Abstract
Background
The dispersal ability of queens is central to understanding ant life-history evolution, and plays a fundamental role in ant population and community dynamics, the maintenance of genetic diversity, and the spread of invasive ants. In tropical ecosystems, species from over 40 genera of ants establish colonies in the stems, hollow thorns, or leaf pouches of specialized plants. However, little is known about the relative dispersal ability of queens competing for access to the same host plants.
Methodology/Principal Findings
We used empirical data and inverse modeling—a technique developed by plant ecologists to model seed dispersal—to quantify and compare the dispersal kernels of queens from three Amazonian ant species that compete for access to host-plants. We found that the modal colonization distance of queens varied 8-fold, with the generalist ant species (Crematogaster laevis) having a greater modal distance than two specialists (Pheidole minutula, Azteca sp.) that use the same host-plants. However, our results also suggest that queens of Azteca sp. have maximal distances that are four-sixteen times greater than those of its competitors.
Conclusions/Significance
We found large differences between ant species in both the modal and maximal distance ant queens disperse to find vacant seedlings used to found new colonies. These differences could result from interspecific differences in queen body size, and hence wing musculature, or because queens differ in their ability to identify potential host plants while in flight. Our results provide support for one of the necessary conditions underlying several of the hypothesized mechanisms promoting coexistence in tropical plant-ants. They also suggest that for some ant species limited dispersal capability could pose a significant barrier to the rescue of populations in isolated forest fragments. Finally, we demonstrate that inverse models parameterized with field data are an excellent means of quantifying the dispersal of ant queens.
Introduction
The approximately 14,000 species of ants (family Formicidae) account for roughly one-third of the world's insect biomass [1]. The dispersal ability of foundress queens is central to understanding ant life-history evolution, and also plays a fundamental role in ant population and community dynamics, the maintenance of genetic diversity, and the spread of invasive ant species [2], [3], [4], [5]. However, with the exception of a few well-studied species [5], [6], little is known regarding the distances queens typically disperse when they leave their colonies to found new nests or the maximum distances they are capable of dispersing (but see e.g., [4], [7], [8]). This is because techniques commonly used to track dispersing animals (e.g., mark-recapture methods, radio transmitters) are rarely applicable to ants given their size, the structural complexity of the habitat through which they disperse, and the difficulty in identifying and surveying all potential nesting sites. Though genetic techniques for estimating dispersal appear promising [4], [8], their application may be limited owing to their stringent assumptions and challenges in sampling intensively enough to accurately estimate dispersal.
The dominance of ants is particularly pronounced in the tropics, where in addition to their numerical superiority they are critical predators, herbivores, ecosystem engineers, and agricultural pests [1]. Species from at least 40 genera of tropical ants also establish colonies in the specialized stems, hollow thorns, leaf pouches, or petioles of plants known as ‘myrmecophyes’; these ants defend host-plants against herbivores and prune encroaching vegetation [9], [10]. Multiple ant species often vie for the same species of host-plant [11], [12], and vacant plants in which queens can establish colonies are a limiting resource for which there is intense competition [13], [14], [15]. Theory suggests that interspecific differences in the dispersal capability of ant queens play a key role in the maintenance of diversity in these communities, either via tradeoffs between dispersal ability and other life-history traits (e.g., competitive ability, colony fecundity), or from the interaction of dispersal limitation with spatial heterogeneity in host-plant density (reviewed in [3]). Studies in multiple plant-ant systems have demonstrated inequities in the competitive ability of ant queens or colonies [16], [17], plant and colony distribution consistent with habitat partitioning and patch dynamics [18], [19], and patterns of colonization that imply dispersal limitation [8], [12], [20] or interspecific variation in dispersal ability [20], [21]. Nevertheless, drawing general conclusions regarding the importance of dispersal for plant-ant coexistence requires quantitative descriptions of dispersal for multiple ants competing for access to the same host-plants.
The biology of myrmecophytes provides a unique opportunity to circumvent the challenges associated with quantifying ant queen dispersal in other systems. The ant species that nest in these plants do so obligately, and each is associated with a limited subset of plant species [11]. Consequently, all ant colonies in a site, as well as all nesting sites to which queens could potentially disperse, can be readily identified by mapping the distribution of host plants [8], [22]. We mapped all individuals of the understory shrubs Maieta guianensis and Tococa bullifera (both Melastomataceae) in 9 hectares of primary forest in the central Amazon (Figure 1). These two plant species serve as hosts for three species of ant symbionts that nest exclusively in their domatia: Crematogaster laevis, Pheidole minutula, and an undescribed species of Azteca [11], [18], [22]. Crematogaster laevis competes for access to host plants with both Azteca sp. and P. minutula (Figure 2), and it has been hypothesized [21] that superior dispersal ability promotes its persistence in this system despite the inferior competitive ability of queens competing for access to host-plant seedlings [16], the poor defense colonies provides host-plants against herbivores [23], its low rates of colony persistence [18], and the high mortality rates of the host plants it occupies [18]. After mapping all colonies of the three ant species, we transplanted vacant, greenhouse-grown seedlings of their host plants (N = 50 individuals of each species) into the central hectare of the plot and repeatedly surveyed them for colonization by ant queens (see Materials and Methods). These data, coupled with the location and size of established colonies, allowed us to estimate a probability density function describing the spatial redistribution of successfully dispersing queens (i.e., the ‘dispersal kernel’) of each ant species using ‘inverse modeling’ – a technique developed by plant ecologists to estimate the distances seeds are dispersed from fruiting trees [24], [25], [26]. To our knowledge this is the first application of inverse modeling techniques to calculate the dispersal kernels of animals.
