Abstract
The Xenopus laevis oocyte has been the workhorse for the investigation of ion transport proteins. These large cells have spawned a multitude of novel techniques that are unfathomable in mammalian cells, yet the fickleness of the oocyte has driven many researchers to use other membrane protein expression systems. Here, we show that some colonies of Xenopus laevis are infected with three multi-drug–resistant bacteria: Pseudomonas fluorescens, Pseudomonas putida, and Stenotrophomonas maltophilia. Oocytes extracted from infected frogs quickly (3–4 d) develop multiple black foci on the animal pole, similar to microinjection scars, which render the extracted eggs useless for electrical recordings. Although multi-drug resistant, the bacteria were susceptible to amikacin and ciprofloxacin in growth assays. Supplementing the oocyte storage media with these two antibiotics prevented the appearance of the black foci and afforded oocytes suitable for whole-cell recordings. Given that P. fluorescens associated with X. laevis has become rapidly drug resistant, it is imperative that researchers store the extracted oocytes in the antibiotic cocktail and not treat the animals harboring the multi-drug–resistant bacteria.
Introduction
Intact oocytes extracted from Xenopus laevis are a versatile expression system for the structural and functional investigation of ion channels and transporters. Ion transport proteins expressed in oocytes can be readily studied in the whole-cell configuration (two-electrode voltage clamp; TEVC) or in excised patches of membrane by exposing the extracellular side (outside-out) or the intracellular side (inside-out) of the protein (and membrane) to the bath solution in patch clamp recordings (Stühmer, 1998). Additionally, cramming inside-out patches back into the egg reexposes ion transport proteins to intracellular components, allowing for the exploration of ion channel regulation (Stühmer, 1998). Cutting open the oocyte simultaneously provides access to the intercellular milieu and reduces the time necessary to charge the membrane, permitting the measurement of fast ionic and gating charge currents (Kaneko et al., 1998; Stefani and Bezanilla, 1998). The oocyte’s physical properties also afford several experimental advantages. Injection of biopolymers into the oocyte cytoplasm is facile, which enables the precise control of protein subunit expression (Wang and Goldstein, 1995), incorporation of unnatural amino acids (Nowak et al., 1998), and examination of ion transport proteins expressed in bacteria (Maduke et al., 1998) and isolated from native cells (Marsal et al., 1995). The large size of the oocyte also makes single-cell biochemistry possible, enabling the direct comparison of cell surface expression and electrical recordings (Zerangue et al., 1999; Gage and Kobertz, 2004; Rocheleau et al., 2006). Even the pigmented animal pole is advantageous—blocking light from exciting the intracellular components, which facilitates imaging of plasma membrane proteins with epifluorescence (Sonnleitner et al., 2002).
Like any membrane protein expression system, oocytes have a dark side. The vitelline envelope must be removed for many experiments (Stühmer, 1998), and the endogenous currents cause some consternation, limiting the ionic composition of the external solution, the range of usable voltages, and the investigation of a few ion transport proteins (Weber, 1999). In addition, several extrinsic factors can affect egg quality, rendering them unusable for electrophysiological recordings. Frog husbandry conditions such as water quality, population density, and nutrition have been reported to affect oocyte quality (Green, 2002; Godfrey and Sanders, 2004; Delpire et al., 2011). Furthermore, many researchers report experiencing unexplained seasonal variations in oocyte quality even in laboratory environments where light and temperature are strictly controlled (Wu and Gerhart, 1991; Goldin, 1992; Delpire et al., 2011). Once extracted, oocytes are susceptible to microbial contaminations, one of which induces marbling of the oocyte pigment and rapid death (Elsner et al., 2000).
