our efforts to explore the physiological and pathological implications of gangliosides especially for GM3. During the course of study, we demonstrated the molecular pathogenesis of type 2 diabetes and insulin resistance focusing on the interaction between insulin receptor and gangliosides in membrane microdomains and propose a new concept: Life style-related diseases, such as type 2 diabetes, are a membrane microdomain disorder caused by aberrant expression of gangliosides. We also encountered an another interesting aspect indicating the indispensable role of gangliosides in auditory system. After careful behavioral examinations of SAT-I knockout mice, their hearing ability was seriously impaired with selective degeneration of the stereocilia of hair cells in the organ of Corti. This is the first observation demonstrating a direct link between gangliosides and hearing functions.
Keywords: GM3 ganglioside, GM3 synthase (SAT-I), insulin resistance, membrane microdomains (lipid rafts), auditory system, hearing loss
Introduction
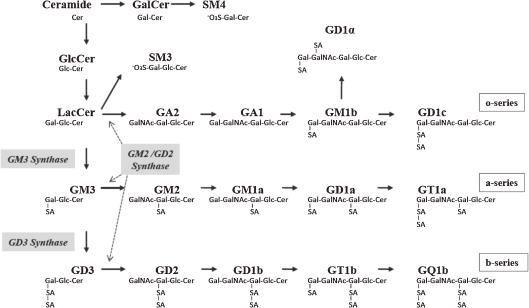
In 1951, the presence of sialic acid at the surface of cell membranes was first demonstrated by Tamio Yamakawa.1) He isolated a sialic acid-containing substance from 100 g of dried ghosts obtained from 10 liters of packed horse erythrocytes and named the glycolipid hematoside.1,2) Hematoside is now also called GM3 ganglioside because this substance is the first product in the biosynthetic pathway of the ganglio-series gangliosides. GM3 serves as a common precursor for complex gangliosides such as the a- and b-series gangliosides (Fig. 1). “Gangliosides expressed in a cell-type specific manner” in the outer leaflet of the cell membranes are expected to interact with various molecules on plasma membranes based on different potentials of non-covalent bonding such as electrostatic and hydrophobic interactions and thereby ganglioside family could participate in various aspects of cellular activities by forming dynamic functional complexes (membrane microdomains or lipid rafts).3,4) Expression levels of cellular gangliosides are known to be influenced by various extracellular stimuli including inflammatory cytokines. Namely, the presence of gangliosides in membrane microdomains is reflecting the characteristics of individual cells under various physiological and pathological environments. Since I was involved in gene cloning of “GM3 synthase” in 1988,5) much efforts of our research group have been dedicated to the elucidation of biological function of GM3. GM3 synthase (CMP-N-acetylneuraminate:lactosylceramide-α-2,3-N-acetylneuraminyltransferase; EC 2.4.99.9), also known by the names SAT-I (used here), ST3GalV, and Siat 9. This review describes our findings on the physiopathological significance of gangliosides especially for GM3 in diabetes and hearing loss.
Figure 1.
Biosynthesis of ganglio-series gangliosides.
1. Gangliosides in type 2 diabetes
A new concept, that ‘‘metabolic disorders, such as type 2 diabetes, are membrane microdomain disorders caused by aberrant expression of gangliosides”, has arisen. By examining this working hypothesis, we demonstrate the molecular pathogenesis of type 2 diabetes and insulin resistance focusing on the interaction between insulin receptor and gangliosides in microdomains and propose the new therapeutic strategy ‘‘membrane microdomain ortho-signaling therapy”.
1-1. GM3 is an inducer of insulin resistance.
Insulin elicits a wide variety of biological activities, which can be categorized into metabolic and mitogenic actions. The binding of insulin to insulin receptor (IR) activates IR internal-tyrosine kinase activity. The activated tyrosine-phosphorylated IR was able to recruit and phosphorylate adaptor proteins such as insulin receptor substrate (IRS). The phosphorylated IRS activates PI3-kinase, resulting in the translocation of glucose transporter 4 (GLUT-4) to plasma membrane to facilitate glucose uptake. This IR–IRS–PI3-kinase signaling cascade is the representative metabolic pathway triggered by insulin. On the other hand, the mitogenic pathway in insulin signaling initiates phosphorylation of Shc by the activated IR and then activates Ras-MAPK signaling.
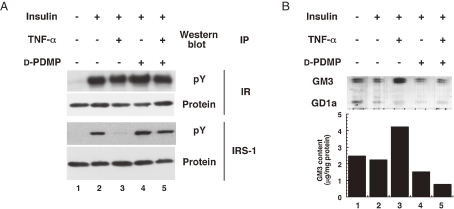
When mouse adipocytes were cultured in low concentrations of TNFα which do not cause generalized suppression of adipocyte gene expression including IRS-1 and GLUT-4, interference of insulin action by TNFα occurred.6) This requires prolonged treatment (at least 72 h), unlike many acute effects of this cytokine. The slowness of the effect suggests that TNFα insulin resistance in adipocytes treated with 0.1 nM TNFα was accompanied by a progressive increase in cell surface GM3. This was reflected by increases in cellular GM3 content, GM3 synthase activity and GM3 synthase mRNA content, indicating that TNFα upregulates GM3 synthesis at the transcriptional level in cultured adipocytes.7) To elucidate whether the increased GM3 in 3T3-L1 adipocytes treated with TNFα is involved in insulin resistance, we used an inhibitor of glucosylceramide synthase, D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (D-PDMP)8) to deplete cellular glycosphingolipids derived from glucosylceramide. D-PDMP proved able to counteract TNF-induced increase of GM3 content in adipocytes and completely normalize the TNF-induced defect in tyrosine phosphorylation of IRS-1 in response to insulin stimulation (Fig. 2A). These findings are supported by the observation that knockout mouse lacking GM3 synthase exhibits enhancement of insulin signaling.9)
Figure 2.
TNFα increases the expression of GM3 and prevention of GM3 synthesis reverses TNFα-induced suppression of insulin signaling in adipocytes. (A) 3T3-L1 adipocytes were cultured in maintenance medium without (lanes 1, 2 and 4) or with (lanes 3 and 5) 0.1 nM TNFα for 96 h and in order to deplete GM3, 20 µM D-PDMP was also included (lanes 4 and 5). Before insulin stimulation (100 nM for 3 min), cells were starved in serum-free media containing 0.5% bovine serum albumin in the absence or presence of TNFα and D-PDMP as above for 8 h. Proteins in cell lysates were immunoprecipitated with antiserum to IR and IRS-1, fractionated by SDS-PAGE, and transferred to Immobilon-P. Western blot was then proved with anti-phosphotyrosine monoclonal antibody, stripped and reproved with antiserum to IR and IRS-1 (B) 3T3-L1 adipocytes were incubated in the absence or presence of TNFα and D-PDMP as in (A) and the ganglioside fraction was visualized by resorcinol staining on HPTLC.
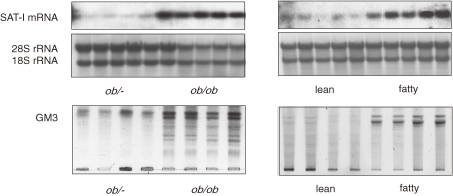
Hotamisligil et al. reported that treatment of adipocytes with TNFα induces an increase in the serine phosphorylation of IRS-1.10) This phosphorylation is an important event since immunoprecipitated IRS-1, which has been serine phosphorylated in response to TNFα, is a direct inhibitor of insulin receptor tyrosine kinase activity. We have shown that TNFα induced serine phosphorylation of IRS-1 in adipocytes was completely suppressed by inhibition of GM3 biosynthesis with D-PDMP treatment, suggesting that the elevated GM3 synthesis induced by TNFα caused the upregulation of serine phosphorylation of IRS-1 (Fig. 2B).7) Since TNF-induced serine phosphorylation of IRS-1 may occur through the activation of a variety of kinases including protein kinase C, c-Jun NH2-terminal kinase, p44/42 kinase, and PI3-kinase, it is important to identify the actual kinase(s) activated by endogenous GM3. Zucker fa/fa rat produced significant levels of TNFα.10) Much less expression was seen in adipose tissues obtained from the lean control animals. Interestingly, these obese-diabetic animals did not show evidence of altered expression of other cytokines, such as IL-1 or IFNγ.10,11) Thus, we were interested in measuring the expression of GM3 synthase mRNA in the epididymal fat of Zucker fa/fa rats and ob/ob mice. Northern blot analysis of GM3 synthase mRNA contents in the adipose tissues from these two typical models of insulin resistance exhibited significantly high levels compared to their lean counterparts (Fig. 3).7)
Figure 3.
Increased GM3 synthase mRNA in adipose tissue of typical rodent models of insulin resistance. Northern blot analysis of GM3 synthase mRNA was performed using total mRNA from adipose tissues of ob/ob mice and Zucker fa/fa rats, and their lean counterparts.
1-2. Caveolae microdomains and insulin signaling.