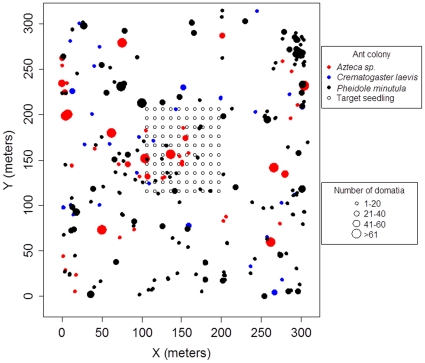
Figure 1. Map of established ant colonies and target seedlings.
Location and size of plants hosting colonies of Azteca sp., Crematogaster laevis, and Pheidole minutula and the location of experimentally planted seedlings (“trap plants”) of Maieta guianensis and Tococa bullifera.
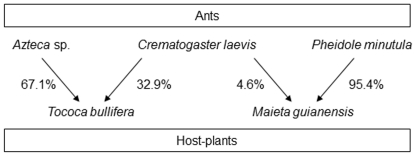
Figure 2. Focal community of ant-plant mutualists.
Graphical depiction of the Amazonian plant-ant community used to quantify dispersal capability of ant queens. Values by arrows are the percentage of host-plants colonized by each species of ant in our 9-ha study site.
Results
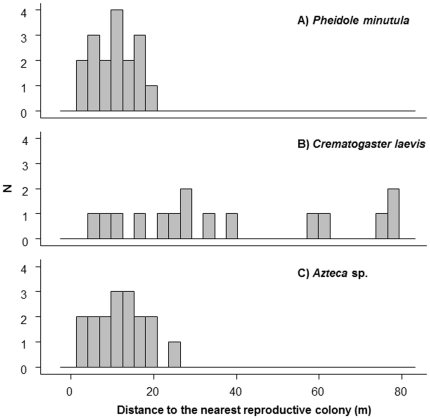
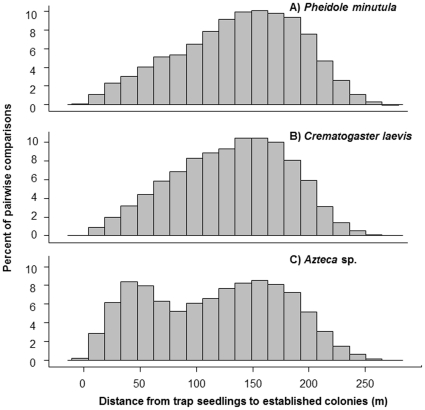
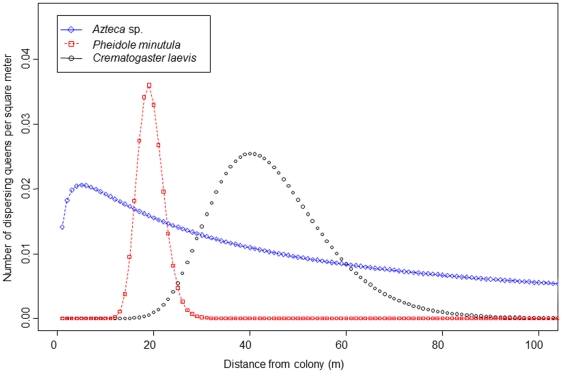
The median distance from colonized seedlings to the nearest potentially reproductive colony was significantly different among ant species (Kruskal-Wallis, H = 13.96, df = 2, p<0.001, Figure 3); experimentally transplanted vacant seedlings (hereafter, “trap plants”, analogous to seed traps used in plant ecology) colonized by Crematogatser laevis queens were significantly further from reproductive colonies than those trap plants colonized by queens of either Azteca sp. or Pheidole minutula (Steele's Nonparametric Multiple Comparison Test [27], Table 1). However, this is not because established C. laevis colonies were located further from trap plants. There was a significant difference among colonies of the different species in their proximity to trap plants (Table 2), but P. minutula colonies were actually further from trap plants than those of C. laevis (average distances from trap plants to colonies: Azteca sp.: 116.09 m±59.84 SD, C. laevis: 130.88 m±60.60 SD, P. minutula: 137.59 m±53.69 SD, Figure 4). Instead, our inverse models suggest C. laevis queens establish colonies furthest from natal colonies. Assuming a log-normal kernel (see Materials and Methods), the modal dispersal and colonization distance of Crematogaster laevis queens is double that of Pheidole minutula queens (40.1 m and 18.9 m, respectively) and eight-fold that of Azteca sp. (5 m; Table 3, Figure 5). The kernels also had very different shapes (Figure 5), suggesting that the maximal colonization distance of C. laevis is approximately 80 m, while queens of Azteca sp. may be capable of infrequent movements in excess of 400 m.
