Abstract
Objective
The relationship between arthritis and fracture was examined in the Women’s Health Initiative (WHI).
Methods
Women were classified into three self-reported groups at baseline: no arthritis (n=83,295), osteoarthritis (OA) (n=63,402), and rheumatoid arthritis (RA) (n=960). Incident fractures were self-reported throughout follow-up. Age-adjusted fracture rates by arthritis category were generated, and Cox-proportional hazards model was used to test the association between arthritis and fracture.
Results
After an average of 7.80 years, 24,137 total fractures were reported including 2,559 self-reported spinal fractures and 1,698 adjudicated hip fractures. For each fracture type, age-adjusted fracture rates were highest in the RA group and lowest in the non-arthritic group. After adjustment for several covariates, report of arthritis was associated with increased risk for spine, hip, and any clinical fractures. Compared to the non-arthritis group, the risk [HR (95% CI)] of sustaining any clinical fracture in the OA group was 1.09 (1.05, 1.13) (p<0.001) and 1.49 (1.26, 1.75) (p<0.001) in the RA group. The risk of sustaining a hip fracture was not statistically increased in the OA group [1.11 (0.98, 1.25)] (p=0.122) compared to the non-arthritis group; however the risk of hip fracture significantly increased [3.03 (2.03, 4.51)] (p<0.001) in the RA group compared to the non-arthritis group.
Conclusion
The increase in fracture risk found in this study confirms the importance of fracture prevention in patients with both RA and OA.
Key Indexing Terms: Arthritis, Epidemiology, Fracture, Postmenopausal Women
INTRODUCTION
With an increasing number of older adults in our society, osteoporosis has become a major public health concern. Fragility fractures, the most devastating outcome associated with osteoporosis, have been shown to lead to increased pain and disability, decreased quality of life (1), and higher mortality rates (2). Age and bone mineral density (BMD) are the primary risk factors associated with osteoporosis and fragility fractures (3), but others noted in FRAX®, the current World Health Organization fracture assessment calculator, include gender, weight, height, history of fractures, history of parental hip fracture, smoking, alcohol use, history of secondary osteoporosis, glucocorticoid (GC) use, and the presence of certain co-morbid conditions, such as rheumatoid arthritis (RA) (1).
RA is a multi-system inflammatory disorder characterized by inflammation and destruction of synovial joints (4). RA patients have lower BMD’s (5–7) and an increased fracture risk compared to non-arthritic controls (8–10). RA affects about 1% of the general population (11), where as osteoarthritis (OA), a commonly used arthritic comparison population in RA studies, affects about 30% of adults; making it the most common arthritic condition.
OA is typically not associated with fractures, and was previously thought of as a “protective” factor for fractures. Studies by Cumming (12), Dequeker (13), and Kanis (14) showed a reduction in fracture risk in OA cases, and studies by Jones (15) and Arden (16) showed no increased or reduced risk in fractures among OA cases. In contrast, Bergink (17) and subsequent study by Arden (18) found an increased risk in their OA cases.
Arthritis, in general, is one of the largest public health concerns for aging populations. In the United States, direct and indirect costs attributable to arthritis and other rheumatic conditions have been estimated to total $128 billion (19), and the number of individuals diagnosed is expected to increase an average of 16% by year 2030 (20). If arthritis, particularly OA, is associated with an increased risk of fractures, then the increasing arthritis prevalence would indicate a potential increase in fracture outcomes and associated complications.
The primary goal of this paper is to investigate fracture risk in a group of multi-ethnic postmenopausal self-reported arthritis cases compared to non-arthritic controls. This paper will also test if the association is modified by ethnicity or GC use.
MATERIALS AND METHODS
The association between arthritis and fracture was evaluated prospectively using data from the Women’s Health Initiative (WHI). The exposure, arthritis status, was self-reported by participants at baseline. The outcome, incident fractures, was reported over the follow-up period. All participants gave written consent to participate in the WHI, and the University of Arizona Institutional Review Board approved this current study.
Women’s Health Initiative (WHI)
The WHI is a nationwide study that investigated the risk factors and preventive strategies of the major contributors to morbidity and mortality in postmenopausal women from the United States: including heart disease, breast and colorectal cancer, and osteoporotic fractures (21). The WHI recruited 161,808 postmenopausal women aged 50 to 79 years from 40 centers across the country to participate in the clinical trials (CT) component, including the hormone therapy trials (HT), dietary modification trial (DM), and the calcium and vitamin D trial (CaD), or the observational study (OS). Details of recruitment strategies and baseline participant information have been previously published (22).
