Abstract
OBJECTIVE
To investigate the relationship between anxiety and event-free survival (i.e., composite endpoint of death, emergency department visits, or hospitalizations) for patients with HF, and examine whether behavioral and physiologic mechanisms mediate any association between anxiety and outcomes.
METHODS
In this longitudinal study, patients with HF completed the anxiety subscale of the Brief Symptom Inventory, and heart rate variability and plasma norepinephrine level were measured. Dietary and medication adherence were measured with a 24-hour urine sodium level and the Medication Event Monitoring System, respectively. Patients were followed at least 1 year for event-free survival.
RESULTS
A total of 147 patients were enrolled. Patients with high anxiety had a shorter (HR 2.2, 95% CI 1.1–4.3, P = .03) period of event-free survival than patients with lower anxiety. Anxiety independently predicted medication adherence (P = .008), which in turn predicted event-free survival (HR 2.0, CI 1.2–3.3, P = .008). The effect of anxiety (P = .17) on event-free survival was less significant when the regression model included both anxiety and medication adherence than when the model only included anxiety (P = .03), indicating that medication adherence mediated the relationship between anxiety and event-free survival.
CONCLUSION
This is the first study to show that medication nonadherence links anxiety and event-free survival for patients with HF. Interventions that reduce anxiety and improve adherence may favorably benefit outcomes.
Over 5,700,000 Americans have heart failure (HF), a chronic condition that contributes to more than 292,000 deaths annually.1 The effects of demographic, clinical, and treatment characteristics on outcomes has been well-studied. Improved outcomes have been associated with use of angiotensin-converting enzyme (ACE) inhibitors,2 beta-adrenergic blockers,3 angiotensin receptor blockers,4 aldosterone inhibitors,5 mechanical circulatory support devices,6 ventricular reconstruction surgery,7 cardiac resynchronization therapy,8 nutritional therapy,9 and disease management.10 Nonetheless, HF hospitalizations continue to rise.11
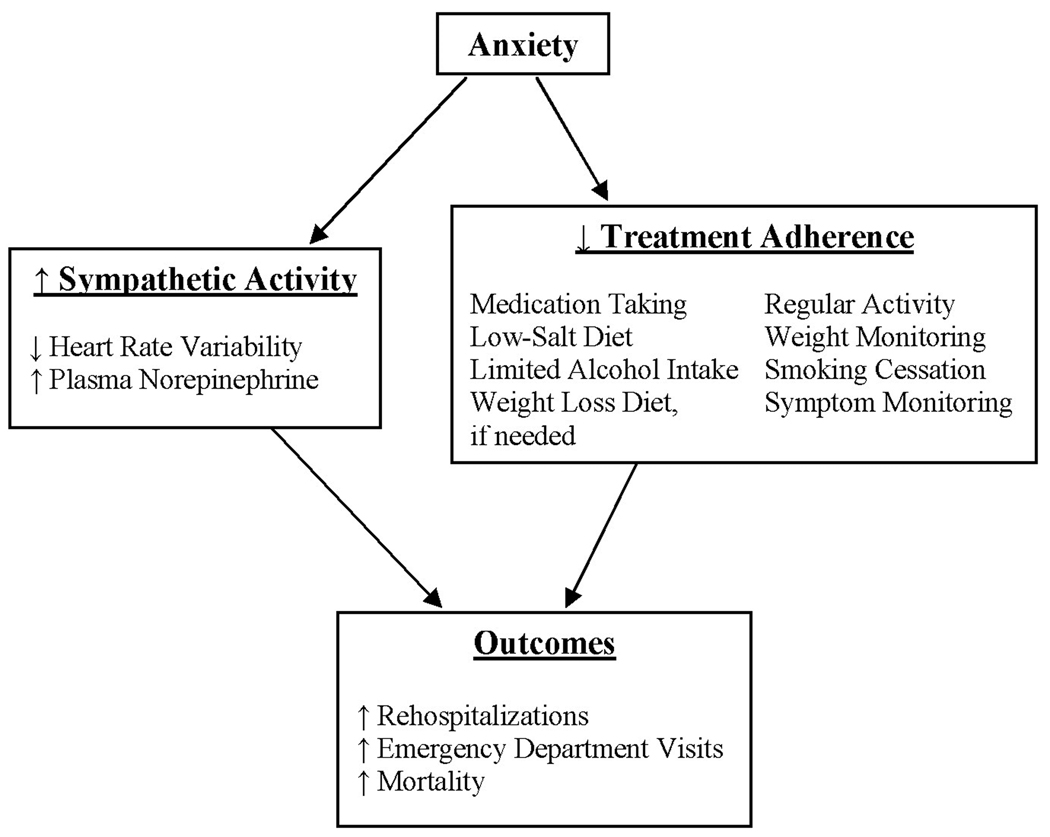
Patients with cardiac disease, including HF, often experience high levels of anxiety.12–17 Anxiety has been linked to adverse outcomes for patients with acute myocardial infarction (AMI) 18,19 but the link between anxiety and outcomes has not been well-established in HF.20 Although the mechanisms whereby anxiety may be associated with cardiac outcomes are unclear,13 evidence suggests that behavioral and physiologic pathways (Figure 1) may link anxiety and adverse outcomes.13
Fig 1.
Proposed physiologic and behavioral pathways linking anxiety and adverse outcomes
One physiologic model accounts for the relationships between psychological factors and heart disease outcomes.21 According to the model, psychological factors, such as anxiety, stimulate sympathetic nervous system (SNS) activity and catecholamine release that, in time, produce harmful consequences. Elevated levels of plasma norepinephrine, the standard biochemical method of assessing severity of SNS activation, predict mortality for patients with HF.22 For patients with AMI, but not healthy persons, elevated plasma norepinephrine levels were positively correlated with anxiety.23 Others demonstrated that although patients with HF had higher baseline sympathetic activity and heart rates than healthy persons, HF patients had still higher sympathetic activity and heart rates during mental stress, which is considered an anxiety equivalent.24 Controlling for physiologic variables, anxious patients with AMI had lower baroreflex cardiac control than nonanxious patients, placing them at increased risk for dysrhythmias.25 Further, it is well-known that patients with HF have reduced heart rate variability (HRV)26 due, in part, to high sympathetic activity.27 Depressed HRV independently predicts morbidity and mortality for patients with HF.28
Others have hypothesized that behavioral mechanisms, such as treatment nonadherence, link anxiety and cardiac disease. Compared to nonanxious persons, those with high anxiety may eat an unhealthy diet, smoke, consume drugs or alcohol, fail to adhere to therapy, or be physically inactive.19,29,30 Anxious patients with AMI experience problems coping with challenges, which may adversely affect treatment adherence and rehabilitation efforts.31 Nonetheless, no investigators have directly examined the hypothesized links of anxiety, SNS arousal, and poor treatment adherence with clinical outcomes for patients with HF.
The purposes of this study were to investigate the relationship between anxiety and event-free survival for patients with HF and examine whether behavioral and/or physiologic mechanisms mediate any association between anxiety and outcomes. We hypothesized that 1) HF patients with high anxiety would have worse event-free survival than patients with low anxiety and 2) SNS arousal and nonadherence to prescribed treatments would mediate the association between anxiety and event-free survival.
MATERIALS AND METHODS
Design
This sub-study was planned a priori as part of a prospective study to assess the relationship between depression and outcomes for patients with HF. The Institutional Review Board approved the study and all subjects gave written informed consent.