Figure 3. Distance from colonized seedlings to the nearest reproductive ant colony.
Histograms of the pairwise distances from each colonized trap plant to the nearest reproductive colony of the ant species that colonized it. A) Pheidole minutula: mean pairwise distance = 10.91 m±5.26 SD, B) Crematogaster laevis: mean pairwise distance = 37.49 m±25.92 SD, C) Azteca sp.: mean pairwise distance = 12.30 m±6.53 SD.
Table 1. Result of Steel's Test comparing the median distance of colonized trap plants to the nearest reproductive colony for all pairwise comparisons of ant species.
| Comparison |
Relative Effect,
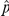 (lower-upper 95% confidence limits)
(lower-upper 95% confidence limits)
|
p value |
| Azteca sp. vs. Crematogaster laevis | 0.82 (0.58–1.07) | 0.005 |
| Azteca sp. vs. Pheidole minutula | 0.45 (0.21–0.68) | 0.87 |
| C. laevis vs. P. minutula | 0.15 (−0.09–0.40) | 0.002 |
Table 2. Nested Analysis of Variance comparing the average distance of trap plants to colonies of the three ant species (Crematogaster laevis, Azteca sp., Pheidole minutula) mapped in our 9 ha study site.
| Source | df | MS | F | P |
| Ant Species | 2 | 496230 | 169.68 | <0.0001 |
| Trap (Ant Species) | 3 | 74 | 0.025 | 0.99 |
| Error | 16694 | 48822953 |
(Nested ANOVA; Main effect of Ant species: F2,16694 = 169.68, P = <0.0001).
Figure 4. Pairwise distances from established colonies to trap plants.
Histograms of the distance from trap plants to colonies for each of the three focal ant species. The X axis shows the percentage of all colony-trap pairwise comparisons. A) Pheidole minutula: mean colony-trap distance = 137.59 m±53.69 SD, B) Crematogaster laevis: mean colony-trap distance = 130.88 m±60.60 SD, C) Azteca sp.: mean colony-trap distance = 116.09 m±59.84 SD.
Table 3. Maximum-likelihood parameter estimates (MLE) and 95% support intervals (SI) for inverse models estimating the dispersal kernels of three mutualist ant species nesting in two species of Amazonian ant-plants.
| Pheidole minutula | Azteca sp. | Crematogaster laevis | |
| Parameter 1 | MLE (lower SI-Upper SI) | MLE (lower SI-Upper SI) | MLE (lower SI-Upper SI) |
| X0 | 18.85 (17.42–20.47) | 5 (5–5.05) | 40.16 (31.54–52.16) |
| Xb | 0.14 (0.09–0.23) | 1.85 (1.21–1.93) | 0.27 (0.15–0.66) |
| a | 1.58 (1.00–4.00) | 11.40 (7.45–22.57) | 14.84 (8.75–23.36) |
| b | 55.07 (36.50–96.20) | 114.15 (−10.00–250.00) | 1.91 (0–10) |
Parameters: X0 = Mode of the log-normal dispersal kernel, Xb = Variance of the log-normal dispersal kernel, a = Slope of the line describing the relationship between plant size and queen production, b = Intercept of the relationship between plant size and queen production.
Figure 5. Dispersal kernels for three species of Amazonian plant-ants.
Dispersal kernels (i.e., probability density functions describing the spatial redistribution of queens around reproductive colonies) for ant queens obligately nesting in Tococa bullifera or Maieta guianensis. These kernels are scaled for a colony housed in a plant of the median size observed in our 9-ha study plot.
Discussion
We were able to estimate the shapes of effective dispersal kernals for queens of three ant species. The dispersal kernel for Crematogaster laevis had the greatest mode, suggesting that it generally disperses further than either ant species with which it competes for access to host plants. However, our results also revealed the potential for long-distance dispersal events by Azteca sp. That potential partner ant species differ significantly in their capacity to disperse to and colonize host-plants plants may help explain patterns of colonization and ant colony distribution previously observed in this [18] and other [19], [28], [29] ant-plant systems. Along with the lack of specialized entrances to domatia (i.e., “lock-and-key” mechanisms, sensu [30]), interspecific differences in dispersal and colonization success may also be important mechanisms inhibiting the evolution of further specialization in ant-plant systems, in which there are often large differences in the quality of defense and host plant fitness associated with different ant partner species [18], [23], [31], [32].