Despite good laboratory practices of both our animals and surgically harvested eggs, we recently observed a decline in oocyte quality. A few days after extraction, late stage oocytes (V and VI) formed black foci on the animal pole similar to the pigmented ring scar formed during wound healing (Gingell, 1970; Merriam and Christensen, 1983; Bement et al., 1999). The afflicted oocytes had negligible electric resting potentials and poor viability. Unsurprisingly, attempts to record from an injected batch of compromised oocytes were futile. Since frog skin harbors bacteria detrimental for oocyte longevity, we initially made hygienic changes in our animal husbandry and egg handling: none of which were effective and the oocytes remained unsuitable for electrophysiology experiments. Similarly, systematic tinkering with the standard antibiotics—penicillin, streptomycin, gentamicin and tetracycline—in the storage media did not prevent black foci formation. To identify the cause of the black foci, we cultured the compromised oocytes and discovered that they were infected with multi-drug–resistant Stenotrophomonas maltophilia, Pseudomonas fluorescens, and Pseudomonas putida. Antibiotic testing showed that all three species of bacteria were susceptible to amikacin and ciprofloxacin, which when included in the oocyte storage media, prevented the appearance of black foci and resulted in oocytes that were usable for electrophysiological recordings.
MATERIALS AND METHODS
Husbandry
X. laevis were ordered from commercial vendors (Nasco, Xenopus 1, and Xenopus Express). Female frogs used for our studies are ordered as adults, with a snout-to-vent length of 9 cm or greater. The primary Animal Medicine Facility at University of Massachusetts Medical School (UMMS) only houses frogs from a single vendor, which was our original and current vendor. Upon arrival, frogs are maintained in static tanks for a clinical, observational, and acclimation period lasting a minimum of 5 d. The maximum housing density of frogs in each static tank is 1 frog per 2 liter of artificial pond water. The water in the static frog housing tanks is changed at least twice weekly. The room is maintained at ∼20°C, with a humidity of 30–70%, and room lights on a 12:12-h light/dark cycle. Frogs are each fed 5–10 pellets of Frog Brittle (Nasco) twice weekly. At the end of the clinical observational period, healthy frogs are released to the standard colony housing area. Colony frogs are housed in either a continuous flow-through system (PharmHouse XLS Rack System; Pharmacal) or a recirculating frog housing system (X-Mod; Marine Biotech). Room environmental conditions and feeding schedules remain the same. The water supplied to the frog housing area is first passed through a 5-μm particulate filter and reverse osmosis system (HP 1200; CUNO/Water Factory Systems) before entering an intermediate holding tank. Water in the holding tank is treated with a commercial artificial lake salt additive (Cichlid Lake Salt; Seachem Laboratories Inc.) at a concentration of 5.5 g per 37.85 liter of water. While in the holding tank, the water is continually circulated through a 15-W ultraviolet light system until it is released. Water conditions in both colony systems are targeted at the following levels: water temperature, 17–19°C; pH, 6.6–7.0; conductivity, 1,550–1,650 μS; ammonia, 0–0.8 PPM; nitrites, 0–0.75 PPM; nitrates, 0–20 PPM. For the blind study, the animals from the three vendors were housed in an ancillary facility in static tanks under the aforementioned environmental and aquatic conditions listed.
Oocyte extraction
Late-stage oocytes (stage V–VI) are removed from the ovaries of adult female frogs in accordance with the procedures described in our UMMS IACUC-approved protocol. Specifically, the female is anesthetized by gradually cooling the frog in an ice water bath at 4°C over 30–45 min. Once anesthetized, the skin on the frog’s abdomen is sterilized by swabbing the surgical site with a 10% povidone-iodine solution. Autoclaved instruments are used for the extraction of ovarian tissue, and harvested oocytes are defolliculated for 30–60 min with 2 mg/ml type 2 collagenase (290 U/mg dw; Worthington Biochemical Corp) in sterile OR2 solution containing: 82.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, and 5 mM Hepes, pH 7.4. The same lot number of type 2 collagenase was used for the entire study, which also contained: 600 U/mg dw caseinase, 4.6 U/mg dw clostripain, 0.51 U/mg dw tryptic activity, based on the manufacturer’s certificate of analysis.
Oocyte storage conditions
After collagenase, isolated oocytes are rinsed with OR2 and stored in ND96-GT storage solution containing: 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Hepes, and 50 mM µg/ml of both gentamicin and tetracycline (Sigma-Aldrich) pH 7.4, at 16°C. Oocytes are checked daily for death and rinsed with ND96 solution with freshly supplemented antibiotics. The ND96-ACT storage solution is supplemented with 50 µg/ml of tetracycline and 100 µg/ml of both amikacin and ciprofloxacin (Sigma-Aldrich). All solutions containing antibiotics should be disposed in accordance with state regulations. For the Massachusetts Department of Environmental Protection, the solution is mixed with a chemical absorbent powder (kitty litter), autoclaved, and incinerated.