Sample and setting
Adult outpatients from a university-based medical center and its clinics and a private hospital located in the midwestern United States were recruited for the study. Providers referred patients to the researchers, and the researchers posted signs and fliers about the study in outpatient clinics. Patients were eligible to enroll if they had a confirmed diagnosis of chronic HF as confirmed by a physician using established criteria widely used by researchers.32,33 In addition, all eligible patients had undergone evaluation of HF, were on stable doses of drug therapy, had not been referred for urgent heart transplantation, and read and spoke English. Patients were excluded if they had: 1) HF due to valvular heart disease, myocarditis or other inflammatory processes, or pregnancy, 2) a history of cerebral vascular accident within the past 3 months or chronic major sequelae such as inability to communicate or persistent hemiplegia or paraplegia, 3) an AMI infarction within the past 3 months, 4) coexisting terminal illness, 5) a major psychiatric disorder, such as schizophrenia, diagnosed by a clinician and that interfered with the patient’s ability to answer questions, engage in a longitudinal study, or care for oneself, and 6) impaired cognition. Patients with symptoms of depression or anxiety were not excluded.
Measures
Anxiety
Anxiety was assessed using the anxiety subscale of the Brief Symptom Inventory (BSI).34 The 6–item anxiety subscale includes brief descriptions of psychological symptoms that are associated with anxiety. Using a 0 (“not at all”) to 4 (“extremely”) scale, participants rate their level of distress concerning these symptoms. The six items are averaged. The averaged score quantifies the patient’s level of anxiety and can range from 0 to 4. Higher scores denote higher anxiety. The normative value for healthy adults is 0.35 ± 0.45; however, normative data are unavailable for patients with HF or other cardiac disorders.34 The subscale contains no physiologic indices of anxiety, such as heart rate or diaphoresis, that could spuriously overestimate measurement of anxiety in patients with physical disease. Evidence supporting the validity of each subscale of the BSI has been reported.34 Investigators have reported Cronbach’s α ranging from .85 to .90 when studying patients with cardiac disease.18,35,36 In this study, the Cronbach α coefficient for the BSI was 0.85.
SNS arousal
Heart rate variability is a noninvasive measure of autonomic nervous system tone. Patients with HF typically exhibit decreased HRV related to high levels of sympathetic activity and decreased parasympathetic activity.27,37 Heart rate variability was measured in the time and frequency domains,38 and assessed from data obtained from 3-channel 2-hour Holter (Del Mar DigiCorder 483) electrocardiogram (ECG) recordings. A 2-hour recording period is long enough to accurately measure and assess HRV.39 The recordings were scanned in semi-automatic mode (operator verification of all beat types) on a Del Mar 373 Holter Analysis System Scanner (Del Mar Avionics, Irvine, CA). The data from these recordings included time of beat, type of beat, serial RR intervals (also termed normal-to-normal [NN] intervals) in milliseconds (ms), and standard deviation of normal sinus RR intervals in 5-minute epochs for the 2-hour recording period. To demonstrate inter- and intra-rater reliability, two members of the study team reviewed the recordings and scanned them twice. The members achieved identical findings for 90% of the recordings. When results differed, the members rescanned the recordings to review and resolve areas of disagreement.
Time-domain analyses are statistical calculations of RR intervals. Time-domain measures included standard deviation of all NN intervals for a selected time period (SDNN) and square root of the mean squared differences of successive NN intervals (RMSSD).38
For frequency-domain (or spectral) analysis, fast Fourier transformation was used to apportion the HRV signal into its frequency components and to quantify the power of these components.38 Two frequency bands are of clinical interest: 1) low frequency (LF) band (0.04– 0.15 Hz), and 2) high frequency band (0.15–0.4 Hz).38 In humans, LF and high frequency peak frequencies are commonly centered around about 0.1 Hz and 0.25 Hz, respectively. The area under the curve of each frequency band represents the power within that band. Total power represents the variability of the entire signal and is obtained by summing the powers of each frequency band. Low frequency and high frequency power also were “normalized” (i.e., expressed as a percentage of total power).38 Reliability and validity of this method for reflecting autonomic nervous system tone in cardiac patients have been demonstrated.38 To perform frequency-domain analysis, the recording period was divided into consecutive 5-minute epochs.38 An instantaneous HR function was sampled at 256-ms intervals and smoothed using a 584-ms boxcar filter.40 When artifact or any non-normal complex occurred, the preceding and succeeding RR intervals were excluded from the analysis and the instantaneous HR function was estimated by linear interpolation.38 The entire 5-minute segment was excluded from analysis if more than 95% of the RR intervals were not NN intervals. The mean NN interval was subtracted from the sampled HR data and a Hanning window applied.40 A fast Fourier transform was then computed, resulting in an absolute 5-minute power spectrum.
Plasma norepinephrine, a second measure of SNS arousal used in this study, is a standard biochemical method of assessing severity of SNS activation, and has been shown to predict mortality in HF.22 To ensure valid measurement, the blood sample was obtained under the following conditions to control for factors that extraneously affect norepinephrine release: 1) blood was drawn through a catheter that was placed at least 30 minutes prior to sampling in order to avoid pain and anxiety associated with venipuncture, 2) patients remained in the same semi-fowlers position for 30 minutes prior to the blood draw, 3) patients were asked to refrain from caffeine, alcohol, and nicotine for at least 8 hours prior to data collection, 4) the room was comfortably warm and quiet with minimal interruptions, and 5) data collection was conducted at the same time of day (1300–1500).41 Blood was collected into centrifuge blood tubes containing ethylenediaminetetraacetic acid (an anticoagulant), immediately placed on ice, and centrifuged at 4°C. Specimens were stored in single-use aliquots at −80°C until time of analysis. Plasma concentrations were determined using the Bi-Cat ELISA kit (American Laboratory Products Company, Windham, NH). Mean intra- and inter-assay coefficients of variation for the laboratory were < 8%, indicating reproducible and valid measurement.
Adherence
Objective evidence of dietary adherence was measured using 24-hour urine sodium levels. Patients were given urinals, urine collection hats, and urine jugs as well as written and oral instructions regarding how to collect urine for 24 hours. Patients were instructed to record the time and amount of each void in a urination log. Either a member of the research team visited the patient’s home to pick up the 24-hour urine collection, or the patient returned the collection to a member of the research team, usually in conjunction with another appointment. In either case, the team member reviewed the urine log for completeness with the patient.
A 2–3 gram sodium diet is recommended for patients with mild HF.42 Dietary sodium is absorbed and subsequently secreted. Urinary sodium represents approximately 86%43 to 95%44 of sodium intake depending primarily on amount of perspiration. Most patients with HF do not engage in strenuous physical activity that produces significant perspiration; therefore, urinary sodium represents approximately 95% of sodium intake. Thus, a urinary sodium of 2.85 grams (123 mmol), which is 95% of 3 grams, was used to define adherence to a sodium restricted diet.
