Abstract
Nevirapine resistance mutations arise commonly following single or extended-dose nevirapine (ED-NVP) prophylaxis to prevent mother-to-child transmission (PMTCT) of human immunodeficiency virus (HIV), but decay within 6–12 months of single-dose exposure. Use of ED-NVP prophylaxis in infants is expected to rise, but data on decay of nevirapine resistance mutations in infants in whom ED-NVP failed remain limited. We assessed, in Ethiopian infants participating in the Six-Week Extended Nevirapine (SWEN) Trial, the prevalence and persistence of nevirapine resistance mutations at 6 and 12 months following single-dose or up to 6 weeks of ED-NVP, and correlated their presence with the timing of infection and the type of resistance mutations. Standard population genotyping followed by high-throughput cloning were done on dried blood spot samples collected during the trial. More infants who received ED-NVP had nevirapine resistance detected by standard population genotyping (high frequencies) at age 6 months compared with those who received single-dose nevirapine (SD-NVP) (58% of 24 vs. 26% of 19, respectively; p = 0.06). Moreover, 56% of ED-NVP-exposed infants with nevirapine resistance at age 6 months still had nevirapine resistance mutations present at high frequencies at age 1 year. Infants infected before 6 weeks of age who received either SD- or ED-NVP were more likely to have Y181C or K103N; these mutations were also more likely to persist at high frequencies through 1 year of age. HIV-infected infants in whom ED-NVP prophylaxis fails are likely to experience delayed clearance of nevirapine-resistant virus in the first year of life, which in turn places them at risk for early selection of multidrug-resistant HIV after initial therapy with nonnucleoside reverse transcriptase inhibitor-based regimens.
Introduction
A single peripartum dose of nevirapine reduces mother-to-child transmission of human immunodeficiency virus (HIV) by nearly 50%.1,2 This risk can be reduced further when combinations of antiretroviral drugs are used.3,4 However, risk of mother-to-child transmission of HIV continues postnatally if the infant is breastfed, and nearly 40% of new HIV infections in children each year (estimated at 370,000 in 2009) occur through breastfeeding.5 Randomized controlled clinical trials, however, show that giving nevirapine daily to breastfeeding infants of HIV-infected women significantly reduces the risk of HIV transmission.3,6,7 The World Health Organization (WHO) now recommends extended daily nevirapine during and for 1 week after cessation of breastfeeding as one strategy to prevent mother-to-child HIV transmission in resource-constrained settings.8 In nonbreastfeeding infants in these settings, nevirapine given daily to the infant for 4–6 weeks is also an alternate prophylactic regimen to daily zidovudine.8
One effect of nevirapine prophylaxis is the ease with which resistance occurs,9–11 which in turn may compromise therapy with nonnucleoside reverse transcriptase inhibitor-containing regimens.12–15 Following single-dose nevirapine (SD-NVP) prophylaxis, approximately 36% of women and 53% of infants develop nevirapine resistance to high levels in blood that can be identified with standard genotyping.16 Though these resistance mutations decay from detection over time,9,17,18 they can remain archived in replication-competent forms in up to 8% of women after a median of 2 years of drug exposure.19 Longitudinal studies in infants who develop nevirapine resistance with SD-NVP prophylaxis show that nevirapine resistance remains detectable by standard population genotyping in approximately 0–20% of infants retested between 6 months and 1 year of age,9,20,21 with one study reporting 50% of eight children maintaining nevirapine resistance through 12 months of age.22
In the Six-Week-Extended Nevirapine (SWEN) Trial, in which breastfed Ethiopian, Indian, and Ugandan infants were randomized to receive SD-NVP or up to 6 weeks of daily nevirapine (ED-NVP), a 46% reduction in HIV transmission was observed.6 In this trial, among the infants who received ED-NVP prophylaxis and were diagnosed with HIV infection by age 6 weeks, a high proportion (up to 92%) developed nevirapine-resistant virus at high frequencies compared with up to 50% of those who received only a single dose of nevirapine.20,21 To date, longitudinal studies assessing decay of nevirapine resistance mutations arising following failed ED-NVP prophylaxis are limited.9,20,21 In the SWEN study, 100% of seven Ugandan infants who received ED-NVP, and were diagnosed with HIV infection by 6 weeks of age who had nevirapine resistance, maintained nevirapine resistance mutations at high frequencies through 6 months of age compared with 16.7% of those who received only the single-dose regimen.20 In the Indian infants, 40% of five HIV subtype C-infected infants had persistence of nevirapine resistance mutations at high frequencies through 1 year of age compared with 20% of five single-dose exposed infants.21 These studies were limited by small sample sizes and heterogeneity in the timing of infection and age at repeat genotyping. We conducted a longitudinal study of decay of nevirapine resistance mutations following SD- or ED-NVP prophylaxis in Ethiopian subtype C HIV-infected infants enrolled in the SWEN Trial who survived through 1 year of age but in whom antiretroviral therapy was not yet available for treatment.
