Abstract
The last decade saw the advent of increasingly realistic epidemic models that leverage on the availability of highly detailed census and human mobility data. Data-driven models aim at a granularity down to the level of households or single individuals. However, relatively little systematic work has been done to provide coupled behavior-disease models able to close the feedback loop between behavioral changes triggered in the population by an individual's perception of the disease spread and the actual disease spread itself. While models lacking this coupling can be extremely successful in mild epidemics, they obviously will be of limited use in situations where social disruption or behavioral alterations are induced in the population by knowledge of the disease. Here we propose a characterization of a set of prototypical mechanisms for self-initiated social distancing induced by local and non-local prevalence-based information available to individuals in the population. We characterize the effects of these mechanisms in the framework of a compartmental scheme that enlarges the basic SIR model by considering separate behavioral classes within the population. The transition of individuals in/out of behavioral classes is coupled with the spreading of the disease and provides a rich phase space with multiple epidemic peaks and tipping points. The class of models presented here can be used in the case of data-driven computational approaches to analyze scenarios of social adaptation and behavioral change.
Introduction
Understanding human behavior has long been recognized as one of the keys to understanding epidemic spreading [1], [2], which has triggered intense research activity aimed at including social complexity in epidemiological models. Age structure [3], human mobility [4]–[16] and very detailed data at the individual level [17]–[20] are now incorporated in most of the realistic models. However, much remains to be done. Models based on social mobility and behavior [21], [22] have shown to be valuable tools in the quantitative analysis of the unfolding of the recent H1N1 pandemic [21], [22], but it has become clear that societal reactions coupling behavior and disease spreading can have substantial impact on epidemic spreading [2], [23] thus defining limitations of most current modeling approaches [24]. Societal reactions can be grouped into different classes. First, there are changes imposed by authorities through the closure of schools, churches, public offices, and bans on public gatherings [25]–[28]. Second, individuals self-initiate behavioral changes due to the concern induced by the disease [29]–[36]. Behavioral changes vary from simply avoiding social contact with infected individuals and crowded spaces [37] to reducing travel [38], [39] and preventing children from attending school. In all cases we have a modification of the spreading process due to the change of mobility or contact patterns in the population. In general, these behavioral changes may have a considerable impact on epidemic progression such as the reduction in epidemic size and delay of the epidemic peak.
Several studies have been carried out in order to evaluate the impact and role that organized public health measures have in the midst of real epidemics [26]–[28]. However, only a few recent attempts have considered self-induced behavioral changes individuals adopt during an outbreak in order to reduce the risk of infection. In some approaches individual behaviors were modeled by modifying contact rates in response to the state of the disease [27], [29], [30], [36], [40]. In others new compartments representing individual responses were proposed [31], [33], [35]. Finally, in some studies the spread of information in the presence of the disease was explicitly modeled and coupled with the spreading of the disease itself [32]. However, we are still without a formulation of a general behavior-disease model.
In this study we propose a general framework to model the spread of information concerning the epidemic and the eventual behavioral changes in a single population. The emergent infectious diseases that we consider throughout the manuscript resemble the natural history of an acute respiratory infection with a short duration of infectiousness and have mild impact on the health status of individuals in that healthy status is recovered at the end of the infectious period. We modify the classic susceptible-infected-recovered (SIR) model [41] by introducing a class of individuals,  , that represents susceptible people who self-initiate behavioral changes that lead to a reduction in the transmissibility of the infectious disease. In other words, this class models the spread of ‘fear’ associated with the actual infectious disease spread [35], [42]. Individuals who fear the disease self-initiate social distancing measures that reduce the transmissibility of the disease. The spread of fear depends on the source and type of information to which individuals are exposed [32], [43]. We classify the general interaction schemes governing the transitions of individuals into and out of
, that represents susceptible people who self-initiate behavioral changes that lead to a reduction in the transmissibility of the infectious disease. In other words, this class models the spread of ‘fear’ associated with the actual infectious disease spread [35], [42]. Individuals who fear the disease self-initiate social distancing measures that reduce the transmissibility of the disease. The spread of fear depends on the source and type of information to which individuals are exposed [32], [43]. We classify the general interaction schemes governing the transitions of individuals into and out of 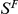 by considering behavioral changes due to different information spreading mechanisms, i.e., belief-based versus prevalence-based and local versus global information spreading mechanisms. We provide a theoretical and numerical analysis of the various mechanisms involved and uncover a rich phenomenology of the behavior-disease models that includes epidemics with multiple activity peaks and transition points. We also show that in the presence of belief-based propagation mechanisms the population may acquire a collective ‘memory’ of the fear of the disease that makes the population more resilient to future outbreaks. This abundance of different dynamical behaviors clearly shows the importance of the behavior-disease perspective in the study of realistic progressions of infectious diseases and provides a chart for future studies and scenario analyses in data-driven epidemic models.
by considering behavioral changes due to different information spreading mechanisms, i.e., belief-based versus prevalence-based and local versus global information spreading mechanisms. We provide a theoretical and numerical analysis of the various mechanisms involved and uncover a rich phenomenology of the behavior-disease models that includes epidemics with multiple activity peaks and transition points. We also show that in the presence of belief-based propagation mechanisms the population may acquire a collective ‘memory’ of the fear of the disease that makes the population more resilient to future outbreaks. This abundance of different dynamical behaviors clearly shows the importance of the behavior-disease perspective in the study of realistic progressions of infectious diseases and provides a chart for future studies and scenario analyses in data-driven epidemic models.
Methods
Epidemic model and basic assumptions
In order to describe the infectious disease progression we use the minimal and prototypical SIR model. This model is customarily used to describe the progression of acute infectious diseases such as influenza in closed populations where the total number of individuals 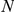 in the population is partitioned into the compartments
in the population is partitioned into the compartments 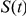 ,
, 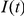 and
and 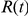 , denoting the number of susceptible, infected and recovered individuals at time
, denoting the number of susceptible, infected and recovered individuals at time  , respectively. By definition it follows
, respectively. By definition it follows 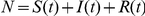 . The model is described by two simple types of transitions represented in Figure 1. The first one, denoted by
. The model is described by two simple types of transitions represented in Figure 1. The first one, denoted by 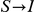 , is when a susceptible individual interacts with an infectious individual and acquires infection with transmission rate
, is when a susceptible individual interacts with an infectious individual and acquires infection with transmission rate 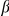 . The second one, denoted by
. The second one, denoted by 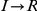 , occurs when an infected individual recovers from the disease with rate
, occurs when an infected individual recovers from the disease with rate 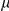 and is henceforth assumed to have permanent immunity to the disease. The SIR model is therefore described by the two following reactions and the associated rates:
and is henceforth assumed to have permanent immunity to the disease. The SIR model is therefore described by the two following reactions and the associated rates:
| (1) |
| (2) |
While the 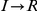 transition is itself a spontaneous process, the transition from
transition is itself a spontaneous process, the transition from 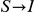 depends on the structure of the population and the contact patterns of individuals. Here we consider the usual homogeneous mixing approximation that assumes that individuals interact randomly among the population. According to this assumption the larger the number of infectious individuals among one individual's contacts the higher the probability of transmission of the infection. This readily translates in the definition of the force of infection in terms of a mass action law [44],
depends on the structure of the population and the contact patterns of individuals. Here we consider the usual homogeneous mixing approximation that assumes that individuals interact randomly among the population. According to this assumption the larger the number of infectious individuals among one individual's contacts the higher the probability of transmission of the infection. This readily translates in the definition of the force of infection in terms of a mass action law [44], 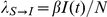 that expresses the per capita rate at which susceptible individuals contract the infection. In order to simulate the SIR model as a stochastic process we can consider a simple binomial model of transition for discrete individuals and discrete times. Each member of the susceptible compartment at time
that expresses the per capita rate at which susceptible individuals contract the infection. In order to simulate the SIR model as a stochastic process we can consider a simple binomial model of transition for discrete individuals and discrete times. Each member of the susceptible compartment at time  has a probability
has a probability 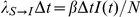 during the time interval between
during the time interval between  and
and 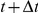 to contract the disease and transfer to the infected state at time
to contract the disease and transfer to the infected state at time 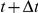 , where
, where 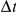 is the unitary time scale considered that we have set to
is the unitary time scale considered that we have set to 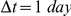 in simulations. As we assume to have
in simulations. As we assume to have 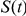 independent events occurring with the same probability, the number of newly infected individuals
independent events occurring with the same probability, the number of newly infected individuals 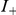 generated during the time interval
generated during the time interval 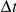 is a random variable that will follow the binomial distribution
is a random variable that will follow the binomial distribution 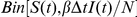 . Analogously, the number of newly recovered individuals
. Analogously, the number of newly recovered individuals  at time
at time  is a random variable that will obey the binomial distribution
is a random variable that will obey the binomial distribution 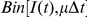 , where the number of independent trials is given by the number of infectious individuals
, where the number of independent trials is given by the number of infectious individuals 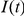 that attempt to recover and the probability of recovery in the time interval
that attempt to recover and the probability of recovery in the time interval 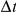 is given by the recovery probability
is given by the recovery probability 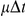 . In this processes we recognize that the stochastic variables define a Markov chain [45], [46] of stochastic events
. In this processes we recognize that the stochastic variables define a Markov chain [45], [46] of stochastic events 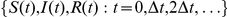 in which the current state of the system is determined only by the state of the system at the previous time steps. Formally, we can indeed write the following Markov chain relations:
in which the current state of the system is determined only by the state of the system at the previous time steps. Formally, we can indeed write the following Markov chain relations:
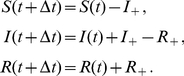 |
(3) |
These equations can be readily used to simulate different stochastic realizations of the epidemic events with the same basic parameters and initial conditions. This allows us to analyze the model's behavior by taking into account statistical fluctuations and noise in the epidemic process. The equations can also be translated into the standard set of continuous deterministic differential equations describing the SIR model by using expected values as
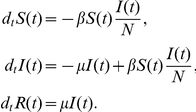 |
The crucial parameter in the analysis of single population epidemic outbreaks is the basic reproductive number 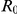 , which counts the expected number of secondary infected cases generated by a primary infected individual. Under the assumption of homogeneous mixing of the population the basic reproductive number of the SIR model is given by
, which counts the expected number of secondary infected cases generated by a primary infected individual. Under the assumption of homogeneous mixing of the population the basic reproductive number of the SIR model is given by
| (4) |
By the simple linearization of the above equations for 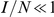 it is straightforward to see that in the single population case any epidemic will spread to a nonzero fraction of the population only if
it is straightforward to see that in the single population case any epidemic will spread to a nonzero fraction of the population only if 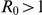 . In this case the epidemic is able to generate a number of infected individuals larger than those who recover, leading to an increase in the overall number of infectious individuals
. In this case the epidemic is able to generate a number of infected individuals larger than those who recover, leading to an increase in the overall number of infectious individuals 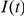 . The previous considerations lead to the definition of a crucial epidemiological concept: the epidemic threshold. Indeed, if the transmission rate is not large enough to allow a reproductive number larger than one (i.e.,
. The previous considerations lead to the definition of a crucial epidemiological concept: the epidemic threshold. Indeed, if the transmission rate is not large enough to allow a reproductive number larger than one (i.e., 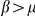 ), the epidemic outbreak will be confined to a tiny portion of the population and will die out in a finite amount of time in the thermodynamic limit of
), the epidemic outbreak will be confined to a tiny portion of the population and will die out in a finite amount of time in the thermodynamic limit of 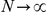 .