Objective evidence of medication adherence was measured using the Medication Event Monitoring System (MEMS). The MEMS (AARDEX, Union City, CA) is a microelectronic device housed within the cap of a medication vial. The MEMS records the date and time that the patient removes the cap from the medication vial and presumably takes the correct dose of the medication. To ensure accuracy, patients documented in a diary additional cap removals related to medication refills or other openings not associated with intake of the medication. The MEMS collects real-time data which are later downloaded to a computer. Evidence supporting the validity of the MEMS to assess HF patients’ adherence with prescribed medications has been reported.45–47 In fact, use of an electronic monitoring device is now the preferred standard method to assess adherence.48 Data were collected for one HF medication (ACE inhibitor, diuretic, beta-adrenergic blocker, digoxin) for each patient. The medication selected for MEMS monitoring was one that the patient took twice per day. If all medications were taken once daily, however, the MEMS was used for the beta-adrenergic blocker. If no beta-adrenergic blocker was prescribed, the MEMS was used for the ACE inhibitor or angiotensin receptor blocker. Patients used the MEMS for 3 months and were unable to access data stored within the MEMS. We defined medication adherence as the percent of days correct doses taken ([total number of days dose taken/total number of days prescribed] × 100). Acceptable adherence was defined as equal to or greater than 88% because Wu and colleagues reported that this level of adherence was most sensitive and specific in predicting event-free survival.49
Procedure
Initial data collection occurred at a research center. Sociodemographic (i.e., age, education level, gender, ethnicity, marital status,) and clinical characteristics (i.e., left ventricular ejection fraction, comorbidities, smoking status, beta-adrenergic blocker use, ACE inhibitor use, New York Heart Association [NYHA] functional class) data were collected by patient interview and medical record review. Patients completed the BSI Anxiety Subscale and questionnaires regarding demographic information. Next, patients were placed in a semi-fowlers position, a saline-filled butterfly access device was inserted, cardiac leads were placed on the patient’s chest, and 2-hour Holter monitoring began. After 30 minutes, a plasma norepinephrine blood sample was drawn. The patient was given written and verbal instructions for how to collect 24-hour urine specimen and use the MEMS medication vial. Patients were given a urine collection set and arrangements were made to obtain the urine specimen.
Three-month data collection
Three months after initial data collection, the patient returned the MEMS device by giving it to a study team member during a home visit, delivering it during a regularly scheduled clinic visit, or mailing it in a prepaid package. Company-provided equipment was used to download MEMS data to a personal computer.
12-month data collection
Twelve months after initial data collection, a study team member reviewed patients’ medical records, the hospital administrative record database, death certificates, and patient diaries, and also interviewed patients and family members to collect data about rehospitalizations, emergency department (ED) visits, and mortality. Patients were followed until 12-month data collection was accomplished for all subjects. Thus, some patients were followed longer than 12 months. The reliability and validity of outcome measures were determined by having all investigators examine the same record and comparing their conclusions. In addition, the accuracy of classifying events was ensured by using multiple methods to determine whether and when an event occurred.
Data analysis
Data were analyzed with SPSS software, version 16.0 (SPSS Inc., Chicago, IL). For all analyses, a P value of ≤ .05 was considered statistically significant. To test the first hypothesis, patients were divided into quartile groups based on their BSI anxiety score. Because these anxiety groups may have differed on some demographic and clinical characteristics that needed to be controlled for statistically, baseline differences were examined using either ANOVA or linear by linear association chi-square tests (for dependent nominal variables), as appropriate. Cox proportional hazard modeling was used to assess whether anxiety predicted event-free survival, while controlling for the potential covariates of age, gender, left ventricular ejection fraction, NYHA classification, ACE inhibitor use, and beta-adrenergic blocker use.
For the second hypothesis, a series of regression models was used to determine whether SNS arousal and/or adherence to prescribed treatments mediated any relationship between high anxiety and event-free survival. Mediation was considered to have occurred if there was a significant relationship between anxiety and event-free survival that became nonsignificant when the mediator was included in the model. The test for mediation followed the steps outlined by Baron and Kenny.50 In summary, the initial step tested whether anxiety predicted event-free survival, as described above to test the first hypothesis. The second step tested whether anxiety predicted each potential mediator (i.e., dietary adherence and medication adherence) using multiple regression. The next step tested whether the mediators predicted event-free survival (using Cox regression), and finally whether anxiety and the potential mediator variables predicted event-free survival when entered together into the model. Mediation is present if, in the final regression, a mediator predicted the outcome and the P value testing the relationship between anxiety and outcomes was less significant than the P value in the first step.
RESULTS
Characteristics of the sample
Characteristics of the sample are summarized in Table 1. The average age of the 147 participants was 61 ± 11 years and nearly one-third were women (30%). Over half the participants were married (61%) and the majority was Caucasian (88%).
TABLE I.
Sample demographic and clinical characteristics (N = 147)
| Characteristic | |
|---|---|
| Age (yrs) | 61 ± 11 |
| Education (yrs) | 13 ± 3 |
| Women, n (%) | 44 (30) |
| Ethnicity | |
| Caucasian, n (%) | 130 (88) |
| Black, n (%) | 16 (11) |
| Hispanic or Latino, n (%) | 1 (1) |
| Marital status | |
| Married/cohabitate, n (%) | 90 (61) |
| Widowed, n (%) | 24 (16) |
| Divorced/separated, n (%) | 19 (13) |
| Single, n (%) | 14 (10) |
| Left ventricular ejection fraction (%) | 35 ± 14 |
| Prior acute myocardial infarction, n (%) | 85 (58) |
| Prior coronary artery bypass grafting, n (%) | 51 (35) |
| Prior hypertension, n (%) | 113 (77) |
| History of diabetes, n (%) | 70 (48) |
| Current smoker, n (%) | 28 (19) |
| Prescribed beta-adrenergic blocker, n (%) | 131 (89) |
| Prescribed angiotensin-converting enzyme inhibitor, n (%) | 106 (72) |
| New York Heart Association classification | |
| I, n (%) | 9 (6) |
| II, n (%) | 47 (32) |
| III, n (%) | 65 (44) |
| IV, n (%) | 22 (15) |
Anxiety and event-free survival
The mean anxiety score was 0.71 ± 0.74, and 79 (54.1%) patients reported higher anxiety than the norm reference of 0.35 for healthy adults. Based on the distribution of anxiety scores, the subjects were stratified into four groups (Table 2), from lowest anxiety to highest anxiety, to facilitate Cox proportional hazard modeling. The four groups are not strict quartiles because 30% of the patients reported no anxiety. There were no differences in sociodemographic or clinical variables among the four anxiety groups.
TABLE II.
