Abstract
Protein synthesis and autophagy work as two opposing processes to control cell growth in response to nutrient supply. The mammalian/mechanistic target of rapamycin complex 1 (mTORC1) pathway, which acts as a master regulator to control protein synthesis, has recently been shown to inhibit autophagy by phosphorylating and inactivating ULK1, an autophagy regulatory protein. ULK1 also inhibits phosphorylation of a mTORC1 substrate, S6K1, indicating that a complex signaling interplay exists between mTORC1 and ULK1. Here, we demonstrate that ULK1 induces multisite phosphorylation of Raptor in vivo and in vitro. Using phospho-specific antibodies we identify Ser855 and Ser859 as being strongly phosphorylated by ULK1, with moderate phosphorylation of Ser792 also observed. Interestingly, ULK1 overexpression also increases phosphorylation of Raptor Ser863 and the mTOR autophosphorylation site, Ser2481 in a mTORC1-dependent manner. Despite this evidence for heightened mTORC1 kinase activity following ULK1 overexpresssion, mTORC1-mediated phosphorylation of S6K1 and 4E-BP1 is significantly inhibited. ULK1 expression has no effect on protein-protein interactions between the components of mTORC1, but does reduce the ability of Raptor to bind to the substrate 4E-BP1. Furthermore, shRNA knockdown of ULK1 leads to increased phosphorylation of mTORC1 substrates and decreased phosphorylation of Raptor at Ser859 and Ser792. We propose a new mechanism whereby ULK1 contributes to mTORC1 inhibition through hindrance of substrate docking to Raptor. This is a novel negative feedback loop that occurs upon activation of autophagy to maintain mTORC1 inhibition when nutrient supplies are limiting.
Key words: autophagy, mTORC1, raptor, mTOR, S6K1, ULK1, ULK2
Introduction
The mammalian target of rapamycin (mTOR, now called the mechanistic target of rapamycin within higher eukaryotes) is a conserved serine/threonine kinase that regulates many fundamental cellular processes. mTOR, when associated with Raptor, mLST8 and PRAS40, forms mTOR Complex 1 (mTORC1),1–4 which promotes anabolic processes such as protein synthesis, cell growth and cell proliferation. Raptor serves as a scaffold protein within the complex, responsible for identifying and binding mTORC1 substrates.5,6 mTORC1 integrates signals derived from growth factors, energy and nutrients to control the phosphorylation and activity of two key downstream substrates involved in protein translation, namely ribosomal protein S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). Insufficient levels of cellular amino acids downregulate mTORC1 signaling which limits further protein synthesis and activates autophagy, a catabolic process.
Autophagy is an evolutionarily conserved cellular process that drives the trafficking of unwanted proteins and cellular components to lysosomes for degradation upon nutrient limitation (reviewed in ref. 7). By degrading intracellular components, induction of autophagy provides energy as well as structural building blocks such as amino acids required for critical cellular processes and metabolic homeostasis. In yeast, the autophagy-related (Atg) protein, Atg1, forms a complex with Atg13, Atg17, Atg29 and Atg31,8–10 and plays an initial role in inducing autophagy. The mammalian equivalent of this complex consists of ULK1, ATG13, FIP200 and ATG101.11,12 ULK1 is a serine/threonine kinase which promotes autophagy signaling. Recently, it has been shown that mTORC1 associates with the ULK1-ATG13-FIP200 complex through the binding of Raptor with ULK1.13,14 Through Raptor interaction with the ULK1-ATG13-FIP200 complex, mTORC1 phosphorylates both ULK1 and ATG13, which represses ULK1 kinase activity.13,15,16 Thus, in conditions where mTORC1 is active (i.e., when amino acids are plentiful), autophagy is repressed as a consequence of ULK1 inhibition by mTORC1-mediated phosphorylation. Conversely, upon nutrient deprivation and mTORC1 downregulation, mTORC1 no longer mediates inhibitory phosphorylation of ULK1. As a consequence, ULK1 is activated by autophosphorylation, thereby allowing ULK1-mediated phosphorylation of ATG13 and FIP200 and subsequent initiation of autophagy.
Biochemical switching between anabolic processes such as protein synthesis and catabolic processes such as autophagy must be tightly controlled in cells. Two publications revealed that ULK1 negatively regulates S6K1 in Drosophila17,18 and mammalian cells,17 suggesting possible crosstalk between ULK1 and the mTORC1 pathway. This work implies that there exists a complex interplay of signal transduction between mTORC1 and the autophagic ULK1-ATG13-FIP200 complex which coordinates whether protein synthesis or autophagy becomes dominant. To further explore such potential crosstalk, we examined whether ULK1 negatively regulates mTORC1 via a direct mechanism. Upon overexpression of ULK1, we found that ULK1 directly phosphorylates Raptor on multiple sites and inhibits mTORC1 signaling by impeding substrate binding. Therefore, while mTORC1 represses ULK1 and induction of autophagy during nutrient sufficiency, we now propose a novel negative feedback loop whereby ULK1 induces Raptor phosphorylation, impairs substrate docking to Raptor and represses mTORC1 signaling during nutrient limitation.
Results
ULK1 induces phosphorylation of Raptor.
Increasing evidence suggests complex interplay between the mTORC1 pathway and the autophagic ULK1-ATG13-FIP200 complex. Recent data indicate that mTORC1 phosphorylates ULK1 and ATG13, thus repressing autophagy.13,15,16 Two earlier reports indicated the reverse was also true, namely that ULK1 negatively regulated S6K1 (a mTORC1 substrate) in both Drosophila17,18 and mammalian cells.17 This inhibition of S6K1 by ULK1 was not an indirect consequence of ectopic induction of autophagy: rather, Lee et al.17 hypothesized that ULK1 modulates S6K1 activity by affecting S6K1 Thr389-specific kinases or phosphatases. To explore this relationship further, we investigated whether ULK1 could directly modulate mTORC1, the upstream Thr389 kinase of S6K1.
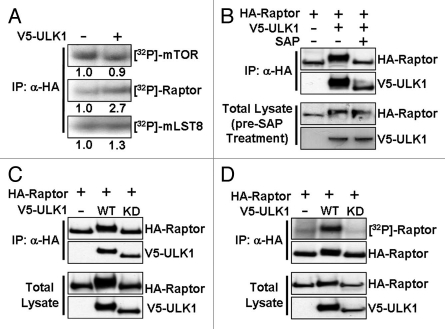
By in vivo radiolabeling HEK293 cells with [32P]-orthophosphate, we analyzed the phosphorylation status of the mTORC1 components, mTOR, Raptor and mLST8 (also known as GβL) following overexpression of ULK1. Expression of ULK1 minimally altered the phosphorylation of mTOR and mLST8, while it increased Raptor phosphorylation by more than 2.5-fold (Fig. 1A). Further experimentation revealed that Raptor undergoes a mobility shift on SDS-PAGE to a slower migrating form when co-expressed with ULK1. We hypothesized that this mobility shift was due to phosphorylation of Raptor either directly or indirectly by ULK1. To test this idea, we immunoprecipitated HA-Raptor which had been co-expressed with V5-ULK1 and then treated the HA-Raptor with shrimp alkaline phosphatase. We found that the mobility shift of Raptor observed in the presence of ULK1 was reversed following treatment with shrimp alkaline phosphatase (Fig. 1B), confirming our hypothesis that protein phosphorylation was responsible for this Raptor mobility shift. To determine whether ULK1 kinase activity is required for Raptor phosphorylation, we generated kinase-dead ULK1 (K46I), which has been shown to fully ablate the activity of ULK1.19 We found that expression of the K46I ULK1 mutant failed to induce a Raptor mobility shift (Fig. 1C), although it maintained its ability to bind to Raptor, thus supporting previous data14 that ULK1 kinase activity is not required for the ULK1-Raptor interaction. The phosphorylation event was further confirmed by in vivo [32P]-radiolabeling of HA-Raptor (Fig. 1D) where we found that expression of wild-type ULK1, but not the kinase-dead mutant, induced Raptor phosphorylation.