.
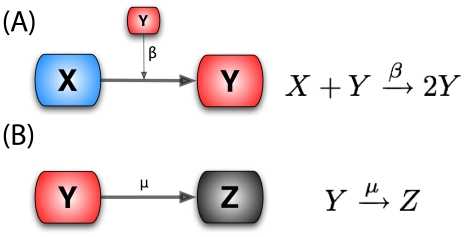
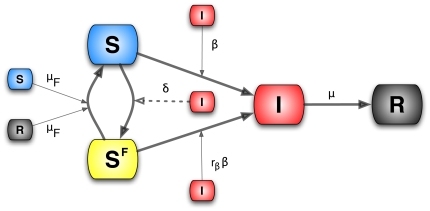
Figure 1. Schematic representation of the two types of transitions that will be recurrent in the paper.
In panel (A) we show the first in which individuals in compartment 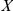 interact with individuals in class
interact with individuals in class 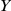 , represented by the small square, becoming
, represented by the small square, becoming 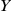 themselves. In general the compartment inducing the transition of individuals in
themselves. In general the compartment inducing the transition of individuals in 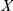 could be any other compartment in the model, e.g.
could be any other compartment in the model, e.g. 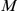 , different from the end-point of the transition. We assume the homogeneous mixing of the population so that the rate at which an individual in
, different from the end-point of the transition. We assume the homogeneous mixing of the population so that the rate at which an individual in 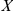 interacts with individuals in
interacts with individuals in 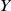 and changes status is simply given by the product of prevalence
and changes status is simply given by the product of prevalence 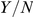 of
of 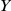 and the transmission rate
and the transmission rate 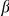 ,
, 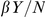 . This type of reaction can be written as
. This type of reaction can be written as 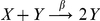 . In the case of the SIR model
. In the case of the SIR model 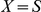 and
and 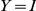 . In panel (B) we show the second type. This is a spontaneous transition with rate
. In panel (B) we show the second type. This is a spontaneous transition with rate 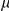 in which an individual in compartment
in which an individual in compartment 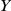 spontaneously moves to compartment
spontaneously moves to compartment 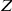 . These types of reactions can be written as
. These types of reactions can be written as 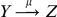 . In the SIR model
. In the SIR model 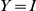 and
and  .
.
In the following we will use binomial stochastic processes to simulate numerically the progression of the epidemics and we will use the continuum limit to provide the analytical discussion of the models.
Coupling epidemic spreading and behavioral changes
We need to classify the source and type of information concerning the disease that people use to conduct their behavior in order to model the coupling between behavioral changes and the disease spread. In other words, while the disease spreads in the population, individuals are exposed (by local contacts, global mass media news, etc.) to information [32] on the disease that will lead to changes in their behavior. This is equivalent to the coupled spread of two competing contagion processes [32], [33], [35]: the infectious disease and the ‘fear of the disease’ contagion processes. The fear of the disease is what induces behavioral changes in the population. For this reason we will assume that individuals affected by the fear of the disease will be grouped in a specific compartment 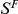 of susceptible individuals. These individuals will not be removed from the population, but they will take actions such as reducing the number of potentially infectious contacts, wearing face masks, and other social distancing measures that change disease parameters. In the following we will consider that self-induced behavior changes have the effect of reducing the transmission rate of the infection, introducing the following reaction:
of susceptible individuals. These individuals will not be removed from the population, but they will take actions such as reducing the number of potentially infectious contacts, wearing face masks, and other social distancing measures that change disease parameters. In the following we will consider that self-induced behavior changes have the effect of reducing the transmission rate of the infection, introducing the following reaction:
| (5) |
with 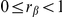 (i.e.,
(i.e., 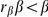 ). The above process corresponds to a force of infection on the individuals affected by the fear contagion
). The above process corresponds to a force of infection on the individuals affected by the fear contagion 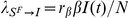 . The parameter
. The parameter 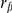 therefore modulates the level of self-induced behavioral change that leads to the reduction of the transmission rate. As the scope of the awareness of the disease or of the adopted behavioral changes is avoidance of infection, we assume that individuals in the
therefore modulates the level of self-induced behavioral change that leads to the reduction of the transmission rate. As the scope of the awareness of the disease or of the adopted behavioral changes is avoidance of infection, we assume that individuals in the 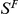 compartment relax their behavioral changes upon infection and return back to their regular social behavior. While the above modeling scheme is a straightforward way to include social distancing in the system, a large number of possible scenarios can be considered in the modeling of the contagion process that induce susceptible individuals to adopt self-induced behavioral changes and transition to the state
compartment relax their behavioral changes upon infection and return back to their regular social behavior. While the above modeling scheme is a straightforward way to include social distancing in the system, a large number of possible scenarios can be considered in the modeling of the contagion process that induce susceptible individuals to adopt self-induced behavioral changes and transition to the state 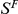 . In particular we consider three main mechanisms:
. In particular we consider three main mechanisms:
- Local, prevalence-based spread of the fear of the disease. In this scenario we assume that susceptible individuals will adopt behavioral changes only if they interact with infectious individuals. This implies that the larger the number of sick and infectious individuals among one individual's contacts, the higher the probability for the individual to adopt behavioral changes induced by awareness/fear of the disease. The fear contagion process therefore can be modeled as
where in analogy with the disease spread,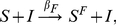
(6) 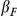 is the transmission rate of the awareness/fear of the disease. This process defines a transition rate for the fear of the disease that can be expressed by the usual mass-action law
is the transmission rate of the awareness/fear of the disease. This process defines a transition rate for the fear of the disease that can be expressed by the usual mass-action law 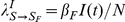 .
. - Global, prevalence-based spread of the fear of the disease. In some circumstances, individuals adopt self-induced behavioral changes because of information that is publicly available, generally through newspapers, television, and the Internet. In this case the local transmission is superseded by a global mechanism in which the news of a few infected individuals, even if not in contact with the large majority of the population, is able to trigger a widespread reaction in the population. In this case the fear contagion process is not well represented by the usual mass action law and has to be replaced by
where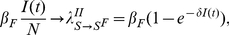
(7) 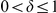 . Figure 2 shows the schematic representation of this.
. Figure 2 shows the schematic representation of this.
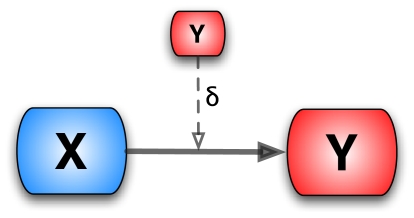
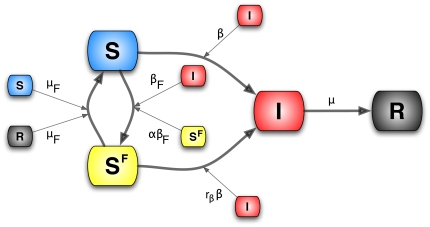
Figure 2. Schematic representation of the third type of interaction discussed.
In this case the transition into compartment 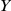 is based on the absolute number of the individuals in the compartment (shown by the small square). In general the inducing compartment could be different (e.g.
is based on the absolute number of the individuals in the compartment (shown by the small square). In general the inducing compartment could be different (e.g. 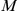 ) than the end-point of the transition.
) than the end-point of the transition.
For small values of 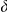 we have a pseudo mass action law [44] of the first order in
we have a pseudo mass action law [44] of the first order in 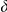 :
:
| (8) |
The above contagion process acts on the whole population even in the case of a very limited number of infectious individuals and the parameter 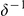 identifies the characteristic number of infected individuals reported by the news above which the fear spreads quickly in the population similarly to a panic wave.
identifies the characteristic number of infected individuals reported by the news above which the fear spreads quickly in the population similarly to a panic wave.
- Local, belief-based spread of the fear of the disease. In addition to the local prevalence-based spread of the fear of the disease, in this case we assume that the fear contagion may also occur by contacting individuals who have already acquired fear/awareness of the disease. In other words, the larger the number of individuals who have fear/awareness of the disease among one individual's contacts, the higher the probability of that individual adopting behavioral changes and moving into the
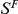 class. The fear contagion therefore can also progress according to the following process:
class. The fear contagion therefore can also progress according to the following process:
where the transmission rate is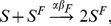
(9) 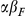 , with
, with  modulating the ratio between the transmission rate by contacting infected individuals and contacting individuals with fear of the disease. The transition rate is defined by the mass-action law
modulating the ratio between the transmission rate by contacting infected individuals and contacting individuals with fear of the disease. The transition rate is defined by the mass-action law 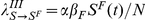 .
.