Mean anxiety score by quartile
| Group | N | Mean BSI Score |
|---|---|---|
| Lowest quartile (0–30%) | 43 | 0.00 ± 0.00 |
| Second quartile (31–50%) | 31 | 0.30 ± 0.13 |
| Third quartile (51–75%) | 33 | 0.84 ± 0.13 |
| Highest quartile (76–100%) | 39 | 1.73 ± 0.51 |
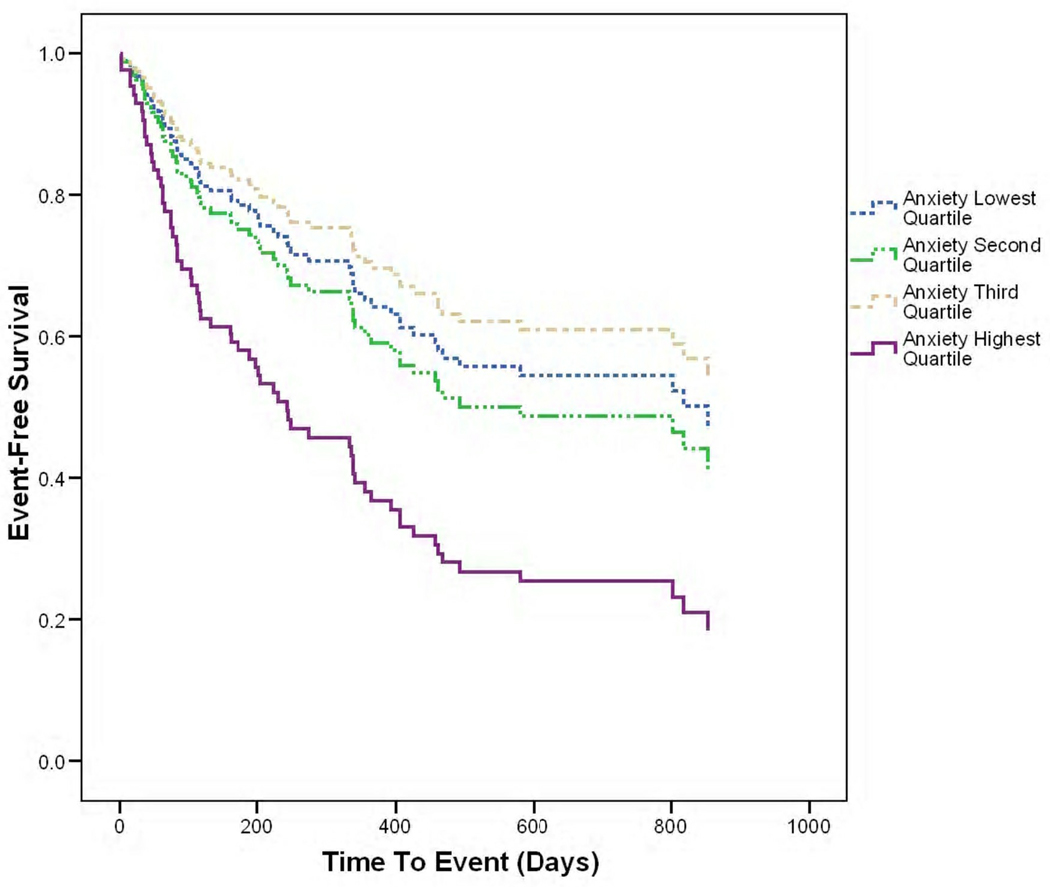
The mean follow-up time to first event was 389 ± 324 days. The first event outcomes shown in Table 3 were used for event-free survival analyses. The data confirmed the first hypothesis. Cox proportional hazard modeling revealed that after adjusting for age, gender, NYHA class, left ventricular ejection fraction, ACE inhibitor use, and beta-adrenergic blocker use, high anxiety independently predicted event-free survival (Figure 2, Table 4). Patients in the highest anxiety group were more likely to visit the ED, be hospitalized, or die compared to those in the three lower anxiety groups (HR 2.2, 95% CI 1.1–4.3, P = .03).
TABLE III.
First event outcomes at the follow-up point
| Outcome | N (%) |
|---|---|
| Heart failure death | 2 (1.4) |
| Other death | 1 (0.7) |
| Heart failure hospitalization | 10 (6.8) |
| Cardiac disease hospitalization | 23 (15.6) |
| Other hospitalization | 29 (19.7) |
| Heart failure emergency department visit | 2 (1.4) |
| Cardiac disease emergency department visit | 4 (2.7) |
Fig 2.
Cox survival plot of anxiety by quartile and event-free survival
TABLE IV.
Adjusted hazard ratios for the prediction of event-free survival from anxiety controlling for age, gender, medication use, left ventricular ejection fraction, and New York Heart Association classification (N = 147)
| Hazard Ratio |
95% Confidence Interval |
P Value |
|
|---|---|---|---|
| Age | 1.015 | .991–1.039 | .22 |
| Gender | .851 | .490–1.480 | .57 |
| Prescribed angiotensin-converting enzyme inhibitor | .626 | .351–1.118 | .11 |
| Prescribed beta-adrenergic blocker | .472 | .218–1.022 | .06 |
| Left ventricular ejection fraction | .987 | .968–1.007 | .22 |
| New York Heart Association classification | 1.2 | .712–2.023 | .49 |
| Second anxiety quartile* | 1.146 | .523–2.511 | .73 |
| Third anxiety quartile* | .814 | .383–1.729 | .59 |
| Fourth anxiety quartile* | 2.167 | 1.091–4.304 | .03 |
Compared to lowest quartile of anxiety
Omnibus test of model coefficients χ2 = 23.79, P = .005
SNS arousal as mediator
The mean plasma norepinephrine level was .36 ± .23 nmol/L. Norepinephrine level was not related to anxiety, adherence, or all measures of HRV except SDNN (Table 5).
TABLE V.
Correlations between anxiety level, plasma norepinephrine level, dietary adherence, medication adherence, and measures of heart rate variability
| Anxiety LevelA | Dietary AdherenceB |
Medication AdherenceC |
Plasma NorepinephrineD |
|
|---|---|---|---|---|
| Anxiety level (n = 146) |
------------ | .04; p = .67 | .18; p =.04 | −.10; p = .26 |
| Dietary adherence (n = 141) |
.04; p = .67 | ------------ | −.15; p = .11 | −.05; p = .57 |
| Medication adherence (n = 135) |
.18; p =.04 | −.15; p = .11 | ------------ | −.01; p = .96 |
| Plasma norepinephrine level (n = 143) |
−.10; p = .26 | −.05; p = .57 | −.01; p = .96 | ------------ |
| SDNN (n = 65) | −.13; p = .30 | −.01; p = .97 | −.17; p = .22 | −.26; p = .03 |
| RMSSD (n = 65) | −.21; p = .08 | −.17; p = .18 | .08; p = .56 | −.08; p = .50 |
| LF nu (n = 65) | .10; p = .41 | .15; p = .26 | −.22; p = .12 | .07; p = .61 |
| HF nu (n = 65) | −.10; p = .41 | −.15; p = .26 | .22; p = .12 | −.07; p = .61 |
| Log LF (n = 65) | .09; p = .49 | .15; p = .25 | −.14; p = .30 | −.17; p = .18 |
| Log HF (n = 65) | −.003; p = .98 | .06; p = .64 | −.01; p = .95 | −.20; p = .12 |
| Log LF/HF ratio (n = 65) |
.15; p = .24 | .15; p = .25 | −.21; p = .13 | .01; p = .95 |
Measured at the continuous level
Reflected by 24 hour urine sodium excretion
Adherence as the percent of days correct doses taken
Measured at the continuous level
LF = low frequency; HF = high frequency; nu = normalized units; RMSSD = square root of the mean squared differences of successive NN intervals; SDNN = standard deviation of all NN intervals
Heart rate variability monitoring was conducted for 65 subjects but contraindicated for the remaining subjects, who had atrial fibrillation or paced cardiac rhythms. As expected, the distribution of LF and high frequency power was positively skewed; therefore, these values were log transformed from the 0.04–0.15 Hz and 0.15–0.4 Hz frequency bands, respectively. The time-domain and frequency-domain measurements of HRV are shown in Table 6. No measures of HRV, except SDNN as mentioned above, were correlated with anxiety, adherence, or norepinephrine level (Table 5).
TABLE VI.
Mean time-domain and frequency-domain measurements of heart rate variability (N = 65)
| Time-Domain | |
| SDNN (ms) | 56 ± 26 |
| RMSSD (ms) | 32 ± 20 |
| Frequency-Domain | |
| LF | 248.9 ± 392.1 |
| HF | 241.4 ± 339.5 |
| LF nu | 49.8 ± 21.1 |
| HF nu | 50.2 ± 21.1 |
| Log LF | 4.6 ± 1.4 |
| Log HF | 4.9 ± 1.2 |
| Log LF/HF ratio | 1.0 ± 0.2 |
LF = low frequency; HF = high frequency; nu = normalized units; RMSSD = square root of the mean squared differences of successive NN intervals; SDNN = standard deviation of all NN intervals
Given the lack of association between both anxiety and plasma norepinephrine level, and anxiety and HRV (Table 5), the data did not support the hypothesis that SNS arousal mediates the association between anxiety and event-free survival.
