Abstract
During voltage-dependent activation in Shaker channels, four arginine residues in the S4 segment (R1–R4) cross the transmembrane electric field. It has been proposed that R1–R4 movement is facilitated by a “gating charge transfer center” comprising a phenylalanine (F290) in S2 plus two acidic residues, one each in S2 and S3. According to this proposal, R1 occupies the charge transfer center in the resting state, defined as the conformation in which S4 is maximally retracted toward the cytoplasm. However, other evidence suggests that R1 is located extracellular to the charge transfer center, near I287 in S2, in the resting state. To investigate the resting position of R1, we mutated I287 to histidine (I287H), paired it with histidine mutations of key voltage sensor residues, and determined the effect of extracellular Zn2+ on channel activity. In I287H+R1H, Zn2+ generated a slow component of activation with a maximum amplitude (Aslow,max) of ∼56%, indicating that only a fraction of voltage sensors can bind Zn2+ at a holding potential of −80 mV. Aslow,max decreased after applying either depolarizing or hyperpolarizing prepulses from −80 mV. The decline of Aslow,max after negative prepulses indicates that R1 moves inward to abolish ion binding, going beyond the point where reorientation of the I287H and R1H side chains would reestablish a binding site. These data support the proposal that R1 occupies the charge transfer center upon hyperpolarization. Consistent with this, pairing I287H with A359H in the S3–S4 loop generated a Zn2+-binding site. At saturating concentrations, Aslow,max reached 100%, indicating that Zn2+ traps the I287H+A359H voltage sensor in an absorbing conformation. Transferring I287H+A359H into a mutant background that stabilizes the resting state significantly enhanced Zn2+ binding at −80 mV. Our results strongly support the conclusion that R1 occupies the gating charge transfer center in the resting conformation.
INTRODUCTION
In the voltage-gated Shaker K+ channel, the probability of opening increases from ∼10−7 to nearly 1 over a range of <100 mV (Islas and Sigworth, 1999). This exquisite sensitivity to voltage is conferred by positively charged amino acid residues located in the S4 transmembrane segment. In response to membrane depolarization, the side chains of four S4 arginine residues (R1–R4) in each of the four channel subunits are transferred some or all of the way across the transmembrane electric field (Fig. 1 A) (Aggarwal and MacKinnon, 1996; Seoh et al., 1996). These conformational changes convert the voltage sensor domain from its resting state, in which S4 adopts its most inward position, to its fully activated state, in the process transferring ∼13 e0 across the transmembrane electric field (Schoppa et al., 1992; Aggarwal and MacKinnon, 1996; Seoh et al., 1996; Islas and Sigworth, 1999). How the charged residues cross the transmembrane field is not well understood.
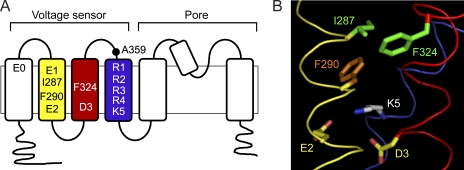
Figure 1.
The gating charge transfer center (Tao et al., 2010). (A) The membrane topology of one subunit of the tetrameric Shaker K+ channel is shown. The approximate positions of conserved charged residues and I287, F290, F324, and A359 in the voltage sensor domain are indicated. Conserved charged residues are labeled using the following generic nomenclature: E0, E247 in S1; E1, E283 in S2; E2, E293 in S2; D3, D316 in S3; R1, R362 in S4; R2, R365 in S4; R3, R368 in S4; R4, R371 in S4; K5, K374 in S4. The S2, S3, and S4 transmembrane segments are shown in yellow, red, and blue, respectively, for comparison to B. (B) The charge transfer center consists of F290 (orange) and E2 in S2 (yellow/red) and D3 in S3 (yellow/red), and is occupied by K5 in S4 (gray/blue) in the Kv1.2/Kv2.1 paddle chimera x-ray structure (Long et al., 2007). Also shown are I287 in S2 and F324 in S3 (green), which correspond to residues that form a naturally occurring binding site for extracellular divalent cations in eag (Silverman et al., 2000; Lin et al., 2010). I287 and F324 are located extracellular to the charge transfer center. Ribbons representing the backbone atoms of S2, S3, and S4 are shown in yellow, red, and blue, respectively. Backbone atoms and the indicated side chains were extracted from the 2r9r x-ray structure and labeled according to the Shaker sequence (Long et al., 2007). In the chimera, the F324 position is occupied by a tyrosine residue, which was mutated in silico using PyMOL (v1.3; The PyMOL Molecular Graphics System; Schrödinger LLC). The figure was made using PyMOL.
Recently, Tao et al. (2010) proposed that three highly conserved residues, corresponding to F290 and E293 (E2) in S2 and D316 (D3) in S3 in Shaker, constitute a “gating charge transfer center” that facilitates the passage of S4 arginine residues across the transmembrane electric field during activation (Fig. 1, A and B). This charge transfer center is occupied by a lysine residue (K5 in S4) in the x-ray structure of a Kv1.2/Kv2.1 paddle chimera, which depicts the voltage sensor domain in a fully activated or inactivated conformation (Fig. 1 B) (Long et al., 2007). Tao et al. (2010) showed that mutating F290 to tryptophan (F290W) preferentially enhances lysine (K5) occupation of the charge transfer center, stabilizing the activated conformation. In contrast, mutating R1 to lysine and K5 to arginine in the presence of F290W (F290W+R1K+K5R) slows ON gating charge movement and significantly shifts its voltage dependence in the depolarized direction, consistent with stabilizing the resting conformation (Tao et al., 2010). From these results, Tao et al. suggested that R1 occupies the charge transfer center in the resting state.