Imaging
Intact oocytes images were captured using SPOT imaging software (Diagnostic Imaging Inc.) using a dissecting scope equipped with a color digital camera. Images were processed in Photoshop (Adobe) and adjustments were limited to levels and cropping. For electron microscopy images of oocytes, the samples were fixed by immersion in 2.5% (vol/vol) glutaraldehyde in Na Cacodylate buffer (pH 7.2) for a minimum of 2 h at room temperature. The fixed oocytes were then washed three times in the same buffer. After the third wash, the eggs were dehydrated through a graded series of ethanol to 100%, and then Critical Point Dried in liquid CO2. The eggs were then affixed with silver conductive paste to the surface of aluminum scanning electron microscopy stubs and sputter coated with Au/Pd (80/20). Examination of the specimens was performed using an FEI Quanta 200 FEG MK II scanning electron microscope at 10 Kv accelerating voltage. For imaging of individual bacteria cultured from infected oocytes, the bacteria were stained with 1% uranyl acetate and spread onto a carbon stabilized formvar support film. Images were captured with a Philips CM 10 TEM at 7900X using an 80KV accelerating voltage.
Bacterial and fungal cultures
All cultures were submitted to a commercial diagnostic laboratory (IDEXX Preclinical Research Services). Bacterial culture samples were streaked onto 10% sheep blood agar with Columbia Blood Agar Base or MacConkey agar and were incubated at 21°C and 37°C for a minimum of 4–6 d. Samples submitted for fungal culture were streaked on Inhibitory Mold agar and incubated for 21 d.
Histopathology
Oocyte samples were fixed in 10% buffered formalin before preparation with hematoxylin and eosin stain for histopathological evaluation. The histopathology slides were submitted to and read by a board certified, contracted veterinary pathologist (Histo-Scientific Research Laboratories).
RESULTS
As in with many laboratories that use extracted Xenopus oocytes, our frog husbandry, surgical procedures, and oocyte handling were an amalgam of established procedures, documented prophylactic measures, and old wives tales (see Materials and methods for complete details). Our X. laevis were housed in circulating tanks, fed diligently by animal medicine staff, and bathed in a perfect circadian 12 h of light per day. We extracted the oocytes using sterilized instruments following our IACUC-approved surgical procedure, which enables survival surgeries to minimize animal usage. The extracted oocytes were defolliculated with collagenase and stored in standard ND96 buffer supplemented with gentamicin in a 16°C incubator. Many years ago, we observed the “marbled egg” phenotype described by Elsner et al. (2000); therefore, we included a sterile skin preparation with 10% povidone-iodine solution to our surgical procedure and added tetracycline to the oocyte storage media. With this combination of animal husbandry, surgical procedures and antibiotic cocktail, 9 out of 10 surgeries afforded experiment-quality oocytes that would last nearly a week with very little effort (daily media change).
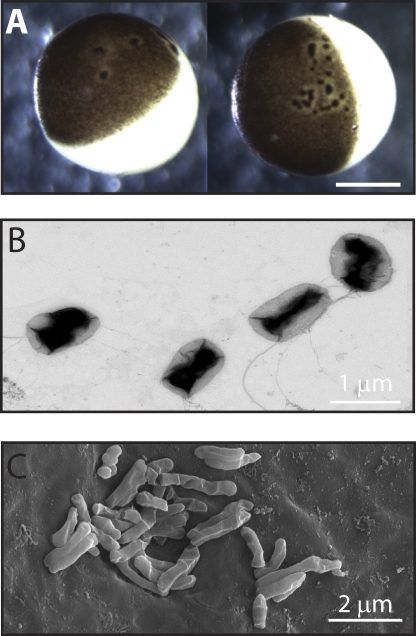
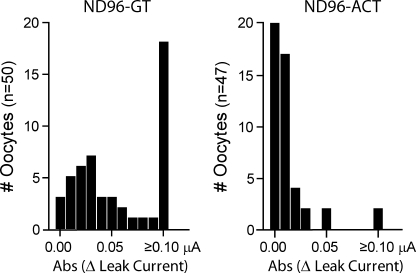
About 2 yr ago, we frequently observed that an entire batch of extracted eggs would develop multiple black foci surrounded by an unpigmented halo on the animal pole (Fig. 1 A). The black foci were reminiscent of the pigmented scar ring formed after microinjection (Merriam and Christensen, 1983; Bement et al., 1999), but would appear on both uninjected and injected eggs 3–4 d after extraction. In addition to morphological changes, these eggs had weak membranes that easily ruptured with typical handling. More distressingly, voltage-clamped, compromised oocytes rapidly developed a large, nonspecific leak current (>0.1 μA) within 2 min of establishing a holding potential (–80 mV; Fig. 2), which continued to increase until the oocytes became unclampable.
