Abstract
The authors report a case of a 55-year-old Caucasian woman who received autologous bone marrow stem cell transplantation 3 years after a subcortical stroke. She exhibited positive cognitive changes 6 months and 1 year after the surgery without rehabilitation. The blood flow changes, measured with SPECT, were statistical significant in prefrontal areas. During the presurgical neuropsychological assessment, the patient presented a critical speech reduction, reflected in impaired performance in verbal fluency, vocabulary and in each task which required overt verbal response. One year later, she showed improvement in mental flexibility, receptive language, phonological fluency, verbal memory and auditory verbal memory. Positive cognitive changes in verbal and executive functions seem to be contingent on increased blood flow in prefrontal areas. Posterior neuropsychological evaluation 3 and 5 years after transplantation did not show deterioration of the cognitive improvement.
Background
This is the only report on cognitive improvement after bone marrow stem cell (BMSC) transplantation in humans. A previous open-label human trial of implanted LBS-Neurons (Layton BioScience, Sunnyvale, California, USA) for chronic stroke using cultured cells reported cognitive changes 6 months after surgery, and fluorodeoxyglucose positron emission tomography (FDG-PET) data demonstrated a relationship between relative regional metabolic changes and clinical performance measures.1
Case presentation
A 55-year-old Caucasian woman presented with a middle cerebral artery occlusion that resulted in left unilateral lesions of the pyramidal tract, the anterior arm and knee of the internal capsule, the extreme and external capsules, the insular lobe, claustrum, thalamus and striatum, the inferior parietal and temporal lobes and the inferior longitudinal fasciculus.
The patient at presurgical or baseline evaluation presented motor and sensory deficits and residual arm and leg paralysis in the right hemibody evaluated by neurological scales. One year later, she obtained better performance at NIH Stroke Scale (all items) 3.4%; Barthel Index 10%; Scandinavian Stroke Scale (all items) 13.4%; Tinetti Scale (gait and equilibrium) 12.5%; and Medical Research Council Stroke Scale (motor defects) 2.5% (for a detailed report see table 1).
Table 1.
Results of neurological and functional scales
| Scale | Presurgical | 6 Months after | 12 Months after | Improvement (%) |
|---|---|---|---|---|
| NIH Stroke scale (NIHSS) item 5 (arm) | 2 | 2 | 2 | 0 |
| NIHSS item 6 (leg) | 2 | 1 | 1 | 0 |
| NIHSS (all items) | 13 | 12 | 12 | 3.4 |
| Scandinavian Stroke Scale (SSS) (arm) | 0 | 0 | 0 | 0 |
| SSS (leg) | 4 | 5 | 5 | 0 |
| SSS (all items) | 29 | 35 | 36 | 13.4 |
| Tinetti Scale (gait and equilibrium) | 7 | 9 | 9 | 12.5 |
| Medical Research Council Stroke Scale (motor defects) | 48 | 50 | 50 | 2.5 |
| Ashworth Scale (rigidity) | 8 | 7 | 7 | 6.2 |
| Barthel Index | 75 | 75 | 85 | 10 |
For a detailed description of the scales employed in this analysis, see the table 4 of the original paper (Suárez-Monteagudo et al, 2009).
She finished the secondary school and was professionally active at the moment of the stroke (3 years before this intervention) working as a secretary. After stroke, she discontinued work, remaining at home partially doing some domestic chores. She was married at the moment of the intervention but her husband died 3 years after transplantation. Now, she is living with a daughter and granddaughter.
The neuropsychological assessment at the baseline reflected language impairments characterised by a critical speech reduction, poor performance in verbal fluency, phonological paraphasias which increased with repetition, and articulation deficits. This verbal deficiency and the paralysis of the right and dominant hand negatively influenced her performance at the majority of the tests, but in some of them she reached the control group (ie, Wisconsin Card Sorting Test (WCST) and the Auditory Verbal Learning Test (AVLT)-recognition).
Investigations
Neuropsychological assessment
The battery comprised the following tests: Wechsler Adult Intelligence Scale (WAIS)-r (full scale FSIQ, verbal VIQ and performance PIQ); Wisconsin Sorting Card Test (categories sorted, total and perseverate errors); Peabody Picture Vocabulary Test receptive language (errors); Wechsler Memory Scale (memory); Trail Making Part A: attention (time completion), Part B: mental flexibility (time completion) and B−A (difference in seconds); Rey Complex Figure Test: visospatial/construction, non-verbal memory (copy, immediate recall); Rey AVLT: verbal learning and memory (recognition, immediate and delayed recall); Verbal Fluency: phonological and semantic.