In contrast, a variety of evidence suggests that R1 is extracellular to F290, in the vicinity of E1 or I287 in S2, in the resting state (Starace and Bezanilla, 2004; Tombola et al., 2005; Campos et al., 2007). R1 mutations generate gating pore currents carried by protons or monovalent cations (Starace and Bezanilla, 2004; Tombola et al., 2005). These currents, which flow through the voltage sensor domain rather than the central ion-selective pore, are activated by membrane hyperpolarization and are thought to occur in the resting conformation. Mutating E1 in S2 alters the amplitude of gating pore currents, suggesting that R1 is close to E1, above F290, at rest (Tombola et al., 2005). In addition, the R1C mutation in Shaker forms disulfide bonds in the closed state with I241C in S1 or I287C in S2 (Campos et al., 2007). I241 and I287 are located extracellular to the charge transfer center in the paddle chimera structure (Long et al., 2007). These results have been incorporated into models for the resting conformation in which R1 is located above F290, in proximity to E1 in S2 (Yarov-Yarovoy et al., 2006; Campos et al., 2007; Pathak et al., 2007; Khalili-Araghi et al., 2010). This corresponds to the approximate position of R4 in the chimera structure (Long et al., 2007). A recent computational study was able to account for a single-channel gating charge of ∼12–12.7 e0 by converting the paddle chimera structure into a resting state in which R1 in S4 forms a salt bridge with E1 in S2 (Khalili-Araghi et al., 2010).
An important caveat is that most of the experimental evidence supporting current models for the resting state was obtained using R1 mutations (Starace and Bezanilla, 2004; Tombola et al., 2005; Campos et al., 2007). Without the positively charged guanidinium group at the R1 position, S4 may not move as far inward in the mutants as it does in the wild-type channel. However, concerns also apply to the results of Tao et al. (2010) because the combined mutations F290W+R1K+K5R may pull S4 into a deeper position than it normally occupies at rest. Alternatively, the enhanced interaction between F290W and R1K could occur with R1K located above F290W rather than below in the charge transfer center. Therefore, the existing data do not resolve the location of R1 in the resting state.
To investigate the resting position of R1, we have taken advantage of a water-filled crevice that allows extracellular divalent cations to access natural and engineered binding sites in the voltage sensor domain (Silverman et al., 2000, 2004; Tang et al., 2000; Lin et al., 2010). Previously, we showed that mutating I287 in S2 and F324 in S3 to aspartate in Shaker recapitulates a naturally occurring Mg2+-binding site found in eag (Lin et al., 2010). In I287D+F324D, Mg2+ shifts the voltage dependence of the delay before channel opening and the initial component of gating charge movement in the depolarized direction. These results support the conclusion that Mg2+ binds to and stabilizes the resting state (Silverman et al., 2004; Bannister et al., 2005; Lin et al., 2010). Importantly, the binding site positions 287 and 324 are located extracellular to the gating charge transfer center, as shown in the crystal structure of the paddle chimera (Fig. 1 B).
We now report that R1 in S4 is located in proximity to I287 in an intermediate closed conformation and moves further inward to occupy the gating charge transfer center in the resting state. Our results support a mechanism for Shaker activation in which R1 and K5 are located in the charge transfer center in the resting and activated conformations, respectively, and R2, R3, and R4 traverse this region during voltage-dependent gating.
MATERIALS AND METHODS
Site-directed mutations were generated in Shaker–inactivation removed (IR; Δ6-46) using QuikChange (Agilent Technologies) and confirmed by sequencing (Hoshi et al., 1990). RNA was transcribed in vitro using the mMessage mMachine T7 Ultra kit (Invitrogen) and injected into oocytes isolated from Xenopus laevis frogs. Animal procedures were approved by the Chancellor’s Animal Research Committee at UCLA. 1–2 d after injection, ionic currents were recorded at room temperature using a two-electrode voltage clamp (OC-725; Warner Instruments) (Timpe et al., 1988; Papazian et al., 1991). The bath solution contained 96 mM NaCl, 2 mM KCl, 0.5 mM CaCl2, and 5 mM HEPES, pH 7.5, with or without 50 nM to 200 µM ZnSO4, as indicated. Linear leak and capacitive currents were subtracted using a P/−4 protocol.
Activation kinetics were characterized by fitting current traces with one or the sum of two exponential functions using the equations: y = A[1−exp(−t/τact)]+A0 or y = Afast[1−exp(−t/τfast)]+Aslow[1−exp(−t/τslow)]+A0, where A corresponds to amplitude and τ corresponds to time constant. A0 is the initial amplitude, and t is time. To estimate the half-maximal effective concentration of Zn2+ ([Zn2+]1/2), A or τ values were plotted versus [Zn2+]. Datasets were fitted with a rectangular hyperbolic function of the form: y = P1*[Zn2+]/(P2 + [Zn2+]), where y corresponds to A or τ, P1 corresponds to the maximum value of A or τ, and P2 corresponds to [Zn2+]1/2.