Defining arthritis status
The WHI health assessment form was used to identify arthritis status at baseline. The participants were asked, “Did your doctor ever tell you that you have arthritis?” with responses of yes or no. Women responding “yes” were then asked “What type of arthritis do you have?” with responses of “rheumatoid arthritis” and “other/do not know”. For this study, the arthritis exposure variable consists of three categories: 1) non-arthritic control group, including the women who answered “no” to the initial arthritis question; 2) OA group, including those women answering “yes” to the initial arthritis question and answering “other/do not know” on the arthritis type question; and 3) RA group, those women reporting RA as arthritis type plus one of the commonly used rheumatologic treatment medications.
Wright and colleagues previously published that the other/do not know group serves as the proxy for OA (23), and Walitt and colleagues found that the combination of self-report and medication had the highest positive predictive value (62.2%) for defining RA within the WHI compared to self-report alone (24). Women were excluded if they did not respond to the initial or follow-up arthritis question, if they reported RA but did not report one of the treatment medications of interest, or if they reported other rheumatologic or inflammatory arthritic conditions including lupus or ulcerative colitis.
Fracture ascertainment
The participants self-reported clinical fractures during periodic medical updates (every six months for women participating in the CT, and yearly for the women participating in the OS). The WHI collected information on fractures of the upper and lower arm, elbow, spine, tailbone, hip, upper and lower leg, and foot, but excluded fractures of the ribs, sternum, skull or face. All fractures reported in the CT and all hip fracture (CT and OS) were adjudicated by review of radiologic reports or medical records by centrally trained and masked physicians (25). The fractures of interest in this analysis included total (all types of fracture), spine, and hip.
Covariates
Variables associated with arthritis and/or fractures were considered as possible covariates including: age, race/ethnicity, body mass index (BMI), education, income, physical activity, hospitalizations, number of falls in the previous year, smoking status, alcohol use, hormone use status, parental fracture >40 years, calcium and vitamin D intake, depression score, years since menopause, personal fracture after 55 years, joint replacements, general health score, and use of certain medications (phenobarbital, anticonvulsants, anti-Parkinsonian drugs, antidepressants, anti-anxiety drugs, thyroid medicatoions, thiazolidinediones, proton pump inhibitors, thiazide diruetics, statins, bisphosponates, calcitonins, non steroidal anti-inflammatory drugs, estrogens, heparin, and selective-estrogen receptor modulators).
All covariates were assessed at baseline. Height and weight were measured using standardized procedures by WHI clinical staff, and were used to calculate BMI (kg/m2). Race/ethnicity was classified into 6 categories: American Indian or Alaskan Native, Asian or Pacific Islander, African American, Hispanic/Latino, White (not of Hispanic origin), or other. Women reported highest level of education completed, if they had been hospitalized in the last 2 year (yes or no), fracture at the age of 55 or older (yes or no), and the number of times they fell to the ground in the past 12 months (0, 1, 2, ≥3). Summary variables were generated based on questions regarding parental fractures (yes or no), physical activity (metabolic equivalence units (METs) per week), hormone use (never, past, or current user), smoking status (non, past, or current smoker), and alcohol use (non, past, or current drinker). Years since menopause was calculated based on reported last menstrual period. Questions from the Rand 36-Item Health Survey were used to compute a general health construct, and questions from the center for epidemiological studies depression scale (CES-D) were used to calculate a depression score. Dietary calcium and vitamin D amounts generated from food frequency questionnaire data were combined with amounts reported from supplemental use to generate total calcium and vitamin D variables. Binary variables for each class of drugs were used. Bisphosphonates and calcitonin were combined to create an osteoporosis medication summary variable. Variables related to the WHI design, such as clinical trial assignment (not randomized, placebo, or intervention), were also included as covariates.
Statistical analysis
Descriptive statistics by arthritis group were performed using analysis of variance (ANOVA) for continuous variables and chi-squared tests for categorical variables. Age-adjusted fractures rates and 95% confidence intervals (95% CI) by arthritis group were calculated using direct standardization. Cox-proportional hazards models were used to test difference in risk of fracture between groups. Days from randomization to fracture served as the event time, and days from randomization to last contact served as the censoring time for those who did not fracture. Marginal analyses were performed for each covariate, which was included in the full model if the covariate was significant p<0.2 at the 0.05 alpha level in a 2-sided test. Backward elimination techniques were used to produce the final model, including all variables statistically (p<0.05) or biologically significant. Survival estimates were generated to graphically portray group differences in fracture risk. Ethnicity and GC interactions were tested using cross-product interaction terms (for example arthritis*ethnicity) and stratified analyses. All analyses were performed in STATA v. 10 (Statacorp, College Station, TX).