The fear/awareness contagion process is not only defined by the spreading of fear from individual to individual, but also by the process defining the transition from the state of fear of the disease back to the regular susceptible state in which the individual relaxes the adopted behavioral changes and returns to regular social behavior. We can imagine a similar reaction 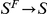 on a very long time scale in which individuals lose memory of the disease independent of their interactions with other individuals and resume their normal social behavior. This would correspond to spontaneous recovery from fear as proposed by Epstein et al.
[35]. However, our social behavior is modified by our local interactions with other individuals on a much more rapidly acting time-scale. We can therefore consider the following processes:
on a very long time scale in which individuals lose memory of the disease independent of their interactions with other individuals and resume their normal social behavior. This would correspond to spontaneous recovery from fear as proposed by Epstein et al.
[35]. However, our social behavior is modified by our local interactions with other individuals on a much more rapidly acting time-scale. We can therefore consider the following processes:
| (10) |
and
| (11) |
We can then define two mass-action laws: 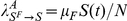 and
and 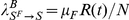 . These mimic the process in which the interaction between individuals with fear and without fear, susceptible or recovered, leads the individual with fear to resume regular social behavior. Both processes, occurring with rate
. These mimic the process in which the interaction between individuals with fear and without fear, susceptible or recovered, leads the individual with fear to resume regular social behavior. Both processes, occurring with rate 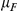 , tell us that the larger the number of individuals who adopt regular social behavior among one individual's contacts, the higher the probability for the individual to relax any behavioral changes and resume regular social behavior. The two interactions translate into a unique mass action law:
, tell us that the larger the number of individuals who adopt regular social behavior among one individual's contacts, the higher the probability for the individual to relax any behavioral changes and resume regular social behavior. The two interactions translate into a unique mass action law: 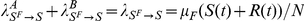 . The fear contagion process is therefore hampered by the presence of large numbers of individuals acting normally. The spreading of fear is the outcome of two opposite forces acting on society, but is always initially triggered by the presence of infectious individuals [27], [32], [33], [35], [36]. In Table 1 we report all the infection and recovery transitions for the disease and fear contagion dynamics and the corresponding terms and rates. We will use those terms to characterize different scenarios of interplay between the information and disease spreading processes. Unless specified otherwise the numerical simulations will be performed by individual-based chain binomial processes [45], [46] in discrete time and the analytical discussion will consider the continuous deterministic limit. In the comparison between the analytic conclusions with the numerical simulations we will always make sure to discuss the differences due to stochastic effects such as the outbreak probability at relatively small values of the reproductive number
. The fear contagion process is therefore hampered by the presence of large numbers of individuals acting normally. The spreading of fear is the outcome of two opposite forces acting on society, but is always initially triggered by the presence of infectious individuals [27], [32], [33], [35], [36]. In Table 1 we report all the infection and recovery transitions for the disease and fear contagion dynamics and the corresponding terms and rates. We will use those terms to characterize different scenarios of interplay between the information and disease spreading processes. Unless specified otherwise the numerical simulations will be performed by individual-based chain binomial processes [45], [46] in discrete time and the analytical discussion will consider the continuous deterministic limit. In the comparison between the analytic conclusions with the numerical simulations we will always make sure to discuss the differences due to stochastic effects such as the outbreak probability at relatively small values of the reproductive number 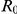 . In the following discussion
. In the following discussion 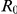 will refer to the basic reproductive number of the SIR model unless specified otherwise.
will refer to the basic reproductive number of the SIR model unless specified otherwise.
Table 1. In this table we show all the transitions and their rates used in the three different models.
| Transition | Transition rate | Equation flow term | Dynamical model | |
| Disease |
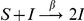
|
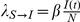
|
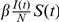
|
Models I,II,III |
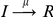
|
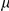
|
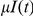
|
Models I,II,III | |
| Behavior |
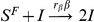
|
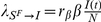
|
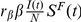
|
Models I,II,III |
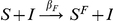
|
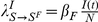
|
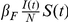
|
Models I,II,III | |
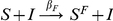
|
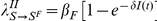
|
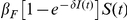
|
Model II | |
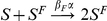
|
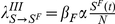
|
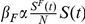
|
Model III | |
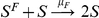
|
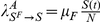
|
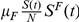
|
Models I,II,III | |
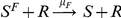
|
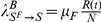
|
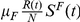
|
Models I,II,III |
In the last column we write the model in which the transition has been used. For example, the first transition 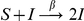 is related to the disease dynamic and has been used in all three models.
is related to the disease dynamic and has been used in all three models.
Results
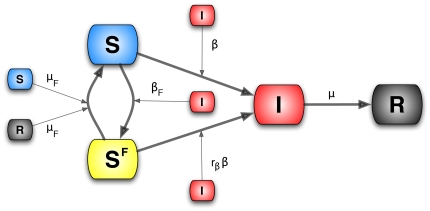
Model I: Local, prevalence-based spread of the fear of the disease
The first model (Model I) we consider is the coupling of the SIR model with local prevalence-based spread of the fear of the disease. The coupled behavior-disease model is described by the following set of equations:
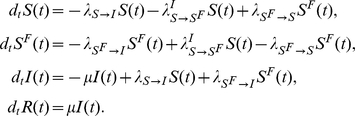 |
A schematic representation of the model is provided in Figure 3. Considering Table 1 we can write down all the terms,
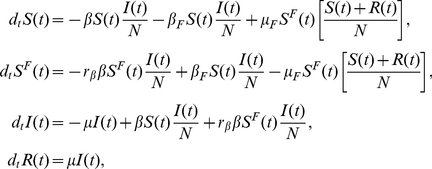 |
(12) |
in which
| (13) |
meaning that the total number of individuals in the population does not change. In acute diseases, the time scale of the spreading is very small with respect to the average lifetime of a person, allowing us to ignore birth and death processes and the demographic drift of the population. This is also the time scale over which it is more meaningful to consider the effect of the spread of behavioral changes. Diseases with a longer time scale may be equally affected by behavioral changes emerging especially as cultural changes toward certain social behavior – for instance sexual habits in the presence of a sexually transmitted disease with a long latency period – but in this case the demography of the system should be taken into account.
Figure 3. Model I Schematic representation of Model I.
To explain the equations we can simply consider the negative terms. In particular the first term of the first equation in Eq. (12) takes into account individuals in the susceptible compartment  who through interaction with infected individuals become sick. The second term takes into account individuals in the susceptible compartment
who through interaction with infected individuals become sick. The second term takes into account individuals in the susceptible compartment 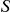 who through interaction with infected individuals change their own behavior. The first term of the second equation takes into account individuals in compartment
who through interaction with infected individuals change their own behavior. The first term of the second equation takes into account individuals in compartment 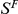 who through interaction with infected individuals become sick. It is important to remember that the transmission rate for people in compartment
who through interaction with infected individuals become sick. It is important to remember that the transmission rate for people in compartment 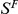 is reduced by a factor
is reduced by a factor 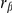 due to the protection that they gain on account of membership in this class. The last term in the second equation takes into account people in compartment
due to the protection that they gain on account of membership in this class. The last term in the second equation takes into account people in compartment 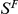 who through interaction with healthy individuals,
who through interaction with healthy individuals, 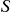 , and recovered ones,
, and recovered ones, 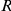 , normalize their behavior and move back to compartment
, normalize their behavior and move back to compartment 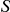 . The first term in the third equation takes into account the spontaneous recovery of sick individuals.
. The first term in the third equation takes into account the spontaneous recovery of sick individuals.
It is natural to assume that in the beginning of the disease spreading process the population is fully susceptible except for the infectious seeds, which means that we can set 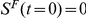 . At this point the behavioral response is not active yet. If the disease proceeds to spread much faster than fear contagion, then the model reduces to the classic SIR with basic reproductive number
. At this point the behavioral response is not active yet. If the disease proceeds to spread much faster than fear contagion, then the model reduces to the classic SIR with basic reproductive number 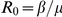 . In this case the initial spread is well described by
. In this case the initial spread is well described by 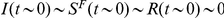 . The number of individuals in the compartment
. The number of individuals in the compartment 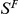 is of the same order of infectious and recovered individuals. From the conservation of the number of individuals follows
is of the same order of infectious and recovered individuals. From the conservation of the number of individuals follows 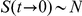 . Since
. Since 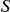 is the leading order, all the terms in the equations like
is the leading order, all the terms in the equations like 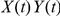 in which both
in which both 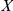 and
and 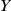 are different from
are different from 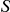 can be considered as second order. Using this approximation we can linearize the system and reduce the equations to first-order ordinary differential equations that are easy to integrate. In particular for
can be considered as second order. Using this approximation we can linearize the system and reduce the equations to first-order ordinary differential equations that are easy to integrate. In particular for 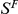 we can write
we can write
| (14) |
which has the following solution:
| (15) |
For 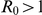 fear will spread in the population since the condition
fear will spread in the population since the condition 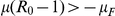 is always satisfied. The growth of the fear contagion is due to the spread of the infection in the population.
is always satisfied. The growth of the fear contagion is due to the spread of the infection in the population.
When fear spreads much faster than the disease, 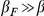 , everyone quickly becomes scared and our model reduces to an SIR model with a reduced reproductive ratio
, everyone quickly becomes scared and our model reduces to an SIR model with a reduced reproductive ratio 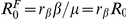 that is dominated by the characteristics of the
that is dominated by the characteristics of the 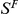 compartment.
compartment.