Figure 1.
ULK1 induces phosphorylation of Raptor. (A) HEK293 cells transfected with Myc-mTOR, HA-Raptor, HA-mLST8 in addition to V5-tagged ULK1 (where indicated), were radiolabeled with [32P]-orthophosphate. After lysis, mTOR/Raptor/mLST8 was immunoprecipitated using anti-HA antibodies and phosphorylation of mTORC1 components assessed by autoradiography. (B) HA-Raptor and V5-ULK1 were co-expressed in HEK293 cells and Raptor was purified by HA-immunoprecipitation. One sample was treated with SAP, as indicated. Western blots were performed to determine the mobility of Raptor. (C) HEK293 cells were co-transfected with HA-Raptor and either wild-type (WT) or kinase dead (KD) V5-ULK1. Following HA-immunoprecipitation, samples were assessed for Raptor mobility by western blot. (D) As in (C) but cells were radiolabeled with [32P]-orthophosphate prior to lysis and immunoprecipitation. Phosphorylation of Raptor was determined by autoradiography.
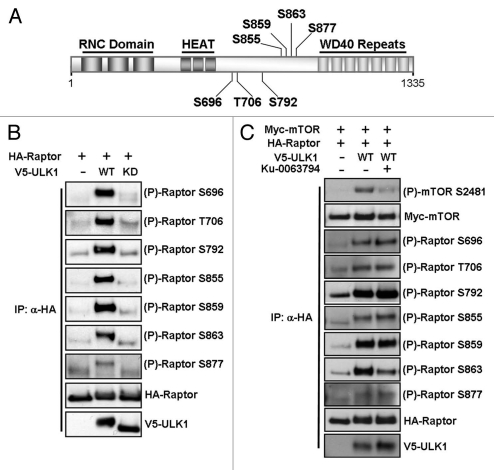
The importance of Raptor phosphorylation in response to AMPK signaling, MAPK signaling, mTORC1 activation or during cell cycle progression has recently come to light, with a variety of Raptor phosphorylation sites being identified.20–26 To explore which sites were being phosphorylated in response to ULK1 overexpression, we employed a series of phospho-specific Raptor antibodies, directed towards the sites highlighted in Figure 2A. ULK1 promoted in vivo phosphorylation of Ser696, Thr706, Ser792, Ser855, Ser859 and Ser863 and weakly promoted the phosphorylation of Ser877 (Fig. 2B). Importantly, wild-type but not kinase-dead ULK1 promoted these phosphorylation events, indicating that the kinase activity of ULK1 is required for Raptor phosphorylation.
Figure 2.
ULK1 induces phosphorylation of Raptor on multiple sites in 293 cells. (A) A schematic showing the structure of Raptor, including the Raptor N-terminal conserved (RNC) domains, 3 HEAT repeats and 7 C-terminal WD40 repeats. The location of the phosphorylation sites examined in this study is shown in the central region of the protein. (B) HEK293 cells were transfected with HA-Raptor and either wild-type (WT) or kinase dead (KD) V5-ULK1. Following an HA immunoprecipitation, samples were assessed for phosphorylation of Raptor using site-specific antibodies. Total Raptor and ULK1 are shown as controls. (C) HEK293 cells were transfected with Myc-mTOR, HA-Raptor and either wild-type (WT) or kinase dead (KD) V5-ULK1 and treated with 1 µM Ku-0063794 for 3 h prior to lysis, where indicated. Immunoprecipitations and western blots were performed as in (B) with total mTOR, Raptor and ULK1 shown as controls.
As Raptor Ser863 is phosphorylated by mTOR and positively regulates mTORC1,22,24 it was surprising to see that the negative regulator, ULK1, caused robust phosphorylation of this site. To investigate this further, we performed the experiment as in Figure 2B, but with the addition of a specific mTOR kinase inhibitor, Ku-0063794.27 Addition of 1 µM Ku-0063794 for 3 h inhibited Raptor Ser863 phosphorylation in ULK1 overexpressing cells showing that this phosphorylation event was mTOR dependent (Fig. 2C and 3rd lane). We also observed that the mTOR autophosphorylation site at Ser2481 was phosphorylated when ULK1 was overexpressed, which was reversed upon Ku-0063794 treatment (Fig. 2C). This autophosphorylation site is reflective of mTORC1 catalytic activity,28 suggesting that overexpression of ULK1 was increasing the catalytic activity of mTORC1 in vivo. Addition of the mTORC1 inhibitor had no effect on ULK1-mediated phosphorylation of the other Raptor phosphorylation sites examined.
To determine whether these sites are phosphorylated directly by ULK1 or as an indirect consequence of signaling downstream of ULK1, we performed an in vitro ULK1 kinase assay against purified GST-Raptor. In our ULK1 kinase assay, we observe high levels of ULK1 autophosphorylation as well as [32P]-radiolabel incorporation into ATG13, a known ULK1 substrate, only in the presence of wild-type ULK1 (Fig. 3A). We also detected phosphorylation of Raptor in the same assay as observed by [32P]-radiolabel incorporation into Raptor and a Raptor mobility shift (Fig. 3A). To determine which sites of Raptor were being directly phosphorylated by ULK1, we subjected in vitro ULK1-phosphorylated Raptor to the panel of phospho-Raptor antibodies (Fig. 3B). This revealed that Ser792, Ser855, Ser859, Ser863 and Ser877 were being directly phosphorylated by ULK1. Phosphorylation of Ser859 was the strongest, while Ser792, Ser863 and Ser877 only showed a modest increase in phosphorylation. There was no change in the phosphorylation status of Ser696 or Thr706 in this in vitro ULK1 assay, suggesting that these sites are not directly phosphorylated by ULK1.
Figure 3.
ULK1 phosphorylates ATG13 and Raptor in vitro. (A) An in vitro ULK1 kinase assay was performed by immunoprecipitation of either wild-type (WT) or kinase dead (KD) V5-ULK1 followed by incubation with substrate (either purified GST-ATG13 or GST-Raptor) for 45 min in the presence of γ-[32P]-ATP. [32P]-radiolabel incorporation into substrates was determined by autoradiography. (B) The assay was performed as in (A) using GST-Raptor as substrate and cold ATP. Samples were assessed for Raptor phosphorylation using site-specific phospho-Raptor antibodies. Total Raptor and ULK1 are shown as controls.