To estimate the delay before channel opening, the fitted exponential function was extrapolated to the zero current level; the delay was defined as the difference between this time point and the start of the pulse (Perozo et al., 1994; Lin et al., 2010). To characterize the steady-state properties of activation, conductance was calculated from steady-state current amplitudes at a variety of test voltages and normalized to the maximum value obtained during the experiment. Datasets were fitted with a Boltzmann equation of the form: g/gmax = (A1−A2)/{1+exp[(V−V1/2)/slope]}+A2.
Values for kinetic and steady-state parameters are provided as mean ± SEM. Statistical significance was evaluated using a one-way ANOVA followed by Student’s t test.
RESULTS
I287H and F324H form a Zn2+-binding site extracellular to the charge transfer center
To investigate the resting position of R1 in S4, we mutated I287 to histidine (I287H), paired it with histidine mutations of key residues in the voltage sensor domain, and determined the effect of extracellular Zn2+ on channel activation. To verify that I287H can be accessed by Zn2+, we mutated I287 and F324, which are analogous to the binding site residues in eag, to histidine and investigated the effects of extracellular Zn2+ on channel kinetics (Silverman et al., 2000; Lin et al., 2010). The addition of 1 µM Zn2+ dramatically slowed channel opening (Fig. 2, A and B). The half-maximal effective concentration of Zn2+ ([Zn2+]1/2) in I287H+F324H was 120 nM (Fig. 2 C). In contrast, Zn2+ had little or no effect on activation kinetics in the Shaker-IR parent channel or the I287H or F324H single mutants (Fig. 2 D).
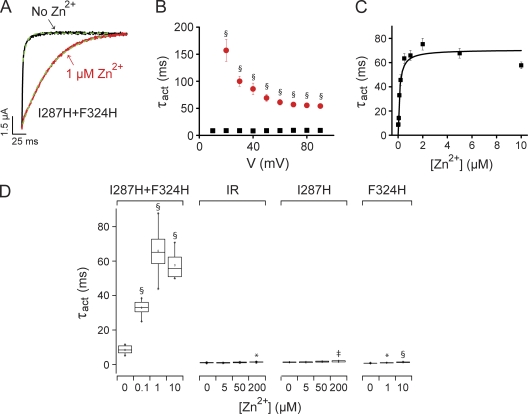
Figure 2.
Extracellular Zn2+ slows activation in I287H+F324H channels. (A) Currents were evoked by depolarizing from −80 to +60 mV in the absence (black) or presence (red) of 1 µM Zn2+. Traces were fitted with single-exponential functions (dashed green lines) to provide activation time constants (τact). (B) τact values measured at test voltages ranging from +10 to +90 mV in the absence (black squares) or presence (red circles) of 1 µM Zn2+ differed significantly (§, P < 0.0005) at all voltages. Data are shown as mean ± SEM (n = 13). At +60 mV, τact values were 66 ± 4 ms and 8.6 ± 0.6 ms with and without 1 µM Zn2+, respectively. (C) Values of τact at +60 mV were plotted versus Zn2+ concentration and fitted with a rectangular hyperbola (black line) to determine [Zn2+]1/2, which was 0.12 µM (n = 6–13). (D) The box plot shows τact values measured at +60 mV in the indicated concentrations of Zn2+ for I287H+F324H, the Shaker-IR parent channel, and the I287H and F324H single-mutant channels. Mean values of τact that differed significantly from no Zn2+ are indicated: *, P < 0.05; ‡, P < 0.005; §, P < 0.0005 (n = 2–13).
In I287H+F324H, Zn2+ increased the delay before channel opening and shifted the dependence of the delay on prepulse voltage in the depolarized direction (Fig. 3, A–C). In contrast, Zn2+ had no significant effect on the conductance–voltage curve (Fig. 3 C). The delay before channel opening arises from nonrate-limiting conformational changes of the voltage sensor domain that occur early in the activation pathway, including transitions from the resting state to the penultimate closed state (Perozo et al., 1994; Lin et al., 2010). The effect of Zn2+ on the delay indicates that the ion can access the site at rest and that binding stabilizes the resting conformation (Lin et al., 2010).
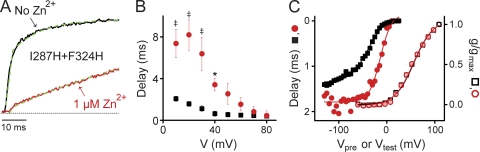
Figure 3.
Extracellular Zn2+ delays pore opening in I287H+F324H channels. (A) Representative current traces, evoked by depolarizing from −80 to +40 mV in the absence (black) or presence (red) of 1 µM Zn2+, are shown on an expanded scale to illustrate the effect of Zn2+ on the delay before pore opening. Delays were determined by extrapolating fitted single-exponential functions (dashed green lines) to the zero current level (dashed gray line) (Perozo et al., 1994; Lin et al., 2010). (B) The delay before pore opening was measured at test voltages ranging from +10 to +80 mV in the absence (black squares) or presence (red circles) of 1 µM Zn2+. Delay values differed significantly in the presence and absence of Zn2+, as indicated (*, P < 0.05; ‡, P < 0.005). Values are shown as mean ± SEM (n = 4–10). At +40 mV, delays measured with and without Zn2+ were 3.4 ± 0.6 ms and 0.6 ± 0.2 ms, respectively. (C) Zn2+ shifts the dependence of the delay on prepulse potential in the depolarized direction. Delay values have been plotted on an inverted scale as a function of prepulse potential. Delays were measured in the absence (black squares) or presence (red circles) of 1 µM Zn2+ by stepping from the holding potential of −80 mV to prepulse voltages ranging from −130 to 25 mV for 20 ms before depolarizing to +60 mV. Delay data were fitted with single Boltzmann functions (solid lines). Values of V1/2 and slope were −39 ± 1 mV and 18 ± 1 mV, and −14 ± 3 mV and 12 ± 2 mV, respectively, in the absence and presence of Zn2+ (n = 6–8). Normalized conductance values, measured as a function of test potential in the absence (open black squares) or presence (open red circles) of Zn2+, are shown on the same axes. Conductance data fitted with single Boltzmann functions (solid lines) with (red) or without (black) Zn2+ did not differ significantly. Values of V1/2 and slope were 44 ± 1 mV and 17 ± 1 mV, and 43 ± 1 mV and 18 ± 1 mV, respectively, in the absence and presence of Zn2+ (n = 5). For clarity, error bars have been omitted from the figure.