Materials and Methods
Clinical samples
Clinical samples from Ethiopian infants participating in the SWEN trial were assessed.6 In the SWEN trial, infants born to HIV-infected women in Ethiopia, India, and Uganda who planned to breastfeed were randomized to receive SD-NVP followed by daily multivitamins or SD-NVP followed by ED-NVP and multivitamins starting on day 8 to up to day 42 of life.6 Eligibility criteria, informed consent procedures, and primary outcome have been previously described.2 There were 772 live births from 780 Ethiopian women enrolled in the SWEN trial from February 2001 to March 2007 who met eligibility for the (pure) intent-to-treat analysis.6 Blood samples were collected frequently on Ethiopian infants (within 72 h of birth, at weeks 2, 6, and 14, and at months 6, 9, and 12) during the main trial for estimating mode and timing of HIV infection. HIV RNA in plasma was used to diagnose infection as previously reported.6 Infants were assigned to one of the following infection categories in the main trial based on the time of first positive HIV RNA polymerase chain reaction (PCR): in utero (positive HIV RNA by day 7), peripartum, or early-breastfeeding (positive from day 7 through week 14).
HIV-infected infants received care and treatment based on local clinical care guidelines, but none of the infants in this study received antiretroviral therapy prior to HIV genotyping: antiretroviral therapy became routinely available after 2005. Follow-up genotyping was performed at 1 year of age in infants who had nevirapine resistance detected by standard population genotyping at age 6 months.
HIV genotyping from dried blood spots, high-throughput cloning, and sequencing
Total nucleic acids were extracted from two whole spots of dried blood saved on Whatman filter paper (GE Healthcare, Wauwatosa, WI) using NucliSens silica-based magnetic nucleic acid extraction methods (Biomerieux, Boxtel, NL) eluted into 60 μl of elution buffer. cDNA was synthesized from 10 μl of total nucleic acid extract using our previously published gene-specific primers23 from which a 663-base pair (bp) region of HIV reverse transcriptase (HIV-RT) encoding known sites of nevirapine resistance was amplified with an in-house, genotyping assay.23 For the 18 samples that were negative by the in-house genotyping assay, a repeat genotype was performed on cDNA with different primers that amplify shorter fragments of HIV-RT (425 and 366 base pairs)24 and encoding for the relevant drug resistance sites. Amplicons were electrophoresed on a 1.5% agarose gel, purified using a column-based method (Qiagen, Valencia, CA), and sequenced with HIV sequence-specific primers23 on an ABI 3730 capillary electrophoresis instrument (Applied Biosystems, San Jose, CA).
Patient sequences were aligned using Sequencher 4.9 sequencing software (Gene Codes, Ann Arbor, MI) with HIV subtype C Ethiopian reference sequences obtained from the Los Alamos database.25 Genotypic resistance mutations were based on the recommendations from the Stanford Genotypic Resistance Database26 and the International AIDS Society–USA Drug Resistance Mutation list.27 Substitutions at the following eight amino acid positions of HIV-RT were considered as NVP resistance mutations: L100I, K101E/P, K103N/S, V106A/M, V108I, Y181C/I/V, Y188C/L/H, or G190A/S/E.
Clonal frequencies of nevirapine resistance mutations were assessed using high-throughput cloning (TOPO Cloning, Invitrogen, Carlsbad, CA) and sequencing (up to 96 clones per subject) on an ABI 3730 capillary electrophoresis instrument (Agencourt Bioscience Corporation, Beverly, MA).21 Cloning, however, was performed only in patient samples where the larger 663-bp HIV-RT fragment was amplified. Nevirapine resistance mutations identified by standard population genotyping were classified as high-frequency resistance and those requiring cloning for detection as low-frequency resistance. Follow-up genotyping was performed on infants who had nevirapine resistance detected by standard population genotyping at 6 months of age and had available dried blood spots (DBS) at age 12 months.