Nonadherence as mediator
Urinary sodium levels from the 24-hour urine specimen were 196.27 ± 92.26 mmol/day, which computes to an average daily sodium intake of 4512.18 ± 2121.11 mg/day. Of the 141 patients (95.9%) with a useable 24-hour urine sample, 24% had a computed sodium intake of ≤ 3 gms. There was no association between anxiety and dietary adherence, as measured by the 24-urine sodium level (Table 5), thus, we did not use dietary adherence in any further mediation tests.
MEMS data were available for 135 patients (91.8%). Subjects used the MEMS for one of their prescribed medications for an average of 94 ± 18 days. Based on date and time data from the MEMS, patients took the correct number of doses on 80.6% ± 22.8% days. Individually, 56% of patients took the correct number of doses on ≥ 88% of the days that they used the MEMS. Medication adherence was correlated with anxiety (Table 5).
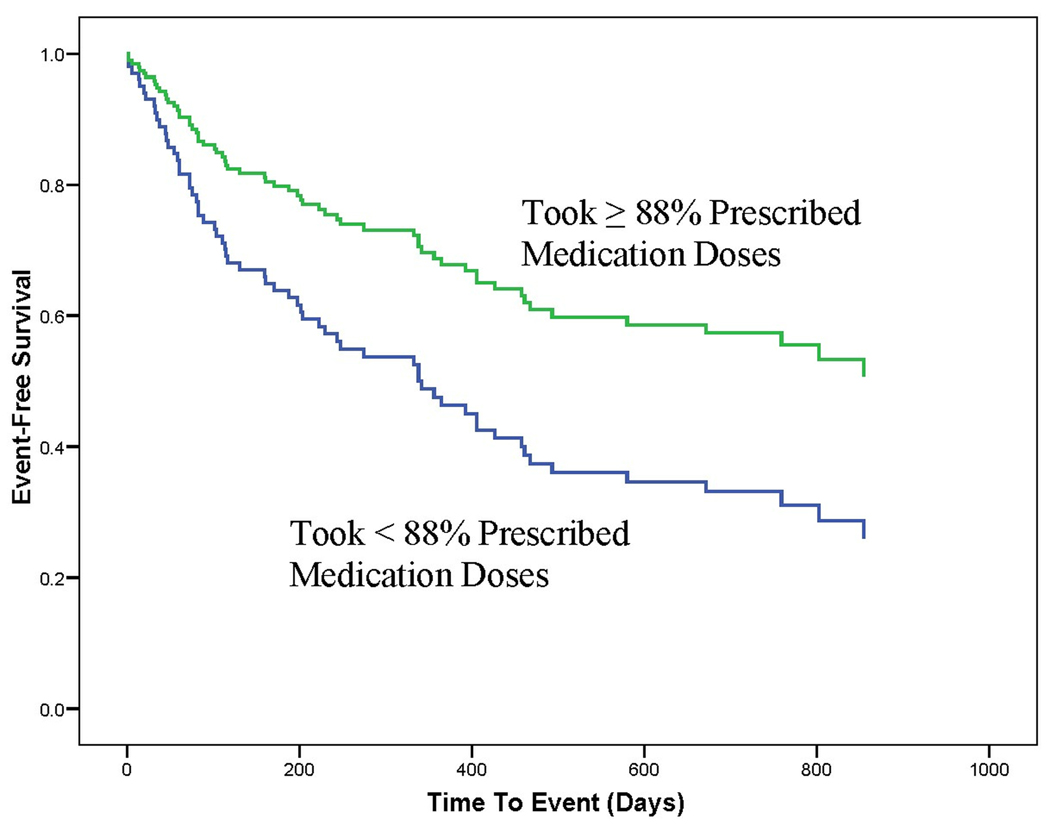
The data regarding objectively-measured medication adherence demonstrated that medication adherence mediated the relationship between anxiety and event-free survival, thus partially supporting the second hypothesis. To demonstrate a mediation effect of medication adherence, it is first necessary for anxiety to predict event-free survival. This was evident in the Cox regression reported above and shown in Figure 2. Further demonstration of mediation requires evidence that anxiety predict medication adherence. The regression analysis reported in Table 7 demonstrates this relationship. It is then necessary to demonstrate a relationship between medication adherence and event-free survival. Figure 3 shows that after adjusting for age, gender, NYHA class, left ventricular ejection fraction, ACE inhibitors, and beta-adrenergic blockers, medication adherence independently predicted, event-free survival (P = 0.001). Patients who took the correct number of doses on less than 88% of days were twice (CI 1.2–3.3, P = .008) as likely to experience the combined endpoint. The final step in demonstrating mediation is to show, in a regression that includes both anxiety and medication adherence, that the effect of anxiety on event-free survival has an associated P value that is higher (less significant) than the P value for the relationship between anxiety and event-free survival when anxiety is entered as a predictor without adherence. Table 4 and Table 8 show that medication adherence mediated the relationship between anxiety and poorer HF outcomes because the effect of anxiety on event-free survival was less significant (P = .17) when the regression model included both anxiety and medication adherence than when the model only included anxiety (P = .03).
TABLE VII.
Multiple regression of demographic, clinical, and anxiety variables associated with medication adherence (percentage days correct number of prescribed doses taken) (N = 147)
| Predictor Variables |
Step F |
Step P Value |
R2 | Adjusted R2 |
F for R2 Change |
P Value for R2 Change |
Standardized Beta |
Beta P Value |
|---|---|---|---|---|---|---|---|---|
| Step 1 | 2.64 | .052 | .06 | .04 | 2.64 | .052 | ||
| Age | .07 | .44 | ||||||
| Gender | −.10 | .27 | ||||||
| NYHA class | −.20 | .02 | ||||||
| Step 2 | 3.61 | .008 | .10 | .08 | 6.20 | .01 | ||
| Age | .01 | .91 | ||||||
| Gender | −.09 | .33 | ||||||
| NYHA class | −.17 | .06 | ||||||
| BSI anxiety core | −.22 | .01 | ||||||
BSI = Brief Symptom Inventory; NYHA = New York Heart Association
Fig 3.
Cox survival plot of medication adherence and event-free survival
TABLE VIII.
Adjusted hazard ratios for the prediction of event-free survival from anxiety and medication adherence controlling for age, gender, medication use, left ventricular ejection fraction, and New York Heart Association classification
| Hazard Ratio |
95% Confidence Interval |
P Value |
|
|---|---|---|---|
| Age | 1.007 | .982–1.032 | .60 |
| Gender | .902 | .487–1.670 | .74 |
| Prescribed angiotensin-converting enzyme inhibitor | .538 | .293–.988 | .05 |
| Prescribed beta-adrenergic blocker | .442 | .181–1.078 | .07 |
| Left ventricular ejection fraction | .988 | .966–1.010 | .29 |
| New York Heart Association classification | 1.141 | .632–2.050 | .66 |
| Second anxiety quartile* | .884 | .336–2.321 | .80 |
| Third anxiety quartile* | .952 | .413–2.194 | .91 |
| Fourth anxiety quartile* | 1.682 | .795–3.560 | .17 |
| Medication adherence | 1.345 | 1.060–1.708 | .02 |
Compared to lowest quartile of anxiety
Omnibus test of model coefficients χ2 = 29.73, P = .001
DISCUSSION
To our knowledge, this is the first study to show that anxiety predicts event-free survival for patients with HF, and that medication adherence behavior mediates this relationship. The findings are unique because, unlike others,51,52 we found that high anxiety independently predicted clinical outcomes. Although anxiety has been shown to contribute to poor outcomes for patients with other cardiovascular disorders,18,19 this new evidence shows that high anxiety and nonadherence to prescribed medications helps explain untoward outcomes for patients with HF that cannot be fully accounted for by sociodemographic or clinical variables. These data indicate that it is critical for clinicians to assess anxiety in all patients with HF.