RESULTS
Of the 161,808 women enrolled in the WHI, 147,657 were not missing arthritis information and did not report lupus or ulcerative colitis. Of that, 83,295 (56.4%) were included in the non-arthritic control group. Of the women who reported arthritis, 63,402 (43.0%) were placed in the OA group, and 960 (0.65%) women met the criteria for the RA group. All other women were excluded from analyses.
Differences in baseline demographic and lifestyle variables were present by arthritis group. The OA and RA groups were significantly older than the non-arthritic control group, with the OA group being on average 2.92 years older (bonferonni p-value <0.001) than the non-arthritic control group, and the RA group being on average 2.86 years older (bonferroni p-value <0.001) than the non-arthritic controls. The arthritis groups had a larger percentage of African Americans compared to the non-arthritic control group (RA: 13.2%, OA: 9.3%, non-arthritic control: 8.2%). In a post-hoc chi-squared test, the percentage of African Americans in the OA was found to be significantly higher in the OA group compared to the non-arthritis group (p<0.001), and similarly the percentage of African Americans in the RA group was significantly higher (p<0.001) than the non-arthritis group, The OA group had the largest mean weight (75.5 kg) followed by the RA group (73.2 kg) and the non-arthritic control group (71.7 kg). The weight of the OA group was significantly higher than the non-arthritis group (bonferroni p-value <0.001) and the RA group (bonferroni p-value <0.001), and the RA weight was significantly higher than the non-arthritis group (bonferroni p-value 0.025). There were statistically significant differences in the percentage of hospitalization the last 2 years (RA: 28.0%,OA: 18.4%, no arthritis: 11.6%) (overall p<0.001) and history of fracture at ≥55 years (RA: 20.0%, OA18.8%, no arthritis14.1%) (overall p<0.001). Complete descriptive information with overall ANOVA and chi-squared test p-values can be found in Tables 1 and 2.
Table 1.
Baseline Characteristics of Categorical Variables by Arthritis Status
| No Arthritis* (n=83,295) |
OA* (n=63,402) |
RA* (n=960) |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Baseline Age Group | ||||||
| 50–59 | 33,804 | 40.58 | 15,378 | 24.25 | 240 | 25.00 |
| 60–69 | 35,446 | 42.55 | 30,248 | 47.71 | 448 | 46.67 |