Statistical methods
Nevirapine resistance mutations present exclusively at high (detected by standard population genotyping) or low frequencies (required high-throughput cloning for detection) were analyzed in infants randomized to receive ED-NVP or only SD-NVP prophylaxis. The proportion of infants who had nevirapine resistance detected at 6 months of age and whose timing of infection differed was compared between the two groups (ED-NVP or SD-NVP) using Fisher's exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Similarly, the proportion of deaths at 6 months and 1 year of age in infants with and without nevirapine resistance detected by standard population genotyping at 6 months of age was compared using Fisher's exact test. Data analyses were performed using STATA software, version 10.0 (StataCorp LP, College Station, TX).
Results
In the SWEN Trial, 76 Ethiopian infants were diagnosed with HIV infection in the first 14 weeks of life: 55 survived to the age of 6 months of whom 46 (84% of surviving infants) had DBS samples available for HIV drug resistance testing. Among the 46 infants, 39% were identified as infected in utero (10 ED-NVP-exposed and 8 SD-NVP-exposed infants) and 61% by 14 weeks of age through either peripartum or early breastmilk transmission (16 ED-NVP- and 12 SD-NVP-exposed infants; Table 1). Genotyping was successful in 93% of the 46 infants (24 ED-NVP and 19 SD-NVP-exposed infants; Table 1). In two ED-NVP-exposed infants who were infected in utero, and one SD-NVP-exposed infant infected through peripartum/early breastfeeding, HIV genotyping was unsuccessful. These three infants were excluded from the data shown in Table 1. The median duration of nevirapine prophylaxis for the 24 infants who received ED-NVP and in whom genotyping was successful was 30 days (IQR: 29, 31 days; Table 1). Despite being infected in utero, infants who were assigned to the ED-NVP regimen also received a median of 31 days of nevirapine (IQR: 26–32 days) reflective of the time needed to receive nucleic acid testing required for infant HIV diagnosis. The median plasma viral load in ED-NVP-exposed infants surviving to age 6 months was lower at 5.8 log10 HIV RNA copies/ml (IQR: 5.3, 5.9) compared with 6.1 (IQR: 5.8, 6.3) log10 HIV RNA copies/ml in SD-NVP-exposed infants (p = 0.03). The median CD4+ T cell counts were similar in the two groups [1479 cells/mm3 (IQR: 850, 2329) and 1544 cells/mm3 (IQR: 470, 2246), respectively; p = 0.84; Table 1].
Table 1.
Patient Characteristics
| Characteristics | SWEN (n = 24) | sdNVP (n = 19) | p-valuea |
|---|---|---|---|
| Estimated mode and timing of infection [n (%)] | |||
| In utero (diagnosed ≤1 week) | 8 (27.6%) | 8 (33.3%) | 0.65 |
| Peripartum/early-breastfeeding (diagnosed >1–14 weeks) | 16 (55.2%) | 11 (45.8%) | 0.50 |
| Male [n (%)] | 14 (48.3%) | 10 (41.7%) | 0.78 |
| Median log10 viral load at 6 months of age for infants infected by 14 weeks of age [HIV RNA copies/ml (IQR)] | 5.8 (5.3–5.9) | 6.1 (5.8–6.3) | 0.03 |
| Median CD4 count at 6 months of age for infants infected by 14 weeks [cells/mm3 (IQR)] | 1479 (850–2329) | 1544 (470–2246) | 0.84 |
| Median days of study drug (IQR) | |||
| In utero (diagnosed ≤1 week) | 30.5 (25.5–31.5) | n/a | |
| Peripartum/early-breastfeeding (diagnosed >1–14 weeks) | 30 (30–31.5) | n/a | |
| Median duration of exclusive breastfeeding [weeks (IQR)] | 5.9 (1.7–24.4) | 8.9 (3.8–14.3) | 0.46 |
Fisher's exact test was used to compare categorical variables and Wilcoxon rank sum test for continuous variables.
SWEN, up to 6 weeks of extended-dose nevirapine; sdNVP, single-dose nevirapine; IQR, interquartile range; n/a, not applicable.