Caveolae are a subset of membrane microdomains particularly abundant in adipocytes.12,13) Critical dependence of the insulin metabolic signal transduction on caveolae/microdomains in adipocytes has been demonstrated.14,15) Disruption of microdomains by cholesterol extraction with methyl-β-cyclodextrin resulted in progressive inhibition of tyrosine phosphorylation of IRS-1 and activation of glucose transport in response to insulin although autophosphorylation of IR and activation of MAP kinase were not impaired.12) Similarities between these cell culture results and the findings in many cases of clinical insulin resistance suggest a potential role for microdomains in the pathogenesis of this disorder.16) Couet et al. demonstrated the presence of a caveolin binding motif (fXXXXfXXf) [f: an aromatic amino acid, X: any amino acid] in the β subunit of IRs that could bind to the scaffold domain of caveolin.17) Moreover, mutation of this motif resulted in the inhibition of insulin signaling.18) Indeed, mutations of the IRβ-subunit have been found in type 2 diabetes patients.19)
Lisanti’s laboratory reported the caveolin-1 (CAV1)-null mice developed insulin resistance when placed on high fat diet.20) Interestingly, insulin signaling, as measured by IR phosphorylation and its downstream targets, was selectively decreased in the adipocytes of these animals while signaling in both muscle and liver cells was normal. This signaling defect was attributed to a 90% decrease in IR protein content in the adipocytes, with no changes in mRNA levels, indicating that CAV1 serves to stabilize the IR protein.15,20) These studies clearly indicate the critical importance of the interaction between caveolin and IR in executing successful insulin signaling in adipocytes. Although the direct interaction between CAV1 and IR has been shown as described above, studies of the presence of IRs in DRM (detergent resistant membranes) have provided conflicting data.21–25) Saltiel and colleagues found that insulin stimulation of 3T3-L1 adipocytes was associated with tyrosine phosphorylation of CAV1.26) However, since only trace levels of IR were recovered in the DRM in assays with a buffer of 1% Triton X-100, they speculated on the presence of intermediate molecule(s) bridging IR and caveolin.24) Gustavsson et al. also observed the dissociation of IRs from caveolin-containing DRM after treatments of 0.3% and 0.1% Triton X-100.21) It has been reported that a comparison of protein and lipid contents of DRM prepared with a variety of detergents indicated considerable differences in their ability to selectively solubilize membrane proteins and to enrich sphingolipids and cholesterol over glycerophospholipids and that Triton X-100 was the most reliable detergent.27) Therefore, we performed a flotation assay with a wide range of Triton X-100 concentrations to identify the protein of interest which might weakly associate with DRM. In an assay system containing less than 0.08% Triton X-100, we were able to show that in normal adipocytes IRs can localize to first time, the presence of IR in DRM.28) The accumulation of GM3 and cholesterol and of raft proteins (caveolin, flotillin and fyn) was confirmed the DRM obtained with 0.08% Triton X-100. These results suggest the existence of distinct and variety of rafts consisted of functional proteins having different binding affinity and specificity to molecules constitutively associated with DRM.
As summarized in Table 1, there are many lines of evidence demonstrating that the localization of IRs in caveolae microdomains is essential for successful metabolic signaling of insulin.
Table 1.
Localization of insulin recptor (IR) in caveolae microdomains is essential for the metabolic signaling of insulin
| Function | Evidence | Reference |
|---|---|---|
| Direct binding of IR and caveolin-1 | IR has caveolin binding domain | 17 |
| Coimmunoprecipitation of IR and caveolin | 18 | |
| Colocalization of IR and caveolin-1 | IR and caveolin in light-density fractions by sucrose density floatation assay | 28 |
| Fluorescence microscope | 21 | |
| Electron microscope | 45, 46 | |
| Signaling | Stimulation of caveolin-1 tyrosine phosphorylation by insulin | 23, 26 |
| Caveolin deficient mice show insulin resistance due to accelerated degradation of IR in adipose tissue | 20 | |
| Cholesterol depletion disrupts caveolae and metabolic signaling of insulin | 12 | |
| Increased GM3 eliminates IR from DRM and inhibits IR-IRS-1 signaling | 28, 29 | |
1-3. Insulin resistance as a membrane microdomain disorder.
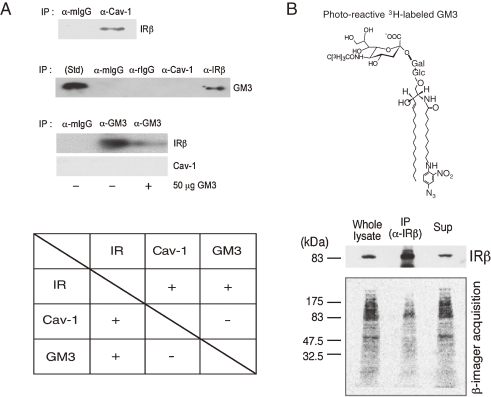
In a state of insulin resistance induced in adipocytes by TNFα we presented evidence that the transformation to a resistant state may depend on increased ganglioside GM3 biosynthesis following upregulated GM3 synthase gene expression. Additionally, GM3 may function as an inhibitor of insulin signaling during chronic exposure to TNFα.7) Since GSL, including GM3, are important components of DRM, we have pursued the possibility that increased GM3 levels in DRM confer insulin resistance upon TNF-treated adipocytes. We examined the effect of TNFα on the composition and function of DRM in adipocytes and demonstrated that increased GM3 levels result in the elimination of IRs from the DRM while raft marker proteins such as caveolin and flotillin remain in the DRM.28) Although the localization of IRs to DRM may be maintained by the association with CAV1 as mentioned above, the excess accumulation of GM3 in the DRM may weaken IR–caveolin interaction. Therefore, to examine interactions among IR, CAV1, and GM3 in 3T3-L1 adipocytes, we initially performed coimmunoprecipitation assays. CAV1 has a scaffolding domain to which IR and other functional transmembrane proteins bind through a caveolin binding domain in their cytoplasmic region.17,18) As expected from another study,18) IR was co-precipitated with CAV1 (Fig. 4A). GM3 was coprecipitated with IR but not with CAV1 (Fig. 4A, upper panel). In addition, IR but and GM3, but there is no interaction between GM3 and CAV1, suggesting that IR can form distinct complexes with each. Alternatively, the amount of IRs coprecipitated with GM3 was greatly diminished by the presence of GM3, confirming the specific binding ability of the anti-GM3 antibody to GM3 in the immunoprecipitation medium (Fig. 4A, lower panel). We next examined GM3–protein interactions occurring within the plasma membrane of living cells by performing a cross-linking assay using a photoactivatable radioactive derivative of GM3 (Fig. 4C). Adipocytes were preincubated with [3H]GM3(N3), then irradiated to induce cross-linking of GM3. Target proteins were then separated by SDS/PAGE and visualized by autoradiography. A broad range of radioactivity reflecting GM3–protein complexes could be detected from 80 kDa to 200 kDa, suggesting a close association between GM3 and a variety of cell surface proteins, including IR. Moreover, a specific radioactive band corresponding to the 90-kDa IRβ-subunit was immunoprecipitated with anti-IRβ antibodies, confirming the direct association of GM3 and IR. Therefore, we found that IR form complexes with CAV1 and GM3 independently in 3T3-L1 adipocytes.29) Lipids are asymmetrically distributed in the outer and inner leaflets of plasma membranes. In typical mammalian cells, most acidic phospholipids are located in the inner leaflet, and only acidic glycosphingolipids such as sulfatides and gangliosides are in the outer. The binding of proteins to lipid membranes is often mediated by electrostatic interactions between the proteins’ basic domains and acidic lipids. Gangliosides, which bear sialic acid residues, exist ubiquitously in the outer leaflet of the vertebrate plasma membrane. GM3 is the most abundant ganglioside, and the primary ganglioside found in adipocytes.30) Glycosphingolipids, including gangliosides, is oriented at a defined angle to the axis of the ceramide.31) In addition, GM3 spontaneously forms clusters with its own saturated fatty acyl chains, regardless of any repulsion between the negatively charged units in the sugar chains.32) Thus, GM3 clusters with other cell surface gangliosides such as GEM generate a negatively charged environment just above the plasma membrane. Conversely, IR has a sequence in its transmembrane domain, homologous among mammals, that allows presentation of the basic amino acid lysine (IR944) just above transmembrane domain. Therefore, during lateral diffusion an electrostatic interaction between the lysine residue at IR944 and the GM3 cluster could occur due to their proximity on the plasma membrane (Fig. 5A). We previously developed GM3-reconstituted cells by stably transfecting the GM3 synthase (SAT-I) gene into GM3-deficient cells (Fig. 4B, left panel).33) Using the fluorescence recovery after photobleaching (FRAP) technique, we examined the mobility of IR in the plasma membranes of GM3-reconstituted (GM3(+)) cells and mock (GM3(−)) cells expressing equal levels of CAV1 (Fig. 5B, right panel inset). The mobility of IR-GFP expressed in the GM3(+) cells was statistically (10%) higher than that in the GM3(−) cells (Fig. 5B, right panel), providing further evidence that GM3 is able to enhance IR mobility by dissociating the CAV1 and IR complex in living cells.
Figure 4.
The insulin receptor forms distinct complexes with caveolin-1 (CAV1) and GM3 in adipocytes. (A) Co-immunoprecipitation assay of Cav1 and IR. Post nuclear supernatants (PNS) of whole precipitates were subjected to SDS-PAGE followed by immunoblotting with an anti-IRβ antibody. GM3 associates with IR but not with Cav1. Upper panel: PNS were immunoprecipitated with an anti-Cav1 antibody, an anti-IRβ antibody, or an anti-mouse or anti-rat IgG. The precipitates were subjected to TLC followed by immunostaining with the anti-GM3 antibody M2590. Lower panel: Immunoprecipitation was performed with the anti-GM3 antibody DH2, in the presence or absence of 50 µg GM3, or with anti-mouse IgG. The precipitates were then subjected to SDS-PAGE followed by immunoblotting with an anti-IRβ or anti-Cav1 antibody. (B) Cross-linking assay of GM3 and IR. Adipocytes were treated with photoactivatable 3H-labeled GM3, washed, and then irradiated. Cells were then lysed and subjected to immunoprecipitation with an anti-IRβ antibody. PNS, anti-IRβ immunoprecipitates (IP), and the supernatant from the immunoprecipitation (Sup) were subjected to SDS-PAGE, followed by immunoblotting with an anti-IRβ antibody and autoradiography.29)
Figure 5.