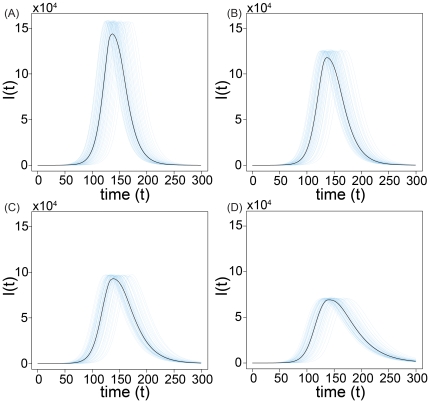
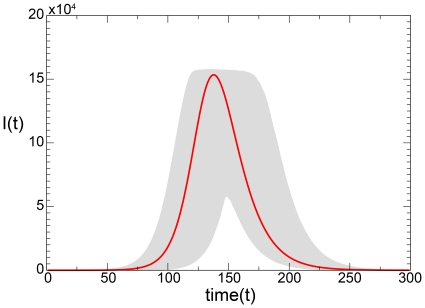
By considering both stochastic simulations of the model and direct integration of the equations, we explored numerically the intermediate regime between these two limits, i.e. 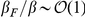 . The spread of the fear of infection contagion in this regime does not significantly affect the timing of the disease spread, as showed in Figure 4. In this figure the stochastic fluctuations are demonstrated by
. The spread of the fear of infection contagion in this regime does not significantly affect the timing of the disease spread, as showed in Figure 4. In this figure the stochastic fluctuations are demonstrated by 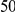 individual realizations and compared with the median profiles obtained by considering
individual realizations and compared with the median profiles obtained by considering 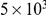 different stochastic realizations. The deterministic solution of the equation for
different stochastic realizations. The deterministic solution of the equation for 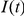 , obtained by direct integration of the equations, is well inside the
, obtained by direct integration of the equations, is well inside the 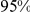 reference range of our stochastic simulations as shown in Figure 5. In this region of the model's phase space fear simply produces a mild reduction in the epidemic size.
reference range of our stochastic simulations as shown in Figure 5. In this region of the model's phase space fear simply produces a mild reduction in the epidemic size.
Figure 4. Model I for 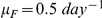 ,
, 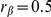 ,
, 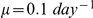 ,
, 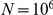 and
and 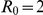 .
.
We show the medians of 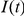 , evaluated using
, evaluated using 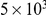 stochastic runs for the baseline (SIR model without fear of contagion) and three realizations of the model for different values of
stochastic runs for the baseline (SIR model without fear of contagion) and three realizations of the model for different values of 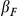 . In particular in panel (A) we show the baseline SIR model with the same disease parameters. In panel (B) we set
. In particular in panel (A) we show the baseline SIR model with the same disease parameters. In panel (B) we set 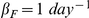 . In panel (C) we set
. In panel (C) we set 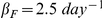 . In panel (D) we set
. In panel (D) we set 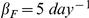 . It is clear how the peak time is the same for all the scenarios and how the number of infected individuals at peak is reduced as
. It is clear how the peak time is the same for all the scenarios and how the number of infected individuals at peak is reduced as 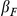 increases.
increases.
Figure 5. Model I fixing 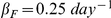 ,
, 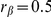 ,
, 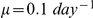 and
and 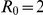 .
.
We compare the solution of the deterministic equations (red solid line) with the 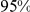 reference ranges of our stochastic solutions. Here we consider
reference ranges of our stochastic solutions. Here we consider 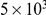 runs that produced at least an epidemic size of
runs that produced at least an epidemic size of 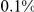 of the population (
of the population (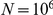 ).
).
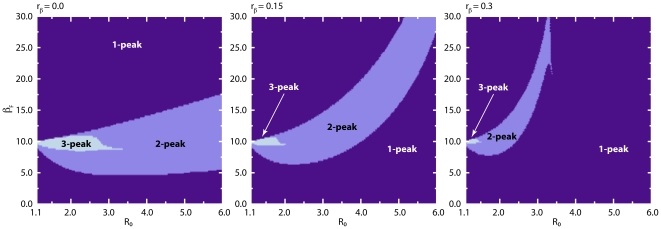
By increasing the value of 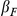 it is possible to find a region of parameters characterized by multiple peaks. In Figure 6 we show
it is possible to find a region of parameters characterized by multiple peaks. In Figure 6 we show 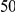 stochastic runs and the median profile obtained from
stochastic runs and the median profile obtained from 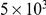 runs for a set of parameters associated with multiple peaks. After the first wave of infection individuals leave the compartment
runs for a set of parameters associated with multiple peaks. After the first wave of infection individuals leave the compartment 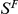 and return to the susceptible state in which they are less protected from the disease. The second wave manifests if the number of infected individuals at this stage is not too small and if there is still a large enough pool of individuals susceptible to the infection. A closer inspection of the parameter space by numerical integration of the deterministic equations yields very rich dynamical behavior. Figure 7 displays the phase diagram of the model on
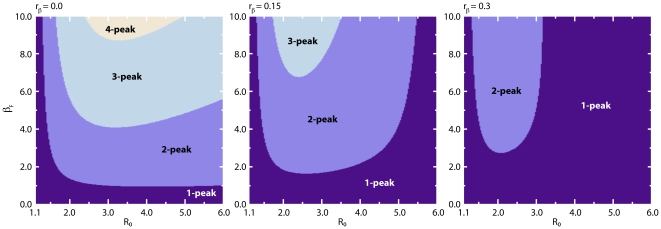
and return to the susceptible state in which they are less protected from the disease. The second wave manifests if the number of infected individuals at this stage is not too small and if there is still a large enough pool of individuals susceptible to the infection. A closer inspection of the parameter space by numerical integration of the deterministic equations yields very rich dynamical behavior. Figure 7 displays the phase diagram of the model on 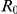 -
-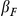 plane regarding different number of disease activity peaks for a set of model parameters. As
plane regarding different number of disease activity peaks for a set of model parameters. As 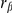 increases, the region in which multiple peaks are encountered shifts to smaller values of
increases, the region in which multiple peaks are encountered shifts to smaller values of 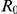 and larger values of
and larger values of 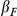 . Fixing
. Fixing 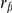 , increasing values of
, increasing values of 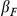 increase the number of infection peaks while an increase in
increase the number of infection peaks while an increase in 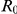 leads to a decrease in the number of peaks. It is interesting to note that adding a simple modification to the basic SIR model leads to scenarios with more than one peak. This is important not only from a mathematical point of view (existence of states characterized by multiple and unstable stationary points in the function
leads to a decrease in the number of peaks. It is interesting to note that adding a simple modification to the basic SIR model leads to scenarios with more than one peak. This is important not only from a mathematical point of view (existence of states characterized by multiple and unstable stationary points in the function 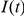 ) but also for practical reasons; in historical data from the 1918 pandemic multiple epidemic peaks were observed [26]–[28]. By increasing the value of
) but also for practical reasons; in historical data from the 1918 pandemic multiple epidemic peaks were observed [26]–[28]. By increasing the value of 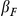 to larger and larger values, the spread of the fear contagion becomes increasingly rapid with respect to the spread of the disease. It is natural to think in this regime that the reproductive number of the disease is characterized by the
to larger and larger values, the spread of the fear contagion becomes increasingly rapid with respect to the spread of the disease. It is natural to think in this regime that the reproductive number of the disease is characterized by the 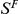 class. We then have two different scenarios:
class. We then have two different scenarios:
Figure 6. Model I Multiple waves of infection.
Fixing 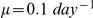 ,
, 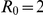 ,
, 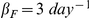 ,
, 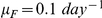 ,
, 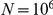 and
and 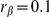 we show
we show 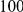 stochastic runs of the infected profiles and the median evaluated considering
stochastic runs of the infected profiles and the median evaluated considering 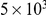 runs in which the epidemic size is at least
runs in which the epidemic size is at least 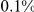 of the population.
of the population.
Figure 7. Model I Phase diagram of infection waves on 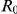 -
-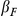 plane.
plane.
We display the regions of parameter space on 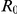 -
-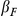 plane exhibiting different number of disease activity peaks for three different values of
plane exhibiting different number of disease activity peaks for three different values of 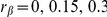 , where we have fixed
, where we have fixed 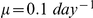 ,
, 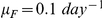 and
and 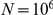 . The phase diagram has been obtained by numerical integration of the deterministic equations in Eq. (12).
. The phase diagram has been obtained by numerical integration of the deterministic equations in Eq. (12).
If
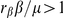 , then the epidemic size is given by that of an SIR model with
, then the epidemic size is given by that of an SIR model with 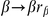 ;
;If
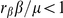 , then fear completely stops the spreading of the disease.
, then fear completely stops the spreading of the disease.
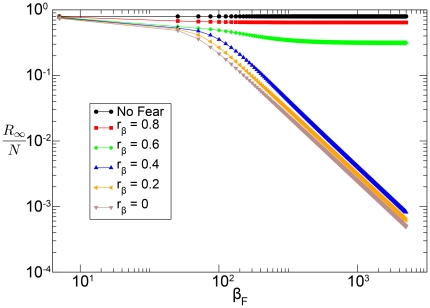
This is confirmed in Figure 8 in which we plot the proportion of recovered individuals at the end of the epidemic, which is evaluated by the integration of the deterministic equations. We consider different values of 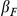 and
and 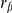 and hold fixed the other parameters. It is clear that for very large values of
and hold fixed the other parameters. It is clear that for very large values of 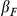 the spreading of the disease is characterized by the reproductive number
the spreading of the disease is characterized by the reproductive number 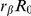 .
.
Figure 8. Model I fixing 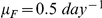 ,
, 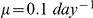 ,
, 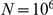 and
and 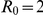 we evaluate the normalized epidemic size
we evaluate the normalized epidemic size 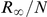 for different values of
for different values of 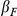 and
and 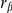 through direct integration of the equations.
through direct integration of the equations.
Once the product 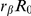 is smaller than unity, then the epidemic size goes to
is smaller than unity, then the epidemic size goes to 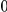 as
as 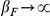 .
.
At the end of the disease epidemic the system enters the so-called ‘disease-free’ stage. This region of the phase space is described by
| (16) |
This regime can be easily derived by setting 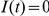 in the set of Eqs. (12). The system is then reduced to
in the set of Eqs. (12). The system is then reduced to
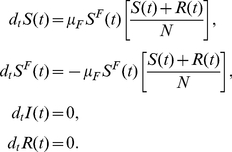 |
(17) |
From the last equation it is clear that 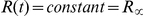 , and the first and second equations are equivalent. It is then possible to find the solution for
, and the first and second equations are equivalent. It is then possible to find the solution for 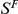 and
and 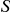 by using the conservation of individuals. In particular the equation to solve is
by using the conservation of individuals. In particular the equation to solve is
| (18) |
By integrating this equation directly it is easy to show that fear disappears exponentially:
| (19) |
In the stationary state, for 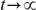 , the system reaches the disease- and fear-free equilibrium:
, the system reaches the disease- and fear-free equilibrium:
| (20) |
There is no possibility of an endemic state of fear. Fear can only be produced by the presence of infected people. As soon as the infection dies out, fearful people recover from their fear by interacting with all the susceptible and recovered individuals and become susceptible themselves.