Extracellular Zn2+ binds to I287H+R1H in a state-dependent manner
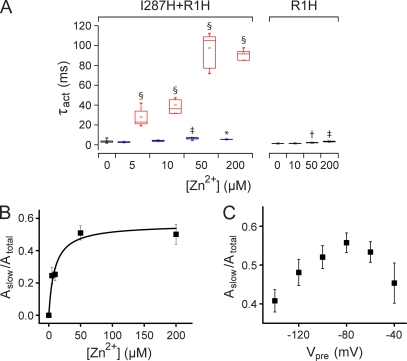
Previous studies suggest that R1 in S4 is in proximity to E1 and I287 in S2 in the resting conformation (Starace and Bezanilla, 2004; Tombola et al., 2005; Campos et al., 2007). To probe the state dependence of proximity between I287 and R1, we made the I287H+R1H double mutant and recorded ionic currents in the presence and absence of extracellular Zn2+ (Fig. 4). The addition of Zn2+ generated a slow kinetic component of activation that was prominent in the presence of a saturating concentration of Zn2+ (50 µM; Fig. 4 A). As a result, currents recorded in Zn2+ were not well fitted by a single-exponential component (Fig. 4 B). In contrast, Zn2+ had little effect on activation kinetics in the R1H single mutant (see Fig. 5 A).
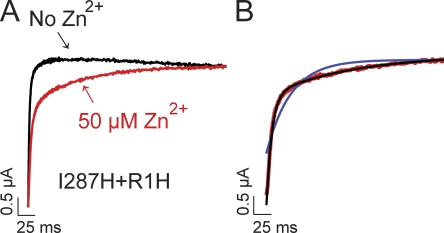
Figure 4.
Zn2+ induces a slow component of activation in I287H+R1H channels. (A) Currents were evoked by depolarizing from −80 to +60 mV in the absence (black) or presence (red) of 50 µM Zn2+. (B) Current trace obtained at +60 mV in the presence of Zn2+ (red) has been fitted with one (blue line) or the sum of two (black line) exponential functions.
Figure 5.
A subset of I287H+R1H voltage sensors binds Zn2+ at −80 mV. (A) The box plot shows τact values measured at +60 mV in the indicated concentrations of Zn2+ for I287H+R1H and the R1H single-mutant channel. I287H+R1H current traces were fitted with a single-exponential function in the absence of Zn2+ (black box, τact) and the sum of two exponential functions in the presence of Zn2+ (blue boxes, τfast; red boxes, τslow). R1H current traces were fitted with a single-exponential function in the presence and absence of Zn2+ (black boxes, τact). Mean values of τact, τfast, or τslow that differed significantly from no Zn2+ are indicated: *, P < 0.05; †, P < 0.01; ‡, P < 0.005; §, P < 0.0005 (n = 3–13). (B) The normalized amplitude of the slow component of activation measured at +60 mV in I287H+R1H channels has been plotted versus Zn2+ concentration (n = 4–7). The data were fitted with a rectangular hyperbola (black line) to obtain values for [Zn2+]1/2 and Aslow,max, which were 9.4 µM and 0.56, respectively. (C) Aslow,max was measured as a function of prepulse voltage in the presence of a saturating Zn2+ concentration (200 µM; n = 4–7). The membrane was stepped from −80 mV to prepulse voltages ranging from −140 to −40 mV for 1 s before depolarizing to +80 mV.
Zn2+-binding conformation in I287H+R1H is not the resting state
I287H+R1H current traces were fitted with the sum of two exponential functions to obtain estimates for the time constants, τfast and τslow, and their amplitudes, Afast and Aslow (Fig. 5 A). Normalized Aslow values were plotted versus Zn2+ concentration (Fig. 5 B). The data were fitted with a rectangular hyperbola to provide values for [Zn2+]1/2 (9.4 µM) and Aslow,max, the normalized maximum slow component amplitude. Interestingly, Aslow,max approached ∼56% at saturating Zn2+ concentrations (Fig. 5 B). This result strongly suggests that at −80 mV, the holding potential during the experiment, only a fraction of the voltage sensors are in a conformation in which I287H and R1H are in proximity and able to form a binding site for extracellular Zn2+.