The lysine residue IR944 is essential for the interaction of IR with GM3. (A) Schematic representation of the proposed interaction of the lysine residue at IR944, which is located just above the transmembrane domain, and GM3 at the cell surface. (B) Enhanced mobility of IR in GM3-enriched membrane. Left panel: Glycosphingolipid (GSL) analysis of GM3-reconstituted cells (GM3(+)) and mock cells (GM3(−)). GSLs extracted from these cells, corresponding to 1 mg of cellular protein, were separated on HPTLC plates and stained with resorcinol-HCl reagent, to visualize gangliosides, or with cupric acetate-phosphoric acid reagent and GM3(+) cells expressing equal levels of caveolin-1 (Cav1) (inset). (C) Specificity of the interaction between lysine at IR944 and GM3 by FRAP analyses. Upper panel: Schematic structure of IR-GFP mutants in which the lysine at IR944 is replaced with basic and neutral amino acids. Lower panel: Fluorescence recovery of IR-GFP mutants in GM3(+) and GM3(−) cells.
The binding of between IR and CAV1 has been studied in detail.18) To similarly understand interactions between IR and GM3 have not been analyzed. We constructed several mutants of IR in which the lysine at IR944 was replaced with the basic amino acid, arginine, or with the neutral amino acid valine, serine, or glutamine. The fluorescence recovery of to those of IR(WT) and IR(K944R) in GM3(+) cells. However, in GM3(−) cells, no such difference in the mobility between IR(wt) and IR(K944S) was observed (Fig. 5C). This demonstrates that the lysine in the wild type is essential for its binding to GM3 due to its basic charge.29)
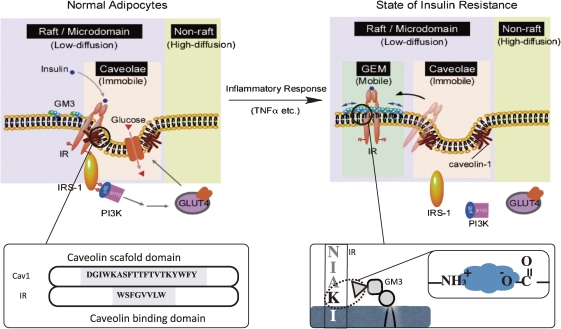
IR may be constitutively resident in caveolae via its binding to the scaffolding domain of CAV1 through the caveolin binding domain in its cytoplasmic region.17) In fact, there are many supporting data showing that binding of IR and CAV1 is necessary for successful insulin metabolic signaling (Table 1). Iwabuchi et al. demonstrated that caveolae and GEM could be separated by an anti-CAV1 antibody.34) In adipocytes the localization of IR in the caveolae is interrupted by elevated levels of the endogenous ganglioside GM3 during a state of insulin resistance induced by inflammatory response (e.g. TNFα).7) Our latest study has proven a mechanism, at least in part, in which the dissociation of the IR–CAV1 complex is caused by the interaction of a lysine residue, located just above the transmembrane domain in IRβ-subunit, and the increased GM3 clustered at the cell surface by live cell studies using FRAP techniques (Fig. 5C).29) Here, we proposed mechanism behind the shift of IR from the caveolae to the GEM in adipocytes during a state of insulin resistance. Shown is a schematic representation of raft/microdomains comprising caveolae and non-caveolae rafts such as GEM (Fig. 6).
Figure 6.
Proposed mechanism behind the shift of insulin receptors from the caveolae to the glycosphingolipid-enriched microdomains (GEM) in adipocytes during a state of insulin resistance. A schematic representation of raft/microdomains comprising caveolae and non-caveolae rafts such as GEM. Caveolae and GEM reportedly can be separated by an anti-CAV1 antibody. IR may be constitutively resident in caveolae via its binding to the scaffolding domain of CAV1 through the caveolin-binding domain in its cytoplasmic region. Binding of IR and CAV1 is necessary for successful insulin metabolic signaling (Table 1). In adipocytes the localization of IR in the caveolae is interrupted by elevated levels of the endogenous ganglioside GM3 during a state of insulin resistance induced by TNFα.29) The present study has proven a mechanism, at least in part, in which the dissociation of the IR/CAV1 complex is caused by the interaction of a lysine residue at IR944, located just above the transmembrane domain, and the increased GM3 clustered at the cell surface.
1-4. Serum GM3 levels as a new biomarker of metabolic syndrome.
GM3 is the major ganglioside present in serum and is known to be associated with serum lipoproteins.35) However, there have been no studies examining a relationship between serum GM3 levels and adiposity indices, as well as between serum GM3 levels and metabolic risk variables.36) Serum GM3 levels were higher in hyperglycemic patients (1.4-fold), hyperlipidemic patients (1.4-fold) and hyperglycemic patients with hyperlipidemia (1.6-fold), than in normal subjects. In addition, serum GM3 levels were significantly increased in type 2 diabetic patients with severe obesity (visceral fat area >200 cm2, BMI >30). The GM3 level was positively correlated with LDL-c (0.403, P = 0.012) in type 2 diabetes mellitus, but not affected by blood pressure. In addition, the high levels of small dense LDL (>10 mg/gl) were associated with the elevation of GM3. Serum GM3 levels were affected by glucose and lipid metabolism abnormalities and by visceral obesity. Interestingly, small dense LDL is reportedly associated with the development of atherosclerosis,37–39) and GM3 has been detected in atherosclerotic lesions.40,41) Thus, our findings provide evidence that GM3 may be a useful marker for the management of metabolic syndrome including insulin resistance, as well as for the early diagnosis of atherosclerosis. We are in the process to expanding the number of patients suffered from various stages of metabolic disorders to measure GM3 levels in serum as well as in insulin responsive organs such as muscle and adipose tissues.
1-5. A possible therapeutic intervention of metabolic syndrome by inhibiting ganglioside synthesis.
Critical involvement of GM3 in insulin resistance and metabolic syndrome including type 2 diabetes has now became evident based on the following key observations; (i) TNFα increases the expression of GM3 in adipocytes and the TNFα-induced insulin resistance is prevented by treatment with a glucosylceramide synthase inhibitor, D-PDMP, and decreases GM3 contents.7) Recently, an improved PDMP analog42,43) and another type of glucosylceramide synthase inhibitor,44) were proven to have therapeutic value by oral administration in diabetic rodent models. These glucosylceramide synthase inhibitors were able to improve insulin sensitivity and glucose homeostasis in rodent models of obesity;42–44) (ii) GM3 contents increase in the adipose tissue of Zucker fa/fa rats and ob/ob mice, which are typical rodent models of obesity7) and diet induced obesity. Indeed, when we fed high fat diet for 10 weeks to wild type mice and SAT-I deficient mice, both mice became obese similarly. Nevertheless, in the obese SAT-I deficient mice, the expression levels of TNFα in mesenteric adipose tissues was significantly lower than those in the obese B6 mice, providing the evidence that the enhancement of GM3 levels in adipose tissue under obese conditions is upstream and causal event in the progression of metabolic syndrome (unpublished observation); (iii) insulin sensitivity is enhanced in SAT-I deficient mice;9) (iv) the accumulation of GM3 in insulin resistance results in dissociation of the insulin receptor from caveolae;28) (v) dissociation of the insulin receptor from caveolae is caused by electrostatic interaction between GM3 and the lysine residue (Lys-944) located just above the transmembrane of the insulin receptor.29) Thus these data substantiate a rationale for designing novel therapies against type 2 diabetes and related diseases based on inhibition of ganglioside biosynthesis.29) Here I have summarized the distinct pathway of metabolic disorder including the aberrant expression of GM3 (Fig. 7).
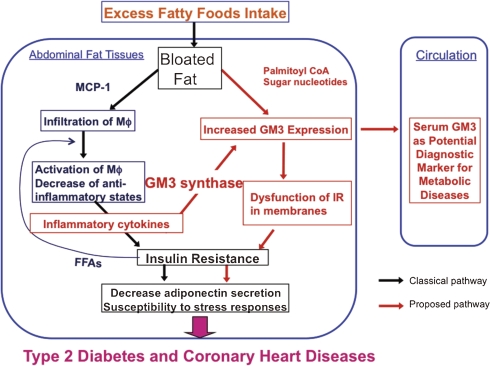
Figure 7.
Insulin resistance through increased GM3 levels in metabolic syndrome. MCP-1: monocyte chemotactic protein-1; Mφ: macrophage.
2. Gangliosides in auditory system and hearing loss
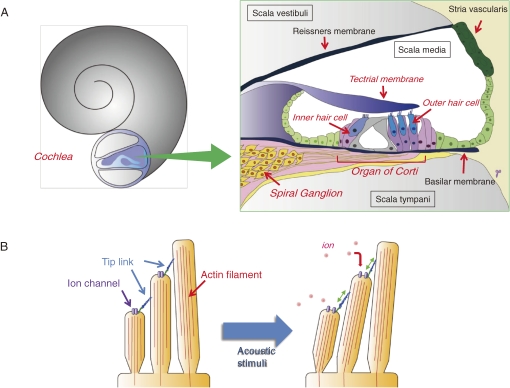
Sounds from the outside world propagate through the external and middle ears then stimulate the inner ear. The mammalian inner ear contains the sensory organ known as the “cochlea” (Fig. 8). The organ of Corti sits in the scala media, which houses hair cells arrayed on the basilar membrane along the length of cochlea duct. The cochlea harbors about 15,000 sensory hair cells, which are the mechanoreceptors of hearing. These cells are responsible for translating auditory stimuli into electrical energy that can be perceived by the nervous system (Fig. 8). Hair cells are sensitive to mechanical and chemical insults, so they can be easily acoustical overstimulation, and by the effects of aging. The impairment and loss of hair cells in the cochlea are responsible for a significant proportion of sensorineural hearing impairment. While cells in many organs (such as the skin and gut epithelium) are constantly replenished throughout life, other organs such as the liver are even capable of regeneration following injury, no hair cell replacement or cell proliferation occurs in the mammalian cochlea. This inability is the major reason for the difficulties faced in the fundamental treatment of hearing loss.47)
Figure 8.