ULK1 represses mTORC1 signaling.
It is known that phosphorylation of Raptor by RSK,21 ERK1/2,26 AMPK20 or during mitosis23 can modulate the activity of mTORC1. Therefore, to determine whether ULK1-mediated phosphorylation of Raptor could modulate mTORC1 signaling, we analyzed the activity of the downstream substrate S6K1 and the phosphorylation of 4E-BP1 in the presence or absence of ULK1 overexpression.
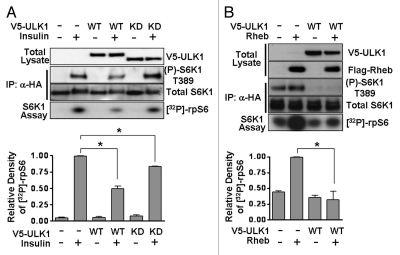
S6K1 activity was determined by analyzing the incorporation of [32P]-radiolabel into a ribosomal protein S6 (rpS6) peptide. We found that insulin-induced S6K1 phosphorylation on Thr389 was reduced and S6K1 activity was repressed by 51 ± 6% when ULK1 was overexpressed (Fig. 4A). Rheb-induced S6K1 phosphorylation and activity was also significantly inhibited following ULK1 expression (Fig. 4B). These results support previous data indicating that ULK1 strongly inhibits S6K1 phosphorylation in nutrient sufficient cells.17 Interestingly, we found that the kinase-dead mutant, which has no phosphorylation capacity but still binds to Raptor, was also able to repress S6K1 activity by 17 ± 1%, (Fig. 4A).
Figure 4.
ULK1 reduces S6K1 phosphorylation and activity. (A) HEK293 cells were transfected with HA-S6K1 with or without V5-ULK1, serum-starved overnight and stimulated with 100 nM insulin for 30 min prior to lysis where indicated. HA-S6K1 was immunoprecipitated and used in an in vitro kinase assay against recombinant rpS6 peptide. [32P]-radiolabel incorporation into rpS6 was determined by autoradiography. S6K1 phosphorylation was determined using phospho-Thr389 antibody. The densitometry of the phospho-rpS6 autorads from the three experiments is shown in the graph (mean ± SD). Statistical significance was analyzed using a student's t-test, *p < 0.01. (B) A S6K1 assay was performed as in (A) but in the presence or absence of Flag-Rheb expression instead of insulin stimulation. S6K1 activity was determined by Thr389 phosphorylation of S6K1 and [32P]-radiolabel incorporation into rpS6. The densitometry of the [32P]-radiolabeled rpS6 autorads from the three experiments is shown in the graph (mean ± SD). Statistical significance was analyzed using a student's t-test, *p < 0.05.
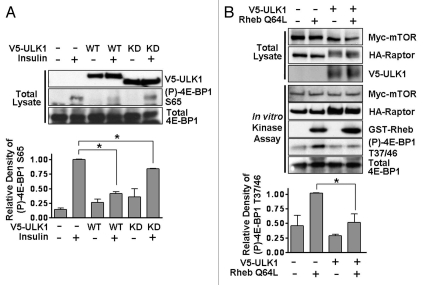
To analyze the effect that ULK1 may be having on another mTORC1 target, we determined 4E-BP1 phosphorylation in cells following wild-type or kinase-dead ULK1 expression. We found a similar profile to that seen for S6K1 phosphorylation, with wild-type ULK1 repressing Ser65 phosphorylation of 4E-BP1 by 58 ± 4% and kinase-dead ULK1 causing around 16 ± 1% repression (Fig. 5A). An in vitro mTORC1 kinase assay was also used to determine the level of mTORC1-mediated 4E-BP1 phosphorylation in response to ULK1 overexpression. Myc-mTOR/HA-Raptor with or without V5-ULK1 was transiently transfected into HEK293 cells and the mTOR/Raptor complex immunoprecipitated using an anti-HA antibody. The immunoprecipitates were then incubated with purified GST-4E-BP1, with or without the addition of GST-Rheb Q64L (a constitutively active form of Rheb). This in vitro mTORC1 kinase assay revealed that ULK1 overexpression inhibited the phosphorylation of 4E-BP1 by mTORC1 upon addition of Rheb (Fig. 5B).
Figure 5.
ULK1 inhibits phosphorylation of 4E-BP1 by mTORC1. (A) HEK293 cells were transfected with wild-type or kinase-dead ULK1, serum-starved overnight and then stimulated with 100 nM insulin for 30 min prior to lysis, where indicated. Phosphorylation of 4E-BP1 at Ser65 was analyzed by western blotting. Total 4E-BP1 and ULK1 are shown as controls. Densitometry of phospho-4E-BP1 from independent experiments is shown in the graph (mean ± SD). Statistical significance was analyzed using a student's t-test, *p < 0.01. (B) An in vitro mTORC1 kinase assay was performed by combining immunoprecipitated Myc-mTOR/HA-Raptor complexes, which were expressed with or without V5-ULK1, with recombinant GST-4E-BP1 in the presence or absence of active GST-Rheb (Q64L), as indicated. mTORC1 kinase activity was determined by western blotting using phospho-4E-BP1 (Thr37/46) antibody. The densitometry of the phospho-4E-BP1 blots from the three experiments is shown in the graph (mean ± SD). Statistical significance was analyzed using a student's t-test, *p < 0.05.
Raptor has reduced substrate binding capacity when ULK1 is overexpressed.
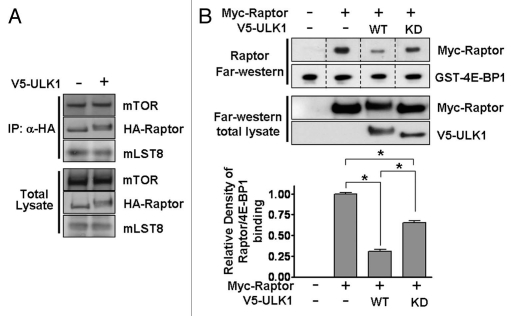
As our data reveals that ULK1 overexpression inhibits mTORC1, we next wanted to investigate the mechanism(s) behind this observation. In other studies, Raptor phosphorylation by multiple positive and negative inputs has not been found to affect mTORC1 formation or substrate binding.21–26 To examine mTORC1 formation, we analyzed the amount of endogenous mTOR and mLST8 bound to HA-Raptor expressed in the presence or absence of V5-ULK1. ULK1 overexpression had no significant effect on mTOR/Raptor/mLST8 protein association within mTORC1 (Fig. 6A). Therefore, ULK1 binding to Raptor and ULK1-induced Raptor phosphorylation is not causing disassembly of mTORC1. In contrast to other studies which used a co-immunoprecipitation approach to assess Raptor binding to substrate, we employed a far-western approach. To do this, we incubated Myc-Raptor-expressing lysates with GST-4E-BP1 immobilized on PVDF membrane and detected the amount of bound Raptor by western blotting. When we employed Raptor from ULK1 expressing cells, we saw a 69 ± 4% loss of substrate binding (Fig. 6B). We also observed a mobility shift of Raptor, indicating high levels of Raptor phosphorylation (Fig. 6B). Additionally, it was observed that expression of kinase-dead ULK1 reduced the binding capacity of Raptor by 35 ± 4% (Fig. 6B and end part), approximately half as inhibitory as overexpression of wild-type ULK1. It therefore appears that hindrance of proper Raptor function through overexpression of ULK1 contributes to the ability of ULK1 to inhibit mTORC1. A reduction in Raptor-mediated substrate docking due to ULK1-Raptor binding would account for the loss of S6K1 activity and 4E-BP1 phosphorylation seen following kinase-dead ULK1 expression observed in both Figures 4A and 5A.