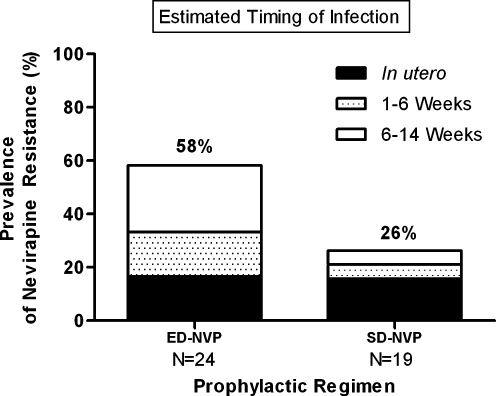
At age 6 months, a higher proportion of ED-NVP-exposed infants diagnosed with HIV infection within the first 14 weeks of life had nevirapine resistance mutations present at high frequencies compared to those who received SD-NVP alone [58% (14/24) vs. 26% (5/19); p = 0.063; Fig. 1). The persistence of nevirapine resistance following ED-NVP was assessed by mode of infection. Fifty percent (4/8) of in utero-infected infants who received ED-NVP, because of a delay in diagnosis, had persistent nevirapine resistance mutations at high frequencies through 6 months of age compared with 38% (3/8) of those who received the SD-NVP regimen (p = 1.0; Fig. 1). The prevalence of high frequencies of nevirapine resistance at age 6 months remained higher among ED-NVP-exposed infants infected through peripartum or early-breastfeeding between birth and 14 weeks of age than those who received SD-NVP [62% (10/16) vs. 18% (2/11); p = 0.047; Fig. 1].
FIG. 1.
Prevalence of nevirapine resistance by standard population genotyping at age 6 months in extended and single-dose nevirapine-exposed infants by timing of infection. ED-NVP, extended dose nevirapine; SD-NVP, single-dose nevirapine.
Since standard population genotyping assays do not provide quantitative information on drug resistance, we examined whether extended doses of nevirapine gave rise to a persistence of higher clonal frequencies of nevirapine resistance mutations than administration of a single dose. This approach was limited to infants who had genotypes derived from the 663-bp product, as high-throughput cloning on multiple short products is less feasible. The clonal frequencies of nevirapine resistance mutations were not different at age 6 months in infected infants who received extended or single-dose nevirapine. Analyses of a median of 69 (IQR: 49, 85) and 76 (IQR: 70, 90) clones in the 11 ED-NVP-exposed infants and five SD-NVP-exposed infants showed a median clonal frequency of nevirapine resistance mutations of 100.0% (IQR: 42.9, 100) and 98.2% (IQR: 74.3, 100), respectively (Table 2).
Table 2.
Types and Clonal Frequencies of Nevirapine Resistance Mutations
| Prophylactic regimen | Subject ID | Age at which HIV infection first detected | Nevirapine resistance mutations at age 6 months (% clonal frequencies) | Follow-up of nevirapine resistance mutations at age 12 months (% clonal frequencies) |
|---|---|---|---|---|
| ED-NVP | 8 | Birth | Y181C (n/a) | Y181C (99) |
| 12 | Birth | Y181C (78)a | Y181C/Y (28) | |
| 14 | Birth | Y181C (41) | ns | |
| 50 | Birth | Y181C (n/a) | ns | |
| 4 | 3 weeks | Y188C (43) | WT [V106A (5)] | |
| 1 | 6 weeks | Y181C (100)a | WT [Y181C (18)] | |
| 6 | 6 weeks | K103N (100) | K103N/K (75) | |
| 13 | 6 weeks | Y181C (100) | Y181C (100) | |
| 2 | 14 weeks | Y188C (40) | WT [K103N (3)] | |
| 7 | 14 weeks | K103N (n/a) | K103N (75) | |
| 11 | 14 weeks | G190A (100) | WT [G190A (6)] | |
| 15 | 14 weeks | V106M (94) | ns | |
| 38 | 14 weeks | Y181C (100) | ns | |
| 44 | 14 weeks | G190A (100) | ns | |
| sdNVP | 9 | Birth | Y181C/Y (74) | Y181C/Y (14)b |
| 27 | Birth | K103N/K (35) | ns | |
| 41 | Birth | Y181C/Y (98) | ns | |
| 51 | 6 weeks | Y188C (100) | ns | |
| 24 | 14 weeks | G190A (100) | ns |
Resistance mutations detected at age 6 months by standard populations genotyping following extended- or single-dose nevirapine prophylaxis.
A second nevirapine resistance mutation was detected at 1.2% clonal frequency.
Two additional mutations were detected after cloning [K103N (9%) and V106A (2%)].
ED-NVP, up to 6 weeks of daily nevirapine; sdNVP, single-dose nevirapine; n/a, not available because only a short PCR product was amplified or cloning was unsuccessful; ns, no sample.
High-throughput cloning was also performed to assess nevirapine resistance at low frequencies in infants who lacked resistance mutations by standard population genotyping at age 6 months. High-throughput clonal analysis was carried out on 16 infants (4 of 10 ED-NVP-exposed and 12 of 14 SD-NVP-exposed infants) in whom nevirapine resistance was not identified by standard population genotyping. Low-frequency nevirapine resistance was detected in 50% of four ED-NVP-exposed infants in a median of 78 (IQR: 55, 83) clones and 42% of 12 SD-NVP-exposed infants in a median of 79 clones (IQR: 58, 83). The median frequencies of nevirapine resistance mutations identified through cloning were 2.1% (IQR: 1.2, 2.9) in the ED-NVP-exposed infants and 2.0% (IQR: 1.4, 4.6%) in the SD-NVP-exposed infants.