In a population of channels, the distribution of voltage sensor conformations is controlled by membrane potential. To determine whether the Zn2+-binding conformation in I287H+R1H corresponds to the resting state, we investigated the effect of prepulse voltage on Aslow,max measured in the presence of a saturating concentration of Zn2+. The membrane was stepped from the holding potential of −80 mV to prepulse voltages ranging from −140 to −40 mV for 1 s before depolarizing to +80 mV. Current traces were fitted with the sum of two exponential functions to estimate Aslow,max. We found that Aslow,max decreased after both depolarizing and hyperpolarizing prepulses (Fig. 5 C). These data indicate that changing the membrane potential in either direction separates R1H and I287H, precluding Zn2+ binding. The decrease in Aslow,max after depolarizing prepulses is readily explained. Depolarization would be expected to move R1 toward the extracellular side of the membrane, away from I287. The more surprising result is that prepulse hyperpolarization also reduced Aslow,max. These data are incompatible with the idea that Zn2+ binds to the resting state in I287H+R1H. In that case, the value of Aslow,max would be expected to increase upon hyperpolarization because more voltage sensors would be drawn down into the resting, ion-binding conformation. In contrast, the decrease in Aslow,max upon hyperpolarization provides strong evidence that R1 moves further inward toward the gating charge transfer center in the resting state. With R1H located below F290, the ion-binding site would be disrupted.
I287H forms a binding site with A359H, located in the S3–S4 loop
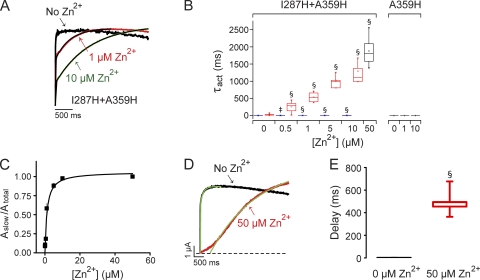
If I287H+R1H voltage sensors are unable to bind Zn2+ in the resting state because R1H is located in the gating charge transfer center, residues in the S3–S4 loop may be pulled into the plane of the membrane in the resting conformation. To investigate this possibility, we made the I287H+A359H double mutant and recorded ionic currents in the presence and absence of extracellular Zn2+. Position A359 is three residues N-terminal to R1 (R362) in Shaker (Fig. 1 A) (Tempel et al., 1987). The addition of a subsaturating concentration of Zn2+ generated a slow kinetic component of activation (Fig. 6 A). Currents recorded in 10 µM Zn2+ or less were not well fitted by a single-exponential component. In contrast, the A359H single mutant was not significantly affected by extracellular Zn2+ (Fig. 6 B).
Figure 6.
Extracellular Zn2+ slows activation and delays pore opening in I287H+A359H channels. (A) At subsaturating concentrations, Zn2+ induced a slow component of activation in I287H+A359H channels. The membrane was depolarized from −80 to +60 mV for 2 s. Representative current traces recorded in the presence or absence of Zn2+ (black, 0 µM; red, 1 µM; green, 10 µM) were scaled to the same amplitude, overlaid, and fitted with one (no Zn2+) or the sum of two (+Zn2+) exponential functions (black lines). (B) The box plot shows τact values measured at +60 mV in the indicated concentrations of Zn2+ for I287H+A359H and the A359H single-mutant channel. I287H+A359H current traces were fitted with the sum of two exponential functions (blue boxes, τfast; red boxes, τslow), except at 50 µM Zn2+, where one component was sufficient (black box, τact). A359H current traces were fitted with a single-exponential function (black boxes, τact). Mean values of τact, τfast, or τslow that differed significantly from no Zn2+ are indicated: ‡, P < 0.005; §, P < 0.0005 (n = 3–25). (C) The normalized amplitude of the slow component of activation measured at +60 mV has been plotted versus Zn2+ concentration (n = 3–10). The data were fitted with a rectangular hyperbola (black line) to obtain values for [Zn2+]1/2 and Aslow,max, which were 1.2 µM and 1.0, respectively. (D) Zn2+ increases the delay before pore opening. Representative current traces, evoked at +60 mV in the absence (black) or presence (red) of a saturating concentration (50 µM) of Zn2+, were fitted with single-exponential functions (green). The fitted functions were extrapolated to the zero current level (dashed line) to estimate the delay (Perozo et al., 1994; Lin et al., 2010). (E) The box plot shows the delay before pore opening measured in the absence (black box) or presence (red box) of 50 µM Zn2+. Mean values obtained at +60 mV were 496 ± 50 ms and 0.8 ± 0.1 ms, with and without Zn2+, respectively, and differed significantly (§, P < 0.0005; n = 5–9).
I287H+A359H current traces were fitted with the sum of two exponential functions (Fig. 6 B). Normalized Aslow values were plotted versus Zn2+ concentration (Fig. 6 C). The data were fitted with a rectangular hyperbola to provide values for [Zn2+]1/2 (1.2 µM) and Aslow,max. In a saturating concentration of Zn2+ (50 µM), Aslow,max reached 100% (Fig. 6 C). These results indicate that Zn2+ binding in I287H+A359H traps the voltage sensor in an absorbing conformation. To bind Zn2+, A359H must be in proximity to I287H. This strongly suggests that, in the ion-binding conformation, R1 has moved further toward the intracellular side of the membrane. These results are consistent with the conclusion that R1 is located in the gating charge transfer center in the resting conformation.
If Zn2+ traps the I287H+A359H voltage sensor in the resting conformation, ion binding would be expected to increase the delay before channel opening. We measured the delay in a saturating concentration of Zn2+ (50 µM) so that activation kinetics could be fitted reasonably well with a single-exponential function (Fig. 6 D). The addition of Zn2+ dramatically increased the delay before pore opening (Fig. 6 E). Because activation was extremely slow in 50 µM Zn2+ (see Fig. 6 B), it was not feasible to study the dependence of the delay on prepulse voltage.