Figure 6.
ULK1 causes loss of Raptor substrate binding without affecting mTORC1 association. (A) HA-Raptor was expressed in the presence or absence of V5-ULK1. Following HA-immunoprecipitation, western blotting was used to probe for the amount of endogenous mTOR and mLST8 co-immunoprecipitating with Raptor. (B) Raptor substrate binding was assessed using a Raptor overlay assay. 750 ng GST-4E-BP1 was resolved by SDS-PAGE and transferred to PVDF membrane. This was incubated with Myc-Raptor-containing lysate and the amount of Myc-Raptor bound to 4E-BP1 determined by western blotting with Myc antibodies. The graph shows the combined densitometry data of Raptor-4E-BP1 binding for three experiments (mean ± SD). Statistical significance was analyzed using a student's t-test, *p < 0.01. Anti-GST and anti-V5 antibodies were used to obtain total levels of GST-4E-BP1 and V5-ULK1 respectively.
ULK1 knockdown increases mTORC1 signaling.
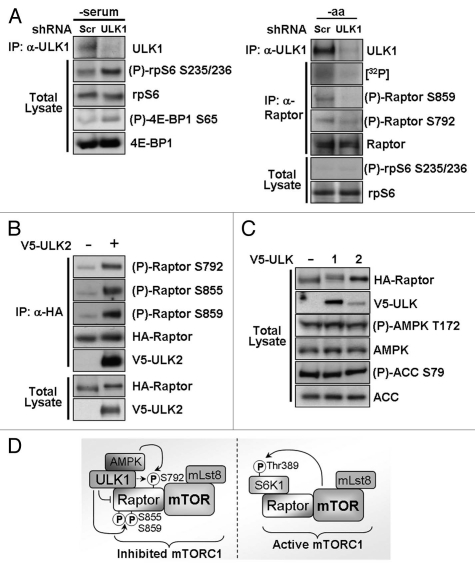
To confirm our findings of ULK1-induced inhibition of mTORC1 by ULK1 overexpression, we knocked down endogenous ULK1 in HEK293 cells using shRNA and determined the effect on mTORC1 substrates. Serum-free conditions were used to reduce basal mTORC1 signaling. The ULK1 shRNA used was efficient in reducing endogenous ULK1 protein expression (Fig. 7A and left part). Loss of ULK1 correlated with an increase in endogenous phospho-rpS6 (analogous to an increase in phospho-S6K1 following ULK1 knockdown, previously observed by Lee et al.17 and Jung et al.15) and endogenous phospho-4E-BP1 (Fig. 7A and left part), thus indicating that loss of ULK1 causes increased signaling through mTORC1. To confirm our findings of ULK1-mediated Raptor phosphorylation, we nutrient deprived HEK293 cells (serum- and amino acid-free conditions) in which ULK1 expression was knocked down by shRNA. We chose to use amino acid-free conditions to reduce inputs from other signaling pathways which are active under full nutrient conditions and known to phosphorylate Raptor in order to better observe ULK1 specific events. We found that [32P]-orthophosphate incorporation into Raptor was markedly reduced with shRNA knockdown of ULK1 (Fig. 7A and right part), supporting our data that ULK1 mediates phosphorylation of Raptor. Additionally, specific phosphorylation of Raptor Ser859 and Ser792 was also reduced (Fig. 7A and right part), confirming our finding that ULK1 is responsible for phosphorylation of these sites. rpS6 was not phosphorylated under amino acid-free conditions, even following ULK1 knockdown, indicating that other amino acid sensors which feed into mTORC1 signaling dominate under these conditions (Fig. 7A and right part).
Figure 7.
Knockdown of endogenous ULK1 prevents Raptor phosphorylation and increases mTORC1 signaling. (A) Endogenous ULK1 expression was knocked down using shRNA and the phosphorylation of endogenous mTORC1 substrates was determined using phospho-specific antibodies (left part). Phosphorylation of Raptor was determined by [32P]-radiolabel incorporation into endogenous Raptor and using phospho-specific antibodies (right part). Scrambled (Scr) shRNA was used as a control. -serum = serum-free DMEM, -aa = amino acid- and serum-free Krebs Ringer Buffer. (B) V5-ULK2 and HA-Raptor were overexpressed in HEK293 cells. Following an HA-immunoprecipitation, phosphorylation of Raptor was determined using site-specific antibodies. Total Raptor levels are shown as a control. (C) Levels of endogenous phospho-AMPK (Thr172) and phospho-ACC (Ser79) were analyzed under normal growth conditions in HEK293 cells which had been transfected with V5-ULK1 or V5-ULK2 24 h prior to lysis. Total AMPK, ACC, HA-Raptor and V5-ULK1 are shown as controls. (D) A schematic diagram showing the proposed mechanism of ULK1-mediated inhibition of mTORC1. ULK1 inhibits signal transduction through mTORC1 (mTOR/Lst8/Raptor protein complex) by interaction with and phosphorylation of Raptor (ULK1 P-sites: Ser855, Ser859 and weakly at Ser792). ULK1 interaction with Raptor interferes with mTORC1 substrate recognition. AMPK, which binds to ULK1, also phosphorylates the inhibitory Ser792 site. Upon repression of autophagy, ULK1 dissociates from Raptor allowing Raptor to efficiently interact and phosphorylate mTORC1 substrates such as S6K1 on Thr389.
ULK2 also phosphorylates Raptor.
There are four known mammalian ULK family members, of which ULK2 shares the greatest homology with ULK1,29 and has also been shown to play a role in autophagy.30 We therefore examined whether ULK2 could phosphorylate Raptor in a similar manner to ULK1. We chose to test phosphorylation of Raptor at Ser855 and Ser859 as these were among the strongest phosphorylated sites in our in vitro ULK1 kinase assay, alongside Ser792, a known negative regulatory site. We observed clear phosphorylation of all three sites following overexpression of ULK2, although the overall mobility shift of Raptor was slightly less pronounced than that seen with ULK1 (Fig. 7B). This suggests that ULK2 may play an overlapping role with ULK1 in the regulation of mTORC1.
Raptor Ser792 is a known site of AMPK phosphorylation,20 and a recent report has implicated AMPK-mediated Raptor phosphorylation as playing an important role in autophagy induction.14 To determine whether ULK1 and ULK2 are acting through AMPK to phosphorylate this site, or acting in a parallel pathway, we analyzed the phosphorylation of AMPK and that of its substrate acetyl-CoA carboxylase (ACC) (Fig. 7C). No differences in either AMPK or ACC phosphorylation could be detected following ULK1 or ULK2 overexpression, suggesting that ULK1/2-induced Raptor Ser792 phosphorylation is independent of AMPK.