Structure of the cochlea and organ of Corti (A) and Sensing and transduction of sounds in the stereocilia (B). Hair cells detect sound through deflection of mechanosensory “stereocilia”, actin-based microvilli-like projections on their apical surface. Each stereocilium is supported by a paracrystalline array of parallel actin filaments. The sound-induced deflections impel tension in the tip links between the stereocilia and stimulate cation-selective mechanotransduction channels present on the stereocilia. This process permits endolymphatic K+ to enter the hair cells, resulting in their electrical excitation.
Many deafness-related genes have been reported, and it is estimated that more than 100 are expressed in the inner ear. These facts suggest that the regulation of the function and maintenance of the inner ear is precise and complex. Identifying the mechanisms active in hearing loss caused by these deafness-related proteins and their encoded proteins, and understanding their interrelationships at the molecular level will could provide a basis for the design of novel practical therapies for sensorineural hearing loss.
Cell-surface glycoconjugates including proteoglycans, glycoproteins, and glycosphingolipids have been suggested to serve important functions in hearing because of their variety and specific expression patterns during the development and maturation of cochlea. However, there has been no definitive proof regarding their involvement in auditory functions. In this review, we provide an overview of the expression of glycoconjugates in tissues of the auditory system, and of their possible involvement in hearing function including our recent findings regarding deafness in SAT-I knockout mice.
2-1. Expression of glycans in the cochlea.
The abundance of glycans in the cochlea was first suggested in the 1980’s in tissue studies using Alcian blue metachromasia, periodate-Schiff staining and ruthenium red staining. Subsequently, the variety and specific expression patterns of some glycoconjugates were demonstrated by newly developed tools such as lectin and antibody detection of specific carbohydrate structures (Table 2). It was reported that the expression levels and distribution of some carbohydrate structures within the cochlea changed significantly during development and maturation. For example in mice at gestation day 19, glycoconjugates strongly positive for staining with wheat germ agglutinin (WGA), which reacts with N-acetylglucosamine and/or sialic acid in glycans, were detected in the apical surface of the outer hair cells (OHC) and the inner hair cells (IHC), but not in the basolateral region. On the day of birth, the strong WGA-positive signal was detected in the intracellular region of the apical side of the epithelial cells, including the hair cells. Furthermore, an intracellular reaction to WGA was also detected in the area of the supporting cells. From postnatal day 10 onward, the intracellular WGA reaction decreased. In another example using Concanavalin A, a lectin that reacts with high mannose-type glycans of glycoproteins, intense staining of the supporting cells and the tectorial membranes (TM) was observed.48) Immunohistology of guinea pig cochlea tissue using a panel of 25 monoclonal antibodies directed to various carbohydrate epitopes identified lacto-series structures localized at specific sites of the TM and the organ of Corti.49)
Table 2.
Expression of glycoconjugates in cochlea
| Expression region | Glycan structure & reactive lectins | Animals | Reference |
|---|---|---|---|
| Organ of Corti | B-, H-blood group antigen | Rat | 64 |
| keratan sulfate | Guinea pig | 65 | |
| chondroitin 4-sulfate | 66 | ||
| GT1b, GM3 | Mouse | 60 | |
| poly-sialic acid | Rat | 67 | |
| Mouse | 68 | ||
| WGA, ConA, SBA, PSA, RCA-II, PHA-E | Mouse | 48 | |
| Tectrial membrane | Lex, Ley, sLex, sialyl-unbranched type2 chain | Guinea pig | 49 |
| chondroitin 4-, or 6-sulfate, keratan sulfate | Gerbil | 69 | |
| keratan sulfate | Chinchilla | 70 | |
| LFA, GSA II, DBA, SJA, VVA, SBA, APA, | Gerbil | 71 | |
| UAE-I, LTA, ConA, LCA, PSA, RCA-I, MPA | Guinea pig | 71 | |
| WGA, DSA, PWM, E-PHA, L-PHA | Rat | 71 | |
| Mouse | 71 | ||
| WGA, APA, UAE-I, RCA120, HPA, ConA | Human | 72 | |
| Stria vascularis | sLex, sialyl-unbranched type2 chain, | Guinea pig | 49 |
| GM3, 9-O-Ac GD3, GD2/GD3 | |||
| heparan sulfate | Chinchilla | 73 | |
| Rat | 74 | ||
| keratan sulfate | Chinchilla | 70 | |
| GM3 | Mouse | 60 | |
| WGA, APA, RCA120, HPA | Human | 72 | |
| WGA, ConA, DBA, PNA, RCA-I, SBA | Rat | 75 | |
| Spiral ganglion | heparan sulfate | Chinchilla | 73 |
| poly-sialic acid | Rat | 67 | |
| Mouse | 68 | ||
| GM3, GD2/GD3 | Guinea pig | 49 | |
| GM3 | Mouse | 60 | |
| Reissners membrane | heparan sulfate | Mouse | 76 |
| Rat | 74 | ||
| WGA, APA, RCA120, HPA, ConA | Human | 72 | |
| Unknown (TLC analysis) | GM3, GM1, GT1b, GQ1b | Chinchilla | 77 |
| GM3, GM1, GM2, GD3, GD1a,b, GT1b, GalCer, SM4 | Rat | 78 | |
| GM3, GM1, GD3, GD1a,b, GT1b, LacCer, GlcCer, SM4, SM3 | Mouse | 60 | |
These distinct and spatiotemporal expression profiles of specific glycoconjugate species in inner ear during development and maturation suggest their critical roles in the auditory system. Details of the molecular functions of each glycoconjugate in hearing are largely unknown. However, as described below, hearing loss has been reported in certain human diseases caused by the mutation of genes involved in glycoconjugate metabolism, and in transgenic mice carrying mutations related to glycoconjugate metabolism.
2-2. Hearing loss in human diseases related to glycoconjugate metabolism.
Hearing loss has been reported in some lysosomal storage diseases (LSDs) resulting from the mutation of enzymes related to intralysosomal hydrolysis of glycoconjugates; such diseases include sialidosis, mucopolysaccharidosis (Morquio syndrome, Sly disease), GM2 gangliosidosis (Sandhoff’s disease), and α- and β-mannosidosis.50–53) Sensorineural defects in the inner ear have been reported in LSD patients, as have conductive abnormalities in the middle and external ears. Hearing loss has also been reported in the hereditary multisystemic disorders termed congenital disorders of glycosylation (CDG) type I, which are caused by a disruption in the production of lipid-linked oligosaccharides necessary for generation of nascent glycoproteins in the endoplasmic reticulum. CDG type Ig (CDG-Ig) is caused by a deficiency of the α-mannosyltransferase. dolichyl-P-mannose:Man-7-GlcNAc-2-PP-dolichyl-α-6-mannosyltransferase, which is encoded by the gene ALG12. In CDG Ig patients, Man-7-GlcNAc-2-PP-dolichol is poorly transferred to protein and so is accumulated. Certain patients with CDG-Ig share many common clinical features, including sensorineural hearing loss, as well as male genital hypoplasia, IgG deficiency, psychomotor retardation, and blindness.54) Although the molecular mechanisms of the sensorineural hearing defects present in LSD and CDG Ig patients are generally unclear, these facts nevertheless indicate that proper metabolism of glycoconjugates is indispensable for the maintenance of hearing ability.
Another example which would seem to support the importance of glycosylation in normal hearing involves the protein clarin-1. Clarin-1 belongs to a superfamily of four-transmembrane proteins that includes the tetraspanin and claudin families. The gene encoding clarin-1 is mutated in some types of Usher syndrome, a human inherited disorder causing deafness and blindness. When expressed in HEK293 cells, clarin-1 localizes to the plasma membrane and becomes concentrated in low density compartments, possibly in some type of microdomains. In addition, clarin-1 reorganizes actin filament structures and induces lamellipodia. In North America, the most prevalent mutation in Usher syndrome III is the clarin-1 N48K mutation, which causes a defect in N-linked glycosylation. N48K mutant proteins are quickly degraded by the proteasomal pathway, resulting in a loss in actin-reorganizing function. Consistent with this phenomenon, in hair cells from mice deficient in clarin-1, F-actin-enriched stereocilia exhibit structural disorganization.55) These observations suggest a possible role for clarin-1 and its glycosylation in the regulation and homeostasis of stereocilia.
2-3. Mice lacking ganglioside GM3 synthase (SAT-I) exhibit complete hearing loss due to selective degeneration of the organ of Corti.
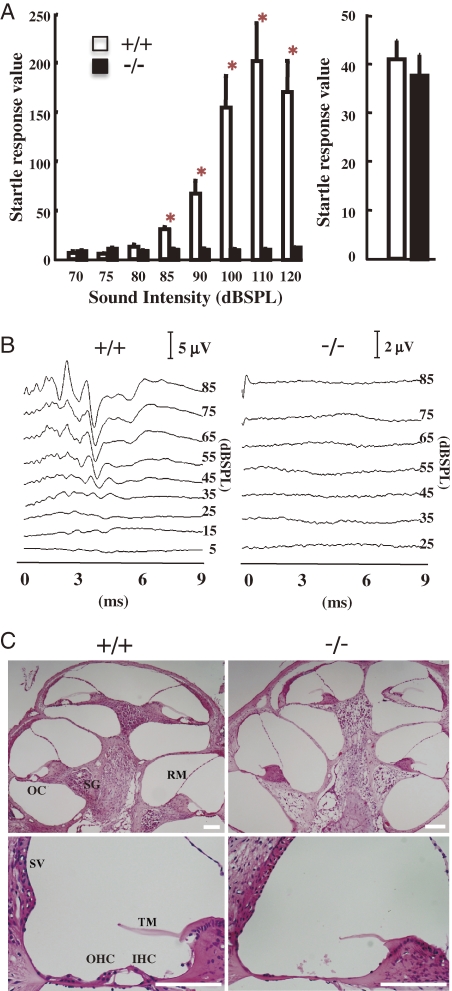
We generated SAT-I-deficient mice and examined their general physiological characteristics. In most tissues of the SAT-I-deficient mice, all of the a-, b- and c-series gangliosides are converted to o-series gangliosides, including GM1b and GD1α, which are produced by bypassing GM3 synthesis (Fig. 1). During observations of their general behavior, we found that SAT-I−/− mice exhibited no acoustic reflex, indicating a loss of hearing. Three-month-old SAT-I+/+ mice exhibited acoustic startle responses to stimuli 85 dB and higher, however the littermate SAT-I−/− mice exhibited no startle reflex in response to any acoustic stimulation (Fig. 9A, left panel). In contrast, all littermate mice demonstrated normal startle responses to air puffs administered to their backs (Fig. 9A, right panel), suggesting an impairment in the hearing ability of mice deficient in SAT-I.