It has previously been suggested [21] that smaller body size, and hence flight muscles, may explain why the Pheidole minutula queens have lower dispersal distances than those of Crematogaster laevis; the same appears to be true in other ant-plant systems [20]. However, the Azteca sp. queens have the lowest modal dispersal distance of these three species, despite being similar in size to C. laevis. Given the potential for long distance dispersal by queens of Azteca sp., we hypothesize that this shorter modal dispersal distance instead reflects their superior efficiency at finding host plants. Testing this hypothesis will be challenging – it remains a mystery how plant-ant queens in flight identify host-plant seedlings against a backdrop of hundreds of other plant species [9], [33]. However, it is likely they use a combination of visual and olfactory cues, as is the case with phytophagous insects [34]. Indeed at short distances, queens have been shown to use volatiles emitted by plants to discriminate host-plants from closely related but non-myrmecophytic species [33], [35], [36]. It may be that Azteca sp. queens have the ability to detect these volatiles at greater distances than their competitors, superior abilities to identify the shape of plants and the characteristic venation patterns of host-plant leaves, or both.
It is notable that the modal ant queen dispersal distances we estimated with inverse models are shorter than the average distances inferred using other techniques [4], [8], [20] and well below the potential dispersal capacity suggested by observations of ants in novel or experimentally created habitat patches [5], [7]. If host plant density is greater in our sites than in other systems, then queens might only be required to disperse short distances to find vacant host plants. A more likely explanation, however, is that previous studies have overestimated dispersal. This could result from not exhaustively mapping all potential source and destination host-plants in a site [8], [20], thereby missing many short-distance dispersal events.
Our study has two important caveats. First, it was conducted entirely during a three month period during the dry season. Little is known regarding the environmental cues that stimulate the nuptial flights of ant queens in tropical forests [37], but the colonization of seedlings by Pheidole minutula in our field sites appears to be closely linked to precipitation ([21]; see also [37] for evidence from Peru of similar seasonality in colonization of Cordia by Allomerus octoarticulatus). If this seasonal variation in host plant colonization by P. minutula is common, then caution should be taken in estimating the total number of colonizations per year using our data. Second, we could be overestimating dispersal distances for all three species if queens arrive at experimental seedlings but left without attempting to colonize them or died prior to entering domatia. The low density of vacant plants [15], [22] probably makes it extremely costly for a queen to disperse again once she has arrived at a host-plant seedling, and extensive field observations indicate that upon arriving at a seedling queens of all three focal taxa immediately shed their wings and attempt to enter domatia (HLV and TJI, personal observation). Some queens will probably die prior to colonizing the seedling on which they land, however, and there is some experimental evidence that P. minutula successfully enters domatia at a higher rate than C. laevis [16]. It is therefore possible that using colonization of trap-plants by queens as a proxy for dispersal means that our results are conservative estimates true dispersal ability, especially for C. laevis. If so, our estimates of dispersal might best be called ‘realized dispersal’, i.e., dispersal followed by successful colonization [8], [20].
In conclusion, our results have implications for the study of plant-ant diversity in tropical ecosystems. First, tropical forests are increasingly fragmented by human activities, which isolates populations of ant-plant partners [22]. The mating system of social insects makes them particularly susceptible to inbreeding [38], and isolated populations are frequently smaller than those in unbroken forest [22]. If the distance separating fragments proves a barrier to dispersal for queens of some species, this will increase the likelihood that isolated populations of ants and their host-plants could suffer the detrimental effects of demographic, environmental, or genetic stochasticity [8]. Second, a critical but rarely documented requirement of some mechanisms that promote coexistence in ant-plant communities is that poorer competitors or habitat specialists are superior dispersers. Our results are consistent with this hypothesis, but also suggest that attempting to categorize species as “good” or “poor” dispersers when testing models of competition-colonization tradeoffs is overly simplistic – is the best disperser the one that has the greatest potential dispersal distance or the one that dispersers further on average? Finally, we show that an inverse modeling approach can help overcome the challenges in quantifying ant dispersal in structurally complex habitats, not the least of which is the difficulty in documenting rare long-distance dispersal events [26].
Materials and Methods
Ethics Statement
All research was conducted with the approval of Brazil's National Council of Scientific and Technological Development (CNPq, Permit Number 276/2005) and the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA, Permit Number 226/2005).
Field Site and data collection
Fieldwork was conducted January–September 2007 in Reserve #1501 of the Biological Dynamics of Forest Fragments Project (BDFFP). This 1,000 ha reserve is located 70 km north of Manaus, Brazil (2°30′S, 60°W) and is embedded in a large (>10,000 ha) expanse of primary forest. The habitat is non-flooded lowland rain forest, with a 30–35 m tall canopy and an understory dominated by stemless palms. Soils in the sites are highly acidic and nutrient poor xanthic farralsols with poor water retention capacity [39]. Annual rainfall ranges from 1,900–3,500 mm per year, and there is a pronounced dry season from June–October [40].