Combining the data from the ULK1 overexpression and knockdown studies, our results indicate that ULK1 can inhibit mTORC1 signaling, promote multisite Raptor phosphorylation and reduce substrate docking to Raptor. Wild-type ULK1 is a more potent inhibitor than kinase-dead ULK1, indicating that ULK1-mediated phosphorylation events play an important role in mTORC1 inhibition. Our proposed mechanism is summarized in Figure 7D.
Discussion
mTORC1 responds to multiple signaling inputs through modulation of its upstream regulator TSC2 and through direct phosphorylation of the mTORC1 component, Raptor. Here we identify ULK1 as a novel kinase of Raptor and show that ULK1 can inhibit substrate binding to Raptor and inhibit mTORC1 signaling. Our findings of ULK1-mTORC1 signaling crosstalk support the initial observations made by Lee et al.17 and Scott et al.18 where they observe that ULK1 negatively regulates S6K1. Upon overexpression of ULK1, we observed robust incorporation of [32P]-radiolabel into Raptor while the phosphorylation status of the other core mTORC1 components, mTOR and mLST8, was largely unaffected. Knockdown of ULK1 confirmed that ULK1 regulates Raptor phosphorylation and loss of ULK1 expression correlated with an upregulation of mTORC1 signaling towards both rpS6 and 4E-BP1. Additionally, kinase-active ULK1 caused a further reduction in mTORC1 signaling and substrate binding than kinase-dead ULK1, indicating that ULK1-mediated phosphorylation events contribute to mTORC1 inhibition. Our data clearly shows a correlation between ULK1-mediated multisite phosphorylation of Raptor and inhibition of mTORC1 signaling. However, the signaling crosstalk between ULK1 and mTORC1 is a complex process. Given this complexity, ULK1-mediated Raptor phosphorylation may be an epiphenomenon. As Raptor is phosphorylated by many positive and negative signaling inputs,20–26 it is likely that the Raptor phosphorylation events observed by either overexpression or knockdown of ULK1 contribute to the regulation of mTORC1. It is highly probable that other factors will contribute to this negative feedback mechanism, which could include phosphorylation of other mTORC1 signaling components or additional inhibitory phosphorylation sites on Raptor.
Our results add to the knowledge of how amino acid supply and autophagy regulate mTORC1 signaling. There are a number of proteins reported to function as amino acid sensors in the mTORC1 pathway (reviewed in ref. 31), including Vps34,32,33 MAP4K3,34 Rag proteins35,36 and RalA,37 which allow mTORC1 to respond to cellular nutrient status. A plentiful supply of amino acids leads to active mTORC1 and repression of autophagy which is brought about, at least in part, by phosphorylation and inactivation of ULK1 by mTORC1.13,15,16 Expression of active Rag proteins has been linked to inhibition of autophagy36 due to their ability to mimic nutrient sufficiency. Our data shows that autophagy signals, through ULK1, feed back to mTORC1 to inhibit its function, thereby repressing protein synthesis and growth when nutrients are limiting. Intriguingly, overexpression of ULK1 increased mTORC1 catalytic activity, as demonstrated by increased phosphorylation of mTOR Ser2481 and Raptor Ser863. However, this occurred concurrently with a decrease in phosphorylation of mTORC1 substrates, suggesting that inhibition of substrate access to mTORC1 by ULK1 is preventing mTORC1 signaling, despite its heightened activity. Interestingly, the ULK1 binding protein, FIP200 also associates with the TSC1-TSC2 complex, which functions upstream of mTORC1, to regulate cell size.38 Clearly multiple levels of crosstalk operate between the mTORC1 and autophagy pathways in order to precisely coordinate cell growth signals depending on nutrient availability.
Although we detected ULK1-induced Raptor Ser696 and Thr706 phosphorylation in vivo, we did not observe phosphorylation of these sites in vitro, suggesting that these are not direct phosphorylation events but are instead a downstream consequence of ULK1 expression. These sites have recently been identified as being phosphorylated by the mitotic kinase cdc2, but do not appear to contribute to alterations in mTORC1 signaling.25 There are conflicting reports in the literature about the activity of autophagy during mitosis. While some groups have reported reduced autophagy in mitotic cells,39,40 another group has reported equal levels of autophagy during mitosis and interphase.41 Therefore it remains to be determined whether ULK1 activity persists during mitosis and could be influencing the action of cdc2. As we identified that Ser696 and Thr706 were phosphorylated in vivo in response to ULK1 expression, it raises an intriguing question of whether ULK1 functions upstream of cdc2. In addition, Ser696 is also an ERK1/2 target,26 suggesting that multiple kinases can phosphorylate these sites.
Ser792 is a known AMPK phosphorylation site on Raptor.20 As a site which negatively regulates mTORC1 signaling it makes sense that Ser792 is phosphorylated in response to ULK1 overexpression. We could not find any evidence that ULK1 overexpression affected AMPK signaling and found that Ser792 could be phosphorylated by ULK1 in vivo and in vitro, indicating that ULK1 may function in parallel to AMPK to phosphorylate this site. However, recent reports have shown that AMPK can bind to the proline-serine rich (PS) domain of ULK1,14 while both ULK1 and ULK2 associate with catalytic and regulatory subunits of AMPK.42 Therefore, we cannot rule out the possibility that AMPK is being co-immunoprecipitated with ULK1 in our in vitro kinase assay which could account for some of the Ser792 phosphorylation seen. The interaction between AMPK and ULK1 is important for ULK1-mediated autophagy and AMPK activation has been demonstrated to recruit 14-3-3 to Raptor when phosphorylated at Ser792, thereby inhibiting mTORC1 activity in the ULK1 complex.14 Together with our data, where ULK1 itself can phosphorylate Raptor and hinder substrate binding, it is likely that both AMPK and ULK1 act in concert to inhibit mTORC1 function during nutrient withdrawal and energy stress. While this article was under review, support for this hypothesis came from two recent publications. The first revealed that AMPK phosphorylates ULK1 and this is required for proper ULK1 function in response to nutrient deprivation.43 A second publication revealed that AMPK could phosphorylate two other sites on ULK1 to promote autophagy under glucose starvation, while mTORC1 activity under nutrient sufficiency phosphorylated ULK1 Ser757 to disrupt ULK1-AMPK association.44 Therefore, coordinated phosphorylation of ULK1 plays a key role in the regulation of this protein.
Of the other sites examined, we found Ser855 and Ser859 to be phosphorylated most strongly in vitro (Fig. 2C), implying that they are directly phosphorylated by ULK1. Overexpression of ULK2 could also phosphorylate these sites (Fig. 7B), suggesting potential redundancy in the function of both ULK1 and ULK2. Despite lower expression of V5-ULK2 compared to V5-ULK1, we were still able to detect a Raptor mobility shift, indicative of multisite phosphorylation (Fig. 7C). The role of ULK2 in autophagy is not yet fully elucidated but it has previously been shown to have the same inhibitory effect on S6K1 phosphorylation as ULK1,17 and we found that ULK2 bound to and phosphorylated Raptor (Fig. 7B). It is likely that ULK2 has an overlapping role with ULK1 and it is possible that the two proteins function in a tissue specific manner to regulate autophagy.