Figure 1.
Xenopus laevis oocytes infected with multi-drug–resistant bacteria. (A) Bright field micrograph of extracted oocytes showing the characteristic black foci and unpigmented halo. Bar, 0.5 mm. (B) Transmission electron micrograph of P. fluorescens cultured from compromised oocytes. (C) Scanning electron micrograph of bacteria on the surface of compromised oocytes.
Figure 2.
Infected oocytes exhibit reduced viability, which can be prevented with amikacin/ciprofloxacin supplementation. Histographs of the absolute change in leak current of oocytes incubated for 4 d in gentamicin/tetracycline (ND96-GT)- or amikacin/ciprofloxacin (ND96-ACT)-supplemented media. Change in leak current at –80 mV was calculated by subtracting the initial current value upon clamping from the value at 2 min. Data are from 2–5 batches of oocytes.
Because oocyte quality can diminish with husbandry conditions (Godfrey and Sanders, 2004; Delpire et al., 2011), we initially switched our frogs to static tanks and changed the salt solution of the artificial pond water; however, these husbandry changes did not prevent the formation of the black foci. To rule out contamination from our animal colony and facility, we ordered new frogs from the same vendor (housing them in a different vivarium) and a batch of surgically harvested oocytes that were directly shipped overnight to the laboratory. Disappointingly, the eggs from the new animals and the mail-ordered oocytes developed black foci and diminished viability 3–4 d after incubation in ND96 storage solution supplemented with gentamicin and tetracycline (ND96-GT). Because the addition of tetracycline to the storage media and increasing sterile technique previously prevented P. fluorescens from killing the oocytes (Elsner et al., 2000), we operated using a surgical drape and tried different combinations of antibiotics, including the more traditional penicillin/streptomycin (100 U/ml) antibiotic combination. However, none of these changes were able to thwart the formation of the black spots.
To determine whether the compromised oocytes were infected, we performed fungal and bacterial cultures on oocytes with black foci. Four batches of oocytes were tested for fungal contamination and all were negative. In contrast, 10 different batches of oocytes were infected with bacteria: 5 were infected with either P. putida or P. fluorescens (Fig. 1 B), and 5 were infected with S. maltophilia. Antibiotic susceptibility data showed that all three bacteria were resistant to gentamicin and tetracycline, as well as many other commonly used antibiotics (Table I). Scanning electron microscopy of the oocyte revealed that the bacteria were clustered on the cell surface (Fig. 1 C).
Table I.