Tococa bullifera (Melastomataceae) is an understory shrub that grows to a maximum height of 2–3 m. It has two pouches at the base of each leaf in which ant queens establish colonies [18], [41]. Maieta guianensis (Melastomataceae), also an understory shrub, grows to a height of 1.5 m [18], [21]. It has highly dimorphic paired leaves, with a pair of foliar pouches at the base of the larger leaves in which ants nest. In our study sites two ant species are associated with M. guianensis; most plants contain colonies of Pheidole minutula (95%), with the remainder occupied by Crematogaster laevis (5%). The ant associates of T. bullifera are an undescribed species of Azteca (67%) and Crematogaster laevis (∼33%) (Figure 2). These frequencies are similar to those reported in previous surveys conducted in our field sites [18]. Although a previous study conducted in our study sites [11] has treated the Azteca species that colonizes T. bullifera and M. guianensis as the same one colonizing the sympatric myrmecophyte Cordia nodosa (Boraginaceae), this appears to be a misidentification resulting from the use of worker morphology to differentiate species. The complex taxonomy of Azteca requires using queens to distinguish species [42]; differences between Azteca queens from C. nodosa and those from T. bullifera in size, coloration, the shape of the propodeum, and the number of propodeal hairs strongly suggest these are distinct species (T. Izzo, unpubl. data). Although seedlings of both plant species can harbor incipient (i.e., non-reproductive) colonies of more than one ant species, adult plants house just a single colony of only one species. In addition to scavenging for insects on the leaf surface, resident ants tend coccids for honeydew inside domatia [43], [44].
From January–July 2007 we demarcated a 9-ha plot at reserve 1501 and then marked and mapped all Maieta guianensis and Tococa bullifera in the plot (Figure 1). For each plant we recorded the identity of its ant resident estimated its size by counting the number of domatia bearing leaves. We mapped a total of 217 M. guianensis (n = 10 with Crematogaster laevis colonies, n = 207 with Pheidole minutula colonies) and 79 Tococa bullifera (n = 26 with C. laevis colonies, n = 53 with Azteca sp. colonies).
Because colony size in Amazonian plant-ants is limited by the number of host-plant domatia [15], we used domatia number as a proxy for colony size. To estimate queen production as a function of colony size we destructively sampled 67 Tococa bullifera with Crematogaster laevis, n = 83 T. bullifera with Azteca sp., n = 87 Maieta guienensis with C. laevis and n = 101 M. guianensis with Pheidole minutula, all from nearby locations outside of the focal study area. Of these, 9, 9, 11, and 36 colonies (respectively), were reproductive. We used these reproductive colonies to estimate the relationship between colony size and queen production (Table 4); linear regression provided a better fit to the data than non-linear models (results not shown).
Table 4. Results of linear regressions testing for a relationship between the number of domatia a plant has and the number of queens counted in that a plant.
| Ant species | Host plant | df | SS | SS | F value | P value | R2 | Regression equation | |
| (regression) | (residual) | ||||||||
| Crematogaster laevis | Maieta guianensis | 1,10 | 18.43 | 2.57 | 71.651 | <0.0001 | 0.88 |
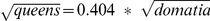
|
|
| Pheidole minutula | Maieta guianensis | 1,35 | 228.54 | 40.46 | 197.72 | <0.0001 | 0.85 |
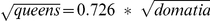
|
|
| Azteca sp. | Tococa bullifera | 1,8 | 30.91 | 3.09 | 80.09 | <0.0001 | 0.91 |
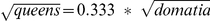
|
|
| Crematogaster laevis | Tococa bullifera | 1,8 | 120.26 | 94.72 | 10.16 | 0.013 | 0.56 |
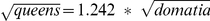
|
|
Note that the intercept of all three regressions is zero because queens are only found in plants with at least one domatium.
We then established an array of greenhouse-grown seedlings in the center of the 9-ha plot (Figure 1). The array was composed of n = 50 M. guianensis (for colonization by Pheidole minutula or Crematogaster laevis) and n = 50 T. bullifera (for colonization by Azteca sp. or C. laevis). Seedlings had at least two fully expanded leaves with domatia and were arranged in a grid with species alternating and plants separated from each other by 10 m. Seedlings of T. bullifera were grown from seeds collected in Reserve 1501 and germinated in a shade house in moist sand; because of the difficulty in germinating M. guianensis seeds we collected vacant M. guianensis seedlings in the reserve and transplanted them to containers filled with local soil and maintained in the same shade-house. From July–September 2007 we surveyed the target seedlings 15, 35, and 90 days after transplanting to record the presence and species identity of queens. All queens found were removed to allow for subsequent colonization, which previous work has shown does not influence the probability of re-colonization [21]. There were n = 17 colonizations by C. laevis, n = 23 by Azteca sp., and n = 25 by P. minutula. Crematogaster laevis colonized n = 15 of its 100 potential host plant seedlings (15%), while Pheidole minutula colonized n = 17 out of 50 (34%) and Azteca sp. colonized n = 17 out of 50 (34%), while the remaining events were repeat colonizations of individual seedlings.