Model II: Global, prevalence-based spread of the fear of the disease
The second fear-inducing process we consider is the spread of the fear contagion through mass-media (Model II). In order to increase ratings mass-media widely advertise the progress of epidemics, causing even the people that have never contacted a diseased person to acquire fear of the disease. In this formulation, even a very small number of infected people is enough to trigger the spread of the fear contagion. To model this we consider a pseudo mass-action law [44] in which the number of infected people is not rescaled by the total population. Hence the absolute number of infected individuals drives the spread. The transition rate peculiar to this model can be written as 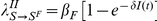 . The equations describing the system read as
. The equations describing the system read as
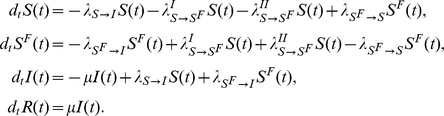 |
A schematic representation of the model is provided in Figure 9. Considering Table 1 we can explicitly introduce all the terms,
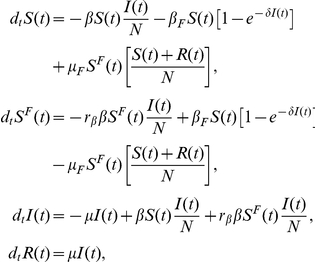 |
yielding that the population size is fixed,
| (21) |
As in the previous model, if the infection spreads faster than the fear contagion, then the reproductive number is simply 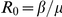 . In the opposite limit it is easy to understand that the reproductive number is
. In the opposite limit it is easy to understand that the reproductive number is 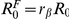 . In this latter limit, if
. In this latter limit, if 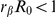 , then the global prevalence-based spread of fear suppresses the spread of the disease. Moreover, in general we will have a reduction in the epidemic size as a function of
, then the global prevalence-based spread of fear suppresses the spread of the disease. Moreover, in general we will have a reduction in the epidemic size as a function of 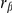 . The early time progression of
. The early time progression of 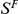 is analogous to that of Model I:
is analogous to that of Model I:
| (22) |
The analogy is due to the fact that as in the first model the transition to 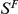 is related only to the presence of infected individuals. Even in this case the condition
is related only to the presence of infected individuals. Even in this case the condition 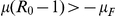 is always satisfied so that if
is always satisfied so that if 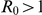 , then fear can spread in the population.
, then fear can spread in the population.
Figure 9. Model II Schematic representation of Model II.
The pseudo mass-action law is represented by the dashed line.
Interestingly, there is a region of the phase space in which this model and Model I are equivalent. In both models the transition to fear is related only to the presence of infected individuals. In the first model we use a mass-action law while in the second we use a pseudo mass-action law. It is possible to relate one of the transmission rates of fear to the other by tuning the parameters. Let us focus our attention on small values of  . We can approximate the transition rate by
. We can approximate the transition rate by
| (23) |
Let us consider the first order term only, i.e., 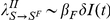 . The relation between the two transmission rates can easily be obtained by imposing
. The relation between the two transmission rates can easily be obtained by imposing
| (24) |
which leads to
| (25) |
where we define 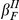 as the rate in the second model, given
as the rate in the second model, given 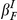 in the first. The above relation guarantees the equivalence of the two models at the first order on
in the first. The above relation guarantees the equivalence of the two models at the first order on 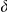 . In the small
. In the small 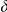 region in which the approximation (23) holds, Model I and II are mathematically indistinguishable for suitable values of the parameters, which indicates that even in the phase space of Model II we have multi-peak regions. These regions, of course, coincide with the regions in the first model.
region in which the approximation (23) holds, Model I and II are mathematically indistinguishable for suitable values of the parameters, which indicates that even in the phase space of Model II we have multi-peak regions. These regions, of course, coincide with the regions in the first model.
The disease-free equilibrium of this model does not allow for an endemic state of fear,
| (26) |
as the transition to fear is induced by the presence of infected individuals only. As soon as the epidemic dies out the in-flow to the 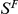 compartment stops, while the out-flow continues to allow people to recover from fear. When the number of infected individuals goes to zero, the media coverage vanishes, as does the fear it causes.
compartment stops, while the out-flow continues to allow people to recover from fear. When the number of infected individuals goes to zero, the media coverage vanishes, as does the fear it causes.
Even in this model the effect of fear results in a reduction of the epidemic size. This reduction is a function of 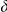 and of all of the parameters. As
and of all of the parameters. As 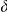 increases the transition into fear becomes faster. Since the people in compartment
increases the transition into fear becomes faster. Since the people in compartment 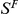 are more protected from the disease, the epidemic size inevitably decreases. While keeping the value of
are more protected from the disease, the epidemic size inevitably decreases. While keeping the value of 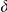 fixed, increasing
fixed, increasing 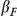 reduces the epidemic size and drives it to its asymptotic value. The asymptotic value of
reduces the epidemic size and drives it to its asymptotic value. The asymptotic value of 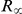 as a function of
as a function of 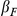 depends on the product
depends on the product 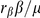 . If this product is bigger than
. If this product is bigger than  , obtained through direct numerical integration of the equations as shown in Figure 10-A, then the asymptotic value is equal to the epidemic size of an SIR model with
, obtained through direct numerical integration of the equations as shown in Figure 10-A, then the asymptotic value is equal to the epidemic size of an SIR model with 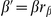 . If the product is smaller than
. If the product is smaller than  , obtained similarly through direct integration of the equation as shown in Figure 10-B, then the asymptotic value is zero; the rate of the spread of awareness is infinitely faster than the spread of the disease. This dynamic can be thought as that of an SIR with a reproductive number smaller than
, obtained similarly through direct integration of the equation as shown in Figure 10-B, then the asymptotic value is zero; the rate of the spread of awareness is infinitely faster than the spread of the disease. This dynamic can be thought as that of an SIR with a reproductive number smaller than  .
.
Figure 10. Model II Reduction of the epidemic size as a function of 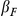 for different values of
for different values of 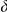 and
and 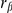 .
.
We fix 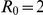 ,
, 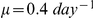 ,
, 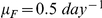 and
and 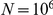 . In panel (A) we assume
. In panel (A) we assume 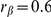 for which
for which 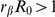 . Increasing the value of
. Increasing the value of 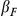 results in an asymptotic value of the epidemic size other than zero. In panel (B) we consider
results in an asymptotic value of the epidemic size other than zero. In panel (B) we consider 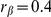 . In this case, instead,
. In this case, instead, 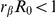 . By increasing the value of
. By increasing the value of 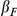 the epidemic size is increasingly reduced. This effect is stronger for bigger values of
the epidemic size is increasingly reduced. This effect is stronger for bigger values of 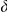 . The values are obtained by numerical integration of the equations.
. The values are obtained by numerical integration of the equations.
Model III: Local, belief-based spread of the fear of the disease
In this section we introduce the last model (Model III) in which we also consider self-reinforcing fear spread which accounts for the possibility that individuals might enter the compartment 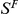 simply by interacting with people in this compartment: fear generating fear. In this model people could develop fear of the infection both by interacting with infected persons and with people already concerned about the disease. A new parameter,
simply by interacting with people in this compartment: fear generating fear. In this model people could develop fear of the infection both by interacting with infected persons and with people already concerned about the disease. A new parameter, 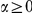 , is necessary to distinguish between these two interactions. We assume that these processes, different in their nature, have different rates. To differentiate them we consider that people who contact infected people are more likely to be scared of the disease than those who interact with fearful individuals. For this reason we set
, is necessary to distinguish between these two interactions. We assume that these processes, different in their nature, have different rates. To differentiate them we consider that people who contact infected people are more likely to be scared of the disease than those who interact with fearful individuals. For this reason we set 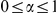 .
.
Let us consider the case of the limit in which no infected individuals are present in the population. The 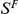 compartment can only grow through the interaction
compartment can only grow through the interaction 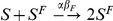 . It is possible to show that in the early stage this can be thought of as an SIS-like model. Let us consider the case in which there are no infected individuals and just one individual in the compartment
. It is possible to show that in the early stage this can be thought of as an SIS-like model. Let us consider the case in which there are no infected individuals and just one individual in the compartment 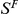 , i.e.,
, i.e., 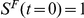 . Considering this limit, the set of equations of Model III could be written as
. Considering this limit, the set of equations of Model III could be written as
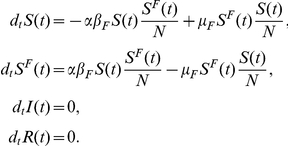 |
We assume that in this early stage all the population is almost fully susceptible  . The equation for
. The equation for  is then
is then
| (27) |
This is the typical early-time term for the ‘infected’ individuals in an SIS model. The spread of fear contagion will start if
| (28) |
This allows us to define the reproductive number of fear by
| (29) |
In isolation, the fear contagion process is analogous to the reproductive number of an SIS or SIR model with transmission rate  . However, in the general case the spread of the fear of infection is coupled with the actual disease spread. The complete set of equations is
. However, in the general case the spread of the fear of infection is coupled with the actual disease spread. The complete set of equations is
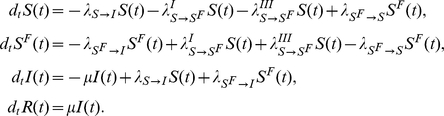 |
A schematic representation of the model is provided in Figure 11. Considering Table 1 we can write all of the terms explicitly,
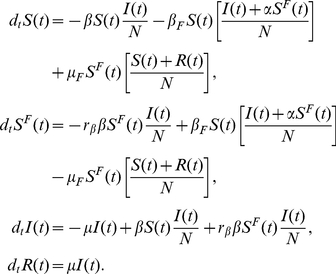 |
(30) |
Also in this model we assume that the population size is fixed,
| (31) |
If we consider the case in which the disease spreads faster than the fear of it, then the reproductive ratio is 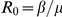 . In the opposite case the reproductive ratio is governed by the compartment
. In the opposite case the reproductive ratio is governed by the compartment 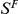 so that
so that 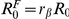 and the epidemic size will be reduced depending on the value of
and the epidemic size will be reduced depending on the value of 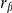 . In this latter case, if
. In this latter case, if 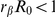 , then the protection from infection gained in the compartment
, then the protection from infection gained in the compartment 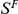 causes the disease to fade out. Following the same linearization strategy shown in previous sections, the early stage of the
causes the disease to fade out. Following the same linearization strategy shown in previous sections, the early stage of the 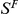 compartment is given by
compartment is given by
| (32) |
Two different regions in the parameter space are then identified: one in which the rate of increase of fear is dominated by its own thought contagion process, 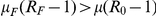 , and one in which the rate of the local belief-based spread is dominated by the disease,
, and one in which the rate of the local belief-based spread is dominated by the disease, 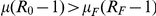 . In the first case the fear spreads independently of the value of
. In the first case the fear spreads independently of the value of 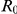 , and the epidemic size will be reduced due to the protection that individuals gain in the
, and the epidemic size will be reduced due to the protection that individuals gain in the 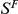 compartment.
compartment.
