Abstract
Interactions between the Bcl-2 family proteins and the mitochondrial fission and fusion machinery regulate cell death in mammals and worms. In Drosophila, the Bcl-2 family proteins have not been shown to be major regulators of cell death. However, emerging evidence suggests that mitochondrial remodeling may be important in Drosophila cell death. We recently demonstrated a series of events that occur during follicle removal in the Drosophila ovary that included mitochondrial remodeling and clustering, followed by uptake and degradation in the follicle cells. Importantly, the Bcl-2 family proteins, mitochondrial dynamics and autophagic proteins regulate these events.
Key words: Drosophila, oogenesis, programmed cell death, mitochondria, Bcl-2, autophagy, apoptosis, caspase, mitochondrial fission and fusion
A balance between pro- and anti-apoptotic Bcl-2 family proteins regulates cell death in mammals and worms. In Drosophila, the major upstream regulators of cell death are the IAP antagonists. However, ovarian cell death occurs independently of the IAP antagonists, pointing to a distinct cell death pathway in oogenesis. Nutritional deprivation leads to the removal of entire follicles or egg chambers during mid-oogenesis, prior to the energetically expensive process of vitellogenesis. Cell death in mid-oogenesis is regulated by an atypical apoptotic cascade which results in caspase and autophagy activation. It is unknown how this cascade is activated. We found that a mitochondrial pathway plays a major role in the activation of cell death in mid-oogenesis.
Drosophila egg chambers consist of three cell types: the oocyte, adjacent nurse cells and surrounding follicle cells. During cell death in mid-oogenesis, the nurse cells die, their remnants are engulfed by the follicle cells, and then the follicle cells die. We characterized mitochondrial changes that occur in the nurse cells during cell death and found a series of events which included mitochondrial remodeling and clustering, followed by uptake and degradation of the mitochondrial clusters in the surrounding follicle cells (Fig. 1). We found that mitochondrial remodeling occurs upstream of caspase activation, but clustering occurs downstream. The Bcl-2 family proteins and mitochondrial fission and fusion machinery regulate this form of cell death, showing an age-dependent increase in the inhibition of cell death.
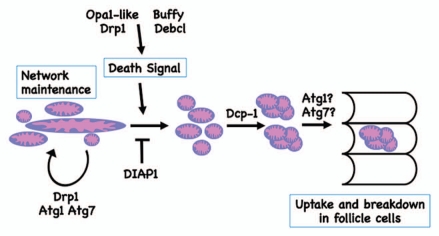
Figure 1.
A model for mitochondrial regulation and clustering during cell death in mid-oogenesis. In healthy egg chambers, mitochondrial networks extend throughout the egg chamber. Upon induction of cell death, mitochondria remodel, followed by the formation of clusters and then uptake by the surrounding follicle cells. Through genetic analysis, we showed that mitochondrial remodeling occurs upstream of effector caspases, and that cluster formation requires the caspase Dcp-1. In addition, the Bcl-2 family proteins, Buffy and Debcl, and mitochondrial fission and fusion regulators, Drp1 and Opa1-like, regulate mitochondrial remodeling as well as activation of cell death. The autophagic proteins, Atg1 and Atg7, are involved in normal mitochondrial network maintenance and remodeling during cell death. Autophagic proteins also may have a role in cluster uptake into the follicle cells, or in the degradation of the nurse cell material within the follicle cells.
Interactions between the Bcl-2 proteins and mitochondrial fission and fusion machinery regulate cell death in mammals and worms. However, to our knowledge, this study is the first to link the Bcl-2 proteins and mitochondrial dynamics in Drosophila. Mitochondrial remodeling during cell death has been described during developmental cell death in Drosophila larval tissues and S2 cells, but in both cases this is in response to IAP binding protein activity. It has been suggested that the IAP binding proteins may act in place of the Bcl-2 family proteins during mitochondrial remodeling in Drosophila. However, it is also possible that the IAP binding proteins may have a role in regulating mitochondrial dynamics in mammals. Notably, many pro-apoptotic factors are localized to mitochondria. Additional interactions within the mitochondria may regulate the initiation of cell death, perhaps even between the Bcl-2 family proteins and the IAP binding proteins.