Figure 9.
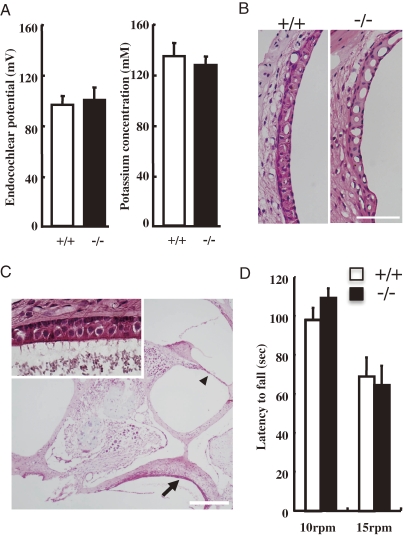
Lack of auditory brain stem response due to the selective degeneration of the organ of Corti. (A) Startle response in 12-week-old SAT-I+/+ (n = 10) and SAT-I−/− (n = 9) mice following acoustic stimuli (70, 75, 80, 85, 90, 100, 110 and 120 dBSPL) or air puff. Data represent the means ± S.E. *P < 0.001; significantly different from the corresponding vehicle-treated group by Student’s t test or the Aspin–Welch test. (B) Auditory brain stem responses (ABR) were obtained from 8-week-old mice (SAT-I+/+ and SAT-I−/− littermates) responding to click stimuli up to 85 dBSPL. Patterns from SAT-I+/+ mice indicate normal ABR; the pattern from a SAT-I−/− mouse reveals no ABR. Representative data for each littermate group (n = 6) are presented. (C) Histology of cochlea. Tissue sections of cochlea in 8-week-old SAT-I+/+ and SAT-I−/− mice were prepared and stained with hematoxylin and eosin. The organ of Corti with outer hair cells (OHC) and inner hair cells (IHC), Reissner’s membrane (RM), tectorial membrane (TM) and stria vascularis (SV) appear to be normal in SAT-I+/+ spiral ganglion (SG) mice. In contrast, the organ of Corti (OC) is completely missing in the cochlea of SAT-I−/− mice. All scale bars represent 100 µm. Representative data for each littermate group (n = 6) are presented.
The auditory brain-stem response (ABR) represents the average surface field potential recorded activity of the auditory neural generators of the lower auditory pathway.56) We performed an ABR study as an objective hearing test on 8-week-old mice using a click sound stimulus of up to 85 dBSPL. Although SAT-I+/+ mice exhibited normal and reproducible ABR peaks, in SAT-I−/− mice all ABR peaks were missing, suggesting that the impaired hearing was due to abnormalities in the cochlear nerve and/or inner ear function (Fig. 9B). Visual observations of 6 to 8-week-old SAT-I littermates indicated no structural differences in the middle and the external ear between the SAT-I+/+ and SAT-I−/− mice. Subsequently, we performed histological examinations of cochlea (Fig. 9C). In SAT-I−/− mice, the organ of Corti was completely missing, although the other regions of cochlea, including the Reissner’s membrane (RM), stria vascularis (SV), and the tectorial membrane (TM), appeared to be normal (Figs. 9C and 10B). Probably, as a consequence of the loss of hair cells, the spiral ganglion (Fig. 9C; SG) was scattered.57)
Figure 10.
Function and histology of Stria vascularis and vestibule in adult SAT-I−/− mice. (A) Endocochlear potential (EP) and potassium ion (K+) concentrations in the endolymph of 6- to 8-week-old SAT-I+/+ (open bar) and SAT-I−/− (closed bar) mice are illustrated. The vertical axis displays mV for EP and mM for K+ concentration. Each bar represents the mean ± S.E. There were no significant differences in the EP or the K+ concentrations between the SAT-I+/+ and SAT-I−/− mice. (B) Microscopic examination of the stria vascularis, stained with hematoxylin and eosin, of 8-week-old SAT-I+/+ (left) and SAT-I−/− (right) mice. No gross difference was detected. Scale bar: 50 µm. (C) Histological examination of the vestibule from an 8-week-old SAT-I−/− mouse. Despite the disappearance of the organ of Corti (arrow head), the layer of hair cells at the sacculus (arrow) and macula statica (the structure faintly stained with eosin above the layer of hair cells) is apparent, and appears to be normal when compared with the control sections of SAT-I+/+ mice (not shown). The inset shows a magnified view of the hair cell layer indicated by the arrow, and demonstrates stereocilia harbored at the apical side of the hair cells. Scale bar: 100 µm. (D) Motor coordination of SAT-I littermates. SAT-I+/+ (n = 10) and SAT-I−/− (n = 11) mice were tested on a rota-rod. The latency to fall was measured at 10 and 15 rpm. Values are expressed as means ± S.E.
We considered the possibility that the degeneration of the organ of Corti in the SAT-I−/− mice might be secondarily induced by changes in the endolymph, an extracellular fluid produced and maintained by the SV that is critical to mechanoelectron transduction.58) To address this question, we next measured the endocochlear potential (EP) and K+ concentrations in the emdolymph. There were no significant differences in EPs and the K+ concentrations between SAT-I+/+ and SAT-I−/− (Fig. 10A), proving that the profound hearing loss in the SAT-I null mice at 8-week-old resulted from the loss of mechano-electro transduction was induced by the primary loss of the organ of Corti, not by the secondary consequence from metabolic changes in the endolymph. In addition, histological studies of the vestibule in the SAT-I−/− mice revealed no abnormality (Fig. 10C), consistent with the normal balance and motor function of the mice when tested on a rota-rod (Fig. 10D).
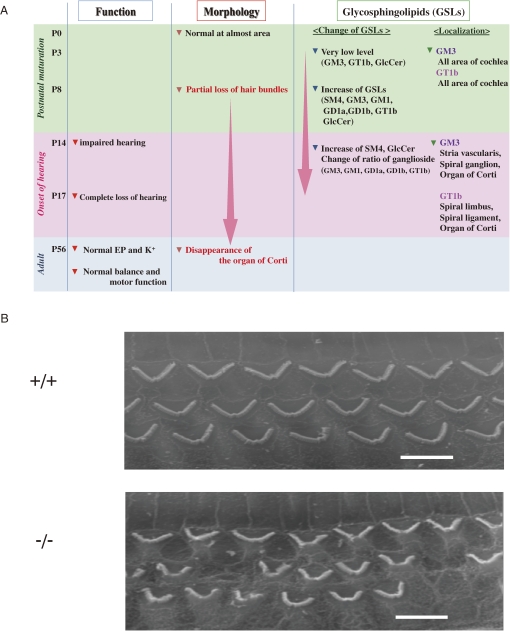
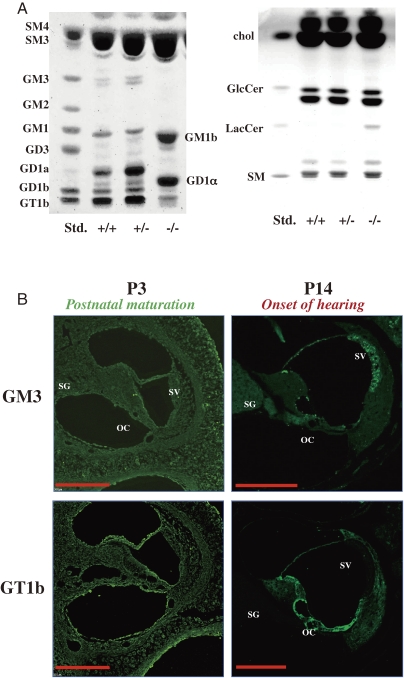
As summarized the phenotypes of SAT-I-deficient mice in Fig. 11A, their hearing ability measured by ABR is seriously impaired at the day of hearing onset, around postnatal day 14, and is completely lost after a few days.59) In the cochlea of these mice, degeneration of the stereocilia of hair cells in the organ of Corti is apparent during the postnatal period of cochlea maturation (Fig. 11B). Normally, various ganglioside species (GM3, GM1, GD1a, GD1b, and GT1b) are expressed in the mouse cochlea. In the inner ear of SAT-I−/− mice, however, only GM1b and GD1α are expressed (Fig. 12A). Interestingly, spatiotemporal changes in localization of individual gangliosides including GM3 and GT1b were observed during the postnatal development and maturation of the normal inner ear. GM3 expressed in almost all regions of cochlea at P3 but on the onset of hearing distinctly localized in stria vascularis, spiral ganglion, and the organ of Corti (Fig. 12B). These observations suggest that the expression of proper gangliosides during the early period of the functional maturation of the cochlea is essential for the acquisition and maintenance of hearing ability.
Figure 11.
Phenotypes of SAT-I-deficient mice in auditory system. (A) Summary of phenotypes of SAT-I−/− mice. (B) SEM images of the three rows of outer hair cells of SAT-I+/+ and SAT-I−/− mice at P14. Scale bars, 5 µm.
Figure 12.