Figure 11. Model III Schematic representation of Model III.
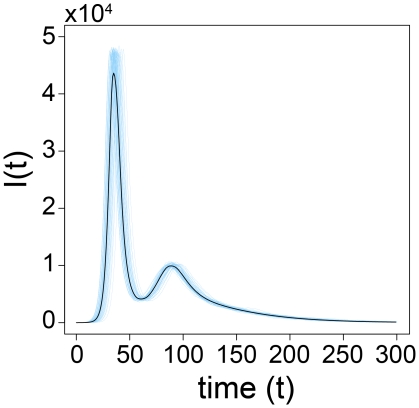
The new interaction, although intuitively simple, significantly complicates the dynamics of the model. In particular within several regions of the parameter space we observe two epidemic peaks as demonstrated in Figure 12. In this figure we plot the medians for two different values of 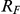 evaluated considering at least
evaluated considering at least 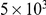 runs in which the epidemic size is at least
runs in which the epidemic size is at least 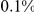 of the population. We also show
of the population. We also show 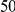 stochastic runs of the model to explicitly visualize the fluctuation among them. This non-trivial behavior can be easily understood. Fear reinforces itself until it severely depletes the reservoir of susceptible individuals, causing a decline in new cases. As a result people are lured into a false sense of security and return back to their normal behavior (recovery from fear) causing a second epidemic peak that can be even larger than the first. Some authors believe that a similar process occurred during the
stochastic runs of the model to explicitly visualize the fluctuation among them. This non-trivial behavior can be easily understood. Fear reinforces itself until it severely depletes the reservoir of susceptible individuals, causing a decline in new cases. As a result people are lured into a false sense of security and return back to their normal behavior (recovery from fear) causing a second epidemic peak that can be even larger than the first. Some authors believe that a similar process occurred during the 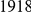 pandemic, resulting in multiple epidemic peaks [26]–[28]. We show in Figure 13 for a set of model parameters the phase diagram of the model on
pandemic, resulting in multiple epidemic peaks [26]–[28]. We show in Figure 13 for a set of model parameters the phase diagram of the model on 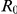 -
-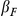 plane regarding different number of disease activity peaks as obtained by numerical integration of the deterministic equations. The figure should be considered as illustrative as we do not have any analytical expression on the sufficient conditions yielding multiple infection peaks.
plane regarding different number of disease activity peaks as obtained by numerical integration of the deterministic equations. The figure should be considered as illustrative as we do not have any analytical expression on the sufficient conditions yielding multiple infection peaks.
Figure 12. Model III Multiple waves of infection.
Fixing 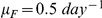 ,
, 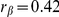 ,
, 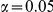 ,
, 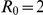 ,
, 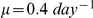 and
and 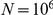 we show
we show 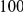 stochastic runs and the medians evaluated considering
stochastic runs and the medians evaluated considering 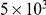 runs for two different values of
runs for two different values of 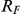 . In panel (A)
. In panel (A) 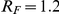 . In panel (B)
. In panel (B) 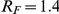 .
.
Figure 13. Model III Phase diagram of infection waves on 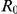 -
-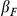 plane.
plane.
We display the regions of parameter space on 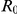 -
-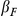 plane exhibiting different number of disease activity peaks for three different values of
plane exhibiting different number of disease activity peaks for three different values of 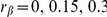 , where we have fixed
, where we have fixed 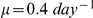 ,
, 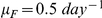 ,
, 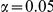 and
and 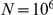 . The phase diagram has been obtained by numerical integration of the deterministic equations in Eq. (30).
. The phase diagram has been obtained by numerical integration of the deterministic equations in Eq. (30).
Residual collective memory of the disease and its effect on epidemic resurgence
At the end of the disease epidemic the system enters the disease-free stage. Setting 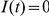 and the epidemic size to
and the epidemic size to 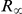 the set of differential equations becomes
the set of differential equations becomes
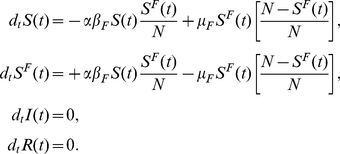 |
(33) |
Conservation of the total number of individuals yields the following differential equation for 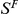 :
:
| (34) |
with the solution
| (35) |
We have defined  as
as
| (36) |
where 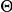 is a time-independent variable and is a function of the parameters of the model. Interestingly, there are two possible disease-free equilibriums. One in which
is a time-independent variable and is a function of the parameters of the model. Interestingly, there are two possible disease-free equilibriums. One in which
| (37) |
where fear dies along with the disease, and the one given by
| (38) |
where fear and behavioral changes persist even after the end of the disease epidemic. The condition 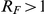 is necessary but not sufficient in order to have an endemic state of fear, while
is necessary but not sufficient in order to have an endemic state of fear, while 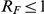 is sufficient to avoid an endemic state of fear. Unfortunately, the parameter
is sufficient to avoid an endemic state of fear. Unfortunately, the parameter  is an implicit function of the whole dynamics through the epidemic size
is an implicit function of the whole dynamics through the epidemic size  .
.
The presence of an endemic state, a societal memory of the disease, and associated fear are quite interesting features of the model induced by fear's self-reinforcement. In Model I transition to the compartment 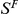 is possible only in the presence of infected individuals. However, in this model fear is able to sustain its presence in the population if the effective reproductive number of the local belief-based spread is larger than unity even if the disease dies out. Unfortunately, this argument cannot be used to fix the range of parameters in the phase space with these properties since any linearization at these stages of the compartments is not suitable. The possibility of having an endemic state of fear indicates that an event localized in time is capable of permanently modifying society with interesting consequences. In the case of a second epidemic, the presence of part of the population already in the compartment
is possible only in the presence of infected individuals. However, in this model fear is able to sustain its presence in the population if the effective reproductive number of the local belief-based spread is larger than unity even if the disease dies out. Unfortunately, this argument cannot be used to fix the range of parameters in the phase space with these properties since any linearization at these stages of the compartments is not suitable. The possibility of having an endemic state of fear indicates that an event localized in time is capable of permanently modifying society with interesting consequences. In the case of a second epidemic, the presence of part of the population already in the compartment 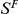 reduces the value of the basic reproduction number. To show this let us consider the differential equation for the infected compartment
reduces the value of the basic reproduction number. To show this let us consider the differential equation for the infected compartment 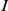 after the re-introduction of the very same infectious virus (meaning that the parameters
after the re-introduction of the very same infectious virus (meaning that the parameters 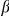 and
and 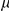 are equal to those of the first infectious disease):
are equal to those of the first infectious disease):
| (39) |
The initial condition of the second disease epidemic could be considered to be the disease-free equilibrium of the first epidemic. By using Eq. (38) we can express the rate equation of the infected compartment during the early stage of the second disease as
| (40) |
Let us define 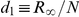 as the proportion of recovered individuals at the end of the first epidemic. In the case of the re-introduction of the disease into the population we will have an outbreak only if the argument in the parenthesis of the above equation is larger than zero, yielding the following condition for the reproductive number
as the proportion of recovered individuals at the end of the first epidemic. In the case of the re-introduction of the disease into the population we will have an outbreak only if the argument in the parenthesis of the above equation is larger than zero, yielding the following condition for the reproductive number 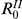 of a second outbreak:
of a second outbreak:
| (41) |
It is worth noting that the societal memory of the first outbreak increases the resistence in the population against the spread of the second outbreak in a non-trivial way. One might be tempted to conclude that the new reproductive number is simply provided by the reproductive number of an SIR model with an equivalent proportion of removed individuals 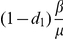 , but this is not the case as we have to factor in the behavioral changes of individuals in the compartment
, but this is not the case as we have to factor in the behavioral changes of individuals in the compartment 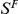 , obtaining
, obtaining
| (42) |
To prove the last inequality we have to show that
| (43) |
or
| (44) |
The expressions on both sides of the above inequality are first-order polynomial functions of 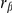 . For
. For 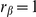 they assume the same value
they assume the same value 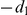 . It is important to stress that in this limit (
. It is important to stress that in this limit (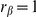 ) the model is indistinguishable from the classical SIR. These two functions can only have one common point which occurs at
) the model is indistinguishable from the classical SIR. These two functions can only have one common point which occurs at 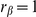 . We will consider only the region in which
. We will consider only the region in which 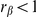 as assumed in our model. To prove our proposition we have to confront the slopes of the functions and show that
as assumed in our model. To prove our proposition we have to confront the slopes of the functions and show that
| (45) |
The polynomial with smaller slope will always be below the other in the relevant region 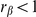 . Eq. (45) can be rewritten as
. Eq. (45) can be rewritten as
| (46) |
which is always satisfied, provided our assumption 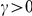 . This is an important result that confirms how an endemic state of behavioral change in the population reduces the likelihood and impact of a second epidemic outbreak. We note that such a state will inevitably fade out on a long time scale. This can be modeled with a spontaneous transition
. This is an important result that confirms how an endemic state of behavioral change in the population reduces the likelihood and impact of a second epidemic outbreak. We note that such a state will inevitably fade out on a long time scale. This can be modeled with a spontaneous transition 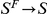 acting on a time scale longer than the epidemic process itself.
acting on a time scale longer than the epidemic process itself.