| 70–79 | 14,045 | 16.86 | 17,776 | 28.04 | 272 | 28.33 |
| Race/Ethnicity | ||||||
| White | 68,699 | 82.68 | 53,153 | 84.05 | 741 | 77.35 |
| Hispanic | 3,573 | 4.30 | 2,060 | 3.26 | 38 | 3.97 |
| African American | 6,817 | 8.20 | 5,857 | 9.26 | 126 | 13.15 |
| Asian | 2,721 | 3.28 | 1,191 | 1.88 | 26 | 2.71 |
| American Indian | 317 | 0.38 | 292 | 0.46 | 9 | 0.94 |
| Unknown | 967 | 1.16 | 686 | 1.09 | 18 | 1.88 |
| Hormone Trial | ||||||
| Not randomized | 68,819 | 82.62 | 53,487 | 84.36 | 878 | 91.46 |
| Intervention | 7,374 | 8.85 | 4,887 | 7.71 | 41 | 4.27 |
| Control | 7,102 | 8.53 | 5,028 | 7.93 | 41 | 4.27 |
| Dietary Modification Trial | ||||||
| Not randomized | 57,310 | 68.80 | 45,779 | 72.20 | 812 | 84.58 |
| Intervention | 10,398 | 12.48 | 7,069 | 11.15 | 59 | 6.15 |
| Control | 15,587 | 18.71 | 10,554 | 16.65 | 89 | 9.27 |
| Calcium and Vitamin D Trial | ||||||
| Not randomized | 63,522 | 76.26 | 50,503 | 79.66 | 866 | 90.21 |
| Intervention | 9,906 | 11.89 | 6,434 | 10.15 | 53 | 5.52 |
| Control | 9,867 | 11.85 | 6,465 | 10.20 | 41 | 4.27 |
| Hospitalized in Last 2 Years | ||||||
| No | 69,909 | 88.43 | 51,453 | 81.59 | 683 | 72.05 |
| Yes | 9,143 | 11.57 | 11,607 | 18.41 | 265 | 27.95 |
| Number of Falls in 12 Months | ||||||
| 0 | 57,007 | 71.34 | 40,530 | 64.10 | 612 | 64.15 |
| 1 | 15,054 | 18.84 | 13,331 | 21.08 | 201 | 21.07 |
| 2 | 5,454 | 6.83 | 6,123 | 9.68 | 97 | 10.17 |
| 3+ | 2,398 | 3.00 | 3,248 | 5.14 | 44 | 4.61 |
| Parental Fracture >40 Years | ||||||
| No | 47,303 | 61.00 | 34,447 | 59.34 | 547 | 62.37 |
| Yes | 30,242 | 39.00 | 23,605 | 40.66 | 330 | 37.63 |
| Fracture at Age 55+ | ||||||
| No | 52,476 | 85.93 | 43,140 | 81.24 | 631 | 80.08 |
| Yes | 8,595 | 14.07 | 9,961 | 18.76 | 157 | 19.92 |
| Smoking Status | ||||||
| Never smoked | 42,597 | 51.69 | 31,502 | 50.34 | 437 | 46.00 |
| Past smoker | 33,770 | 40.98 | 27,124 | 43.35 | 443 | 46.63 |
| Current smoker | 6,046 | 7.34 | 3,947 | 6.31 | 70 | 7.37 |
| Hormone Therapy Use | ||||||
| Never used | 37,413 | 44.95 | 26,774 | 42.27 | 397 | 41.40 |
| Past user | 12,221 | 14.68 | 10,979 | 17.33 | 161 | 16.79 |
| Current user | 33,593 | 40.36 | 25,589 | 40.40 | 401 | 41.81 |
| Osteoporosis Medications Use | ||||||
| No | 81,787 | 98.19 | 61,653 | 97.24 | 892 | 92.92 |
| Yes | 1,508 | 1.81 | 1,749 | 2.76 | 68 | 7.08 |
| Thiazolidinediones Use | ||||||
| No | 83,240 | 99.93 | 63,339 | 99.90 | 958 | 99.79 |