Analysis of gangliosides in mouse inner ear. (A) Thin layer chromatograms of acidic (right panel) and neutral (left panel) glycolipids from the inner ear of 6- to 8-week-old SAT-I+/+, SAT-I+/−, and SAT-I−/− mice. In SAT-I+/+ and SAT-I+/− mice, GM3, GM1, GD1a, GD1b and GT1b are expressed in the inner ear, but these gangliosides are absent in the SAT-I−/− mouse, and the o-series, GM1b and GD1α are compensatorily expressed due to blockage of GM3 synthesis (Fig. 1). (B) Localization of GM3 and GT1b in the cochlea of SAT-I+/+ mice at P3 and P14. At P3, GM3 and GT1b are expressed in almost all regions of the cochlea. At P14, both are expressed in the organ of Corti (OC), however, GM3 is expressed in the SV and SG, but in contrast, GT1b is expressed in regions distinct from GM3, such as the spiral ligament, spiral limbus. All scale bars represent 200 µm.
2-4. Role for microdomains in auditory systems.
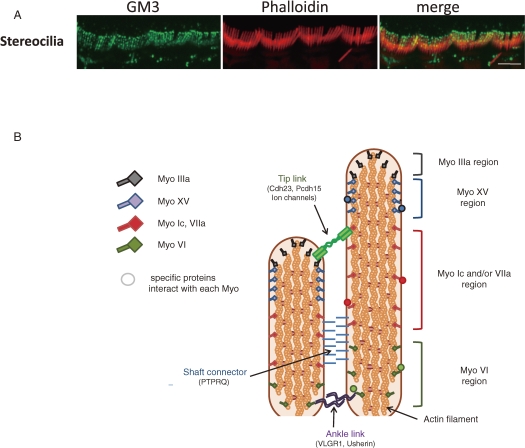
It has been reported that double knockout mice targeted to both GM2/GD2 and GD3 synthases, expressing only GM3, responded to acoustic stimulations.60) Therefore, it is considered that the lack of GM3 may cause the degeneration of the organ of Corti. During the course of studies mentioned in the previous section 2-3, we also found that GM3 may have specific functions in the stereocilia of hair cells. As shown in Fig. 13A, stereocilia of inner hair cells was distinctly stained by anti-GM3 antibody, where the first lesion was occurred in SAT-I−/− mice at P8.59)
Figure 13.
GM3 expresses the stereocilia of hair cells. (A) Whole mount staining of stereocilia of inner hair cells by anti-GM3 antibody and phalloidin. Scale bar represents 5 µm. (B) Diagram of two neighboring stereocilia. Myosins XVa and IIIa are both localized at the tip of each stereocilium, but while myosin XVa localizes to the extreme tip region, myosin IIIa extends further down the stereocilium shaft. Myosin VI localizes at the stereocilium base, and Myosins VIIa and Ic along the shaft. Each myosin interacts and co-localizes with other specific proteins in the stereocilium for the formation of functional networks which links the inside and outside of the plasma membrane. And some membrane glycoprotein are also localized in specific functional region, such as ‘tip link’ (cdh23, Pcdh15, ion channels), shaft connector (PTPRQ) and Ankle links (Usherin, VLGR1).
Hair cells are highly specialized and polarized cells that carry out auditory and vestibular transduction. Stereocilia are microvillus- or filopodium-like cellular processes on the apical surface of hair cells. Each stereocilium is filled with hundreds of crosslinked actin filaments, and certain proteins are specifically expressed in these stereocilia, such as unconventional myosins, Usher proteins, and some deafness-related gene products. These molecules are localized in distinct regions and interact with other specific proteins within the stereocilia to maintain the cells’ mechanoelectro-transduction (Fig. 13B).61) Auditory hair cells must have a system for transporting specific proteins to stereocilia, although none have been identified. One may speculate that some type of functional microdomain like rafts must also exist in apical surface stereocilia, and available data actually support this hypothesis. Several deafness-related gene products, including prestin and clarin-1,62,55) can be biochemically isolated in detergent-resistant membrane microdomains (DRMs/lipid rafts) when over-expressed in conventional cell lines (i.e., HEK293 cells). In addition, some deafness-related gene products are localized in spatially restricted regions of the stereocilia, for example the membrane glycoproteins, PTPRQ, cadherin23, protocadherin15, Usherin, VLGR1. submembrane region of stereocilia. These proteins form a network via specific interactions to form distinct functional regions in stereocilia.
The molecular mechanisms of hearing loss in SAT-I−/− mice remain to be determined. Nevertheless, these findings demonstrate a direct link between gangliosides and hearing functions. We are underway to elucidate functional molecule(s) selectively expressing in the stereocilia interacting with GM3 to maintain its function and integrity using hair cell-like cells derived from embryonic and induced pluripotent stem cells.63)
3. Perspective
The functional roles of GM3 had been examined indirectly by the addition of exogenous GM3 to culture medium or by the depletion of precursor glycosphingolipids using inhibitors of glucosylceramide synthase (reviewed in ref. 79). These results suggest that GM3 may be involved in the transmembrane signaling by regulating growth factor receptor activities and in cell adhesion and motility by interacting with integrins and/or integrin-related molecule(s). However, it has not been conclusively demonstrated that the effect of exogenously added gangliosides or pharmacologically depleted gangliosides reflects the biological function of endogenous GM3.
As described in this review, our research employing GM3 synthase gene and its knockout mice has proved the critical involvement of GM3 in diabetes and hearing function during the past decade. In addition, on going study we are elucidating the indispensable role of GM3 in helper T cell function, because GM3 synthase-deficient mice did not develop asthma (ovoalbumin-induced airway hypersensitivity) (data not shown). Thus, our data substantiate a rationale for designing novel therapies against the metabolic syndrome including type 2 diabetes and immunological disorders such as allergy based on inhibition of GM3 biosynthesis. The extensive reduction of all gangliosides by inhibiting GM3 biosynthesis would carry to important physical and chemical modifications of the all cellular plasma membrane and in particular of lipid microdomains. However, we expect that such extensive depletion of gangliosides will not be necessarily for the treatment of metabolic disorders. When we demonstrated the effectiveness of D-PDMP on the impaired insulin signaling in TNFα-treated 3T3-L1 adipocytes, the normalization of elevated levels of GM3 was enough to ameliorate the state of insulin resistance.7)
Gangliosides expressing cell-type specific manner are expected to interact with various functional molecules on plasma membranes based on different potential of non-covalent bonding such as electrostatic and hydrophobic interactions and thereby ganglioside family could participate various aspects of cellular activities by forming dynamic suprabiomolecular complexes in living cell membranes. Namely, the presence of membrane microdomains (rafts) reflecting characteristics of individual cells. Gangliosides accumulate in microdomains because hydrogen donor and acceptor, and saturated and relatively long acyl chains compared to those of phospholipids should be exist in their ceramide backbone to accelerate their self aggregation. Moreover, since gangliosides expose sialic acid residue to outside of outer leaflet membranes, it is very interesting to know their true physiological counterparts for electrostatic interactions in microdomains. Comprehensive study to elucidate the functional supra-biomolecular complex consisting of gangliosides and functional proteins in microdomains should generate a novel concept and strategy “membrane microdomains ortho-signaling therapy”.
Acknowledgements
The author thanks all the collaborators concerned with the studies described in this review article, who are listed as the co-authors of the paper. This work was supported by grants from Core Research for Evolutional Science and Technology (CREST) of and Technology to promote multidisciplinary research projects, and Mizutani Foundation for Glycoscience.
Abbreviations
- IR
insulin receptor
- IRS-1
insulin receptor substrate-1
- CAV1
caveolin-1
- DRM
detergent resistant membranes
- D-PDMP
D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
- GEM
glycolipid enriched membrane microdomains
Profile
Jin-ichi Inokuchi was born in Fukuoka, Japan, 1953. He received his Ph.D. in 1984 at the Dept. of Biochemistry, Faculty of Pharmaceutical Sciences, Fukuoka University. In 1985 he moved to Mental Health Research Institute, University of Michigan as a postdoctoral fellow and Fulbright Senior Scientist. In this period he contributed in particular to the development of the inhibitor of glucosylceramide synthase (PDMP). In 1992 he became a Manager of Exploratory Group and Head of Glycolipid Section, Tokyo Research Institute, SEIKAGAKU CORPORATION. In 1998 he moved to Hokkaido University as an associate professor. Research projects within his group are focused on the elucidation of physiopathological roles of membrane microdomains in metabolic diseases, immunological functions, CNS and sensory functions. In 2003, he became a principal investigator of Core Research for Evolution Science and Technology (CREST), Japan Science and Technology Agency entitled “Molecular pathogenesis of type 2 diabetes via insulin signaling in membrane microdomains”. In 2006, he became a full professor of Division of Glycopathology at Institute of Molecular Biomembranes and Glycobiology, Tohoku Pharmaceutical University.