Susceptibility of bacteria found in Xenopus oocytes
| Antibiotic | Bacterial susceptibility (MIC) | |
| Pseudomonas sp. | S. maltophilia | |
| Amikacin | S 4 (5/5) | S 4 (5/5) |
| Amoxicillin/Clavulanic Acid | R ≥ 32 (5/5) | R ≥ 32 (5/5) |
| Amoxicillin | R ≥ 32 (5/5) | R ≥ 32 (5/5) |
| Cefazolin | R (5/5) | R (5/5) |
| Cefixime | R (5/5) | R (5/5) |
| Cefotaxime | R (5/5) | R (5/5) |
| Cefpodoxime | R (5/5) | R (5/5) |
| Ceftazidime | S ≤ 8 (3/5), R (1/5), I 16 (1/5) | S ≤ 8 (5/5) |
| Ceftiofur | R ≥ 8 (5/5) | R ≥ 8 (5/5) |
| Ceftriaxone | R (4/5), I (1/5) | R (4/5), I (1/5) |
| Cefuroxime | R (5/5) | R (5/5) |
| Cefalexin | R ≥ 32 (5/5) | R ≥ 32 (5/5) |
| Chloramphenicol | R ≥ 32 (5/5) | R ≥ 32 (5/5) |
| Ciprofloxacin | S ≤ 0.05 (5/5) | S ≤ 0.05 (5/5) |
| Difloxacin | R (5/5) | R (5/5) |
| Enrofloxacin | R ≥ 2 (2/5), I 1 (3/5) | R ≥ 2 (5/5) |
| Gentamicin | R ≥ 16 (5/5) | R ≥ 16 (5/5) |
| Imipenem | S (5/5) | S (5/5) |
| Marbofloxacin | R (2/5), I (3/5) | R (4/5), I (1/5) |
| Ofloxacin | R (3/5), I (2/5) | R (5/5) |
| Orbifloxacin | R (5/5) | R (5/5) |
| Piperacillin | I 64 (4/5), I 128 (1/5) | I 64 (5/5) |
| Tetracycline | R ≥ 16 (5/5) | R ≥ 16 (5/5) |
| Ticarcillin | R ≥ 256 (5/5) | R ≥ 256 (5/5) |
| Tobramycin | R ≥ 16 (5/5) | R ≥ 16 (5/5) |
Bacterial culture results from 10 samples of affected oocytes. Both P. fluorescens and P. putida (Pseudomonas sp.) had identical bacterial susceptibility. S, susceptible; I, intermediate; R, resistant. Minimum inhibitory concentration (MIC) is represented in micrograms/milliliter. Results without MIC values were performed by disc testing. The numbers in parentheses represent the number of colonies with the indicated susceptibility/the total number of oocyte samples that grew that species of bacteria.
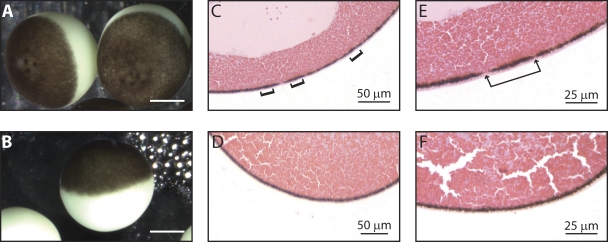
Based on our bacterial culture and sensitivity findings, we modified the antibiotic supplementation in the storage solution from gentamicin and tetracycline to amikacin and ciprofloxacin. At low levels of these antibiotics, 50 µg/ml, the black foci still formed on the animal pole of the oocytes; therefore, we increased the concentration to 100 µg/ml of amikacin and ciprofloxacin (ND96-ACT), which prevented black foci on a batch of oocytes. To rule out dumb luck, we harvested five batches of oocytes from different animals and stored the eggs in either ND96-GT or ND96-ACT, which was changed daily. Cultures were then performed on the oocytes after 4 d of incubation in the different antibiotic cocktails. Four out of five batches of oocytes grew multi-drug–resistant bacteria when stored in ND96-GT: three grew a Pseudomonas species and one grew S. maltophilia. Oocytes in the four samples with bacterial growth all developed the characteristic black spots associated with decreased electric resting potential and viability (Fig. 3 A). In contrast, the oocyte cohorts incubated in ND96-ACT did not grow any bacteria. Moreover, these oocytes did not exhibit any evidence of morphological or physiological abnormalities after 4 d in the storage solution (Fig. 3 B) and were useful for electrophysiological studies (Fig. 2).
Figure 3.
Amikacin/ciprofloxacin supplementation prevents black foci formation. Bright-field micrographs of extracted oocytes incubated for 4 d in tetracycline/gentamicin-supplemented (ND96-GT; A) or amikacin/ciprofloxacin-supplemented media (ND96-ACT; B). Bars, 0.5 mm. Histopathology slices of a compromised oocyte (C) and a healthy oocyte (D), magnified in E and F, respectively. Brackets denote areas of increased pigmentation; arrows indicate areas devoid of pigment molecules, both of which were only observed in infected oocytes.