| Yes | 55 | 0.07 | 63 | 0.10 | 2 | 0.21 |
| Previous Joint Replacement | ||||||
| No | 77,918 | 99.20 | 58,604 | 93.29 | 773 | 81.28 |
| Yes | 628 | 0.80 | 4,218 | 6.71 | 178 | 18.72 |
OA: Osteoarthritis
RA: Rheumatoid arthritis
all variables significantly different between the three groups at p<0.001, with the exception of thiazolidinediones (p=0.020)
Table 2.
Baseline Characteristics of Continuous Variables by Arthritis Status
| No Arthritis* (n=83,295) |
OA * (n=63,402) |
RA* (n=960) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (yrs) | 61.89 | 7.14 | 64.81 | 7.01 | 64.75 | 7.12 |
| Height (cm) | 162.00 | 6.58 | 161.50 | 6.73 | 161.10 | 6.76 |
| Weight (kg) | 71.74 | 15.94 | 75.45 | 17.71 | 73.18 | 17.78 |
| Body Mass Index (kg/m2) | 27.19 | 5.49 | 28.78 | 6.28 | 28.10 | 6.40 |
| Years Since Menopause | 13.60 | 9.36 | 16.90 | 9.62 | 16.71 | 9.97 |
| Total Calcium Intake (mg) | 1,148.00 | 750.00 | 1,207 | 734.10 | 1,264.00 | 837.60 |
| Total Vitamin D Intake (mg) | 8.92 | 6.87 | 9.66 | 7.12 | 9.88 | 6.84 |
| CES-D Depression Score | 0.03 | 0.11 | 0.05 | 0.14 | 0.05 | 0.14 |
| Total Physical Activity per Week (METS) | 13.35 | 14.34 | 11.53 | 12.92 | 9.58 | 11.46 |
| General Health Construct | 78.52 | 16.00 | 70.03 | 18.07 | 57.26 | 20.35 |
OA: Osteoarthritis
RA: Rheumatoid arthritis
All variables significantly different between the three groups at P<0.001
Fractures in the WHI
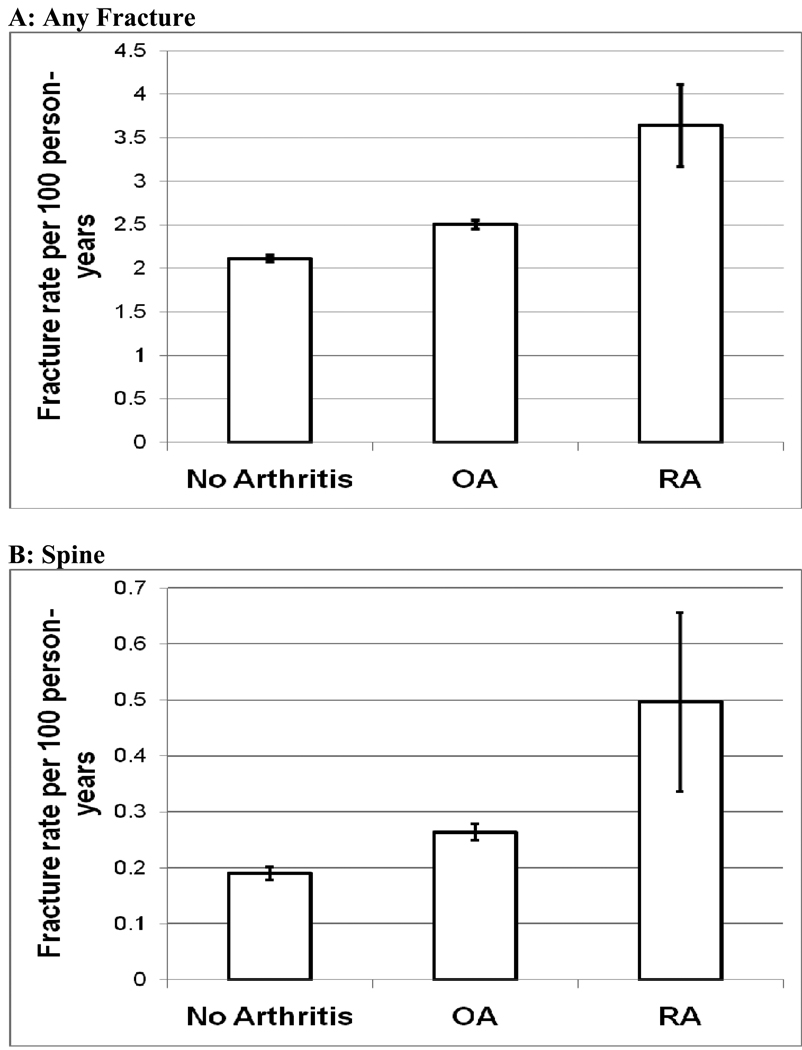
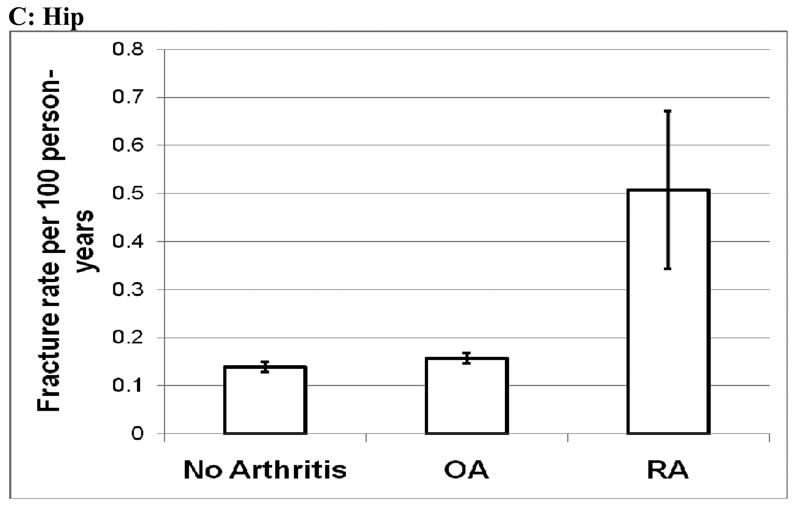
As of March 2008, the women were followed for a mean (SD) of 7.80 (1.54) years, and 24,137 clinical fractures of any type; 2,559 fractures of the spine; and 1,698 hip fractures were reported in the study population (Table 3). The age-adjusted rate (95% CI) per 100 person-years for sustaining total fractures (fracture of any type) was 2.11 (2.08, 2.15) in the non-arthritic control group, increasing to 2.51 (2.46, 2.55) in the OA group, and 3.64 (3.17, 4.11) in the RA group (Figure 1). The age-adjusted spine fracture rate increased from 0.19 to 0.26 per 100 person-years between the control group and the OA group, and increased again to 0.50 per 100 person-years in the RA group. There was no difference in hip fracture rates between the control group and the OA group (0.14/100 vs. 0.16/100), but there was an increase in the hip fracture rate in the RA group (0.51/100) (Figure 1).