Disruption of autophagy was also found to alter mitochondrial dynamics in oogenesis. Autophagy-deficient flies show abnormal mitochondrial networks in both healthy and dying egg chambers. In wild-type healthy egg chambers, mitochondria are evenly distributed throughout the egg chamber, but in Atg1 and Atg7 mutants, mitochondria form large masses. In Atg1 mutants, mitochondrial masses are also observed during cell death. Intriguingly, these phenotypes are similar to those observed in mitochondrial fission mutants. These findings indicate that autophagy is necessary for proper mitochondrial network maintenance in the fly ovary. The abnormal mitochondrial networks may result from disrupted mitochondrial removal in Atg mutants. Alternatively, autophagy proteins could play a more direct role in regulating mitochondrial networks by affecting mitochondrial fission and fusion.
Autophagy-deficient egg chambers show other defects in the cell death process during oogenesis. Previously it has been shown that Atg mutant egg chambers fail to become TUNEL-positive despite showing normal chromatin condensation, suggesting a specific defect in DNA fragmentation. Our study provides evidence that degenerating Atg mutant nurse cells may not be engulfed properly by the surrounding follicle cells. Furthermore, follicle cells show a possible defect in breakdown of the ingested nurse cell cytoplasm. These data suggest that the autophagic machinery plays a role in the delivery and/or breakdown of nurse cell remnants by the engulfing follicle cells. Whether the effects on DNA fragmentation or engulfment are related to mitochondria remains to be determined.
Both autophagy and Bcl-2 family genes were found to alter mitochondrial dynamics and cell death in the fly ovary. In mammals, anti-apoptotic Bcl-2 family proteins interact with the autophagic protein Beclin 1, preventing the activation of autophagy. This places anti-apoptotic Bcl-2 family proteins as negative regulators of autophagy. Interestingly, the Drosophila Bcl-2 family proteins, Buffy and Debcl, were both identified as contributing to autophagy in an RNAi screen in S2 cells. In the ovary, Buffy and Debcl act pro-apoptotically. Also in the ovary, the caspase Dcp-1 promotes autophagy. This suggests that pro-apoptotic pathways may contribute to autophagy, from the evidence in Drosophila, while anti-apoptotic proteins such as mammalian Bcl-2 prevent autophagy.
An unusual feature of cell death in mid-oogenesis is the formation of the mitochondrial clusters. Mitochondrial aggregates or clusters form prior to mitophagy in mammalian cells. There are several candidates that could regulate clustering. During mitochondrial clustering in mitophagy, mitochondrial proteins are ubiquitinated by the E3 ubiquitin ligase Parkin, followed by clustering mediated by the ubiquitin adaptor protein p62. Drosophila parkin and clueless mutants have abnormal mitochondrial networks in healthy mid-oogenesis egg chambers, similar to the mitochondrial networks observed in healthy egg chambers from autophagy mutants in our study. Further analysis of p62 and parkin may provide insight into the regulation and structure of the clusters that form during ovarian cell death.
The role of autophagy in cell death is controversial, and may depend on the tissue being studied. In the Drosophila salivary gland, autophagy and apoptosis function together during cell death, while the ovary provides a distinct model where germline cell death is coupled with phagocytosis by the follicle cells. Our study indicates that autophagy may influence the cell death process in diverse ways, but the precise mechanisms remain to be uncovered.
Acknowledgements
This work was supported by NIH grant R01 GM060574 to K.M. and NICHD Training grant 2T32 HD007387 to E.T.
Punctum to: Tanner EA, Blute TA, Brachmann CB, McCall K. Bcl-2 proteins and autophagy regulate mitochondrial dynamics during programmed cell death in the Drosophila ovary. Development. 2011;138:327–338. doi: 10.1242/dev.057943.