The Y181C and K103N nevirapine resistance mutations were the most common detected at high frequencies in both groups. The Y181C persisted in 50% (7/14) of ED-NVP-exposed infants and 40% (2/5) of SD-NVP-exposed infants (Table 2), whereas, the K103N mutation was present in 14% (2/14) of ED-NVP-exposed infants and 20% (1/5) of SD-NVP-exposed infants (Table 2). Nevirapine resistance mutations other than Y181C and K103N were found to persist at high frequencies through 6 months of age in both ED-NVP-exposed and SD-NVP-exposed infants but less frequently, and these included Y188C, G190A, and V106M (Table 2). Nevirapine resistance mutations detected exclusively at low frequencies were heterogeneous. The Y181C and V106A mutations were detected in the two ED-NVP-exposed infants. The K103N (n = 1), V108I (n = 2), Y181C (n = 1), and V106A in combination with Y188C were detected in the SD-NVP-exposed infants.
The types of nevirapine resistance mutations persisting at high frequencies at 6 months of age in ED-NVP-exposed and SD-NVP-exposed infants differed by timing of infection. Infants identified as HIV infected within the first 6 weeks of life were more likely to have Y181C compared to those infected after 6 weeks of age. Sixty-seven percent (8/12) of infants who were diagnosed with HIV infection by 6 weeks of age had Y181C persisting through 6 months of age compared with 14% (1/7) of infants diagnosed after 6 weeks of age (Table 2; p = 0.057).
Longitudinal assessment of the extent to which nevirapine resistance persists at high frequencies through the first year of life following ED-NVP prophylaxis was performed in 11 of 14 ED-NVP-exposed infants. Only one of the five SD-NVP-exposed infants with resistance detected at high levels at age 6 months had repeat samples available for testing at 1 year of age. Genotyping was successful in 10 of 12 (83.3%) follow-up samples (9 of 11 from ED-NVP-exposed infants and the one sample from the SD-NVP-exposed infant). Among the nine ED-NVP-exposed infants with amplifiable genotypes, 56% (5/9) still maintained nevirapine resistance to high frequencies, which was almost exclusively with either Y181C (n = 3) or K103N (n = 2) (Table 2). The one SD-NVP-exposed infant with follow-up genotyping was infected in utero, and also had Y181C persisting at 1 year of age (Table 2). In the four ED-NVP-exposed infants in whom nevirapine-resistant virus was no longer present at high levels, low-frequency nevirapine resistance mutations remained detectable by cloning in all, and were identical to those present at age 6 months in two (Table 2).
The effect of the persistence of nevirapine resistance mutations at high frequencies through 6 months of age on viral loads, CD4+ T cell counts, and survival through 1 year of age was examined in infants exposed to either intervention. Viral loads and CD4+ T cell counts at age 6 months were not different among surviving infants with and without nevirapine resistance present at high frequencies. The median viral load was 5.87 (IQR: 5.46, 6.0) log10 HIV-RNA copies/ml among infants with nevirapine resistance and 5.87 (IQR: 5.8, 6.3) log10 HIV-RNA copies/ml for those without high-frequency nevirapine resistance, p = 0.44, and median CD4+ T cell counts were 1449 (IQR: 470, 2000) and 1586 IQR: 654, 2625) cell/mm3, respectively (p = 0.64). Survival to 1 year of age was similar for infants maintaining nevirapine resistance through 6 months of age at high frequencies compared to those without this level of resistance [73% (11/15) vs.75% (9/12); p = 1.0).
Discussion
Recent studies in SD-NVP-exposed children have shown that the prevalence of nevirapine resistance, assessed with standard population genotyping and therefore present at high frequencies, in those initiating highly active antiretroviral therapy (HAART) at a median of 8.4 or 9 months of age is between 12% and 19%, respectively,12,15 and has direct relevance for use of nevirapine for treatment of infants.12,15 A similar prevalence (14%) is also reported in SD-NVP-exposed women studied at a median of 17 months following nevirapine exposure.14 In this study of subtype C HIV-infected infants who received ED-NVP to prevent breastmilk HIV transmission, nearly 60% still maintained nevirapine resistance at high frequencies in blood through 6 months of age and 36% through 1 year of age. These findings suggest that daily doses of nevirapine not only results in selection of nevirapine resistance in almost all infants who become infected despite the intervention,20,21 but may also prolong the decay of these resistant variants in the first year of life.