To more closely examine the morphological changes induced by the multi-drug–resistant bacteria, we histopathologically compared the infected and uninfected oocytes. Black foci on the surface of infected oocytes correlated to areas with an increased number of pigmentation granules (Fig. 3 C), surrounded by a region with diminished pigmentation granules (Fig. 3 E). Alternatively, oocytes stored in the ND96-ACT media had continuously smooth membranes with a uniform distribution of pigment granules (Fig. 3, D and F).
Finally, we attempted to identify the source of the multi-drug–resistant bacteria. Although we were able to repeatedly culture multi-drug–resistant bacteria from oocytes incubated for 4 d in ND96-GT (Table I), in general, most cultures sampled from oocytes directly harvested from the animals were negative for bacterial growth. However, one sample did grow a multi-drug–resistant strain of P. putida. The harvested oocytes from this frog that were not used for culture were collagenased and stored in ND96-GT, where they developed multifocal to coalescing black foci on their surfaces. After 4 d of incubation, cultures from these oocytes demonstrated continued infection with multi-drug–resistant P. putida.
Because only one animal’s oocytes were sufficiently infected to test positive for multi-drug–resistant bacteria immediately after surgery, we next tested for a contamination source in our laboratory and vivarium. Cultures from our collagenase and OR2 solutions grew no bacteria. Biofilm and water samples from our aquarium grew four ubiquitous bacteria: Pseudomonas pseudoalcaligenes, Aeromonas hydrophila, Corynebacterium sp. and Alcaligenes sp.—all of which were not multi-drug resistant and sensitive to gentamicin and tetracycline. Four skin swabs from nine colony frogs also grew bacteria (Acinetobacter baumannii and Aeromonas hydrophilia) that were similarly sensitive to most antibiotics, including tetracycline and gentamicin. Cultures from the skin of these nine frogs after a surgical preparation with povidone-iodine were all negative, as were the cultures of their coelomic cavities. Having tested all conceivable sources of contamination (and to have experimental quality oocytes during the “black dot” pandemic), we performed a blind study: our animal medicine facility ordered five frogs from three different vendors (our original vendor and two additional vendors), which were housed in separate static tanks in an ancillary animal facility. After performing 15 surgeries and storing the eggs in ND96-GT for 4 d, we only observed the black foci on oocytes extracted from animals (4 out of 5) ordered from one vendor, which was confirmed by animal medicine to be the original vendor. In total, these data suggest that the animal’s ovarian tissue and/or oocytes are the likely source of the multi-drug–resistant bacteria.
DISCUSSION
We have shown that X. laevis harbor multi-drug–resistant bacteria that infect entire batches of surgically harvested oocytes stored in standard antibiotic-supplemented media. By including amikacin and ciprofloxacin in the storage media (ND96-ACT), we were able to prevent black foci formation to afford experimental quality oocytes. In all of our experiments with amikacin- and ciprofloxacin-supplemented media, tetracycline was also included in the storage solutions because we did not know at the time that P. fluorescens had become resistant to tetracycline. Based on the antibiotic susceptibility of the bacteria that we cultured from oocytes (Table I), amikacin and ciprofloxacin should be sufficient. Nonetheless, we have yet (mostly out of fear) to eliminate tetracycline from our antibiotic cocktail. Given the strong correlation between antibiotic susceptibility and the appearance of the black foci, we believe these multi-drug–resistant bacteria are responsible for the diminished resting potential and poor viability oocytes that we observed. Although we could switch to another vendor and use ND96-GT, which is cheaper than ND96-ACT ($9 versus $12 USD for a 1-liter solution), we consider the quality of extracted oocytes from our original vendor’s animals to be far superior, provided they are not infected with multi-drug–resistant bacteria.