The neuropsychological assessment was performed before surgery (baseline) and 6 months, 1, 3 and 7 years after surgery (follow-up) and was carried out by neuropsychologists who were aware that the patient was part of the open-label exploratory study.
The individual scores were processed using two methods:
-
(A)
Z scores: The quantitative data obtained from neuropsychological tests and scales were normalised using a method proposed by Crawford and Howell2 to estimate the abnormality of the patient’s test scores when compared with a control sample of healthy subjects. The patient’s performance was converted to a z score based on the mean and SD of the control sample and this z is referred to a table of areas under the normal curve. With this approach, the control sample is treated as though it were a population. This procedure tested whether the patient’s score is significantly below that of controls, and provides a point estimate of the abnormality of the score. The z was calculated using a group of 10 healthy subjects similar in age and educational level to the patient. These normative ‘z’ scores were employed to assess the individual deficits and to calculate the differences between patients and controls, using the corresponding p value (two-tailed T distribution).
-
(B)
Summary of change (SC): In order to compare the global performance of the patient before and after the surgery, we calculated the percent change of individual raw score which was defined as SC=log10((baseline_score-follow-up_score)/baseline_score) of every neuropsychological variable. The SC or global change score quantified, in each case, the magnitude and direction of the global cognitive change. This global cognitive score is convenient because it reflected the final individual improvement (positive value) or deterioration (negative value) independent of the clinical and personal characteristics because it compares the same individual in two conditions. The logarithm was employed to unify in single scale the different outcomes of the neuropsychological variables (time in seconds, number of errors, number of exemplars or categories, etc).
MRI acquisition
High-resolution MRI study (T1 MPRAGE) was performed during the presurgical evaluation with a 1.5 T Symphony scanner (Siemens, Erlangen, Germany): TR=11.9 ms, TE=3.9 ms, NEX= 1, flip angle= 15°, slice thickness =1 mm, field of view= 25 cm and matrix size = 256×256.
SPECT acquisition and preprocessing
Perfusion SPECT scanning was carried out during the presurgical evaluation, 12 and 20 months after transplantation. All studies were performed using a double-head SPECT system (DST Xli; Sopha Medical Vision, Buc, France) equipped with ultra-high-resolution fan-beam collimators. The measured tomographic resolution for 99mTc was 8.5 mm in the centre of the image at the fixed radius of rotation of 150 mm. The imaging was started 10 min after injection of 555 MBq of 99mTc-ethyl cysteinate dimer into the antecubital vein of the right arm under resting condition (supine, eyes open, dimly lit quiet room). Projection data were obtained for each camera in a 128×128 format for 64 angles at 26 s/angle. Total counts were equal or greater than 5×106. A Butterworth filter was used for image back-projection reconstruction of SPECT images (cut-off frequency= 0.026 cycle/cm and order= 7). Attenuation correction was performed using Chang’s method with attenuation coefficient μ=0.085 cm.
Before data analysis, the SPECT volumes acquired before and after surgery were scaled by the mean value per voxel of the global brain counts and co-registered onto their corresponding MRI dataset, using statistical parametric mapping software SPM5 of the Wellcome Department of Cognitive Neurology, UK.3 Two difference images were obtained by subtraction of the presurgical from the postsurgical SPECT (12 and 20 months, respectively). The difference images were thresholded at 2 SD, above and below the mean value per voxel of the global brain counts of the corresponding difference image, to highlight regions of perfusion increase or decrease. The identified regions were set as volume of interest (VOI). The mean relative counts value per voxel of the identified VOIs was then calculated for the three original SPECT images of the patient.
Treatment
The patient was part of an open study of five chronic stroke patients reported by our group,4 which provided evidence on the safety and tolerance of the BMSC transplantation and showed recovery of motor functions documented by neurological scales.
The total number of cells implanted varied from 14 to 55 million and was related to individual parameters of viability and survival rate of cells and was not assigned to the subjects a priori, but was determined by the patient’s individual conditions.
Her therapy consisted of 48 million BMSCs implanted along several tracts around perilesional areas of the left hemisphere using stereotactic surgery.
She was selected for this study according to pre-established inclusion/exclusion criteria4 and followed up for 6 months and 1, 3 and 5 years. During the first year after surgery, she did not receive other restorative intervention or rehabilitation. The presurgical pharmacological treatment was maintained unaltered during the postsurgical follow-up period.