Modeling framework
We used inverse models [26], [45] parameterized with the observational and experimental data described above to characterize the colonization of host plants by queens of our three focal species. This method assumes that observed spatial variation in colonization of host plants by queens is a multiplicative function of queen production, which is based on the size of potential queen sources (i.e., host-plant size), and local dispersal, which is modeled with a dispersal kernel that accounts for proximity of the sources to experimental host seedlings. For thorough reviews of inverse models and their construction, assumptions, and application see [24], [26], [45].
The total number of dispersing queens, t, produced by a colony was estimated as a linear function of the number of domatia its host plant has as follows:
| (1) |
where the parameter a determines the steepness in the increase in queen production with the number of domatia, and b determines the intercept of the domatia-queen production relationship.
We used a lognormal dispersal function, which considerable empirical and theoretical work has found to be the most appropriate function for a variety of dispersal mechanisms including animal movement ([46], [47], [48], [49], reviewed in 22). The kernel takes the form:
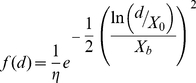 |
(2) |
where d is the observed distance between the colony and the vacant host plant seedling, X 0 is the distance at which maximum recruitment occurs (i.e., the mode of the dispersal kernel), X b determines the breadth or spread of the dispersal kernel, and η is a normalization constant equal to the arcwise integration of the dispersal kernel [25].
Combining local queen production Q and the dispersal kernel results in a model for the potential number of queens in trap plant i over the course of our sampling interval:
| (3) |
where tk is the number of queens of k = 1…n colonies within the maximal dispersal distance (in meters) suggested by our model in the 9 ha plot, dik is the distance from host plant i to source plant k, and f() is the lognormal dispersal kernel. For all analyses we assumed that the expected density of queens in a host plant follows a negative binomial distribution, reflecting the high degree of clumping observed in the data [50]. We used simulated annealing, a global optimization algorithm, to find the parameter values that maximized the likelihood of observed recruitment densities. We also calculated asymptotic 95% support limits for all the parameters. These Analyses were conducted using R v2.9.2 statistical software [51] and the packages “Likelihood 1.3” and “NeighLikeli 1.0”, as were all statistical analyses.
Acknowledgments
We thank Scott Powell, W. Getz, and three anonymous reviewers for comments on the manuscript and Emilia Zoppas de Albuquerque, Wesley Dáttilo and Osmaildo Ferreira da Silva for assistance in the field. The BDFFP staff provided invaluable logistical help; this is publication 582 in the BDFFP technical series. Data used in this paper have been archived at Dryad (www.datadryad.org): doi:10.5061/dryad.h6t7g.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by Grants DEB-0453631 and DEB-0452720 from the US National Science Foundation (http://nsf.gov). Publication of this article was funded in part by the University of Florida Open-Access Publishing Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wilson EO, Hölldobler B. The rise of the ants: A phylogenetic and ecological explanation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7411–7414. doi: 10.1073/pnas.0502264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hölldobler B, Wilson EO. The ants. Cambridge, MA: Belknap Press of Harvard University Press; 1990. [Google Scholar]
- 3.Palmer TM, Stanton ML, Young TP. Competition and coexistence: exploring mechanisms that restrict and maintain diversity within mutualist guilds. American Naturalist. 2003;162:S63–S79. doi: 10.1086/378682. [DOI] [PubMed] [Google Scholar]
- 4.Suni SS, Gordon DM. Fine-scale genetic structure and dispersal distance in the harvester ant Pogonomyrmex barbatus. Heredity. 2010;104:168–173. doi: 10.1038/hdy.2009.124. [DOI] [PubMed] [Google Scholar]
- 5.Markin GP, Dillier JH, Hill SO, Blum MS, Hermann HR. Nuptial flight and flight ranges of the imported fire ant Solenopsis saevissima (Hymenoptera, Formicidae). Journal of the Georgia Entomological Society. 1971;6:145–156. [Google Scholar]