Table 3.
Frequency of Fracture in the WHI and by Arthritis Status
| No Arthritis (n = 83,295) |
OA (n = 63,402) |
RA (n = 960) |
Total Population (n = 147,657) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value | N | % | |
| Any Fracture | 12,411 | 14.9 | 11,488 | 18.1 | 238 | 24.8 | <0.001 | 24,137 | 16.3 |
| Spine | 1,126 | 1.4 | 1,395 | 2.2 | 38 | 4.0 | <0.001 | 2,559 | 1.7 |
| Hip | 775 | 0.9 | 885 | 1.4 | 38 | 4.0 | <0.001 | 1,698 | 1.1 |
OA: Osteoarthritis
RA: Rheumatoid arthritis
Figure 1.
Age-Adjusted Fracture Rates by Arthritis Status
Age-adjusted rates per 100 person-years and 95% confidence intervals (A) Any Fracture; (B) Spine; (C) Hip
OA: Osteoarthritis
RA: Rheumatoid arthritis
Testing the association between arthritis and fracture risk
No significant interaction between ethnicity or GC use was found in the association between arthritis and fracture. Covariates included in the final Cox-proportional hazards model included: age, race/ethnicity, BMI, physical activity, assignment in all clinical trials, hospitalizations, number of falls, smoking status, hormone use, parental fracture >age 40, personal fracture ≥55, total calcium & vitamin D intake, depression score, years since menopause, joint replacements, use of diabetic and osteoporosis medications, and general health score. In comparison to the non-arthritic control group, there was a significant risk for sustaining any type of fracture in both the OA [hazard ratio (95% CI)] [1.09 (1.05, 1.13)] and RA groups [1.49 (1.26, 1.75)] (Table 4). In comparison to the non-arthritic control group, the risk of spine fracture was 1.17 (1.05, 1.29) (p=0.004) and 1.93 (1.29, 2.90) (p= 0.001) in OA and RA groups, respectively (Table 4). No significant increase in hip fracture risk was observed in the OA group [1.11 (0.98, 1.25)] compared to the non-arthritic control group; however, a highly significant increase in hip fracture risk [3.03 (2.03, 4.51)] was observed in the RA group compared to the non-arthritic control group (Table 4).
Table 4.
The Risk of Fracture by Arthritis Group
| No Arthritis (n=83,295) |
OA (n=63,402) |
RA (n=960) |
|||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Any Fracture (n=24,137) | Ref. | 1.09 (1.05, 1.13) | <0.001 | 1.49 (1.26, 1.75) | <0.001 |
| Spine (n=2,559) | Ref. | 1.17 (1.05, 1.29) | 0.004 | 1.93 (1.29, 2.90) | 0.001 |
| Hip (n=1,698) | Ref. | 1.11 (0.98, 1.25) | 0.105 | 3.03 (2.03, 4.51) | <0.001 |
Adjusted for age; race; BMI; physical activity; assignment in the HT trial, DM trial, and CaD trial; hospitalizations; falls; smoking ; hormone use; parental fracture >age 40; calcium & vitamin D intake; depression score; years since menopause; diabetic treatments; osteoporosis medication; general health score; fracture >55; and joint replacements
OA: Osteoarthritis
RA: Rheumatoid arthritis
DISCUSSION
In this large population of postmenopausal women, self-reported arthritis is associated with significant fracture risk increase in women reporting OA and RA. After controlling for several covariates, the RA group had a highly significant increased risk of all fractures studied (HRtotal = 1.49, HRspine = 1.93, HRhip = 3.03) in comparison to the non-arthritis group. Modest significant increases in total (HR = 1.09) and spine (HR = 1.17) fracture risk were seen in the OA group in comparison to the non-arthritis group, but no significant increase in hip fracture risk was seen. The associations found between arthritis and fracture were not modified by race, ethnicity, or glucocorticoids use in this study.
The RA findings from this study are consistent with the literature showing an increased risk of fractures in RA patients (9, 10, 26). Recently, incidence of any, spine and hip fracture in the Consortium of Rheumatology Researchers of North American (CORRONA) Registry were reported to be 3.71, 0.78, and 0.66 per 100 person-years, respectively (27). The age-adjusted fracture rates for the RA group were 3.64, 0.49, and 0.50 per 100 person-years for total, spine, and hip fracture in the WHI, and though the CORRONA registry includes premenopausal women and men, the incidence rates of the nationwide CORRONA registry are comparable to the rates found in the WHI.