Of the seven Raptor phosphorylation sites examined in this study, six were shown previously to be phosphorylated in cells undergoing mitosis.23 However, under these circumstances the phosphorylation of Raptor was found to increase mTORC1 signaling. This contrasts with our findings, suggesting that there may be other as yet unidentified Raptor phosphorylation sites which differentially regulate mTORC1 activity during autophagy or mitosis. Alternatively, the proteins bound to mTORC1 in each circumstance could influence the activatory or inhibitory nature of Raptor phosphorylation.
Rheb is thought to enhance mTORC1 substrate recognition through increased Raptor binding.45,46 It was, therefore, of interest that ULK1 phosphorylated Raptor and reduced its association with 4E-BP1 (Fig. 6B). Overexpression of ULK1 prevented 4E-BP1 phosphorylation in the in vitro mTORC1 kinase assay after addition of active Rheb (Fig. 5B), while S6K1 activation upon Rheb overexpression was completely ablated upon ULK1 expression (Fig. 4B). Our data, therefore, suggest that Raptor phosphorylation by ULK1 opposes Rheb function, by blocking substrate binding with Raptor and thus preventing optimal phosphorylation of these substrates by mTORC1. Additionally, ULK1 may function in a similar manner to PRAS40,3,4,47 by inhibiting mTORC1 through Raptor interaction.
As defects in autophagy are associated with muscle, cardiac and neurodegenerative diseases and cancer (reviewed in ref. 48), finding drugs which target this pathway is of clinical importance. A screen for chemical modulators of autophagy revealed four drugs already approved for clinical use which reversibly inhibited mTORC1 and stimulated autophagy.49 These activators of autophagy inhibited mTORC1 signaling to both S6K1 and 4E-BP1 which is consistent with our data showing that ULK1 regulates the pathway at the level of mTORC1.
Evidently, Raptor phosphorylation is mediated by many upstream kinases which positively or negatively regulate mTORC1 function. It remains to be determined which phosphorylation site or combination of sites create an activatory or inhibitory complex and whether this induces a conformational change or association with alternative mTORC1 binding partners. Further research into Raptor phosphorylation should aid our understanding of the intricacies of mTORC1 regulation.
Materials and Methods
Plasmid details.
Myc-mTOR/pRK5 (Addgene plasmid 1861) and HA-Raptor/pRK5 (Addgene plasmid 8513) plasmids were kindly obtained from Dr. D.M. Sabatini.50 Myc-Raptor was from Addgene (plasmid 1859). GST-Rheb, GST-Raptor and GST-ATG13 were subcloned from Flag-Rheb/pRK7 (a kind gift from Prof. J. Blenis, Harvard University), HA-Raptor/pRK5 and IMAGE clone 5744513 (Source Bioscience LifeSciences) respectively into the pDEST27 vector using the Gateway system (Invitrogen, 11812-013). The Rheb Q64L mutation was introduced by site-directed mutagenesis using Phusion polymerase (Finnzymes, F-530S) for the PCR reaction and transformation into Omnimax 2-T1 cells (Invitrogen, C854003). ULK1 and ULK2 were subcloned from IMAGE clones 3526749 and 5268025 (Source Bioscience LifeSciences), respectively, into pcDNA3.1/nV5-DEST using the Gateway system (Invitrogen, 12290-010). The kinase dead ULK1 mutant, K46I, was made using site-directed mutagenesis as described above.
Antibodies and other biochemicals.
Anti-HA antibody was purchased from Roche (11 867 423 001), anti-Rheb C19 (sc-6341), anti-ULK1 (sc-10900) and anti-mTOR (sc-1549) antibodies from Santa Cruz. Anti-mLST8 (3274S), phospho-4E-BP1 Thr37/46 (9459S) and Ser65 (9456S), total 4E-BP1 (9644), anti-phospho Raptor Ser792 (2083), phospho-mTOR Ser2481 (2974S), phospho-S6K1 Thr389 (9205), total S6K1 (9202), phospho-rpS6 Ser235/236 (2211), total rpS6 (2212), phospho-AMPKα Thr172 (2535), total AMPKα (2603), phospho-ACC Ser79 (3661), total ACC (3676) and ULK1 R600 (4773) antibodies were obtained from Cell Signaling. Anti-V5 (46-0705) and anti-V5-HRP (46-0708) were from Invitrogen. Anti-Myc clone 9E10 (M5546) antibody was from Sigma and anti-GST antibody (05-782) was from Millipore. Anti-Raptor was generated in rabbits against human Raptor derived-‘MESEMLQSPLLGLGEEDEAD’ peptide with C-terminal Cys (Millipore). Anti-phospho Raptor antibodies (Ser696, Thr706, Ser855, Ser859, Ser863 and Ser877) were generated as previously described in reference 24. All chemicals were purchased from Sigma, unless otherwise stated.
Cell culture and transfection.
HEK293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) foetal calf serum, 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen). CaCl2-mediated transfection was carried out as previously described in reference 51. Lipofectamine 2000 transfection was performed according to the manufacturer's protocol (Invitrogen 11668-019). Cells were harvested 24–36 h post-transfection. Cells requiring insulin stimulation were treated with 100 nM insulin (Sigma, I9278) for 30 min prior to lysis. Krebs Ringer buffer (20 mM HEPES (pH 7.4), 115 mM NaCl, 5 mM KCl, 10 mM NaHCO3, 2.5 mM MgCl2, 2.5 mM CaCl2, 4.5 g/l glucose) was used for amino acid starvation experiments.
HA-Raptor immunoprecipitation and SAP treatment.
HEK293 cells were transfected with HA-Raptor with or without V5-ULK1 and lysed in mTORC1 lysis buffer (40 mM HEPES (pH 7.4), 2 mM EDTA, 10 mM β-glycerophosphate, 0.3% (w/v) CHAPS and protease inhibitors). Following removal of insoluble matter by centrifugation for 8 min at 16,000 g, lysates were immunoprecipitated using anti-HA antibody coupled to Protein G-Sepharose beads (GE Healthcare 17-0618-01) for 2 h at 4°C. HA-Raptor immunopreciptates were washed three times in low salt buffer (40 mM HEPES (pH 7.4), 2 mM EDTA, 10 mM β-glycerophosphate, 150 mM NaCl, 0.3% (w/v) CHAPS and protease inhibitors) and once in HEPES/KCl buffer (25 mM HEPES pH 7.4, 20 mM KCl) prior to resuspension in sample buffer (Invitrogen). Samples requiring shrimp alkaline phosphatase (SAP) treatment were lysed in phosphatase lysis buffer (25 mM HEPES (pH 7.4), 1.5 mM MgCl2, 0.3% (w/v) CHAPS and protease inhibitors) and immunoprecipitated as above. HA immunoprecipitates were washed three times in phosphatase lysis buffer before resuspension in phosphatase lysis buffer containing 0.2 U/µl SAP (USB, 70092Y/Z/X) and incubation at 37°C for 1 h. Immunoprecipitates were washed twice more in phosphatase lysis buffer before resuspension in sample buffer.