References
- 1).Yamakawa T., Suzuki S. (1951) The chemistry of the lipids of posthemolytic residue or stroma of erythrocytes. I. Concerning the ether-insoluble lipids of lyophilized horse blood stroma. J. Biochem. 38, 199–212 [Google Scholar]
- 2).Yamakawa T. (2005) Studies on erythrocyte glycolipids. Proc. Jpn. Acad., Ser. B 81, 52–63 [Google Scholar]
- 3).Regina-Todeschini A., Hakomori S.I. (2008) Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta 1780, 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Sabourdy F., Kedjouar B., Sorli S.C., Colié S., Milhas D., Salma Y., Levade T. (2008) Functions of sphingolipid metabolism in mammals—lessons from genetic defects. Biochim. Biophys. Acta 1781, 145–183 [DOI] [PubMed] [Google Scholar]
- 5).Ishii A., Ohta M., Watanabe Y., Matsuda K., Ishiyama K., Sakoe K., Nakamura M., Inokuchi J., Sanai Y., Saito M. (1998) Expression cloning and functional characterization of human cDNA for ganglioside GM3 synthase. J. Biol. Chem. 273, 31652–31655 [DOI] [PubMed] [Google Scholar]
- 6).Guo D., Donner D.B. (1996) Tumor necrosis factor promotes phosphorylation and binding of insulin receptor substrate 1 to phosphatidylinositol 3-kinase in 3T3–L1 adipocytes. J. Biol. Chem. 271, 615–618 [DOI] [PubMed] [Google Scholar]
- 7).Tagami S., Inokuchi J., Kabayama S., Yoshimura H., Kitamura F., Uemura S., Ogawa C., Ishii A., Saito M., Ohtsuka Y., Sakaue S., Igarashi Y. (2002) Ganglioside GM3 participates in the pathological conditions of insulin resistance. J. Biol. Chem. 277, 3085–3092 [DOI] [PubMed] [Google Scholar]
- 8).Inokuchi J., Radin N. (1987) Preparation of the active isomer of 1-phenyl-2-decanoylamino-3-morpholino-1-propanol, inhibitor of murine glucocerebroside synthetase. J. Lipid Res. 28, 565–571 [PubMed] [Google Scholar]
- 9).Yamashita T., Hashiramoto A., Haluzik M., Mizukami H., Beck S., Norton A., Kono M., Tsuji S., Daniotti J.L., Werth N., Sandhoff R., Sandhoff K., Proia R.L. (2003) Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc. Natl. Acad. Sci. USA 100, 3445–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Hotamisligil G.S., Shargill N.S., Spiegelman B.M. (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 [DOI] [PubMed] [Google Scholar]
- 11).Hotamisligil G.S., Spiegelman B.M. (1994) Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes 43, 1271–1278 [DOI] [PubMed] [Google Scholar]
- 12).Parpal S., Karlsson M., Thorn H., Stralfors P. (2001) Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J. Biol. Chem. 276, 9670–9678 [DOI] [PubMed] [Google Scholar]
- 13).Fan J.Y., Carpentier J.L., van Obberghen E., Grunfeld C., Gorden P., Orci L. (1983) Morphological changes of the 3T3–L1 fibroblast plasma membrane upon differentiation to the adipocyte form. J. Cell Sci. 61, 219–230 [DOI] [PubMed] [Google Scholar]
- 14).Bickel P.E. (2002) Lipid rafts and insulin signaling. Am. J. Physiol. Endocrinol. Metab. 282, E1–E10 [DOI] [PubMed] [Google Scholar]
- 15).Cohen A.W., Combs T.P., Scherer P.E., Lisanti M.P. (2003) Role of caveolin and caveolae in insulin signaling and diabetes. Am. J. Physiol. Endocrinol. Metab. 285, E1151–E1160 [DOI] [PubMed] [Google Scholar]
- 16).Virkamaki A., Ueki K., Kahn C.R. (1999) Protein–protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J. Clin. Invest. 103, 931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Couet J., Li S., Okamoto T., Ikezu T., Lisanti M.P. (1997) Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 272, 6525–6533 [DOI] [PubMed] [Google Scholar]
- 18).Nystrom F.H., Chen H., Cong L.N., Li Y., Quon M.J. (1999) Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in transfected Cos-7 cells and rat adipose cells. Mol. Endocrinol. 13, 2013–2024 [DOI] [PubMed] [Google Scholar]
- 19).Imamura T., Takata Y., Sasaoka T., Takada Y., Morioka H., Haruta T., Sawa T., Iwanishi M., Hu Y.G., Suzuki Y., Hamada J., Kobayashi M. (1994) Two naturally occurring mutations in the kinase domain of insulin receptor accelerate degradation of the insulin receptor and impair the kinase activity. J. Biol. Chem. 269, 31019–31027 [PubMed] [Google Scholar]
- 20).Cohen A.W., Razani B., Wang X.B., Combs T.P., Williams T.M., Scherer P.E., Lisanti M.P. (2003) Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am. J. Physiol. Cell Physiol. 285, C222–C235 [DOI] [PubMed] [Google Scholar]
- 21).Gustavsson J., Parpal S., Karlsson M., Ramsing C., Thorn H., Borg M., Lindroth M., Peterson K.H., Magnusson K.E., Strålfors P. (1999) Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J. 13, 1961–1971 [PubMed] [Google Scholar]
- 22).Iwanishi M., Haruta T., Takata Y., Ishibashi O., Sasaoka T., Egawa K., Imamura T., Naitou K., Itazu T., Kobayashi M. (1993) A mutation (Trp1193 → Leu1193) in the tyrosine kinase domain of the insulin receptor associated with type A syndrome of insulin resistance. Diabetologia 36, 414–422 [DOI] [PubMed] [Google Scholar]
- 23).Kimura A., Mora S., Shigematsu S., Pessin J.E., Saltiel A.R. (2002) The insulin receptor catalyzes the tyrosine phosphorylation of caveolin-1. J. Biol. Chem. 277, 30153–30158 [DOI] [PubMed] [Google Scholar]
- 24).Mastick C.C., Saltiel A.R. (1997) Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3–L1 cells. J. Biol. Chem. 272, 20706–20714 [DOI] [PubMed] [Google Scholar]
- 25).Muller G., Jung C., Wied S., Welte S., Jordan H., Frick W. (2001) Redistribution of glycolipid raft domain components induces insulin-mimetic signaling in rat adipocytes. Mol. Cell. Biol. 21, 4553–4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Mastick C.C., Brady M.J., Saltiel A.R. (1995) Insulin stimulates the tyrosine phosphorylation of caveolin. J. Cell Biol. 129, 1523–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Schuck S., Honsho M., Ekroos K., Shevchenko A., Simons K. (2003) Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA 100, 5795–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Kabayama K., Sato T., Kitamura F., Uemura S., Kang B.W., Igarashi Y., Inokuchi J. (2005) TNFα-induced insulin resistance in adipocytes as a membrane microdomain disorder: involvement of ganglioside GM3. Glycobiology 15, 21–29 [DOI] [PubMed] [Google Scholar]
- 29).Kabayama K., Sato T., Saito K., Loberto N., Prinetti A., Sonnino S., Kinjo M., Igarashi Y., Inokuchi J. (2007) Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc. Natl. Acad. Sci. USA 104, 13678–13683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Ohashi M. (1979) A comparison of the ganglioside distributions of fat tissues in various animals by two-dimensional thin layer chromatography. Lipids 14, 52–57 [DOI] [PubMed] [Google Scholar]
- 31).Hakomori S.I. (2002) Inaugural article: the glycosynapse. Proc. Natl. Acad. Sci. USA 99, 225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Sonnino S., Mauri L., Chigorno V., Prinetti A. (2007) Gangliosides as components of lipid membrane domains. Glycobiology 17, 1R–13R [DOI] [PubMed] [Google Scholar]
- 33).Uemura S., Kabayama K., Noguchi M., Igarashi Y., Inokuchi J. (2003) Sialylation and sulfation of lactosylceramide distinctly regulate anchorage-independent growth, apoptosis, and gene expression in 3LL Lewis lung carcinoma cells. Glycobiology 13, 207–216 [DOI] [PubMed] [Google Scholar]
- 34).Iwabuchi K., Handa K., Hakomori S. (1998) Separation of ‘‘glycosphingolipid signaling domain” from caveolin-containing membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J. Biol. Chem. 273, 33766–33773 [DOI] [PubMed] [Google Scholar]
- 35).Senn H.J., Orth M., Fitzke E., Wieland H., Gerok W. (1989) Gangliosides in normal human serum. Concentration, pattern and transport by lipoproteins. Eur. J. Biochem. 181, 657–662 [DOI] [PubMed] [Google Scholar]
- 36).Sato T., Nihei Y., Nagafuku M., Tagami S., Chin R., Kawamura M., Miyazaki S., Suzuki M., Sugahara S., Takahashi Y., Saito A., Igarashi Y., Inokuchi J. (2008) Circulating levels of ganglioside GM3 in metabolic syndrome: a pilot study. Obes. Res. Clin. Pract. 2, 231–238 [DOI] [PubMed] [Google Scholar]
- 37).Austin M.A., Breslow J.L., Hennekens C.H., Buring J.E., Willett W.C., Krauss R.M. (1988) Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 260, 1917–1921 [PubMed] [Google Scholar]
- 38).de Graaf J., Hak-Lemmers H.L., Hectors M.P., Demacker P.N., Hendriks J.C., Stalenhoef A.F. (1991) Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arterioscler. Thromb. 11, 298–306 [DOI] [PubMed] [Google Scholar]
- 39).Tribble D.L., Krauss R.M., Lansberg M.G., Thiel P.M., van den Berg J.J. (1995) Greater oxidative susceptibility of the surface monolayer in small dense LDL may contribute to differences in copper-induced oxidation among LDL density subfractions. J. Lipid Res. 36, 662–671 [PubMed] [Google Scholar]
- 40).Bobryshev Y.V., Lord R.S., Golovanova N.K., Gracheva E.V., Zvezdina N.D., Sadovskaya V.L., Prokazova N.V. (1997) Incorporation and localisation of ganglioside GM3 in human intimal atherosclerotic lesions. Biochim. Biophys. Acta 1361, 287–294 [DOI] [PubMed] [Google Scholar]