General lifestyle and demographic osteoporosis risk factors, such as age, smoking, and physical activity, play a significant role in fracture risk (1), but the primary risk factor for fracture is low BMD. It has been well documented that RA patients have lower BMD at many skeletal sites compared to various control populations (6, 7, 28), and though BMD was not examined in this study, it is highly probable that the associations seen are in part, driven by BMD. A sensitivity analysis in the participants from three WHI clinical centers with available BMD measurements was proposed, however, could not be adequately completed due to the low frequency of fractures (total fractures n=22; spine fractures n=2; hip fractures n=3) in the smaller RA group (n=78).
The risk of sustaining any clinical fracture and a spinal fracture was modestly but significantly increased in the OA group compared to the non-arthritic controls. It is likely that the effects of OA on fracture rate are being underestimated in this study due to the misclassification inherent in self-reporting OA. As previously mentioned, the association between OA and fracture has been mixed in the literature. The most recent study to suggest OA increases the risk of fractures reported by Arden and colleagues found that after adjusting for falls and the use of walking aids, clinician diagnosed knee OA patients had a significant risk for non-vertebral fractures [1.48 (1.00, 2.19)], and no significant association was seen between clinician diagnosed knee OA patients and risk for hip fracture [1.84 (0.78, 4.34)] (18). Though the results of our study are in agreement with the Arden study, the use of clinically diagnosed, site-specific OA patients yielded higher fracture estimates than those found in our study using self-reported OA cases.
In contrast, the most recent study showing a protective effect of OA on fracture risk was a case-control population-based study conducted in Denmark. After adjustment for several variables, Vestergaard and colleagues found an significant risk reduction for any fracture, hip, and spine fractures in participants with OA duration greater than two years (29). Population demographics could be the primary explanation for the difference associations seen between the Vestergaard study and the current, as the Danish population used was almost 20 years younger than the WHI population.
Though a consensus has not been reached, several biological mechanisms have been proposed relating OA to fracture. Like RA, the increase in fracture in OA patients could be driven through a BMD pathway. Studies have shown that BMD in OA populations is typically higher than non-arthritic populations (30–32); therefore, this argument does not provide a good explanation for increases in fracture. Though OA patients have a higher BMD or bone quantity, quality or strength of the bones may be compromised compared to other arthritic and non-arthritic populations. Javaid and colleagues assessed hip structural geometry, as a marker of bone strength, in a group of OA patients and found alterations in geometry precede OA diagnosis (33), suggesting a biological process involved in OA potentially alters bone strength.
Falling is another proposed OA fracture mechanism. OA, especially at sites like the knee and hip, is associated with increased pain, decreased postural stability, and decreased muscle strength, all which have been shown to be significant contributors to fall risk (34–36). Falling is a well-documented risk factor for fractures (1), and early studies have shown that the self-report of OA is associated with increased risk of falls (37, 38). More recently, Foley and colleagues did not see increased risk for falls in knee and hip radiographic OA cases, but did see that report of pain is highly associated with falls and OA patients reported more pain (39).
One last possibility is that our results represent the consequences related to behavioral and physiologic changes that occur in individuals that perceive articular discomfort they classify as arthritis. Self-reported health status has been shown to be an independent risk factor for fractures in many studies (40–44). It is possible that self-reported arthritis in the WHI is a measure of autoperception that encompasses a variety of health domains, such as pain, balance confidence, self-efficacy, and functional status.
Strengths and limitations
This study has several limitations related to the arthritis exposure. Regarding OA, the limitations associated with self-report and the use of a proxy measure of OA within the WHI previously described by Wright and colleagues apply to this analysis (23). Walitt and colleagues also found that self-reported OA in the WHI was very sensitive (95.0%), but not particularly specific (23.4%), and only had fair agreement between self-reported OA and chart review (kappa = 0.23) (unpublished data). The potential for the moderate amount of misclassification in the OA group would bias the results of this study to the null. People experiencing joint pain due to a previous injury, have other soft-tissue condition such as tendonitis, or other non-inflammatory arthritic conditions, may report having OA though not clinically diagnosed. This could also lead to a moderate amount of misclassification, again, biasing the estimates towards the null. Not having site specific or radiographically confirmed OA cases is another limitation of this study. Fracture risk is probably different for persons with OA of the hip compared to persons with knee, hand, or spine OA. The OA affected area may have a higher BMD, whereas regions without OA have normal or low BMD, potentially altering overall fracture risk.