Endogenous Raptor and ULK1 immunoprecipitations.
Cells were lysed and immunoprecipitated using the protocol for HA-Raptor but using anti-Raptor antibody (University of Dundee) for immunoprecipitation for 6 h rather than anti-HA antibody. Cells for ULK1 immunoprecipitation were lysed in Buffer B (40 mM HEPES (pH 7.5), 120 mM NaCl, 1 mM EDTA, 10 mM pyrophosphate, 10 mM β-glycerophosphate, 50 mM NaF, 1.5 mM Na3VO4, 0.3% (w/v) CHAPS plus protease inhibitors) and immunoprecipitated using anti-ULK1 antibody (Santa Cruz) and Protein G-Sepharose. Beads were washed three times in Buffer B prior to addition of sample buffer (Invitrogen).
mTORC1 kinase assay.
Myc-mTOR/HA-Raptor transfected cells were lysed in mTORC1 lysis buffer and insoluble material removed by centrifugation. Myc-mTOR and HA-Raptor were co-immunoprecipitated from the lysate using anti-HA antibody, incubated at 4°C for 2 h. Protein G-Sepharose beads were added for an additional 1 h. Beads were then washed once in low salt buffer, twice in high salt buffer (40 mM HEPES (pH 7.4), 2 mM EDTA, 10 mM β-glycerophosphate, 400 mM NaCl, 0.3% (w/v) CHAPS and protease inhibitors) and twice in HEPES/KCl buffer. GST-Rheb Q64L was purified from transfected cells by lysis in Rheb lysis buffer (40 mM HEPES (pH 7.4), 10 mM β-glycerophosphate, 10 mM pyrophosphate, 5 mM MgCl2, 0.3% (w/v) CHAPS and protease inhibitors (but no DTT)). GST-Rheb Q64L was captured using a GST beads and a spin trap module (Thermo Scientific 69725), washed three times in Rheb lysis buffer, then Rheb storage buffer (20 mM HEPES (pH 8), 200 mM NaCl, 5 mM MgCl2) and finally eluted in 10 mM reduced glutathione made up in Rheb storage buffer. GST-Rheb Q64L was diluted in mTORC1 Kinase Assay Buffer (25 mM HEPES (pH 7.4), 20 mM KCl, 10 mM MgCl2), then added to the mTOR/Raptor complexed beads. A preincubation at 30°C for 5 min was performed before the addition of start buffer (25 mM HEPES (pH 7.4), 10 mM MgCl2, 140 mM KCl, 0.5 mM ATP and 150 ng dephosphorylated recombinant GST-4E-BP1 protein). Reactions were performed at 30°C for 30 min with constant shaking and stopped by the addition of NuPAGE sample buffer (Invitrogen, NP0007).
S6K1 assay.
HEK293 cells were transfected with HA-S6K1 with or without V5-ULK1 (wild-type or kinase dead) and Flag-Rheb (as indicated in the figures) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Four h post-transfection, cells were changed to serum-free medium and incubated overnight prior to insulin stimulation (100 nM insulin for 30 min) where required. Cells were lysed using 10 mM K2PO4 (pH 7.4), 1 mM EDTA pH 7.05, 5 mM EGTA, 10 mM MgCl2, 50 mM β-glycerol phosphate, 1 mM Na3VO4 and 2 mM DTT, plus protease inhibitors. Lysates were incubated for 2 h at 4°C with anti-HA antibody coupled to protein G-Sepharose beads. HA immunoprecipitates were washed once each with Buffer A (10 mM Tris (pH 7.2), 1% (v/v) nonidet P-40, 0.5% (v/v) sodium deoxycholate, 100 mM NaCl, 1 mM EDTA and protease inhibitors), Buffer B (10 mM Tris (pH 7.2), 0.1% (v/v) nonidet P-40, 0.5% sodium deoxycholate, 1 M NaCl, 1 mM EDTA, plus protease inhibitors) and ST Buffer (50 mM Tris-HCl (pH 7.2), 5 mM Tris-base, 150 mM NaCl, plus protease inhibitors). S6K1 complexed beads were incubated for 10 min at 30°C in 20 mM HEPES (pH 7.2), 10 mM MgCl2, 50 µM unlabeled ATP, 5 µCi of γ-[32P]ATP (Perkin Elmer Life Sciences) and 3 ng/µl PKI, in the presence of recombinant GST-rpS6 peptide (32 final amino acids of ribosomal protein S6). Reactions were subjected to SDS-PAGE, and the relative levels of [32P]-labeled GST-rpS6 were determined by autoradiography.
Western blotting.
Samples were prepared in sample buffer (Invitrogen) and heated at 70°C for 10 min before separation and transfer of proteins to polyvinylidene difluoride (PVDF) membranes (Millipore, IPVH00010) using the Novex system (Invitrogen). Membranes were blocked in 3% (w/v) dry milk powder/Tris buffered saline 0.1% (v/v) Tween (TBS-T), then probed using the required primary antibody and horse radish peroxidase (HRP)-conjugated secondary antibody (Sigma). Proteins were visualized using the enhanced chemiluminescent (ECL) solution (GE Healthcare, RPN2132) and Hyperfilm (Fujifilm 47410 08389). Western blots shown are representative of at least three independent experiments.
Far western (Raptor overlay assay).
Raptor-containing lysates were prepared by co-transfection of wild-type or mutant Raptor with or without V5-ULK1, followed by lysis in Raptor lysis buffer (50 mM β-glycerophosphate (pH 7.4), 1 mM EDTA, 1 mM EGTA, 1% (v/v) Triton X-100 plus protease inhibitors) after 48 h and removal of insoluble material by centrifugation at 4°C for 8 min at 16,000 g. 750 ng of recombinant GST-4E-BP1 protein was resolved by SDS-PAGE and transferred to PVDF membrane. Following blocking in 5% (w/v) dry milk powder/TBS-T, the membrane was incubated overnight at 4°C in 5% dry milk powder made up in Raptor lysis buffer and containing 10% (v/v) overexpressed Raptor lysate. The membrane was then washed and probed with anti-Myc antibody followed by processing and visualization using the final steps of the standard western blotting protocol.
ULK1 knockdown.
Lipofectamine transfection mixtures containing 2.5 µg scrambled shRNA or ULK1 shRNA (MISSION shRNA 1-1064slcl, Sigma) were prepared according to the manufacturer's protocol. These were added dropwise to 35 mm tissue culture plates containing DMEM and 10% (v/v) FCS. Trypsinised HEK293 cells were then added to the plates and incubated at 37°C for 24 h. Media was then changed and the plates incubated for a further 48 h prior to lysis.
In vivo radiolabeling.
To [32P]-radiolabel either the mTOR/Raptor/mLst8 protein complex or endogenous Raptor in vivo, HEK293 cells (after being transiently transfected on 35 mm plates) were washed and incubated in 2 ml of phosphate-free medium containing 0.2 mCi [32P]-orthophosphate for 4 h. These cells were then harvested as normal using mTORC1 lysis buffer. HA-Raptor and HA-mLST8 associated with Myc-mTOR were immunoprecipitated for 2 h with anti-HA antibody bound to protein G-Sepharose and washed as described for the mTORC1 kinase assay. For analysis of endogenous Raptor phosphorylation upon ULK1 knockdown, anti-Raptor antibody was used instead.