Outcome and follow-up
Six months and 1 year after surgery, her verbal skills showed improvements in receptive language, phonological fluency and auditory verbal memory and learning.
The full-scale IQ (WAIS-r) increased more than 1 SD due to verbal subscale increase. Relative to the baseline, the speed of processing on the Trail Making A and B and the difference (B−A) improved with completion times almost half after surgery, suggesting more effective attention and mental flexibility. Additionally, errors on the WCST decreased 50%. There were no changes in performance of visuospatial function and non-verbal memory. Her improvements in several tests reached the control group (see z and raw scores in table 2), for example, the immediate and delayed recall of the AVLT.
Table 2.
Neuropsychological results
| Presurgical | 6 Months | 12 Months | 3 Years | 5 Years | |
|---|---|---|---|---|---|
| Raw data | |||||
| REY copy | 30 | 32 | 30 | 35 | 35 |
| REY mem | 8 | 6 | 8 | 12 | 11 |
| WCST categ | 6 | 6 | 6 | 6 | 5 |
| WCST error | 36 | 14 | 18 | 19 | 36 |
| WCST pers | 7 | 3 | 9 | 6 | 6 |
| Phonol F | 1 | 1 | 2 | 5 | 2 |
| Semant F | 5 | 5 | 5 | 5 | 8 |
| Peab_err | 49 | 29 | 23 | 20 | 17 |
| AVLT recog | 13 | 12 | 13 | 14 | 13 |
| AVLT delay | 1 | 4 | 4 | 7 | 10 |
| AVLT imm | 2 | 3 | 5 | 4 | 4 |
| Trail A | 56 | 32 | 48 | 57 | 22 |
| Trail B | 85 | 104 | 63 | 66 | 84 |
| Trail B−A | 29 | 72 | 15 | 9 | 62 |
| WMS | 50 | 66 | 74 | 78 | 62 |
| FSIQ | 55 | 73 | 85 | 88 | 79 |
| VIQ | 0 | 64 | 74 | 78 | 70 |
| PIQ | 70 | 88 | 102 | 104 | 95 |
| Z scores | |||||
| REY copy | −18.6574382 | −12 | −19 | −2.84605 | −2.84605 |
| REY mem | −3.44210626 | −3.9 | −3.4 | −2.58692 | −2.80072 |
| WCST categ | 0.176166066 | 0.62 | 0.62 | 0.616581 | 0.176166 |
| WCST error | 0.019933665 | 0.9 | 0.74 | 0.697678 | 0.019934 |
| WCST pers | 0.125217581 | 0.63 | −0.1 | 0.250435 | 0.250435 |
| Phonol F | −2.80536088 | −2.8 | −2.6 | −1.79442 | −2.55263 |
| Semantic | −4.92950302 | −4.9 | −4.9 | −4.9295 | −4.10792 |
| Peab_err | −8.67182386 | −4.1 | −2.7 | −2.00119 | −1.31112 |
| AVLT recog | 0.297672009 | 0.03 | 0.3 | 0.568283 | 0.297672 |
| AVLT delay | −4.07645465 | −2.7 | −2.7 | −1.32828 | 0.045803 |
| AVLT imm | −2.02590976 | −1.6 | −0.7 | −1.14508 | −1.14508 |
| Trail A | −5.2043495 | −1.6 | −4 | −5.35433 | −0.10499 |
| Trail B | −4.24496804 | −6 | −2.2 | −2.44866 | −4.15043 |
| Trail B−A | −1.47979848 | −7.7 | 0.55 | 1.421767 | −6.26738 |
| WMS | −3.77367041 | −2.8 | −2.3 | −2.11231 | −3.06166 |
| FSIQ | −5.25303147 | −3.8 | −2.8 | −2.58611 | −3.31345 |
| VIQ | −10.4972244 | −5 | −4.1 | −3.72766 | −4.43099 |
| PIQ | −4.06263456 | −2.5 | −1.4 | −1.20281 | −1.95982 |
The boldface identifies the individual z score under the control group level (n=10), p=0.05, Z=−1.92.
In order to compare the intrasubject global performance before and after the surgery, we calculated the percent change of individual raw scores, which was defined as SC of every neuropsychological variable. The SC or global change score quantified in her case was always positive: 6 months (SC=3.37), 1 year (SC=4.9), 3 years (SC=5.6) and 5 years (SC=5.54) after transplantation.