The multiple black foci on the animal poles of infected oocytes are highly reminiscent of the pigment changes seen during the process of oocyte healing after puncture: an increased region of pigmentation surrounded by a halo of diminished pigmentation (Gingell, 1970; Merriam and Christensen, 1983). Amphibian oocytes heal by closure of an actomyosin-based purse string (Bement et al., 1999). During this process, cytoplasmic pigment granules coalesce to form a dense spot, referred to as a scar, which can be seen on the cell surface. This scar is most visible on the surface of the animal pole of the oocyte because of the large amount of pigmentation granules; however, similar processes of healing can be observed on the vegetal pole (Merriam and Christensen, 1983). The striking similarity between wound healing scars and the black foci we observed in compromised oocytes suggests that the bacteria on the oocyte cell surface (Fig. 1 C) are puncturing the membrane to enter the egg. The continual repair of multiple membrane ruptures is consistent with the observed low electric resting potential and cytoplasm leakage from the oocytes.
It is concerning that tetracycline was previously effective against P. fluorescens when identified as a contaminant of X. laevis oocytes (Elsner et al., 2000), but is now ineffective. Although it may be tempting to prophylactically treat the frogs or the frog housing system with amikacin and ciprofloxacin to prevent oocyte contamination, this is not an appropriate course of action. Overuse and inappropriate dosing of antibiotics are considered to be a major cause of the emergence of antibiotic-resistant bacteria (Sosa, 2007). For amphibian medicine, it is a major issue because there is remarkably sparse pharmacokinetic information on safe and effective antibiotic dosing of many species, including X. laevis (Reavill, 2001; Wright and Whitaker, 2001; Green, 2010). Coincidentally, tetracycline is one of a few antibiotics that has accumulated a modest amount of dosing guidelines for this species. It is unknown if tetracycline is used with any regularity at the vendor’s facility for treatment of health conditions in the frogs. If so, its use with X. laevis may have contributed to the development of tetracycline-resistant P. fluorescens. Alternatively, our observation of multi-drug–resistant P. fluorescens may simply be a reflection of the same global shifts in antibiotic resistance observed in the human health industry, which is presumed to be caused by years of antibiotic use. With so few antibiotic options left for treating the Pseudomonas and Stenotrophomonas species associated with oocyte pathology in X. laevis, judicious use of antibiotics in vitro, rather than in vivo, is warranted.
Acknowledgments
We are indebted to Dr. Charles Sagerström for the use of his dissecting microscope, and to Dr. Gregory Hendricks and Dr. Lara Strittmatter for their valuable assistance with bacterial imaging.
This work was supported by a grant to W.R. Kobertz from the National Institutes of Health (DC-007669).
The authors declare that they have no competing financial interests.
Christopher Miller served as editor.
References
- Bement W.M., Mandato C.A., Kirsch M.N. 1999. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr. Biol. 9:579–587 10.1016/S0960-9822(99)80261-9 [DOI] [PubMed] [Google Scholar]
- Delpire E., Gagnon K.B., Ledford J.J., Wallace J.M. 2011. Housing and husbandry of Xenopus laevis affect the quality of oocytes for heterologous expression studies. J. Am. Assoc. Lab. Anim. Sci. 50:46–53 [PMC free article] [PubMed] [Google Scholar]
- Elsner H.A., Hönck H.H., Willmann F., Kreienkamp H.J., Iglauer F. 2000. Poor quality of oocytes from Xenopus laevis used in laboratory experiments: prevention by use of antiseptic surgical technique and antibiotic supplementation. Comp. Med. 50:206–211 [PubMed] [Google Scholar]