These results align with past findings which indicated that patients with HF have high levels of anxiety. In fact, patients with HF have been shown to be more anxious than patients with other cardiac disorders.53,54 Sources of anxiety for patients with HF may include progressive and debilitating physical symptoms with poor long-term prognosis; complex medication, dietary, and activity treatment regimens; comorbidities, recurring hospitalization, hopelessness and loss of control, failure of usual coping mechanisms, isolation from family and friends, frustrations with a complicated healthcare system, financial worries, and fear of death.
The rates of nonadherence to prescribed medications for patients in this study, consistent with previous research reports,55,56 reveals that individual patient adherence remains problematic. Measured objectively for 3 months, only 56% of patients took the correct number of medication doses on 88% or more of the days that they used the MEMS. Anxiety predicted medication adherence. Nonadherence may be related to disagreements about the treatment regimen, side effects of medications, complex dosing schedules, multiple medications, concerns about medication effectiveness, improved symptoms, attitude about the importance of the medication, cost, and self-care deficit, all of which may be associated with anxiety.57,58 Although the exact mechanism by which anxiety is related to adherence remains unknown, anxiety may impair cognition, learning, energy, motivation, and patients’ willingness and ability to adhere to treatment.31 Further research is needed to elucidate how anxiety, a complex and distressing emotion with psychological, physiological, behavioral, and cognitive manifestations impacts adherence to medications or other interventions that are commonly prescribed for patients with HF.
The objective measure of dietary adherence, the 24-hour urine sodium level, did not predict outcomes or mediate the relationship between anxiety and outcomes. This may be because we measured urine sodium level once. In contrast, we measured medication adherence for 3 months.
Unlike others, we found no relationship between anxiety and measures of SNS arousal. Likewise, HRV arousal did not predict outcomes, likely due to the small number of patients eligible for HRV monitoring. In addition, most patients in this study were prescribed beta-adrenergic blockers and ACE inhibitors, which have been shown to affect HRV and perhaps diminish ANS and neurohormonal disturbances.59,60 Although beta-adrenergic blockers have been shown to affect HRV,60 we enrolled patients who took these medications because beta-adrenergic blockers are the standard of care for patients with HF.42,61 Researchers have been criticized for enrolling patients who are not representative of the typical patient with a given condition.62 Thus, to avoid this shortfall, which would diminish our ability to generalize findings to the typical patient with HF, we enrolled patients who took beta-adrenergic blockers. In addition, it would have been unethical to discontinue these therapies, and difficult to enroll sufficient numbers of patients who, for whatever reason, do not take these medications.
Practice guidelines for the management of HF mention the importance of medication adherence but ignore the assessment and management of anxiety, and do not include detailed recommendations for how to improve medication adherence.42 Hundreds of clinical trials have been conducted to test interventions designed to improve adherence for patients with cardiovascular or other chronic disorders. In summary, physician-supervised, nurse-mediated, home-based management strategies; patient education and discharge planning; reminder cards, calls or alarms; medication container design, improved patient communication with clinicians, improved dosing schedules, extended clinic hours, prescription of once-daily formulations or “polypills,” positive reinforcement, electronic monitoring systems, social support, and patient-centered medication instructions have been shown to improve medication adherence and/or outcomes.58,63–65 Patients in a previous study who are married were more likely to be adherent than patients without a spouse, indicating that interventions designed to improve adherence should include the patient’s spouse.66 Where the guidelines fall short, however, is that, at best, they encourage clinicians to consider the patient’s psychologic state when deciding how to educate and manage patients. Absent are recommendations to assess anxiety or initiate interventions to reduce anxiety. Similarly, recently published review and clinical papers contain a plethora of strategies that clinicians can use to improve adherence but omit mention of anxiety.57,58,67 Importantly, our findings indicate anxiety predicts medication adherence, which in turn mediates the relationship between high anxiety and worse outcomes.
LIMITATIONS
Four limitations of this study have the greatest impact on the findings. First, more subjects than expected had atrial fibrillation or paced cardiac rhythm, preventing HRV analysis for these subjects. Second, we used the documented admission diagnosis in each subject’s medical record for first event outcomes. We did not anticipate a large number of nonspecific diagnoses, such as edema and shortness of breath, that were consistent with a HF diagnosis but not documented as such. As a result, we classified more subjects than expected as “other hospitalization.” Third, the sample size was small but nevertheless adequate to demonstrate a relationship between anxiety and event-free survival. We recommend that future investigators conduct a larger study to confirm or refute our findings and to test the best strategies to improve adherence. Fourth, although the ethnicity of the sample reflects the patient population where the study was conducted, the sample lacks racial diversity.
CONCLUSIONS
We found that anxiety predicted event-free survival for patients with HF, and that medication adherence mediated this relationship. Although causality cannot be inferred from our data, the results of this study suggest that it may be important for clinicians to objectively assess medication adherence for patients with HF, and to consider customizing interventions according to patient needs and preferences. Assessment of medication adherence should be included in clinical practice guidelines for management of patients with HF. Nearly all patients used the MEMS without difficulty and were accepting of it. It may not be feasible or cost-effective for clinicians to objectively assess medication adherence for all patients. Nonetheless, our findings indicate that clinicians are obligated to pay special attention to medication adherence, especially for highly anxious patients and those who frequently require hospitalization or visit the ED. If nonadherence is suspected, the MEMS is an attractive assessment method. Further, the approximate $100 cost of the MEMS is justified if nonadherence is corrected and hospitalization or ED visits are avoided.
In summary, the mediation effect of nonadherence to prescribed medications on the relationship between anxiety and outcomes adds to the body of research. The astute assessment of anxiety and medication adherence merit increased attention from clinicians who manage patients with HF because medication adherence helps explain how anxiety is associated with adverse outcomes. Although we did not conduct a randomized trial to test intervention strategies for patients with differing levels of anxiety, findings from this study indicate a need for such research, particularly for patients with the highest levels of anxiety. Interventions designed to reduce anxiety and improve adherence to prescribed medications may improve outcomes.
Acknowledgments
Grants and Financial Support:
TriService Nursing Research Program Grant N05-001
Center Grant 1P20NR010679 from National Institute of Nursing Research
American Association of Critical-Care Nurses Phillips Medical Research Award
University of Kentucky General Clinical Research Center (M01RR02602)
This study was funded from the NIH/NINR; therefore I remind the publishers of the Heart & Lung about the NIH Public access policy. Journal acknowledges that Author retains the right to provide a copy of the final manuscript to the NIH, upon acceptance for Journal publication, and for public archiving in PubMed Central as soon as possible but no later than 12 months after publication by Journal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Institution at which the Research was Performed: University of Kentucky
Disclaimer Statement: This research was sponsored by the TriService Nursing Research Program, Uniformed Services University of the Health Sciences; however, the information or content and conclusions do not necessarily represent the official position or policy of, nor should any official endorsement be inferred by, the TriService Nursing Research Program, Uniformed Services University of the Health Sciences, the Department of Defense, or the U.S. Government.