- 6.Pinto SRR, Mendes G, Santos AMM, Dantas M, Tabarelli M, et al. Landscape attributes drive complex spatial microclimate configuration of Brazilian Atlantic forest fragments. Tropical Conservation Science. 2010;3:389–402. [Google Scholar]
- 7.Simberloff DS, Wilson EO. Experimental zoogeography of islands: the colonization of empty islands. Ecology. 1969;50:278–296. [Google Scholar]
- 8.Türke M, Fiala B, Linsenmair KE, Feldhaar H. Estimation of dispersal distances of the obligately plant-associated ant Crematogaster decamera. Ecological Entomology. 2010;35:662–671. [Google Scholar]
- 9.Heil M, McKey D. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annual Review of Ecology Evolution and Systematics. 2003;34:425–453. [Google Scholar]
- 10.Trager MD, Bhotika S, Hostetler JA, Andrade GV, Rodriguez-Cabal MA, et al. Benefits for plants in ant-plant protective mutualisms: A meta-analysis. PLoS ONE. 2010;5:e14308. doi: 10.1371/journal.pone.0014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonseca CR, Ganade G. Asymmetries, compartments and null interactions in an Amazonian ant-plant community. Journal of Animal Ecology. 1996;65:339–347. [Google Scholar]
- 12.Stanton ML, Palmer TM, Young TP. Competition-colonization trade-offs in a guild of African Acacia-ants. Ecological Monographs. 2002;72:347–363. [Google Scholar]
- 13.Yu DW, Wilson HB, Pierce NE. An empirical model of species coexistence in a spatially structured environment. Ecology. 2001;82:1761–1771. [Google Scholar]
- 14.Longino JT. Geographic variation and community structure in an ant-plant mutualism: Azteca and Cecropia in Costa Rica. Biotropica. 1989;21:126–132. [Google Scholar]
- 15.Fonseca CR. Amazonian ant-plant interactions and the nesting space limitation hypothesis. Journal of Tropical Ecology. 1999;15:807–825. [Google Scholar]
- 16.Izzo TJ, Bruna EM, Vasconcelos HL, Inouye BD. Cooperative colony founding alters the outcome of interspecific competition between Amazonian plant-ants. Insectes Sociaux. 2009;56:341–345. [Google Scholar]
- 17.Palmer TM, Young TP, Stanton ML, Wenk E. Short-term dynamics of an acacia ant community in Laikipia, Kenya. Oecologia. 2000;123:425–435. doi: 10.1007/s004420051030. [DOI] [PubMed] [Google Scholar]
- 18.Vasconcelos HL, Davidson DW. Relationship between plant size and ant associates in two Amazonian ant-plants. Biotropica. 2000;32:100–111. [Google Scholar]
- 19.Yu DW, Davidson DW. Experimental studies of species-specificity in Cecropia-ant relationships. Ecological Monographs. 1997;67:273–294. [Google Scholar]
- 20.Yu DW, Wilson HB, Frederickson ME, Palomino W, De la Colina R, et al. Experimental demonstration of species coexistence enabled by dispersal limitation. Journal of Animal Ecology. 2004;73:1102–1114. [Google Scholar]
- 21.Vasconcelos HL. Ant colonization of Maieta guianensis seedlings, an Amazon ant-plant. Oecologia. 1993;95:439–443. doi: 10.1007/BF00321000. [DOI] [PubMed] [Google Scholar]
- 22.Bruna EM, Vasconcelos HL, Heredia S. The effect of habitat fragmentation on communities of mutualists: Amazonian ants and their host plants. Biological Conservation. 2005;124:209–216. [Google Scholar]
- 23.Bruna EM, Lapola DM, Vasconcelos HL. Interspecific variation in the defensive responses of obligate plant-ants: experimental tests and consequences for herbivory. Oecologia. 2004;138:558–565. doi: 10.1007/s00442-003-1455-5. [DOI] [PubMed] [Google Scholar]
- 24.Canham CD, Uriarte M. Analysis of neighborhood dynamics of forest ecosystems using likelihood methods and modeling. Ecological Applications. 2006;16:62–73. doi: 10.1890/04-0657. [DOI] [PubMed] [Google Scholar]
- 25.Ribbens E, Silander JA, Pacala SW. Seedling recruitment in forests: calibrating models to predict patterns of tree seedling dispersion. Ecology. 1994;75:1794–1806. [Google Scholar]
- 26.Nathan R, Muller-Landau HC. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends in Ecology & Evolution. 2000;15:278–285. doi: 10.1016/s0169-5347(00)01874-7. [DOI] [PubMed] [Google Scholar]
- 27.Steele RGD. Some rank sum multiple comparison tests. Biometrics. 1961;17:539–552. [Google Scholar]
- 28.Debout GDG, Dalecky A, Ngomi A, Mckey DB. Dynamics of species coexistence: maintenance of a plant-ant competitive metacommunity. Oikos. 2009;118:873–884. [Google Scholar]
- 29.Yu DW, Wilson HB. The competition-colonization trade-off is dead; long live the competition-colonization trade-off. American Naturalist. 2001;158:49–63. doi: 10.1086/320865. [DOI] [PubMed] [Google Scholar]