Discontinuous transition in the epidemic prevalence
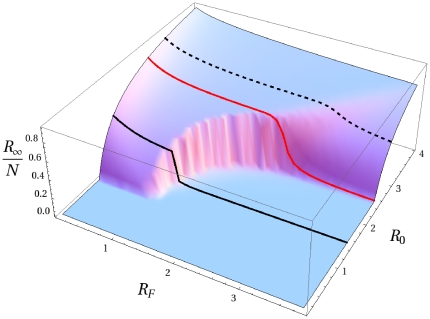
A further interesting characteristic of this model resides in the reduction of the epidemic size as shown in Figure 14. In this plot we show 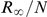 , evaluated through direct integration of the equations, as a function of
, evaluated through direct integration of the equations, as a function of 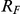 and
and 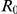 , keeping fixed the other parameters. In this case the self-reinforcement mechanism creates a more complicated phase space that allows for a jump in the epidemic size as
, keeping fixed the other parameters. In this case the self-reinforcement mechanism creates a more complicated phase space that allows for a jump in the epidemic size as 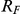 increases above a critical value
increases above a critical value 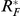 (see the black solid line in Figure 14). This behavior, typical of the first-order phase transitions in cooperative systems, signals a drastic change in the dynamical properties of the behavior-disease model. If
(see the black solid line in Figure 14). This behavior, typical of the first-order phase transitions in cooperative systems, signals a drastic change in the dynamical properties of the behavior-disease model. If 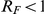 , then obviously the fear of the disease is not able to affect a large fraction of the population and the disease spreads as usual in the population, affecting at the end of its progression
, then obviously the fear of the disease is not able to affect a large fraction of the population and the disease spreads as usual in the population, affecting at the end of its progression 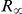 individuals. If
individuals. If 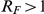 we face two different scenarios or two different regions of
we face two different scenarios or two different regions of 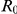 separated by the red solid line in Figure 14:
separated by the red solid line in Figure 14:
Figure 14. Model III Reduction of the epidemic size as a function of 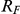 and
and 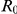 .
.
Fixing 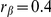 ,
, 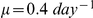 ,
, 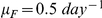 ,
, 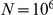 , and
, and 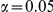 . The three lines are curves of
. The three lines are curves of 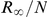 as a function of
as a function of 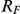 , keeping
, keeping 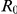 constant. We select three different values of
constant. We select three different values of 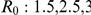 which correspond to solid black, red, and dashed lines, respectively. The value
which correspond to solid black, red, and dashed lines, respectively. The value 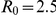 is a special case that leads to
is a special case that leads to 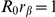 . It divides the phase space in two different regions. All the values of
. It divides the phase space in two different regions. All the values of 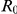 below are characterized by
below are characterized by 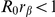 . In this case for large values of
. In this case for large values of 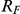 the model is reduced to an SIR with reproductive number
the model is reduced to an SIR with reproductive number 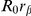 below
below  and the epidemic is halted. Interestingly, this behavior starts in an intermediate regime of
and the epidemic is halted. Interestingly, this behavior starts in an intermediate regime of 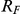 . There is a critical value
. There is a critical value 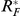 of
of 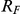 above which (i.e.,
above which (i.e., 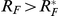 ) the epidemic size is zero. This transition happens with a jump, as shown by the solid black line. All the values of
) the epidemic size is zero. This transition happens with a jump, as shown by the solid black line. All the values of 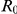 above
above 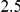 are instead characterized by
are instead characterized by 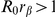 . Also in this case the model is reduced to an SIR with reproductive number
. Also in this case the model is reduced to an SIR with reproductive number 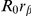 for large values of
for large values of 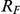 , but in this case this value is above
, but in this case this value is above  . This results in a epidemic size that is always non-zero. In this region of parameters no jumps are present (see the dashed line). The values shown in the plot are computed through numerical integration of the equations.
. This results in a epidemic size that is always non-zero. In this region of parameters no jumps are present (see the dashed line). The values shown in the plot are computed through numerical integration of the equations.
In the case that
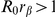 (i.e., the dashed line in Figure 14) the generation of a finite fraction of individuals in the
(i.e., the dashed line in Figure 14) the generation of a finite fraction of individuals in the 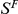 compartment is not able to halt the epidemic. The behavioral changes are not enough to bring the reproductive number below the epidemic threshold and
compartment is not able to halt the epidemic. The behavioral changes are not enough to bring the reproductive number below the epidemic threshold and 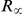 decreases smoothly because of the epidemic progress with a progressively lower effective reproductive number.
decreases smoothly because of the epidemic progress with a progressively lower effective reproductive number.If
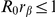 , (i.e., the black solid line in Figure 14) the individuals that populate the
, (i.e., the black solid line in Figure 14) the individuals that populate the 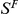 compartment keep the spread of the epidemic below the threshold. In principle, the state
compartment keep the spread of the epidemic below the threshold. In principle, the state 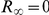 and
and 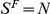 would be possible. In general, the process needs to start with infectious individuals that trigger the first transitions
would be possible. In general, the process needs to start with infectious individuals that trigger the first transitions 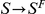 and therefore a small number of
and therefore a small number of 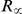 individuals are generated. However, there will be a
individuals are generated. However, there will be a 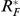 at which the growth of the fear contagion process is faster than the growth of the epidemic with a small
at which the growth of the fear contagion process is faster than the growth of the epidemic with a small 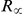 . At this point the fear contagion process is accelerated by the growth of individuals in
. At this point the fear contagion process is accelerated by the growth of individuals in 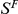 while the epidemic spread is hampered by it. The
while the epidemic spread is hampered by it. The 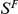 is quickly populated by individuals while the epidemic stops, generating a very small number of
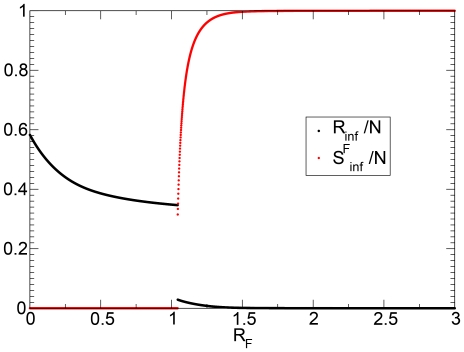
is quickly populated by individuals while the epidemic stops, generating a very small number of 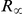 . This generates a jump in the amount of individuals that experience the infection as a function of
. This generates a jump in the amount of individuals that experience the infection as a function of 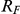 . This is clearly illustrated by Figure 15 where the behavior of both quantities
. This is clearly illustrated by Figure 15 where the behavior of both quantities 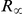 and
and 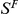 is plotted close to the transition point. The value at which the transition occurs also depends on the other parameters of the model including
is plotted close to the transition point. The value at which the transition occurs also depends on the other parameters of the model including 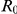 and
and 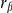 .
.
Figure 15. Model III 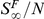 and
and 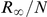 for
for 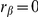 ,
, 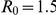 ,
, 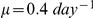 ,
, 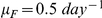 ,
, 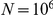 , and
, and 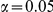 .
.
The values are obtained by numerical integration of the equations.
The extremely rich phase space of this model is important for two reasons: i) we have a strong reduction in the cumulative number of infected individuals associated with discontinuous transition; ii) in the case of a second epidemic the memory of the system shifts the reproductive number towards smaller values. These are very interesting properties of the model due to the self-reinforcing mechanism that clearly creates non-trivial behaviors in the dynamics. We have tried different analytical approaches to get more insight into the phase transition. Unfortunately, the discontinuous transition is triggered by model behavior out of the simple linearized initial state and it is extremely difficult to derive any closed analytical expression. An analytic description is beyond the scope of the present classification of behavior-disease models and is the object of future work on the model.
