Abstract
In the last decade, the low-density lipoprotein receptor-related protein 5 (LRP5) gene, coding for a coreceptor in the canonical Wnt signalling pathway, has been shown to play an important role in regulating bone mass and to be involved in the pathogenesis of several bone disorders. Here we describe a patient who presented with a clinical picture of Autosomal Dominant Osteopetrosis type I (ADO I), in whom we could identify the first deletion in the LRP5 gene causing increased bone mass. This mutation caused the in-frame deletion of two amino acids in the fourth blade of the first propeller of the protein, namely the highly conserved glycine at position 171 and the following glutamate residue. In vitro studies suggested that the pathogenic effect of this novel mutation could be due to a decreased inhibition of Wnt signalling by the antagonistic proteins sclerostin and Dickkopf-1, encoded respectively by the SOST and DKK1 genes, in the presence of mutated LRP5. Our results highlight an increasing molecular heterogeneity in LRP5-related bone diseases.
Keywords: Bone, Osteosclerosis, Osteopetrosis, LRP5, Wnt signalling
Highlights
► We report the first deletion of 2 amino acid residues in LRP5 in an ADO I patient. ► This suggests an increasing molecular heterogeneity in LRP5-related bone diseases. ► This deletion causes decreased inhibition of Wnt signalling by sclerostin and DKK1. ► ADO I and ADO II are clinically and molecularly distinct entities. ► No impairment of the immune system has been documented in ADO I patients.
Introduction
At the end of the eighties, Bollerslev and Andersen reviewed a large group of patients affected by Autosomal Dominant Osteopetrosis (ADO) and, on the basis of radiological and biochemical findings, suggested that two different types of ADO existed [1]. More recently this clinical observation was supported by the results of molecular investigations in patients, which showed that monoallelic defects in low-density lipoprotein receptor-related protein 5 (LRP5) gene caused human ADO I, in which the long bones and the skull are mainly affected, while mutations in a single allele of the chloride channel 7 (ClCN7) gene were responsible for ADO II, in which an increased rate of bone fractures is reported. Both LRP5 and ClCN7 proteins are involved in signalling pathways or cellular processes which are crucial in bone metabolism as demonstrated by the range of bone diseases arising from different mutations in either encoding gene. In particular, biallelic loss of function mutations in LRP5 are responsible for the autosomal recessive osteoporosis pseudoglioma syndrome (OPPG; MIM 259770) [2–4]; on the contrary, monoallelic mutations in LRP5, initially thought to lead to a gain of function of the protein product, cause a range of phenotypes inherited in an autosomal dominant way and characterised by increased bone density. These are endosteal hyperostosis (MIM 144750), osteosclerosis (MIM 144750), dominant osteopetrosis (MIM 607634), van Buchem disease type 2 (VBCH2; MIM 607636) and high bone mass syndrome (HBM; MIM 601884) [4–9]. In addition, studies in different populations have suggested that LRP5 could be a susceptibility gene for osteoporosis and fracture risk [10,11].
The specific clinical picture is strongly related to the LRP5 domain affected by the mutation. So far, the LRP5 mutations reported to have an activating effect on Wnt signalling are all missense mutations and clustered in the first β-propeller domain of the protein. Biochemical studies showed that their effect is likely due to a reduced inhibition of the canonical Wnt pathway by sclerostin and Dickkopf-1 [12–17].
Here we report for the first time the identification and biochemical characterization of a mutation in the LRP5 gene leading to a two amino acid deletion in the protein, found in a patient affected by ADO I.
Materials and methods
Case report
The patient herein described is a 56 year old woman of Caucasian origin, presenting with an ADO I phenotype. The diagnosis was made on the basis of radiological examinations, performed at menopause due to generalized bone pain, which she had been suffering from for many years. Increased bone density mainly involved skull base, mandible and legs.