Densitometry and statistical analysis.
Densitometry was performed where indicated in the figures using ImageJ v1.43 software. Student's t-test was used for statistical analysis, with p < 0.05 taken to be significant.
Acknowledgements
This research was supported by the Association for International Cancer Research Career Development Fellowship [No. 06-914/915] to A. Tee. D. Fingar was supported by an NIH-RO1 (DK-078135) and an American Diabetes Association Jr. Faculty Award. The authors declare no conflict of interests.
Abbreviations
- mTORC1
mammalian target of rapamycin complex 1
- ULK
uncoordinated-51-like kinase
- Raptor
regulatory-associated protein of mTOR
- S6K1
ribosomal protein S6 kinase 1
- 4E-BP1
eukaryotic translation initiation factor 4E-binding protein 1
- PRAS40
proline-rich AKT1 substrate 1
- ATG
autophagy related
- FIP200
focal adhesion protein of 200 kD
- HEK
human embryonic kidney
- AMPK
5′-AMP-activated protein kinase
- Ser
serine
- Thr
threonine
- GST
glutathione S-transferase
- RSK
ribosomal protein S6 kinase
- ERK
extracellular signal-regulated kinase
- Rheb
ras homologue enriched in brain
- PVDF
polyvinylidene fluoride
- shRNA
short hairpin ribonucleic acid
- ACC
acetyl-CoA carboxylase
- TSC
tuberous sclerosis complex
- SAP
shrimp alkaline phosphatase
References
- 1.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with Raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 2.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 3.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signaling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 4.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 6.Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated Raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol. 2003;13:797–806. doi: 10.1016/s0960-9822(03)00329-4. [DOI] [PubMed] [Google Scholar]
- 7.Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol. 2010;298:776–785. doi: 10.1152/ajpcell.00507.2009. [DOI] [PubMed] [Google Scholar]
- 8.Funakoshi T, Matsuura A, Noda T, Ohsumi Y. Analyzes of APG13 gene involved in autophagy in yeast, Saccharomyces cerevisiae. Gene. 1997;192:207–213. doi: 10.1016/s0378-1119(97)00031-0. [DOI] [PubMed] [Google Scholar]
- 9.Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabeya Y, Noda NN, Fujioka Y, Suzuki K, Inagaki F, Ohsumi Y. Characterization of the Atg17-Atg29-Atg31 complex specifically required for starvation-induced autophagy in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2009;389:612–615. doi: 10.1016/j.bbrc.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5:973–979. doi: 10.4161/auto.5.7.9296. [DOI] [PubMed] [Google Scholar]
- 12.Mercer CA, Kaliappan A, Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy. 2009;5:649–662. doi: 10.4161/auto.5.5.8249. [DOI] [PubMed] [Google Scholar]
- 13.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010;5:15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SB, Kim S, Lee J, Park J, Lee G, Kim Y, et al. ATG1, an autophagy regulator, inhibits cell growth by negatively regulating S6 kinase. EMBO Rep. 2007;8:360–365. doi: 10.1038/sj.embor.7400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott RC, Juhász G, Neufeld TP. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr Biol. 2007;17:1–11. doi: 10.1016/j.cub.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan EY, Longatti A, McKnight NC, Tooze SA. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol. 2009;29:157–171. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of Raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrière A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, et al. oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated Raptor phosphorylation. Curr Biol. 2008;18:1269–1277. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Lawrence JC, Sturgill TW, Harris TE. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of Raptor by mTOR. J Biol Chem. 2009;284:14693–14697. doi: 10.1074/jbc.C109.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramírez-Valle F, Badura ML, Braunstein S, Narasimhan M, Schneider RJ. Mitotic Raptor promotes mTORC1 activity, G2/M cell cycle progression and internal ribosome entry site-mediated mRNA translation. Mol Cell Biol. 2010;30:3151–3164. doi: 10.1128/MCB.00322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster KG, Acosta-Jaquez HA, Romeo Y, Ekim B, Soliman GA, Carriere A, et al. Regulation of mTOR Complex 1 (mTORC1) by Raptor Ser863 and multisite phosphorylation. J Biol Chem. 2010;285:80–94. doi: 10.1074/jbc.M109.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwinn DM, Asara JM, Shaw RJ. Raptor is phosphorylated by cdc2 during mitosis. PLoS One. 2010;5:9197. doi: 10.1371/journal.pone.0009197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, et al. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J Biol Chem. 2011;286:567–577. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Martínez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, et al. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem. 2010;285:7866–7879. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, et al. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signaling inputs, substrates and feedback mechanisms. Cell Signal. 2009;21:827–835. doi: 10.1016/j.cellsig.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 33.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, et al. Amino acids mediate mTOR/Raptor signaling through activation of class 3 phosphatidylinositol-3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Findlay GM, Yan L, Procter J, Mieulet V, Lamb RF. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signaling. Biochem J. 2007;403:13–20. doi: 10.1042/BJ20061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind Raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maehama T, Tanaka M, Nishina H, Murakami M, Kanaho Y, Hanada K. RalA functions as an indispensable signal mediator for the nutrient-sensing system. J Biol Chem. 2008;283:35053–35059. doi: 10.1074/jbc.M805822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gan B, Melkoumian ZK, Wu X, Guan KL, Guan JL. Identification of FIP200 interaction with the TSC1-TSC2 complex and its role in regulation of cell size control. J Cell Biol. 2005;170:379–389. doi: 10.1083/jcb.200411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eskelinen EL, Prescott AR, Cooper J, Brachmann SM, Wang L, Tang X, et al. Inhibition of autophagy in mitotic animal cells. Traffic. 2002;3:878–893. doi: 10.1034/j.1600-0854.2002.31204.x. [DOI] [PubMed] [Google Scholar]
- 40.Furuya T, Kim M, Lipinski M, Li J, Kim D, Lu T, et al. Negative regulation of Vps34 by Cdk mediated phosphorylation. Mol Cell. 2010;38:500–511. doi: 10.1016/j.molcel.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Xie R, Nguyen S, Ye M, McKeehan WL. Robust autophagy/mitophagy persists during mitosis. Cell Cycle. 2009;8:1616–1620. doi: 10.4161/cc.8.10.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egan DF, Shackelford DB, Mihaylova MM, Gelino SR, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunlop EA, Dodd KM, Seymour LA, Tee AR. Mammalian target of rapamycin complex 1-mediated phosphorylation of eukaryotic initiation factor 4E-binding protein 1 requires multiple protein-protein interactions for substrate recognition. Cell Signal. 2009;21:1073–1084. doi: 10.1016/j.cellsig.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 46.Sato T, Nakashima A, Guo L, Tamanoi F. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009;284:12783–12791. doi: 10.1074/jbc.M809207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24524. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 48.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balgi AD, Fonseca BD, Donohue E, Tsang TCF, Lajoie P, Proud CG, et al. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS One. 2009;4:7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and Raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 51.Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol. 2002;12:632–639. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]