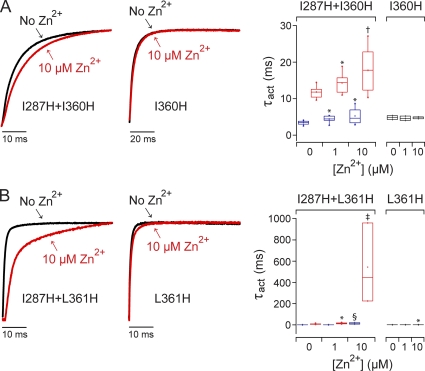
We also investigated the effect of Zn2+ on activation kinetics in I287H+I360H and I287H+L361H (Fig. 7). I360 and L361 are located between A359 and R1 (Tempel et al., 1987). With or without Zn2+, the activation kinetics of I287H+I360H and I287H+L361H were best fitted by the sum of two exponential components, providing estimates for τfast and τslow. We found that 10 µM Zn2+ increased τslow substantially in I287H+L361H, whereas 10 µM Zn2+ had a less dramatic effect on τslow in I287H+I360H (Fig. 7, A and B). Because L361 is immediately adjacent to R1 in the linear sequence, the ion-binding conformation in I287H+L361H may be similar to that in I287H+R1H. The finding that I287H is able to form Zn2+-binding sites with A359H, L361H, and R1H suggests that the directions of these side chains are not rigidly fixed. This may reflect the conformational flexibility of the S4 segment, which can adopt either 310- or α-helical secondary structures (Long et al., 2007).
Figure 7.
Effects of Zn2+ on channels containing I287H paired with histidine mutations in the S3–S4 loop. (A; left) I127H+I360H double mutant. (Middle) I360H single mutant. (B; left) I127H+L361H double mutant. (Middle) L361H single mutant. Currents were evoked by pulsing from −80 to +60 mV in the absence (black) or presence of 10 µM Zn2+ (red). Representative current traces have been scaled to the same amplitude and overlaid. At right, the box plots show τact values measured at +60 mV in the indicated concentrations of Zn2+ for (A) I287H+I360H and the I360H single-mutant channel or (B) I287H+L361H and the L361H single-mutant channel. Double-mutant current traces were fitted with the sum of two exponential functions (blue boxes, τfast; red boxes, τslow). Single-mutant current traces were fitted with a single-exponential function (black boxes, τact). Mean values of τact, τfast, or τslow that differed significantly from no Zn2+ are indicated: *, P < 0.05; †, P < 0.01; ‡, P < 0.005; §, P < 0.0005 (n = 3–10).
Stabilizing the resting state enhances Zn2+ binding in I287H+A359H
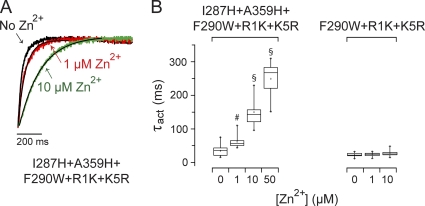
If I287H+A359H binds Zn2+ at rest, stabilizing the resting state should enhance binding. To test this prediction, we transferred the I287H+A359H binding site into the F290W+R1K+K5R triple-mutant background, which stabilizes the resting voltage sensor conformation (Tao et al., 2010). In the triple-mutant background, Zn2+ binding to the I287H+A359H site significantly slowed channel activation (Fig. 8 A). In contrast to I287H+A359H in the wild-type background, activation kinetics were well fitted by a single-exponential component, even in subsaturating concentrations of Zn2+ (Fig. 8, A and B). Therefore, at −80 mV, a large fraction of the voltage sensors were in the ion-binding conformation, that is, with A359H and I287H in proximity to each other. This is compatible with the finding that the bulk of the gating charge in F290W+R1K+K5R moves at voltages positive to −70 mV (Tao et al., 2010). This indicates that most voltage sensor domains are in the resting state at −80 mV, which corresponds to the ion-binding conformation in I287H+A359H.
Figure 8.
Zn2+ slows activation in I287H+A359H+F290W+R1K+K5R channels. (A) Currents were evoked by depolarizing to +60 mV in the absence or presence of Zn2+: black, 0 µM; red, 1 µM; green, 10 µM. Traces have been scaled to the same amplitude and overlaid. Currents were fitted with one exponential component (black lines) to obtain values for τact. (B) The box plot shows values of τact measured at +60 mV in I287H+A359H+F290W+R1K+K5R or the F290W+R1K+K5R control channel as a function of Zn2+ concentration. Mean values of τact that differed significantly from no Zn2+ are indicated: #, P < 0.001; §, P < 0.0005 (n = 6–15).
DISCUSSION
Our results strongly support the conclusion that R1 in the Shaker S4 segment occupies the gating charge transfer center in the resting voltage sensor conformation (Fig. 9 A). To investigate the position of R1, we took advantage of the I287H mutation, which can be used to generate Zn2+-binding sites in or near the pathway of S4 movement (Silverman et al., 2000; Lin et al., 2010). Ion binding directly modulates voltage sensor conformational changes (Tang et al., 2000; Silverman et al., 2004; Bannister et al., 2005; Lin et al., 2010). Importantly, binding sites involving I287H are accessible in the resting state and are located extracellular to the charge transfer center. It is worth noting that the relative positions of I287 and the charge transfer center are not likely to change during voltage-dependent activation because I287 and F290 are separated by only two residues in the linear sequence (Tempel et al., 1987).