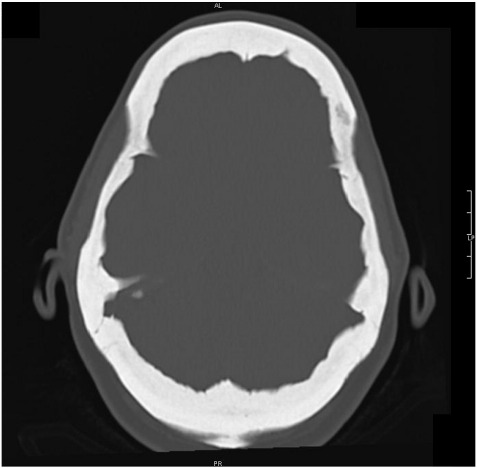
No fractures were reported. At 16 years of age, she experienced complete and sudden blindness of the left eye, whose origin was not investigated. At 50 years of age, she had an infection of the right ear and subsequently monolateral impairment of the hearing capacity arose. At 55 years of age, ophthalmological and audiometric examinations demonstrated reduction of the visual capacity also of the right eye and worsening of the auditory problems. A CT scan performed after diagnosis showed a generalised thickening of the skull (Fig. 1) and restriction of both optical and auditory canals; in addition the patient referred frequent headaches.
Fig. 1.
Axial view of a CT scan of our patient. A generalized thickening of the skull is evident.
Biochemical studies revealed normal values for serum calcium, phosphorus, 1,25(OH)2D3 and bone-specific alkaline phosphatase (ALP), while PTH was slightly increased.
The patient's father, her daughter and two paternal aunts were all diagnosed as osteopetrotic on the basis of X-rays, but it has not been possible to confirm this diagnosis at a molecular level or to perform further evaluations in any of them.
Samples and mutation analysis
DNA sample from the patient was obtained after receiving informed consent. Investigation has been approved by the Local Ethic Committee. Genomic DNA was extracted from PBL by standard techniques; mutation analysis of the LRP5 gene (AF283320) was performed as previously described [2].
Expression constructs and in vitro mutagenesis
The deletion found in the proband (g.69547_69552delGGTGAG) was introduced in untagged full-length human WT LRP5 construct (obtained from Dr. Matthew Warman, Howard Hughes Medical Institute, Orthopaedic Research Laboratories, Boston, MA; [2]) using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) with forward primer 5′-CTGGACAGACTGGACGCCCCGGATTG-3′ and reverse primer 5′-CAATCCGGGGCGTCCAGTCTGTCCAG-3′.
The inserted sequence was verified for the presence of the mutation and absence of PCR errors by DNA sequencing.
A mouse Wnt1-V5 expression construct was provided by Dr. Bart Williams (Van Andel Research Institute, Grand Rapids, MI), a mouse mesdc-2 expression construct was provided by Dr. Bernadette Holdener (State University of New York, Stony Brook, NY), a human DKK1-FLAG expression construct was provided by Dr. Sergei Sokol (Mount Sinai School of Medicin, New York, NY), a mouse amino terminal HA-tagged Sost (HA-mSost) expression construct was obtained from Dr. Matt Warman (Howard Hughes Medical Institute, Orthopaedic Research Laboratories, USA) and Dr. Bert Vogelstein (Johns Hopkins University School of Medicine, Baltimore, MD) kindly provided the Topflash Wnt reporter construct (pGL3-OT). The Renilla Luciferase construct pRL-TK was purchased from Promega Corporation (Madison, WI) [9].
Cell culture, transfection and luciferase reporter assays
HEK293T and Saos-2 cells were grown in DMEM supplemented with 10%v/v FBS (Invitrogen, San Diego, CA). Twenty-four hours prior to transfection, cells were plated at 1.25 × 105 cells/well in 24-well plates. Cells were transfected using Fugene 6 (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. Transfection and luciferase reporter assay were performed as previously described [9].
Statistical analysis
Data are expressed as mean values ± SD. Comparison between two measurements for a single experiment was performed using a Student's t-test. Values of p < 0.05 were considered significant. Statistical tests were provided by the SPSS 15.0 software package (IBM Corporation, Somers, NY).