Contributor Information
Marla J. De Jong, Executive Director, TriService Nursing Research Program, Uniformed Services University of the Health Sciences.
Misook L. Chung, College of Nursing, University of Kentucky.
Jia-Rong Wu, College of Nursing, University of Kentucky.
Barbara Riegel, University of Pennsylvania, Philadelphia.
Mary Kay Rayens, College of Nursing, University of Kentucky.
Debra K. Moser, Professor and Gill Endowed Chair, Co-Director, RICH Heart Program, Director, Center for Biobehavioral Research in Self-Management, College of Nursing, University of Kentucky.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Prospective evaluation of beta-blocker use at the time of hospital discharge as a heart failure performance measure: results from OPTIMIZE-HF. J Card Fail. 2007;13:722–731. doi: 10.1016/j.cardfail.2007.06.727. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 6.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a mechanical left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 7.Athanasuleas CL, Stanley AW, Jr, Buckberg GD, Dor V, DiDonato M, Blackstone EH. Surgical anterior ventricular endocardial restoration (SAVER) in the dilated remodeled ventricle after anterior myocardial infarction. J Am Coll Cardiol. 2001;37:1199–1209. doi: 10.1016/s0735-1097(01)01119-6. [DOI] [PubMed] [Google Scholar]
- 8.Bradley DJ, Bradley EA, Baughman KL, Berger RD, Calkins H, Goodman SN, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730–740. doi: 10.1001/jama.289.6.730. [DOI] [PubMed] [Google Scholar]
- 9.Kuehneman T, Saulsbury D, Splett P, Chapman DB. Demonstrating the impact of nutrition intervention in a heart failure program. J Am Diet Assoc. 2002;102:1790–1794. doi: 10.1016/s0002-8223(02)90384-6. [DOI] [PubMed] [Google Scholar]
- 10.Whellan DJ, Hasselblad V, Peterson E, O'Connor CM, Schulman KA. Metaanalysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005;149:722–729. doi: 10.1016/j.ahj.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Liu L. A new epidemic of heart failure in the United States: findings from the National Hospital Discharge Surveys, 1980–2006. Circulation. 2008;118:S1092. doi: 10.1016/j.ijcard.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Cully JA, Johnson M, Moffett ML, Khan M, Deswal A. Depression and anxiety in ambulatory patients with heart failure. Psychosomatics. 2009;50:592–598. doi: 10.1176/appi.psy.50.6.592. [DOI] [PubMed] [Google Scholar]
- 13.Januzzi JL, Jr, Stern TA, Pasternak RC, DeSanctis RW. The influence of anxiety and depression on outcomes of patients with coronary artery disease. Arch Intern Med. 2000;160:1913–1921. doi: 10.1001/archinte.160.13.1913. [DOI] [PubMed] [Google Scholar]
- 14.De Jong MJ, Chung ML, Roser LP, Jensen LA, Kelso LA, Dracup K, et al. A five-country comparison of anxiety early after acute myocardial infarction. Eur J Cardiovasc Nurs. 2004;3:129–134. doi: 10.1016/j.ejcnurse.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Cully JA, Jimenez DE, Ledoux TA, Deswal A. Recognition and treatment of depression and anxiety symptoms in heart failure. Prim Care Companion J Clin Psychiatry. 2009;11:103–109. doi: 10.4088/pcc.08m00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung ML, Moser DK, Lennie TA, Rayens MK. The effects of depressive symptoms and anxiety on quality of life in patients with heart failure and their spouses: testing dyadic dynamics using Actor-Partner Interdependence Model. J Psychosom Res. 2009;67:29–35. doi: 10.1016/j.jpsychores.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser DK, Dracup K, Evangelista LS, Zambroski CH, Lennie TA, Chung ML, et al. Comparison of prevalence of symptoms of depression, anxiety, and hostility in elderly patients with heart failure, myocardial infarction, and a coronary artery bypass graft. Heart Lung. 2010;39:378–385. doi: 10.1016/j.hrtlng.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moser DK, Dracup K. Is anxiety early after myocardial infarction associated with subsequent ischemic and arrhythmic events? Psychosom Med. 1996;58:395–401. doi: 10.1097/00006842-199609000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Frasure-Smith N, Lesperance F, Talajic M. The impact of negative emotions on prognosis following myocardial infarction: is it more than depression? Health Psychol. 1995;14:388–398. doi: 10.1037//0278-6133.14.5.388. [DOI] [PubMed] [Google Scholar]
- 20.Pelle AJ, Gidron YY, Szabo BM, Denollet J. Psychological predictors of prognosis in chronic heart failure. J Card Fail. 2008;14:341–350. doi: 10.1016/j.cardfail.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosom Med. 1999;61:476–487. doi: 10.1097/00006842-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Anand IS, Fisher LD, Chiang Y-T, Latini R, Masson S, Maggioni AP, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 23.Kohn LM, Sleet DA, Carson JC, Gray RT. Life changes and urinary norepinephrine in myocardial infarction. J Human Stress. 1983;9:38–45. doi: 10.1080/0097840X.1983.9936123. [DOI] [PubMed] [Google Scholar]
- 24.Middlekauff HR, Nguyen AH, Negrao CE, Nitzsche EU, Hoh CK, Natterson BA, et al. Impact of acute mental stress on sympathetic nerve activity and regional blood flow in advanced heart failure: implications for 'triggering' adverse cardiac events. Circulation. 1997;96:1835–1842. doi: 10.1161/01.cir.96.6.1835. [DOI] [PubMed] [Google Scholar]
- 25.Watkins LL, Blumenthal JA, Carney RM. Association of anxiety with reduced baroreflex cardiac control in patients after acute myocardial infarction. Am Heart J. 2002;143:460–466. doi: 10.1067/mhj.2002.120404. [DOI] [PubMed] [Google Scholar]
- 26.Musialik-Lydka A, Sredniawa B, Pasyk S. Heart rate variability in heart failure. Kardiol Pol. 2003;58:10–13. [PubMed] [Google Scholar]
- 27.Burger AJ, Aronson D. Activity of the neurohormonal system and its relationship to autonomic abnormalities in decompensated heart failure. J Card Fail. 2001;7:122–128. doi: 10.1054/jcaf.2001.24964. [DOI] [PubMed] [Google Scholar]
- 28.Aronson D, Mittleman MA, Burger AJ. Measures of heart period variability as predictors of mortality in hospitalized patients with decompensated congestive heart failure. Am J Cardiol. 2004;93:59–63. doi: 10.1016/j.amjcard.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Buselli EF, Stuart EM. Influence of psychosocial factors and biopsychosocial interventions on outcomes after myocardial infarction. J Cardiovasc Nurs. 1999;13:60–72. doi: 10.1097/00005082-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Sirois BC, Burg MM. Negative emotion and coronary heart disease: a review. Behav Modif. 2003;27:83–102. doi: 10.1177/0145445502238695. [DOI] [PubMed] [Google Scholar]
- 31.Rose SK, Conn VS, Rodeman BJ. Anxiety and self-care following myocardial infarction. Issues Ment Health Nurs. 1994;15:433–444. doi: 10.3109/01612849409006919. [DOI] [PubMed] [Google Scholar]