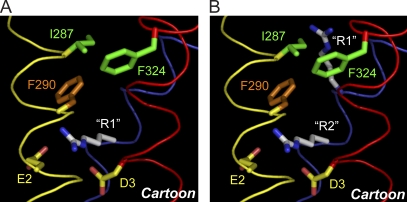
Figure 9.
R1 in S4 occupies the gating charge transfer center in the resting state. (A) Cartoon of the resting conformation shows an arginine representing R1 in the gating charge transfer center. (A and B) Ribbons representing the backbone atoms of S2, S3, and S4 are shown in yellow, red, and blue, respectively. Backbone atoms and side chains corresponding to I287, F290, E2, D3, and F324 (mutated in silico from tryptophan) in Shaker were extracted from the Kv1.2/Kv2.1 paddle chimera x-ray structure (2r9r) (Long et al., 2007). (B) Cartoon of the penultimate closed state shows arginines representing R1 in the vicinity of I287 and R2 in the gating charge transfer center. Other side chains and colored ribbons are the same as in A. The figure was made with PyMOL (v1.3).
We identified an ion-binding conformation in I287H+R1H that was maximally occupied at −80 mV. The fraction of voltage sensors capable of binding Zn2+ was reduced at either more positive or more negative voltages. These data suggest that R1H moves far enough outward or inward to abolish ion binding, going beyond the point where reorientation of the I287H and R1H side chains would be able to reestablish a binding site. It is likely that this requires movement of the S4 backbone. Our results are incompatible with the notion that R1 is close to I287 in the resting state, but are consistent with the idea that R1 and I287 are in proximity in an intermediate closed state in the activation pathway (Fig. 9 B).
In contrast, we found that Zn2+ bound to an absorbing conformation in I287H+A359H. A359 is located in the S3–S4 loop, extracellular to R1. Formation of a binding site between I287H and A359H indicates that the S4 segment has moved inward compared with I287H+R1H, drawing R1 down into the gating charge transfer center. Importantly, our results support the conclusion that R1 occupies the gating charge transfer center during activation of the wild-type channel because entry of R1 into this position does not require the F290W+R1K+K5R triple-mutant combination (Tao et al., 2010). However, transferring I287H+A359H into the triple-mutant background enhanced Zn2+ binding, consistent with the conclusion that the ion-binding conformation corresponds to the resting state.
Extracellular Zn2+ bound to the resting conformation in I287H+F324H and I287H+A359H with [Zn2+]1/2 values of 0.12 and 1.2 µM, respectively. These high apparent affinities suggest that the binding site residues are in atomic proximity of one another at rest (Lainé et al., 2003). In I287H+R1H, [Zn2+]1/2 was larger, 9.4 µM. This lower apparent affinity is compatible with our evidence that I287H+R1H voltage sensors can adopt two or more conformations at −80 mV, only one of which can bind Zn2+. However, we cannot rule out the possibility that the binding site residues are closer to each other in I287H+F324H and I287H+A359H than they are in I287H+R1H.
To initiate outward S4 movement from the resting state, R1 in the charge transfer center must be within the transmembrane electric field. A recent refinement of the Kv1.2 x-ray structure identified a highly conserved hydrophobic layer, ∼10-Å thick, in the voltage sensor domain where the transmembrane electric field is likely to be focused (Chen et al., 2010). This layer comprises 10 residues in S1, S2 (including F290), and S3a, regions that are thought to remain stationary relative to S4 during activation gating. The location of the hydrophobic layer is consistent with the idea that R1 experiences part of the transmembrane field in the resting conformation. Our results are compatible with a mechanism for voltage gating in Shaker in which R1 and K5 occupy the charge transfer center in the resting and activated states, respectively, and R2, R3, and R4 pass through this region during intermediate conformational changes.
The predominant conformation of S4 at negative voltages may be readily affected by R1 mutations, particularly neutralizations. If R1 is replaced by a neutral amino acid, S4 may be less likely to retract as far as it does in the wild-type channel. This could explain why some previous results suggest that R1 is near E1 and I287 in S2 and I241 in S1 in the resting state (Starace and Bezanilla, 2004; Tombola et al., 2005; Campos et al., 2007). In such mutants, R2 may be the primary occupant of the charge transfer center at hyperpolarized potentials (see Fig. 9 B). This would place R2 in the electric field, where it could initiate outward S4 movement upon depolarization (Chen et al., 2010).
Interestingly, S4 segments in a variety of voltage-gated ion channels contain different numbers of positively charged amino acids. For instance, Kv2.1 has a glutamine in the R1 position (Long et al., 2007). Depending on its sequence, the register of S4 in its maximally retracted position may differ. This raises the possibility that the structure of the resting conformation in voltage-gated channels varies depending on which S4 residue occupies the charge transfer center.
Acknowledgments
We are grateful to Dr. Fadi Issa for his comments on the manuscript.
This work was supported by National Institutes of Health grant R01 GM43459 to D.M. Papazian. J.-Y. Hsieh was partially supported by the Jennifer S. Buchwald Graduate Fellowship in Physiology.
Kenton J. Swartz served as editor.