Results
Mutation analysis
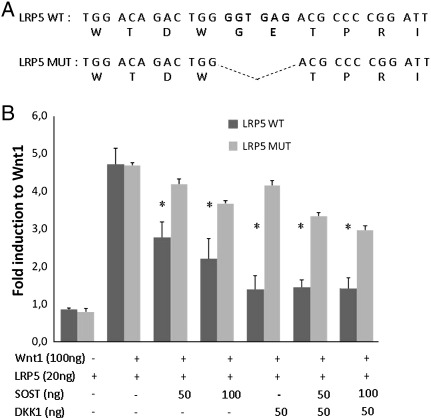
Direct sequencing of the region of interest in the LRP5 gene revealed the presence of an in-frame deletion of six nucleotides (g.69547_69552delGGTGAG; c.511_516delGGTGAG) in exon 3 in one allele, corresponding to two amino acid residues (p.Gly171_Glu172del), while the other allele was normal (Fig. 2A). As has been reported for the other high bone mass-causing mutations, this newly identified one is also located in the first β-propeller domain of the protein, in its amino terminal, extracellular portion. It involves the glycine at position 171, which is highly conserved throughout evolution and between LRP5 and its homologue LRP6, and has been extensively studied. Interestingly, two missense mutations have been already reported at this very same position: a p.Gly171Val, found in a family including phenotypically normal individuals with extremely dense bones [6] and in another kindred with other clinical features: torus palatinus and wide, deep mandible, in addition to increased bone density [5]; and a p.Gly171Arg in a Belgian classical ADO I family [7].
Fig. 2.
A. Sequence information on LRP5 around the new deletion. B. Functional evaluation of canonical Wnt signalling in Saos-2 cells expressing WT or mutant LRP5. Fold induction of luciferase reporter activity in cells is normalized to the fold induction in cells expressing only the Wnt1-V5 expression construct. Bars represent average values ± SD. *: p-value < 0.05.
Functional evaluation
To evaluate the functional effect of this new mutation, wild-type (WT) and mutant (Mut) LRP5 proteins were expressed independently either in the Saos-2 human osteosarcoma cell line or in HEK293T cells along with a luciferase reporter construct.
Co-transfection of LRP5 (either WT or Mut) with Wnt1 resulted in an identical increase in Wnt signalling (Fig. 2B). However, decreased inhibition was observed for the mutant LRP5 after co-transfection with either SOST or DKK1 or a combination of both. Similar results were obtained in HEK293T cells (data not shown). This suggested that the in vivo bone phenotype was caused by a decreased ability of the mutant protein to interact with these two inhibitory molecules.
Discussion
In the last two decades, an increasing amount of genetic data has /INS; clearly demonstrated the role of LRP5 in the regulation of bone homeostasis. In particular, a limited series of mutations has been associated with conditions characterised by increased bone density in humans. In the present work, we report the identification of a new type of mutation in this gene, leading to the in-frame deletion of two amino acids in the LRP5 protein and resulting in a decreased inhibition of Wnt signalling by the extracellular antagonists sclerostin and DKK1. Previously it was shown that a missense mutation of Gly171 results in impaired binding of both sclerostin and DKK1 [9,14], which allows us to assume that deletion of this and its flanking amino acid will have a similar effect. This supports the hypothesis that, besides the third β-propeller domain of LRP5 [10], the first β-propeller domain of the protein also has a critical role in the binding of sclerostin and DKKs. In accordance with the hypothesis raised by Bhat and colleagues [18] the deletion of the Gly171 and Glu172 residues could alter the three-dimensional structure of the receptor, thus determining a reduction in the affinity for its inhibitory ligands. Overall, this disease could be ascribed to a “gain of function” not with regard to the LRP5 protein itself, but to the entire signalling pathway, which turns out to be activated even in the presence of its inhibitory factors.