Nevirapine-resistant virus selected during prophylaxis and present at high frequencies at the start of nevirapine-based HAART can compromise the virological response in women13,14,28,29 and children.12,30 A substantial (83.3%) proportion of SD-NVP-exposed children with resistance detected by standard population genotyping experienced treatment failure by 24 weeks on initial nevirapine-based HAART compared with 18.2% treated with ritonavir-boosted lopinavir HAART. Even children who effectively controlled virus replication with protease inhibitor-based therapy before reintroduction to nevirapine-based HAART, those maintaining nevirapine-resistant virus in plasma at high frequencies (25% or higher) before starting HAART had a 3.5-fold higher risk of virological failure with the switch to nevirapine-based therapy.31 In a recent study, we showed that the level (25% or more of the virus population) at which nevirapine-resistant virus is maintained in plasma following single-dose exposure in children was directly correlated with the frequencies of longer-lived cells with resistant virus.31 It is possible that the higher prevalence of the persistence of high-frequency nevirapine resistance we observed with ED-NVP prophylaxis in infants is related to the generation of a higher frequency of HIV-infected cells with nevirapine resistance than occurs with SD-NVP prophylaxis. In this study, assessment of the burden of archived nevirapine resistance in long-lived cells was not feasible.
The decreasing frequency of nevirapine-resistant virus from plasma within 12–36 months of SD-NVP prophylaxis9,32–34 has clinical relevance for the timing of the reuse of nevirapine for effective treatment in women13,14,28,29,34 and infants.15 The mechanisms underlying the decay of nevirapine-resistant virus from plasma within 1 year of drug exposure are unclear but may reflect the turnover of HIV-infected cells with nevirapine-resistant virus when drug selective pressure is removed. These cells harboring nevirapine-resistant virus are longer-lived but eventually most are cleared, but at a slower rate than activated CD4+ T cells, which produce most of the plasma virus.35 In women who received the SD-NVP prophylactic regimen, replication-competent, nevirapine-resistant proviral genomes were identified for up to 2 years following drug exposure in a small proportion (8%), which supports archiving of nevirapine-resistant variants occurs following nevirapine prophylaxis.19
In this study, most SD- or ED-NVP-exposed children who maintained nevirapine resistance through age 6 months at levels that were detected by standard population genotyping had a virus population that was nearly 100% nevirapine resistant. However, even though 60% of infants with nevirapine resistance at age 6 months still had nevirapine-resistant virus at high frequencies by 1 year of age, wild-type virus became detectable as a mixture with nevirapine-resistant virus in most (80%), suggestive of the establishment of viral reservoirs with wild-type virus.
This study has several limitations. The lack of genotyping at earlier (age 6 weeks) and intermediate time points (age 9 months) would have provided for a more detailed analysis of the kinetics of selection and decay of nevirapine-resistant virus following SD- or ED-NVP prophylaxis in infants, but these samples were not made available for testing. Nevertheless, given that nearly 90% of Ugandan and Indian infants participating in the SWEN Trial who received the ED-NVP and became infected developed nevirapine resistance to high levels by 6 weeks of age, it is likely that most of the infants in this study who received the ED-NVP also developed nevirapine resistance by age 6 weeks. In addition, analysis at 1 year of age in the infants in this study who received ED-NVP allows for comparisons with published studies on nevirapine resistance persisting following SD-NVP exposure. An additional limitation is the lack of available samples in most of the SD-NVP-exposed infants at 1 year of age. Furthermore, a formal sample size assessment to detect a difference across the various comparison groups was not performed for this study, and there may have been some confounding effect from infants with in utero transmission resulting in an underestimate of the difference between those receiving ED-NVP and SD-NVP; however, all available samples were tested.