- Gage S.D., Kobertz W.R. 2004. KCNE3 truncation mutants reveal a bipartite modulation of KCNQ1 K+ channels. J. Gen. Physiol. 124:759–771 10.1085/jgp.200409114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell D. 1970. Contractile responses at the surface of an amphibian egg. J. Embryol. Exp. Morphol. 23:583–609 [PubMed] [Google Scholar]
- Godfrey E.W., Sanders G.E. 2004. Effect of water hardness on oocyte quality and embryo development in the African clawed frog (Xenopus laevis). Comp. Med. 54:170–175 [PubMed] [Google Scholar]
- Goldin A.L. 1992. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 207:266–279 10.1016/0076-6879(92)07017-I [DOI] [PubMed] [Google Scholar]
- Green S.L. 2002. Factors affecting oogenesis in the South African clawed frog (Xenopus laevis). Comp. Med. 52:307–312 [PubMed] [Google Scholar]
- Green S. 2010. The Laboratory Xenopus sp. CRC Press, Boca Raton, FL: 180 pp [Google Scholar]
- Kaneko S., Akaike A., Satoh M. 1998. Cut-open recording techniques. Methods Enzymol. 293:319–331 10.1016/S0076-6879(98)93021-X [DOI] [PubMed] [Google Scholar]
- Maduke M., Williams C., Miller C. 1998. Formation of CLC-0 chloride channels from separated transmembrane and cytoplasmic domains. Biochemistry. 37:1315–1321 10.1021/bi972418o [DOI] [PubMed] [Google Scholar]
- Marsal J., Tigyi G., Miledi R. 1995. Incorporation of acetylcholine receptors and Cl- channels in Xenopus oocytes injected with Torpedo electroplaque membranes. Proc. Natl. Acad. Sci. USA. 92:5224–5228 10.1073/pnas.92.11.5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam R.W., Christensen K. 1983. A contractile ring-like mechanism in wound healing and soluble factors affecting structural stability in the cortex of Xenopus eggs and oocytes. J. Embryol. Exp. Morphol. 75:11–20 [PubMed] [Google Scholar]
- Nowak M.W., Gallivan J.P., Silverman S.K., Labarca C.G., Dougherty D.A., Lester H.A. 1998. In vivo incorporation of unnatural amino acids into ion channels in Xenopus oocyte expression system. Methods Enzymol. 293:504–529 10.1016/S0076-6879(98)93031-2 [DOI] [PubMed] [Google Scholar]
- Reavill D.R. 2001. Amphibian skin diseases. Vet. Clin. North Am. Exot. Anim. Pract. 4:413–440: vi [DOI] [PubMed] [Google Scholar]
- Rocheleau J.M., Gage S.D., Kobertz W.R. 2006. Secondary structure of a KCNE cytoplasmic domain. J. Gen. Physiol. 128:721–729 10.1085/jgp.200609657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnleitner A., Mannuzzu L.M., Terakawa S., Isacoff E.Y. 2002. Structural rearrangements in single ion channels detected optically in living cells. Proc. Natl. Acad. Sci. USA. 99:12759–12764 10.1073/pnas.192261499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa A. 2007. The threat of antibiotic-resistant bacteria and the development of new antibiotics. Antimicrobial Resistance in Bacteria. Amabile-Cuevas C., editor Horizon Bioscience, Norfolk, UK: 7–24 [Google Scholar]
- Stefani E., Bezanilla F. 1998. Cut-open oocyte voltage-clamp technique. Methods Enzymol. 293:300–318 10.1016/S0076-6879(98)93020-8 [DOI] [PubMed] [Google Scholar]
- Stühmer W. 1998. Electrophysiologic recordings from Xenopus oocytes. Methods Enzymol. 293:280–300 10.1016/S0076-6879(98)93019-1 [DOI] [PubMed] [Google Scholar]
- Wang K.W., Goldstein S.A. 1995. Subunit composition of minK potassium channels. Neuron. 14:1303–1309 10.1016/0896-6273(95)90277-5 [DOI] [PubMed] [Google Scholar]
- Weber W. 1999. Ion currents of Xenopus laevis oocytes: state of the art. Biochim. Biophys. Acta. 1421:213–233 10.1016/S0005-2736(99)00135-2 [DOI] [PubMed] [Google Scholar]
- Wright K.M., Whitaker B.R. 2001. Pharmacotherapeutics. Amphibian Medicine and Captive Husbandry. Wright K.M., Whitaker B.R., Krieger Publishing Co, Malabar, FL: 309–330 [Google Scholar]
- Wu M., Gerhart J. 1991. Raising Xenopus in the laboratory. Methods Cell Biol. 36:3–18 10.1016/S0091-679X(08)60269-1 [DOI] [PubMed] [Google Scholar]
- Zerangue N., Schwappach B., Jan Y.N., Jan L.Y. 1999. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 22:537–548 10.1016/S0896-6273(00)80708-4 [DOI] [PubMed] [Google Scholar]