- 30.Brouat C, Garcia N, Andary C, McKey D. Plant lock and ant key: pairwise coevolution of an exclusion filter in an ant-plant mutualism. Proceedings of the Royal Society of London Series B-Biological Sciences. 2001;268:2131–2141. doi: 10.1098/rspb.2001.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanton ML, Palmer TM, Young TP, Evans A, Turner ML. Sterilization and canopy modification of a swollen thorn Acacia tree by a plant-ant. Nature. 1999;401:578–581. [Google Scholar]
- 32.Palmer TM, Doak DF, Stanton ML, Bronstein JL, Kiers ET, et al. Synergy of multiple partners, including freeloaders, increases host fitness in a multispecies mutualism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17234–17239. doi: 10.1073/pnas.1006872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards DP, Hassall M, Sutherland WJ, Yu DW. Assembling a mutualism: ant symbionts locate their host plants by detecting volatile chemicals. Insectes Sociaux. 2006;53:172–176. [Google Scholar]
- 34.Bernays EA, Chapman RF. Host-plant selection by phytophagous insects. New York: Chapman and Hall; 1994. [Google Scholar]
- 35.Dáttilo WFC, Izzo TJ, Inouye BD, Vasconcelos HL, Bruna EM. Volatile recognition by Pheidole minutula (Myrmicinae), an Amazonian ant-plant specialist. Biotropica. 2009;41:642–646. [Google Scholar]
- 36.Blatrix R, Mayer V. Communication in Ant–Plant Symbioses. In: Baluška F, Ninkovic V, editors. Plant Communication from an Ecological Perspective. Springer Berlin Heidelberg; 2010. pp. 127–158. [Google Scholar]
- 37.Frederickson ME. The reproductive phenology of an Amazonian ant species reflects the seasonal availability of its nest sites. Oecologia. 2006;149:418–427. doi: 10.1007/s00442-006-0460-x. [DOI] [PubMed] [Google Scholar]
- 38.Darvill B, O'Connor S, Lye GC, Waters J, Lepais O, et al. Cryptic differences in dispersal lead to differential sensitivity to habitat fragmentation in two bumblebee species. Molecular Ecology. 2010;19:53–63. doi: 10.1111/j.1365-294X.2009.04423.x. [DOI] [PubMed] [Google Scholar]
- 39.Fearnside PM, Leal Filho N. Soil and development from Amazonia: lessons from the Biological Dynamics of Forest Fragments Project. In: Bierregaard RO, Gascon C, Lovejoy TE, Mesquita R, editors. Lessons from Amazonia: the ecology and conservation of a fragmented forest. New Haven: Yale University Press; 2002. pp. 291–312. [Google Scholar]
- 40.Bierregaard RO, Gascon C, Lovejoy TE, Mesquita R, editors. Lessons from Amazonia: the ecology and conservation of a fragmented forest. New Haven: Yale University Press; 2002. 478 p. [Google Scholar]
- 41.Michelangeli FA. A cladistic analysis of the genus Tococa (Melastomataceae) based on morphological data. Systematic Botany. 2000;25:211–234. [Google Scholar]
- 42.Longino JT. Taxonomy of the Cecropia-inhabiting Azteca ants. Journal of Natural History. 1991;25:1571–1602. [Google Scholar]
- 43.Vasconcelos HL. Mutualism between Maieta guianensis Aubl., a myrmecophytic melastome, and one of its ant inhabitants: ant protection against insect herbivores. Oecologia. 1991;87:295–298. doi: 10.1007/BF00325269. [DOI] [PubMed] [Google Scholar]
- 44.Lapola DM, Bruna EM, de Willink CG, Vasconcelos HL. Ant-tended hemiptera in Amazonian myrmecophytes: patterns of abundance and implications for mutualism function. Sociobiology. 2005;46:433–442. [Google Scholar]
- 45.Clark JS, Silman M, Kern R, Macklin E, HilleRisLambers J. Seed dispersal near and far: Patterns across temperate and tropical forests. Ecology. 1999;80:1475–1494. [Google Scholar]
- 46.Tackenberg O. Modeling long-distance dispersal of plant diaspores by wind. Ecological Monographs. 2003;73:173–189. [Google Scholar]
- 47.Nathan R, Katul GG, Horn HS, Thomas SM, Oren R, et al. Mechanisms of long-distance dispersal of seeds by wind. Nature. 2002;418:409–413. doi: 10.1038/nature00844. [DOI] [PubMed] [Google Scholar]
- 48.Greene DF, Johnson EA. Can the variation in samara mass and terminal velocity on an individual plant affect the distribution of dispersal distances? American Naturalist. 1992;139:825–838. [Google Scholar]
- 49.Greene DF, Calogeropoulos C. Dispersal of seeds by animals and wind. In: Bullock J, Kenward R, Hails R, editors. Dispersal ecology. Oxford: Blackwell Press; 2002. pp. 3–23. [Google Scholar]
- 50.Clark JS, Macklin E, Wood L. Stages and spatial scales of recruitment limitation in southern Appalachian forests. Ecological Monographs. 1998;68:213–235. [Google Scholar]
- 51.R Core Development Team. R: A language and environment for statistical computing. 2008. R Foundation for Statistical Computing. Vienna, Austria.