The dominant nevirapine resistance mutations selected during single-dose nevirapine prophylaxis in women and infants differ; the pathogenesis remains unclear.9 K103N is more common in women and Y181C in infants.9 In our study, Y181C remained the dominant mutation in infants who received extended doses of nevirapine and even in those who were infected in utero and had established infection at the time of receipt of the extended prophylactic regimen. This limits the potential for use of newer generations of nonnucleoside reverse transcription inhibitors (NNRTIs) that are inactive against HIV with the Y181C mutation. However, our findings that infants infected after 6 weeks of age, and likely through late breastmilk transmission, were more likely to have infection with resistant virus other than Y181C and K103N, as reported in other studies,21,33,36 and that these mutations were more likely to decay by 1 year suggest that for such children a newer generation NNRTIs may be useful. Importantly, we also found that a substantial proportion (38%) of infants infected in utero who received single-dose nevirapine maintained nevirapine resistance at levels detected by standard population sequencing at 6 months of age compared with 18% of those infected between birth and 14 weeks of age; however, this was not statistically different, but may be limited by the small sample size. Together, these findings highlight differences in the pathogenesis of HIV infection based on timing of infection in children37–39 and emphasize the need to consider timing of infection in the management of HIV-infected children in resource-constrained settings by improving access to HIV testing, including drug resistance testing.
In summary, given the relatively low cost and efficacy of extended doses of nevirapine to prevent mother-to-child HIV transmission (PMTCT), this prophylactic component of PMTCT strategies is likely to increase. In children who become infected despite extended doses of nevirapine, a substantial proportion is likely to maintain nevirapine resistance through the first year of life at levels known to significantly compromise therapy with nevirapine-based HAART, and lead to early selection of multidrug-resistant virus that will likely compromise long-term control of virus replication with second-line regimens. Given that early HAART is lifesaving40 and is recommended for all HIV-infected infants,41 the increased availability of non-NNRTI-containing first-line HAART regimens will be required for minimizing early treatment failure and the selection of additional drug resistance mutations for the few children infected despite nevirapine prophylaxis.
Sequence Data
Nucleotide sequences were submitted to GenBank under accession numbers JF333591–JF336571.
Acknowledgments
We thank the study participants and the Ethiopian SWEN study team for their assistance with the conduct of the study, data collection, and verification. The sequences will be submitted to GenBank; accession numbers will be provided. This work was supported by the Elizabeth Glaser Pediatric AIDS Foundation Scientist Award (D.P.) and National Institutes of Health Grant R01HD057784 (D.P.) and R01-AI38576 (A.R.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Guay LA. Musoke P. Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JB. Musoke P. Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362(9387):859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 3.Chasela CS. Hudgens MG. Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362(24):2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro RL. Hughes MD. Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362(24):2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.2010 Report on the global AIDS epidemic. Internet. 2010. www.unaids.org/globalreport/Global_report.html www.unaids.org/globalreport/Global_report.html
- 6.Bedri A. Gudetta B. Isehak A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: An analysis of three randomised controlled trials. Lancet. 2008;372(9635):300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 7.Kumwenda NI. Hoover DR. Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359(2):119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization: HIV, infant feeding. Revised principles, recommendations, rapid advice. http://whqlibdoc.who.int/publications/2009/9789241598873_eng.pdf. 2009. Jan 8, 2010. http://whqlibdoc.who.int/publications/2009/9789241598873_eng.pdf
- 9.Eshleman SH. Mracna M. Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15(15):1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 10.Jackson JB. Becker-Pergola G. Guay LA, et al. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS. 2000;14(11):F111–F115. doi: 10.1097/00002030-200007280-00001. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JA. Li JF. Morris L, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192(1):16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 12.Coovadia A. Abrams EJ. Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: A randomized controlled trial. JAMA. 2010;304(10):1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockman S. Shapiro RL. Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356(2):135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 14.Lockman S. Hughes MD. McIntyre J, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363(16):1499–1509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palumbo P. Lindsey JC. Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363(16):1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arrive E. Newell ML. Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: A meta-analysis. Int J Epidemiol. 2007;36(5):1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 17.Flys TS. Chen S. Jones DC, et al. Quantitative analysis of HIV-1 variants with the K103N resistance mutation after single-dose nevirapine in women with HIV-1 subtypes A, C, and D. J Acquir Immune Defic Syndr. 2006;42(5):610–613. doi: 10.1097/01.qai.0000221686.67810.20. [DOI] [PubMed] [Google Scholar]