Discussion
We introduced a general framework with different mechanisms in order to consider the spread of awareness of a disease as an additional contagion process. Three mechanisms were proposed. In the first, basic model the social distancing effects and behavioral changes are only related to the fraction of infected individuals in the population. In the second we modeled the spread of awareness considering only the absolute number of infected individuals as might happen in the case that the information the individuals rely on is mostly due to mass media reporting about the global situation. Finally, in the third model we added the possibility that susceptible people will initiate behavioral changes by interacting with individuals who have already adopted a behavioral state dominated by the fear of being infected. This apparently simple interaction allows for the self-reinforcement of fear. We have found that these simple models exhibit a very interesting and rich spectrum of dynamical behaviors. We have found a range of parameters with multiple peaks in the incidence curve and others in which a disease-free equilibrium is present where the population acquires a memory of the behavioral changes induced by the epidemic outbreak. This memory is contained in a stationary (endemic) prevalence of individuals with self-induced behavioral changes. Finally, a discontinuous transition in the number of infected individuals at the end of the epidemic is observed as a function of the transmissibility of fear of the disease contagion. At this stage the study of these properties has been mostly phenomenological and we have focused on minimal models that do not include demographic changes and spontaneous changes in the behavior of individuals such as the fading out of an epidemic over a long time. We should also note that the behavior-disease models we have suggested do not take into account the associated costs of social-distancing measures adopted by individuals, such as societal disruption and financial burden. A game theoretical approach [29], [30] would be well suited in order to account for factors in the decision making process for self-initiated behavioral changes. However, more features added to increase the realism of the models inevitably increase their complexity. Moreover, the non-trivial dynamic behavior of the models emphasizes the importance of calibrating those features by appropriate choices of parameter values. Unfortunately, in many cases we lack the data necessary for calibrating the behavioral models. The availability of real-world, quantitative data concerning behavioral changes in populations affected by epidemic outbreaks is therefore the major roadblock to the integration of behavior-disease models. Any progress in this area certainly has to target novel data acquisition techniques and basic experiments aimed at gathering these data.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work has been partially funded by the NIH R21-DA024259 award and the DTRA-1-0910039 award to AV. The work has been also partly sponsored by the Army Research Laboratory and was accomplished under Cooperative Agreement Number W911NF-09-2-0053. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Laboratory or the U.S. Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferguson N. Capturing human behaviour. Nature. 2007;466:733. doi: 10.1038/446733a. [DOI] [PubMed] [Google Scholar]
- 2.Funk S, Salathé M, Jansen VAA. Modelling the inuence of human behaviour on the spread of infectious diseases: a review. J R Soc Interface. 2010;7:1247–1256. doi: 10.1098/rsif.2010.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epi. 2006;164:936–944. doi: 10.1093/aje/kwj317. [DOI] [PubMed] [Google Scholar]
- 4.Merler S, Ajelli M. The role of population heterogeneity and human mobility in the spread of pandemic inuenza. Proc Roy Soc B: Biol Sci. 2010;277:557–565. doi: 10.1098/rspb.2009.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balcan D, Colizza V, Gonçalves B, Hu H, Ramasco J, et al. Multiscale mobility networks and the large scale spreading of infectious diseases. Proc Natl Acad Sci. 2009;106:21484–2189. doi: 10.1073/pnas.0906910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colizza V, Barrat A, Barthélemy, Valleron MAJ, Vespignani A. Modeling the worldwide spread of pandemic inuenza: Baseline case and containment interventions. PLoS Med. 2007;4:e13. doi: 10.1371/journal.pmed.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colizza V, Barrat A, Barthélemy, Vespignani A. The role of the airline transportation network in the prediction and predictability of global epidemics. Proc Natl Acad Sci USA. 2006;103:2015–2020. doi: 10.1073/pnas.0510525103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollingsworth T, Ferguson N, Anderson R. Will travel restrictions control the international spread of pandemic inuenza? Nat Med. 2006;12:497–499. doi: 10.1038/nm0506-497. [DOI] [PubMed] [Google Scholar]
- 9.Cooper B, Pitman R, Edmunds W, Gay N. Delaying the international spread of pandemic inuenza. PLoS Med. 2006;3:e12. doi: 10.1371/journal.pmed.0030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan S, Wang W, Levin S. The effect of global travel on the spread of sars. Math Biosci Eng. 2006;3:205–218. doi: 10.3934/mbe.2006.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Ginelli F, Hinrichsen H, Livi R, Mukamel D, Torcini A. Contact processes with long-range interactions. J Stat Mech. 2006:P08008. [Google Scholar]
- 12.Hufnagel L, Brockmann D, Geisel T. Forecast and control of epidemics in a globalized world. Proc Natl Acad Sci USA. 2004;101:15124–15129. doi: 10.1073/pnas.0308344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grais RF, Ellis HJ, Glass G. Modeling the spread of annual inuenza epidemics in the us: the potential role of air travel. Health Care Manage Sci. 2004;7:127–134. doi: 10.1023/b:hcms.0000020652.38181.da. [DOI] [PubMed] [Google Scholar]
- 14.Grais RF, Ellis HJ, Glass G. Assessing the impact of airline travel on the geographic spread of pandemic inuenza. Eur J Epidemiol. 2003;18:1065–1072. doi: 10.1023/a:1026140019146. [DOI] [PubMed] [Google Scholar]
- 15.Flahault A, Valleron AJ. A method for assessing the global spread of hiv-1 infection based on air-travel. Math Popul Stud. 1991;3:1–11. doi: 10.1080/08898489209525336. [DOI] [PubMed] [Google Scholar]
- 16.Rvachev LA, Longini IM. A mathematical model for the global spread of inuenza. Math Biosci. 1985;75:3–32. [Google Scholar]
- 17.Longini IM, Nizam A, S X, Ungchusak K, Hanshaoworakul W, et al. Containing pandemic inuenza at the source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 18.Eubank S, Guclu H, Kumar VSA, Marathe MV, Srinivasan A, et al. Modelling disease outbreaks in realistic urban social networks. Nature. 2004;429:180–184. doi: 10.1038/nature02541. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson NM, Cummings DAT, Cauchemez S, Fraser C, Riley S, et al. Strategies for containing an emerging inuenza pandemic in southeast asia. Nature. 2005;437:209. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 20.Chao DL, Halloran ME, Longini IM. Flute, a publicly available stochastic inuenza epidemic simulation model. PLoS Comput Biol. 2010;6:e1000656. doi: 10.1371/journal.pcbi.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balcan D, Hu H, Gonçalves B, Bajardi P, Poletto C, et al. Seasonal transmission potential and activity peaks of the new inuenza a(h1n1): a monte carlo likelihood analysis based on human mobility. BMC Medicine. 2009;7:45. doi: 10.1186/1741-7015-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajardi P, Poletto C, Balcan D, Hu H, Gonçalves B, et al. Modeling vaccination campaigns and the fall/winter 2009 activity of the new a(h1n1) inuenza in the northern hemisphere. Emerging Health Threats. 2009;2:e11. doi: 10.3134/ehtj.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner D. The Science of Fear: How the Culture of Fear Manipulates Your Brain. Plume, New York; 2009. [Google Scholar]
- 24.Vespignani A. Predicting the behavior of techno-social systems. Science. 2009;325:425–428. doi: 10.1126/science.1171990. [DOI] [PubMed] [Google Scholar]
- 25.Cruz-Pacheco G, Duran L, Esteva L, Minzoni A, López-Cervantes M, et al. Modelling of the inuenza a(h1n1) outbreak in mexico city, april-may 2009, with control sanitary. Euro Surveillance. 2009;14:19254. [PubMed] [Google Scholar]
- 26.Hatchett R, Mecher C, M L. Public health interventions and epidemic intensity during the 1918 inuenza pandemic. Proc Natl Acad Sci. 2007;104:7582–7587. doi: 10.1073/pnas.0610941104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bootsma M, Ferguson N. Public health interventions and epidemic intensity during the 1918 inuenza pandemic. Proc Natl Acad Sci. 2007;104:7588–7593. doi: 10.1073/pnas.0611071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markel H, Lipman H, Navarro J, Sloan A, Michalsen J, et al. Nonpharmaceutical interventions implemented by us cities during the 1918–1919 inuenza pandemic. JAMA. 2007;298:6. doi: 10.1001/jama.298.6.644. [DOI] [PubMed] [Google Scholar]
- 29.Fenichel E, Castillo-Chavez C, Ceddia M, Chowell G, Gonzalez Parrae P, et al. Adaptive human behavior in epidemiological models. Proc Natl Acad Sci USA. 2011;108:6306–6311. doi: 10.1073/pnas.1011250108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen F, Jiang M, Rabidoux S, Robinson S. Public avoidance and epidemics: Insights from an economic model. J Theor Biol. 2011;278:107–119. doi: 10.1016/j.jtbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Poletti P, Ajelli M, Merler S. The effect of risk perception on the 2009 h1n1 pandemic inuenza dynamics. PLoS ONE. 2011;6:e16460. doi: 10.1371/journal.pone.0016460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funk S, Gilad E, Watkins C, Jansen V. The spread of awareness and its impact on epidemic outbreaks. Proc Natl Acad Sci. 2009;106:6872–6877. doi: 10.1073/pnas.0810762106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poletti P, Caprile B, Ajelli M, Pugliese A, S M. Spontaneous behavioural changes in response to epidemics. J Theor Biol. 2009;260:31–40. doi: 10.1016/j.jtbi.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Coelho F, Codeço C. Dynamic modeling of vaccinating behavior as a function of individual beliefs. PLoS Comput Biol. 2009;5:e1000425. doi: 10.1371/journal.pcbi.1000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epstein JM, Parker J, Cummings D, Hammond RA. Coupled contagion dynamics of fear and disease: Mathematical and computational explorations. PLoS ONE. 2008;3:e3955. doi: 10.1371/journal.pone.0003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagnoli F, Lio P, Sguanci L. Risk perception in epidemic modeling. Phys Rev E. 2007;76:061904. doi: 10.1103/PhysRevE.76.061904. [DOI] [PubMed] [Google Scholar]
- 37.Crosby AW. America's forgotten pandemic: the inuenza of 1918. Cambridge University Press; 1990. [Google Scholar]
- 38.Campbell GL, Hughes JM. Plague in india: a new warning from an old nemesis. Ann Intern Med. 1995;122:151–153. doi: 10.7326/0003-4819-122-2-199501150-00014. [DOI] [PubMed] [Google Scholar]
- 39.Lau JFT, Yang X, Pang E, Tsui HY, Wong E, et al. Sars-related perceptions in hong kong. Emerg Infect Dis. 2005;11:417–424. doi: 10.3201/eid1103.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goffman W, Newill V. Generalization of epidemic theory an application to the transmission of ideas. Nature. 1964;204:225–228. doi: 10.1038/204225a0. [DOI] [PubMed] [Google Scholar]
- 41.Kermack W, McKendrick A. A contribution to the mathematical theory of epidemics. P Roy Soc Long A Mat. 1927;115:700–721. [Google Scholar]
- 42.Lynch A. Thought contagion as asbract evolution. J Ideas. 1991;2:3–10. [Google Scholar]
- 43.DeFleur ML, Ball-Rokeach S. Theories of Mass Communication. Longman, NY; 1989. [Google Scholar]
- 44.Keeling MJ, Rohani P. Modeling infectious diseases in humans and animals. Princeton University Press; 2008. [Google Scholar]
- 45.Longini IM. Chain binomial models. The Encyclopedia of Biostatistics. 1998;1:593–597. [Google Scholar]
- 46.Halloran ME, Longini IM, Struchiner CJ. Binomial and stochastic transmission models. Design and Analysis of Vaccine Studies. 2010:63–84. [Google Scholar]