The proband herein described was a middle-aged woman who suffered symptoms possibly related to the disease while in her teens, whereas the diagnosis of osteopetrosis was made at menopause when the clinical symptoms had started worsening. Her daughter was found to be osteopetrotic after radiological examination, however she does not present any symptoms. Although it is likely that she carries the same mutation as her mother, confirmation through molecular analysis was not possible. Our data, together with those already reported in the literature, conclude that at variance with ADO II, in which several cases of early onset of the disease are documented [19,20], ADO I symptoms most frequently arise in adulthood, after the first radiological signs. In addition, ADO I patients do not display impairment of the haematological compartment, even though the canonical Wnt signalling is known to play an important role in haematopoiesis, and rarely present visual deficits, while these defects can be very evident in some ADO II patients. Interestingly, our patient did show a complete and abrupt occurrence of blindness at early age, although the exact cause could not be documented at that time (more than 40 years ago). All these findings confirm the original observation of Bollerslev and Andersen [1] that ADO I and ADO II are two distinct entities both from a clinical and molecular point of view.
The canonical Wnt signalling has been reported to regulate key checkpoints in the development of many tissues, and among them, also in lymphopoiesis [21]. Even though in other forms of osteopetrosis both primary and secondary immunological defects have been described, no impairment of the immune system has been documented in ADO I patients, including ours, possibly due to the high redundancy of this pathway.
The identification of a new type of mutation in the LRP5 gene in our patient highlights for the first time the presence of a molecular heterogeneity in LRP5-related diseases characterised by high bone density, which adds to the well-known clinical heterogeneity arising from mutations in this gene.
Conflict of interest
All authors declare no conflicts of interest.
Acknowledgments
This work was supported by E-rare project JTC 2007 OSTEOPETR to AV, Fondazione Cariplo grant to CS, Telethon Foundation (grant GGP10116) to CS, by Ministero della Salute, convenzione 47 (Role of new inflammatory molecules in pregnancy pathologies and in maternal neonatal health) to PV, by the European Commission [HEALTH-F2-2008-201099, TALOS] and by grants from the ‘Fonds voor Wetenschappelijk Onderzoek’ [FWO, G.0065.10N], from the Special Research Funds (BOF TOP and NOI) of the University of Antwerp, all to WVH. EB holds a pre-doctoral specialization scholarship from the “Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen)”.
Edited by: Stuart Ralston
Contributor Information
Alessandra Pangrazio, Email: alessandra.pangrazio@itb.cnr.it.
Eveline Boudin, Email: eveline.boudin@ua.ac.be.
Elke Piters, Email: elke.piters@ua.ac.be.
Giuseppe Damante, Email: damante.giuseppe@aoud.sanita.fvg.it.
Nadia Lo Iacono, Email: nadia.Lo_Iacono@humanitasresearch.it.
Angela Valentina D'Elia, Email: d'elia.angela@aoud.sanita.fvg.it.
Paolo Vezzoni, Email: paolo.vezzoni@itb.cnr.it.
Wim Van Hul, Email: wim.vanhul@ua.ac.be.
Anna Villa, Email: anna.villa@itb.cnr.it.
Cristina Sobacchi, Email: cristina.sobacchi@humanitasresearch.it, cristina.sobacchi@itb.cnr.it.