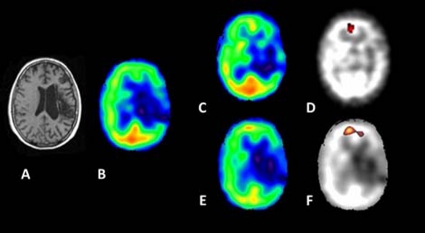
A prefrontal VOI of the left hemisphere (2497 mm3) was defined using the difference image obtained by subtraction of the presurgical from the 12 month postsurgical SPECT with a mean value of 1.01 at the baseline and 1.22 later. A second prefrontal bilateral VOI (12 291 mm3), including the first mentioned, was obtained by subtraction from the second postsurgery SPECT and showed an increase of blood flow with a mean value of 1.43 (see figure 1).
Figure 1.
(A) MRI of the patient. (B) SPECT at baseline. (C) SPECT after 12 months. (D) SPECT parametrical image obtained after subtraction between 12 months and baseline. (E) SPECT after 20 months. (F) Parametrical image after subtraction between 20 months and baseline. The resulting functional parametric images plotted in the native individual space showed an increased blood flow in the left prefrontal area which was more accentuated in the second postoperatory scan at the anterior cingulated gyrus. The brighter colours indicate more intensity of blood flow.
Discussion
Stilley et al reported cognitive improvement in a study where nine subjects with basal ganglia stroke (two controls, seven transplants), all over 2 years poststroke, completed a comprehensive neuropsychological test battery before and 6 months after treatment. The patients received between 5 to 10 million of cultured cells. Four transplanted subjects who had strokes in the non-dominant hemisphere showed marked improvement on the Rey Complex Figure, a test of visuospatial/constructional ability and non-verbal memory, which was the only cognitive domain impaired. The magnitudes of change (more than 2 SD) were beyond that expected of practice or placebo effect. The improvement in visuospatial/skills was considered consistent with the increased FDG uptake on PET scan reported in remote brain locations including the frontal lobe and cerebellum in the same group of patients.5
One of the main limitations reported by Stilley was the restricted length of follow-up. In our case, the patient also had a positive and incremental cognitive change, but in four different times, after transplantation in language and executive functions and two SPECT scans indicating the same cortical pattern of prefrontal increase of blood flow.
Cortical reorganisation after transplantation may be the principle process responsible for recovery of cognitive functions. BMSC is a different kind of cell, but the possible mechanism involved in the therapeutic effect observed could be the migration of transplanted cells reported by Meltzer et al.5 On the other hand, in this case with subcortical aphasia, the improvement of language skills and verbal output seems to be due to indirect effects mediated by fronto-striatal circuits involving white matter tracts subserving language. However, to what extent the functional imaging data correspond to outcome data needs to be evaluated in further studies. This particular cortical area seems to be relevant with the sustained cognitive improvement after transplantation.
We are aware that, while being suggestive, our study was not designed to evaluate causal effect between stem cell implantation and blood flow/cognitive measures. Nevertheless, we believe that this case report merits publications as it describes associations that can be explored in future studies designed to provide causal evidence.
Learning points.
-
▶
Transplantation of BMSCs is safe and had an effective and beneficial influence on the cognitive functions in one left-hemisphere stroke patient.
-
▶
The long-term cognitive outcome, evaluated in a summary of percentage change, was stable and showed a linear increment.
-
▶
The increased blood flow could be an indicator of augmented cerebral plasticity and seems to be an expression of remote and contralesional areas contributing to the global cognitive functioning.
Acknowledgments
The authors would like to thank the patient for agreeing to this case report.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Stilley CS, Ryan CM, Kondziolka D, et al. Changes in cognitive function after neuronal cell transplantation for basal ganglia stroke. Neurology 2004;63:1320–2 [DOI] [PubMed] [Google Scholar]
- 2.Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. Clin Neuropsychol 1998;12:482–486 [Google Scholar]
- 3.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995;2:189–210 [Google Scholar]
- 4.Suárez-Monteagudo C, Hernández-Ramírez P, Alvarez-González L, et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci 2009;27:151–61 [DOI] [PubMed] [Google Scholar]
- 5.Meltzer CC, Kondziolka D, Villemagne VL, et al. Serial [18F] fluorodeoxyglucose positron emission tomography after human neuronal implantation for stroke. Neurosurgery 2001;49:586–91 [DOI] [PubMed] [Google Scholar]