Footnotes
Abbreviations used in this paper:
- IR
- inactivation removed
References
- Aggarwal S.K., MacKinnon R. 1996. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 16:1169–1177 10.1016/S0896-6273(00)80143-9 [DOI] [PubMed] [Google Scholar]
- Bannister J.P.A., Chanda B., Bezanilla F., Papazian D.M. 2005. Optical detection of rate-determining ion-modulated conformational changes of the ether-à-go-go K+ channel voltage sensor. Proc. Natl. Acad. Sci. USA. 102:18718–18723 10.1073/pnas.0505766102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F.V., Chanda B., Roux B., Bezanilla F. 2007. Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc. Natl. Acad. Sci. USA. 104:7904–7909 10.1073/pnas.0702638104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wang Q., Ni F., Ma J. 2010. Structure of the full-length Shaker potassium channel Kv1.2 by normal-mode-based X-ray crystallographic refinement. Proc. Natl. Acad. Sci. USA. 107:11352–11357 10.1073/pnas.1000142107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W.N., Aldrich R.W. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:533–538 10.1126/science.2122519 [DOI] [PubMed] [Google Scholar]
- Islas L.D., Sigworth F.J. 1999. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J. Gen. Physiol. 114:723–742 10.1085/jgp.114.5.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili-Araghi F., Jogini V., Yarov-Yarovoy V., Tajkhorshid E., Roux B., Schulten K. 2010. Calculation of the gating charge for the Kv1.2 voltage-activated potassium channel. Biophys. J. 98:2189–2198 10.1016/j.bpj.2010.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainé M., Lin M.C., Bannister J.P.A., Silverman W.R., Mock A.F., Roux B., Papazian D.M. 2003. Atomic proximity between S4 segment and pore domain in Shaker potassium channels. Neuron. 39:467–481 10.1016/S0896-6273(03)00468-9 [DOI] [PubMed] [Google Scholar]
- Lin M.C., Abramson J., Papazian D.M. 2010. Transfer of ion binding site from ether-à-go-go to Shaker: Mg2+ binds to resting state to modulate channel opening. J. Gen. Physiol. 135:415–431 10.1085/jgp.200910320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.B., Tao X., Campbell E.B., MacKinnon R. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 450:376–382 10.1038/nature06265 [DOI] [PubMed] [Google Scholar]
- Papazian D.M., Timpe L.C., Jan Y.N., Jan L.Y. 1991. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 349:305–310 10.1038/349305a0 [DOI] [PubMed] [Google Scholar]
- Pathak M.M., Yarov-Yarovoy V., Agarwal G., Roux B., Barth P., Kohout S., Tombola F., Isacoff E.Y. 2007. Closing in on the resting state of the Shaker K+ channel. Neuron. 56:124–140 10.1016/j.neuron.2007.09.023 [DOI] [PubMed] [Google Scholar]
- Perozo E., Santacruz-Toloza L., Stefani E., Bezanilla F., Papazian D.M. 1994. S4 mutations alter gating currents of Shaker K channels. Biophys. J. 66:345–354 10.1016/S0006-3495(94)80783-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa N.E., McCormack K., Tanouye M.A., Sigworth F.J. 1992. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 255:1712–1715 10.1126/science.1553560 [DOI] [PubMed] [Google Scholar]
- Seoh S.A., Sigg D., Papazian D.M., Bezanilla F. 1996. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 16:1159–1167 10.1016/S0896-6273(00)80142-7 [DOI] [PubMed] [Google Scholar]
- Silverman W.R., Tang C.-Y., Mock A.F., Huh K.-B., Papazian D.M. 2000. Mg2+ modulates voltage-dependent activation in ether-à-go-go potassium channels by binding between transmembrane segments S2 and S3. J. Gen. Physiol. 116:663–678 10.1085/jgp.116.5.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W.R., Bannister J.P.A., Papazian D.M. 2004. Binding site in eag voltage sensor accommodates a variety of ions and is accessible in closed channel. Biophys. J. 87:3110–3121 10.1529/biophysj.104.044602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starace D.M., Bezanilla F. 2004. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 427:548–553 10.1038/nature02270 [DOI] [PubMed] [Google Scholar]
- Tang C.-Y., Bezanilla F., Papazian D.M. 2000. Extracellular Mg2+ modulates slow gating transitions and the opening of Drosophila ether-à-go-go potassium channels. J. Gen. Physiol. 115:319–338 10.1085/jgp.115.3.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Lee A., Limapichat W., Dougherty D.A., MacKinnon R. 2010. A gating charge transfer center in voltage sensors. Science. 328:67–73 10.1126/science.1185954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel B.L., Papazian D.M., Schwarz T.L., Jan Y.N., Jan L.Y. 1987. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 237:770–775 10.1126/science.2441471 [DOI] [PubMed] [Google Scholar]
- Timpe L.C., Schwarz T.L., Tempel B.L., Papazian D.M., Jan Y.N., Jan L.Y. 1988. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature. 331:143–145 10.1038/331143a0 [DOI] [PubMed] [Google Scholar]
- Tombola F., Pathak M.M., Isacoff E.Y. 2005. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 45:379–388 10.1016/j.neuron.2004.12.047 [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V., Baker D., Catterall W.A. 2006. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K+ channels. Proc. Natl. Acad. Sci. USA. 103:7292–7297 10.1073/pnas.0602350103 [DOI] [PMC free article] [PubMed] [Google Scholar]