- 18.Flys TS. Donnell D. Mwatha A, et al. Persistence of K103N-containing HIV-1 variants after single-dose nevirapine for prevention of HIV-1 mother-to-child transmission. J Infect Dis. 2007;195(5):711–715. doi: 10.1086/511433. [DOI] [PubMed] [Google Scholar]
- 19.Wind-Rotolo M. Durand C. Cranmer L, et al. Identification of nevirapine-resistant HIV-1 in the latent reservoir after single-dose nevirapine to prevent mother-to-child transmission of HIV-1. J Infect Dis. 2009;199(9):1301–1309. doi: 10.1086/597759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Church JD. Omer SB. Guay LA, et al. Analysis of nevirapine (NVP) resistance in ugandan infants who were HIV infected despite receiving single-dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198(7):1075–1082. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moorthy A. Gupta A. Bhosale R, et al. Nevirapine resistance and breast-milk HIV transmission: Effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS One. 2009;4(1):e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinson NA. Morris L. Gray G, et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr. 2007;44(2):148–153. doi: 10.1097/QAI.0b013e31802b920e. [DOI] [PubMed] [Google Scholar]
- 23.Ziemniak C. George-Agwu A. Moss WJ. Ray SC. Persaud D. A sensitive genotyping assay for detection of drug resistance mutations in reverse transcriptase of HIV-1 subtypes B and C in samples stored as dried blood spots or frozen RNA extracts. J Virol Methods. 2006;136(1–2):238–247. doi: 10.1016/j.jviromet.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Simen BB. Simons JF. Hullsiek KH, et al. Low-abundance drug-resistant viral variants in chronically HIV-infected, antiretroviral treatment-naive patients significantly impact treatment outcomes. J Infect Dis. 2009;199(5):693–701. doi: 10.1086/596736. [DOI] [PubMed] [Google Scholar]
- 25.Los Alamos National Laboratory. http://www.hiv.lanl.gov/content/index>. 2008. http://www.hiv.lanl.gov/content/index>
- 26.Standford University HIV Drug Resistance Database. http://hivdb.stanford.edu/ 2008. http://hivdb.stanford.edu/
- 27.Johnson VA. Brun-Vezinet F. Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2009. Top HIV Med. 2009;17(5):138–145. [PubMed] [Google Scholar]
- 28.Jourdain G. Ngo-Giang-Huong N. Le CS, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351(3):229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 29.Chi BH. Sinkala M. Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;21(8):957–964. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palumbo P. Holland B. Dobbs T, et al. Antiretroviral resistance mutations among pregnant human immunodeficiency virus type 1-infected women and their newborns in the United States: Vertical transmission and clades. J Infect Dis. 2001;184(9):1120–1126. doi: 10.1086/323804. [DOI] [PubMed] [Google Scholar]
- 31.Moorthy A. Kuhn L. Coovadia A, et al. Induction therapy with protease-inhibitors may modify the effect of nevirapine resistance on virologic response to nevirapine-based HAART in children who failed prophylaxis. Clin Infect Dis. 2010;52:514–521. doi: 10.1093/cid/ciq161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer S. Boltz V. Martinson N, et al. Persistence of nevirapine-resistant HIV-1 in women after single-dose nevirapine therapy for prevention of maternal-to-fetal HIV-1 transmission. Proc Natl Acad Sci USA. 2006;103(18):7094–7099. doi: 10.1073/pnas.0602033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kassaye S. Lee E. Kantor R, et al. Drug resistance in plasma and breast milk after single-dose nevirapine in subtype C HIV type 1: Population and clonal sequence analysis. AIDS Res Hum Retroviruses. 2007;23(8):1055–1061. doi: 10.1089/aid.2007.0045. [DOI] [PubMed] [Google Scholar]
- 34.Coovadia A. Hunt G. Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48(4):462–472. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perelson AS. Neumann AU. Markowitz M. Leonard JM. Ho DD. HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271(5255):1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 36.Hudelson SE. McConnell MS. Bagenda D, et al. Emergence and persistence of nevirapine resistance in breast milk after single-dose nevirapine administration. AIDS. 2010;24(4):557–561. doi: 10.1097/QAD.0b013e3283346e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohman-Payne B. Slyker JA. Richardson BA, et al. Infants with late breast milk acquisition of HIV-1 generate interferon-gamma responses more rapidly than infants with early peripartum acquisition. Clin Exp Immunol. 2009;156(3):511–517. doi: 10.1111/j.1365-2249.2009.03937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obimbo EM. Wamalwa D. Richardson B, et al. Pediatric HIV-1 in Kenya: Pattern and correlates of viral load and association with mortality. J Acquir Immune Defic Syndr. 2009;51(2):209–215. doi: 10.1097/qai.0b013e31819c16d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Church JD. Mwatha A. Bagenda D, et al. In utero HIV infection is associated with an increased risk of nevirapine resistance in ugandan infants who were exposed to perinatal single dose nevirapine. AIDS Res Hum Retroviruses. 2009;25(7):673–677. doi: 10.1089/aid.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Violari A. Cotton MF. Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antiretroviral therapy for HIV infection in infants and children. Towards universal access. WHO/UNAIDS. 2010. [PubMed]