- 32.Krumholz HM, Amatruda J, Smith GL, Mattera JA, Roumanis SA, Radford MJ, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83–89. doi: 10.1016/s0735-1097(01)01699-0. [DOI] [PubMed] [Google Scholar]
- 33.Hershberger RE, Ni H, Nauman DJ, Burgess D, Toy W, Wise K, et al. Prospective evaluation of an outpatient heart failure management program. J Card Fail. 2001;7:64–74. doi: 10.1054/jcaf.2001.21677. [DOI] [PubMed] [Google Scholar]
- 34.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 35.Moser DK, Dracup K, McKinley S, Yamasaki K, Kim C-J, Riegel B, et al. An international perspective on gender differences in anxiety early after acute myocardial infarction. Psychosom Med. 2003;65:511–516. doi: 10.1097/01.psy.0000041543.74028.10. [DOI] [PubMed] [Google Scholar]
- 36.Kim KA, Moser DK, Garvin BJ, Riegel BJ, Doering LV, Jadack RA, et al. Differences between men and women in anxiety early after acute myocardial infarction. Am J Crit Care. 2000;9:245–253. [PubMed] [Google Scholar]
- 37.Tygesen H, Rundqvist B, Waagstein F, Wennerblom B. Heart rate variability measurement correlates with cardiac norepinephrine spillover in congestive heart failure. Am J Cardiol. 2001;87:1308–1311. doi: 10.1016/s0002-9149(01)01529-6. [DOI] [PubMed] [Google Scholar]
- 38.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 39.Lucreziotti S, Gavazzi A, Scelsi L, Inserra C, Klersy C, Campana C, et al. Five-minute recording of heart rate variability in severe chronic heart failure: correlates with right ventricular function and prognostic implications. Am Heart J. 2000;139:1088–1095. doi: 10.1067/mhj.2000.106168. [DOI] [PubMed] [Google Scholar]
- 40.Atherton JJ, Blackman DJ, Moore TD, Bachmann AW, Tunny TJ, Thomson HL, et al. Diastolic ventricular interaction in chronic heart failure: relation to heart rate variability and neurohumoral status. Heart Vessels. 1998;13:269–277. doi: 10.1007/BF03257231. [DOI] [PubMed] [Google Scholar]
- 41.Latini R, Masson S, Anand I, Judd D, Maggioni AP, Chiang Y-T, et al. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2002;106:2454–2458. doi: 10.1161/01.cir.0000036747.68104.ac. [DOI] [PubMed] [Google Scholar]
- 42.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2001;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 43.Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, et al. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr. 1984;40:786–793. doi: 10.1093/ajcn/40.4.786. [DOI] [PubMed] [Google Scholar]
- 44.Bates CJ, Thurnham DI, Bingham SA, Margetts BM, Nelson M. Biochemical markers of nutrient intake. In: Margetts BM, Nelson M, editors. Design in nutritional epidemiology. 2nd ed. Oxford, England: Oxford University Press; 1997. pp. 170–240. [Google Scholar]
- 45.Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoe AW, Leufkens HG. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail. 2003;9:404–411. doi: 10.1054/s1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 46.Chung ML, Lennie TA, De Jong M, Wu JR, Riegel B, Moser DK. Patients differ in their ability to self-monitor adherence to a low-sodium diet versus medication. J Card Fail. 2008;14:114–120. doi: 10.1016/j.cardfail.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Dunbar-Jacob J, Bohachick P, Mortimer MK, Sereika SM, Foley SM. Medication adherence in persons with cardiovascular disease. J Cardiovasc Nurs. 2003;18:209–218. doi: 10.1097/00005082-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 49.Wu JR, Moser DK, Lennie TA, De Jong MJ, Rayens MK, Chung ML, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. Am Heart J. 2009;157:285–291. doi: 10.1016/j.ahj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 51.Konstam V, Salem D, Pouleur H, Kostis J, Gorkin L, Shumaker S, et al. Baseline quality of life as a predictor of mortality and hospitalization in 5,025 patients with congestive heart failure. Am J Cardiol. 1996;78:890–895. doi: 10.1016/s0002-9149(96)00463-8. [DOI] [PubMed] [Google Scholar]
- 52.Jiang W, Kuchibhatla M, Cuffe MS, Christopher EJ, Alexander JD, Clary GL, et al. Prognostic value of anxiety and depression in patients with chronic heart failure. Circulation. 2004;110:3452–3456. doi: 10.1161/01.CIR.0000148138.25157.F9. [DOI] [PubMed] [Google Scholar]
- 53.Hawthorne MH, Hixon ME. Functional status, mood disturbance and quality of life in patients with heart failure. Prog Cardiovasc Nurs. 1994;9:22–32. [PubMed] [Google Scholar]
- 54.De Jong MJ, Moser DK, An K, Chung ML. Anxiety is not manifested by elevated heart rate and blood pressure in acutely ill cardiac patients. Eur J Cardiovasc Nurs. 2004;3:247–253. doi: 10.1016/j.ejcnurse.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998;80:437–441. doi: 10.1136/hrt.80.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodgers PT, Ruffin DM. Medication nonadherence: Part II-A pilot study in patients with congestive heart failure. Manag Care Interface. 1998;11:67–69. 75. [PubMed] [Google Scholar]
- 57.Albert NM. Improving medication adherence in chronic cardiovascular disease. Crit Care Nurse. 2008;28:54–64. [PubMed] [Google Scholar]
- 58.Hauptman PJ. Medication adherence in heart failure. Heart Failure Reviews. 2008;13:99–106. doi: 10.1007/s10741-007-9020-7. [DOI] [PubMed] [Google Scholar]
- 59.Mann DL, Deswal A, Bozkurt B, Torre-Amione G. New therapeutics for chronic heart failure. Annu Rev Med. 2002;53:59–74. doi: 10.1146/annurev.med.53.082901.104004. [DOI] [PubMed] [Google Scholar]
- 60.Lin L-Y, Lin J-L, Du C-C, Lai L-P, Tseng Y-Z, Huang SK. Reversal of deteriorated fractal behavior of heart rate variability by beta-blocker therapy in patients with advanced congestive heart failure. J Cardiovasc Electrophysiol. 2001;12:26–32. doi: 10.1046/j.1540-8167.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 61.Adams KF, Lindenfeld J, Arnold JM, Baker D, Barnard DH, Baughman KL, et al. HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:e1–e119. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 63.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 64.West JA, Miller NH, Parker KM, Senneca D, Ghandour G, Clark M, et al. A comprehensive management system for heart failure improves clinical outcomes and reduces medical resource utilization. Am J Cardiol. 1997;79:58–63. doi: 10.1016/s0002-9149(96)00676-5. [DOI] [PubMed] [Google Scholar]
- 65.Morrow DG, Weiner M, Young J, Steinley D, Deer M, Murray MD. Improving medication knowledge among older adults with heart failure: a patient-centered approach to instruction design. Gerontologist. 2005;45:545–552. doi: 10.1093/geront/45.4.545. [DOI] [PubMed] [Google Scholar]
- 66.Chung ML, Moser DK, Lennie TA, Riegel B. Spouses enhance medication adherence in patients with heart failure. Circulation. 2006;114 II-518. [Google Scholar]
- 67.Paul S. Hospital discharge education for patients with heart failure: what really works and what is the evidence? Crit Care Nurse. 2008;28:66–82. [PubMed] [Google Scholar]