References
- 1.Bollerslev J., Andersen P.E., Jr. Radiological, biochemical and hereditary evidence of two types of autosomal dominant osteopetrosis. Bone. 1988;9:7–13. doi: 10.1016/8756-3282(88)90021-x. [DOI] [PubMed] [Google Scholar]
- 2.Gong Y., Slee R.B., Fukai N., Rawadi G., Roman-Roman S., Reginato A.M. LDL receptor related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 3.Ai M., Heeger S., Bartels C.F., Schelling D.K. Osteoporosis-Pseudoglioma Collaborative Group. Clinical and molecular findings in osteoporosis-pseudoglioma syndrome. Am J Hum Genet. 2005;77:741–753. doi: 10.1086/497706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levasseur R., Lacombe D., De Vernejoul M.C. LRP5 mutations in osteoporosis-pseudoglioma syndrome and high-bone-mass disorders. Joint Bone Spine. 2005;72:207–214. doi: 10.1016/j.jbspin.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Boyden L.M., Mao J., Belsky J., Mitzner L., Farhi A., Mitnick M.A. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 6.Little R.D., Carulli J.P., Del Mastro R.G., Dupuis J., Osborne M., Folz C. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Wesenbeeck L., Cleiren E., Gram J., Beals R.K., Bénichou O., Scopelliti D. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet. 2003;72:763–771. doi: 10.1086/368277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rickels M.R., Zhang X., Mumm S., Whyte M.P. Oropharyngeal skeletal disease accompanying high bone mass and novel LRP5 mutation. J Bone Miner Res. 2005;20:878–885. doi: 10.1359/JBMR.041223. [DOI] [PubMed] [Google Scholar]
- 9.Balemans W., Devogelaer J.P., Cleiren E., Piters E., Caussin E., Van Hul W. Novel LRP5 missense mutation in a patient with a high bone mass phenotype results in decreased DKK1-mediated inhibition of Wnt signaling. J Bone Miner Res. 2007;22:708–716. doi: 10.1359/jbmr.070211. [DOI] [PubMed] [Google Scholar]
- 10.Balemans W., Van Hul W. The genetics of low-density lipoprotein receptor-related protein 5 in bone: a story of extremes. Endocrinology. 2007;148:2622–2629. doi: 10.1210/en.2006-1352. [DOI] [PubMed] [Google Scholar]
- 11.van Meurs J.B., Trikalinos T.A., Ralston S.H., Balcells S., Brandi M.L., Brixen K. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299:1277–1290. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Wang Y., Li X., Zhang J., Mao J., Li Z. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol. 2004;24:4677–4684. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai M., Holmen S.L., Van Hul W., Williams B.O., Warman M.L. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol. 2005;25:4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellies D.L., Viviano B., McCarthy J., Rey J.P., Itasaki N., Saunders S. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006;21:1738–1749. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- 15.Semenov M.V., He X. LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem. 2006;281:38276–38284. doi: 10.1074/jbc.M609509200. [DOI] [PubMed] [Google Scholar]
- 16.Balemans W., Piters E., Cleiren E., Ai M., Van Wesenbeeck L., Warman M.L. The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations. Calcif Tissue Int. 2008;82:445–453. doi: 10.1007/s00223-008-9130-9. [DOI] [PubMed] [Google Scholar]
- 17.Murrills R.J., Matteo J.J., Bhat B.M., Coleburn V.E., Allen K.M., Chen W. A cell-based Dkk1 binding assay reveals roles for extracellular domains of LRP5 in Dkk1 interaction and highlights differences between wild-type and the high bone mass mutant LRP5(G171V) J Cell Biochem. 2009;108:1066–1075. doi: 10.1002/jcb.22335. [DOI] [PubMed] [Google Scholar]
- 18.Bhat B.M., Allen K.M., Liu W., Graham J., Morales A., Anisowicz A. Structure-based mutation analysis shows the importance of LRP5 beta-propeller 1 in modulating Dkk1-mediated inhibition of Wnt signaling. Gene. 2007;391:103–112. doi: 10.1016/j.gene.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Frattini A., Pangrazio A., Susani L., Sobacchi C., Mirolo M., Abinun M. Chloride channel ClCN7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J Bone Miner Res. 2003;18:1740–1747. doi: 10.1359/jbmr.2003.18.10.1740. [DOI] [PubMed] [Google Scholar]
- 20.Pangrazio A., Pusch M., Caldana E., Frattini A., Lanino E., Tamhankar P.M. Molecular and clinical heterogeneity in CLCN7-dependent osteopetrosis: report of 20 novel mutations. Hum Mutat. 2010;31:E1071–E1080. doi: 10.1002/humu.21167. [DOI] [PubMed] [Google Scholar]
- 21.Staal F.J., Sen J.M. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur J Immunol. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]