- 41).Bobryshev Y.V., Lord R.S., Golovanova N.K., Gracheva E.V., Zvezdina N.D., Prokazova N.V. (2001) Phenotype determination of anti-GM3 positive cells in atherosclerotic lesions of the human aorta. Hypothetical role of ganglioside GM3 in foam cell formation. Biochim. Biophys. Acta 1535, 87–99 [DOI] [PubMed] [Google Scholar]
- 42).Zhao H., Przybylska M., Wu I.H., Zhang J., Siegel C., Komarnitsky S., Yew N.S., Cheng S.H. (2007) Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes 56, 1210–1218 [DOI] [PubMed] [Google Scholar]
- 43).van Eijk M., Aten J., Bijl N., Ottenhoff R., van Roomen C.P., Dubbelhuis P.F., Seeman I., Ghauharali-van der Vlugt K., Overkleeft H.S., Arbeeny C., Groen A.K., Aerts J.M. (2009) Reducing glycosphingolipid content in adipose tissue of obese mice restores insulin sensitivity, adipogenesis and reduces inflammation. PLoS ONE 4, e4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Aerts J.M., Ottenhoff R., Powlson A.S., Grefhorst A., van Eijk M., Dubbelhuis P.F., Aten J., Kuipers F., Serlie M.J., Wennekes T., Sethi J.K., O’Rahilly S., Overkleeft H.S. (2007) Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 56, 1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Karlsson M., Thorn H., Danielsson A., Stenkula K.G., Ost A., Gustavsson J., Nystrom F.H., Strålfors P. (2004) Colocalization of insulin receptor and insulin receptor substrate-1 to caveolae in primary human adipocytes. Cholesterol depletion blocks insulin signalling for metabolic and mitogenic control. Eur. J. Biochem. 271, 2471–2479 [DOI] [PubMed] [Google Scholar]
- 46).Foti M., Porcheron G., Fournier M., Maeder C., Carpentier J.L. (2007) The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3–L1 adipocytes. Proc. Natl. Acad. Sci. USA 104, 1242–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Forge A., Li L., Corwin J.T., Nevill G. (1993) Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 259, 1616–1619 [DOI] [PubMed] [Google Scholar]
- 48).Rueda J., Cantos R., Lim D.J. (2003) Distribution of glycoconjugates during cochlea development in mice: light microscopic lectin study. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 274, 923–933 [DOI] [PubMed] [Google Scholar]
- 49).Hozawa K., Wataya H., Takasaka T., Fenderson B.A., Hakomori S. (1993) Hearing and glycoconjugates: localization of Ley, Lex and sialosyl-Lex in guinea pig cochlea, particularly at the tectorial membrane and sensory epithelia of the organ of Corti. Glycobiology 3, 47–55 [DOI] [PubMed] [Google Scholar]
- 50).Thomas G.H. (2001) Disorders of glycoprotein degradation and structure: α-mannosidosis, β-mannosidosis, fucosidosis, and sialidosis. The Metabolic and Molecular Bases of Inherited Disease 3, 3507–3534 [Google Scholar]
- 51).Fitzgerald J., Verveniotis S.J. (1998) Morquio’s syndrome. A case report and review of clinical findings. N. Y. State Dent. J. 64, 48–50 [PubMed] [Google Scholar]
- 52).Wallace S.P., Prutting C.A., Gerber S.E. (1990) Degeneration of speech, language, and hearing in a patient with mucopolysaccharidosis VII. Int. J. Pediatr. Otorhinolaryngol. 19, 97–107 [DOI] [PubMed] [Google Scholar]
- 53).Ölmez A., Nilssen O., Coşkun T., Klenow H. (2003) Alpha-mannosidosis and mutational analysis in a Turkish patient. Turk. J. Pediatr. 45, 46–50 [PubMed] [Google Scholar]
- 54).Kranz C., Basinger A.A., Güçsavaş-Calikoğlu M., Sun L., Powell C.M., Henderson F.W., Aylsworth A.S., Freeze H.H. (2007) Expanding spectrum of congenital disorder of glycosylation Ig (CDG-Ig): sibs with a unique skeletal dysplasia, hypogammaglobulinemia, cardiomyopathy, genital malformations, and early lethality. Am. J. Med. Genet. A. 143, 1371–1378 [DOI] [PubMed] [Google Scholar]
- 55).Tian G., Zhou Y., Hajkova D., Miyagi M., Dinculescu A., Hauswirth W.W., Palczewski K., Geng R., Alagramam K.N., Isosomppi J., Sankila E.M., Flannery J.G., Imanishi Y. (2009) Clarin-1, encoded by the Usher Syndrome III causative gene, forms a membranous microdomain: possible role of clarin-1 in organizing the actin cytoskeleton. J. Biol. Chem. 284, 18980–18993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Moller A.R., Jannetta P.J. (1982) Comparison between intracranially recorded potentials from the human auditory nerve and scalp recorded auditory brainstem responses (ABR). Scand. Audiol. 11, 33–40 [DOI] [PubMed] [Google Scholar]
- 57).Vetter D.E., Mann J.R., Wangemann P., Liu J., McLaughlin K.J., Lesage F., Marcus D.C., Lazdunski M., Heinemann S.F., Barhanin J. (1996) Inner ear defects induced by null mutation of the isk gene. Neuron 17, 1251–1264 [DOI] [PubMed] [Google Scholar]
- 58).Wangemann P. (2002) K+ cycling and the endocochlear potential. Hear. Res. 165, 1–9 [DOI] [PubMed] [Google Scholar]
- 59).Yoshikawa M., Go S., Takasaki K., Kakazu Y., Ohashi M., Nagafuku M., Kabayama K., Sekimoto J., Suzuki S., Takaiwa K., Kimitsuki T., Matsumoto N., Komune S., Kamei D., Saito M., Fujiwara M., Iwasaki K., Inokuchi J. (2009) Mice lacking ganglioside GM3 synthase exhibit complete hearing loss due to selective degeneration of the organ of Corti. Proc. Natl. Acad. Sci. USA 106, 9483–9488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Kawai H., Allende M.L., Wada R., Kono M., Sango K., Deng C., Miyakawa T., Crawley J.N., Werth N., Bierfreund U., Sandhoff K., Proia R.L. (2001) Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J. Biol. Chem. 276, 6885–6888 [DOI] [PubMed] [Google Scholar]
- 61).Manor U., Kachar B. (2008) Dynamic length regulation of sensory stereocilia. Semin. Cell Dev. Biol. 19, 502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Sturn A.K., Rajagopalan L., Yoo D., Brownell W.E., Pereira F.A. (2007) Functional expression and microdomain localization of prestin in cultured cells. Otolaryngol. Head Neck Surg. 136, 434–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Oshima K., Shin K., Diensthuber M., Peng A.W., Ricci A.J., Heller S. (2010) Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141, 704–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Mollicone R., Trojan J., Oriol R. (1985) Appearance of H and B antigens in primary sensory cells of the rat olfactory apparatus and inner ear. Brain Res. 349, 275–279 [DOI] [PubMed] [Google Scholar]
- 65).Katori Y., Hackney C.M., Furness D.N. (1996) Immunoreactivity of sensory hair bundles of the guinea-pig cochlea to antibodies against elastin and keratan sulphate. Cell Tissue Res. 284, 473–479 [DOI] [PubMed] [Google Scholar]
- 66).Hultcrantz M., Bagger-Sjoback D. (1996) Inner ear content of glycosaminoglycans as shown by monoclonal antibodies. Acta Otolaryngol. 116, 25–32 [DOI] [PubMed] [Google Scholar]
- 67).Simonneau L., Gallego M., Pujol R. (2003) Comparative expression patterns of T-, N-, E-cadherins, β-catenin, and polysialic acid neural cell adhesion molecule in rat cochlea during development: implications for the nature of Kölliker’s organ. J. Comp. Neurol. 459, 113–126 [DOI] [PubMed] [Google Scholar]
- 68).Whitlon D.S., Zhang X. (1997) Polysialic acid in the cochlea of the developing mouse. Int. J. Dev. Neurosci. 15, 657–669 [DOI] [PubMed] [Google Scholar]
- 69).Munyer P.D., Schulte B.A. (1995) Developmental expression of proteoglycans in the tectorial and basilar membrane of the gerbil cochlea. Hear. Res. 85, 85–94 [DOI] [PubMed] [Google Scholar]
- 70).Swartz D.J., Santi P.A. (1997) Immunohistochemical localization of keratan sulfate in the chinchilla inner ear. Hear. Res. 109, 92–101 [DOI] [PubMed] [Google Scholar]
- 71).Gil-Loyzaga P. (1997) Histochemistry of glycoconjugates of the auditory receptor-functional implications. Prog. Histochem. Cytochem. 32, 1–80 [DOI] [PubMed] [Google Scholar]
- 72).Yamashita H., Bagger-Sjöbäck D. (1992) Expression of glycoconjugates in the human fetal cochlea. Acta Otolaryngol. 112, 628–634 [DOI] [PubMed] [Google Scholar]
- 73).Tsuprun V., Santi P. (2001) Proteoglycan arrays in the cochlear basement membrane. Hear. Res. 157, 65–76 [DOI] [PubMed] [Google Scholar]
- 74).Satoh H., Kawasaki K., Kihara I., Nakano Y. (1998) Importance of type IV collagen, laminin, and heparan sulfate proteoglycan in the regulation of labyrinthine fluid in the rat cochlear duct. Eur. Arch. Otorhinolaryngol. 255, 285–288 [DOI] [PubMed] [Google Scholar]
- 75).Khan K.M., Sarfaraz N., Salim Z. (1999) Lectin binding patterns in nonsensory regions of rat cochlea during postnatal development. J. Anat. 194, 497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Torihara K., Morimitsu T., Suganuma T. (1995) Anionic sites on Reissner’s membrane, stria vascularis, and spiral prominence. J. Histochem. Cytochem. 43, 299–305 [DOI] [PubMed] [Google Scholar]
- 77).Santi P.A., Mancini P., Barnes C. (1994) Identification and localization of the GM1 ganglioside in the cochlea using thin-layer chromatography and cholera toxin. J. Histochem. Cytochem. 42, 705–716 [DOI] [PubMed] [Google Scholar]
- 78).Maguchi S., Gasa S., Matsushima J., Saga Y., Kawano M., Makita A. (1991) Glycolipids in rat cochlea. Auris Nasus Larynx 18, 1–8 [DOI] [PubMed] [Google Scholar]
- 79).Inokuchi J., Kabayama K. (2008) Modulation of growth factor receptors in membrane microdomains. Trends Glycosci. Glycotech. 20, 353–371 [Google Scholar]