Regarding the RA classification, the use of medication in the RA definition probably captured true RA cases, but these may represent the more severe cases, potentially overestimating the effect of RA on fracture risk. This study did not take into account incident cases of arthritis and the effect it has on fracture risk, and it also did not also account for the additive or multiplicative effect of having both conditions on fracture risk.
The use of self-reported fracture outcomes can also be seen as a limitation. Sensitivity analyses were performed using adjudicated fractures only. Slight changes in the point estimates were observed with the smaller sample size, however, the overall conclusions did not change. Chen and colleagues found high agreement between self-report and adjudicated fractures in WHI sub-study (45), assuring high quality of the fracture data used in this study.
This study adjusted for several covariates, but was unable to adjust for GC use, as it was used in the definition of the RA group. To test the possible interaction of GCs in the relationship between arthritis and fracture, a categorical variable was created capturing users and non-users in each arthritis group (data not shown). Though no interaction was present, the point estimate of the fracture risk was higher in GC users compared to non-users, and by not adjusting for GCs, the true fracture risk for women not taking GCs was overestimated and the risk was underestimated for women using GCs.
Though limited by the above mentioned factors, there are many strengths of the study. The most notable is the size of the WHI, and the size of each of the exposure groups. Having over 63,000 women in the OA group gave more than adequate power to estimate the effects OA have on fracture outcomes. Though not clinically ascertained, the prevalence of OA in the WHI population was approximately 43%, which is comparable to the 42% prevalence of radiographic OA in the hands, knees, and hips found in the women 60 years and older participating in National Health and Nutrition Examination Survey (NHANES)-III (46). The OA limitations presented would have resulted in estimates being biased toward the null; however, significant association remained in our study. Though not reaching general population prevalence estimates, the RA group sample size was large enough to confirm the association between RA and fracture. The WHI also had a larger percentage of women from minority groups, which allowed for examination of effect modification by race and ethnicity. The women of the WHI were followed on average almost 8 years, ensuring adequate numbers of fracture outcomes, especially for the more rare hip fracture outcome.
Conclusions
Arthritis and osteoporosis are import public health conditions for older adults. OA and RA affect over 25 million adults in the United States and fractures costs billions of health care dollars annually. The increase in fracture risk found in this study confirms the importance of fracture prevention in both patients with RA and OA.
Acknowledgements
Many thanks are extended to the participants of the Women’s Health Initiative, the staff of the Healthy Aging Laboratory, M. Jane Mohler, and Duane Sherrill. This work is a portion of NCW’s dissertation work, which was submitted in partial fulfillment of the requirements for a degree at the University of Arizona.
Funding:
National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR049411-04S1). The WHI program is funded by the National Heart, Lung, and Blood Institute through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Appendix
Short List of WHI Investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA). Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Los Angeles Biomedical Research Institute at Harbor—UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Evelyn Whitlock; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix.
REFERENCES
- 1.Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008 Oct;22(5):671–685. doi: 10.1016/j.beem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Teng GG, Curtis JR, Saag KG. Mortality and osteoporotic fractures: is the link causal, and is it modifiable? Clin Exp Rheumatol. 2008 Sep–Oct;26(5) Suppl 51:S125–S137. [PMC free article] [PubMed] [Google Scholar]
- 3.Kanis JA, Johansson H, Oden A, McCloskey EV. Assessment of fracture risk. Eur J Radiol. 2009 Sep;71(3):392–397. doi: 10.1016/j.ejrad.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005 Mar;4(3):130–136. doi: 10.1016/j.autrev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg MC, Lethbridge-Cejku M, Tobin JD. Bone mineral density and osteoarthritis: data from the Baltimore Longitudinal Study of Aging. Osteoarthritis Cartilage. 2004;12 Suppl A:S45–S48. doi: 10.1016/j.joca.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Kroot EJ, Nieuwenhuizen MG, de Waal Malefijt MC, van Riel PL, Pasker-de Jong PC, Laan RF. Change in bone mineral density in patients with rheumatoid arthritis during the first decade of the disease. Arthritis Rheum. 2001 Jun;44(6):1254–1260. doi: 10.1002/1529-0131(200106)44:6<1254::AID-ART216>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Lodder MC, de Jong Z, Kostense PJ, Molenaar ET, Staal K, Voskuyl AE, et al. Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann Rheum Dis. 2004 Dec;63(12):1576–1580. doi: 10.1136/ard.2003.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum. 2002 Jan;46(1):92–99. doi: 10.1002/1529-0131(200201)46:1<92::AID-ART10057>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Hooyman JR, Melton LJ, 3rd, Nelson AM, O'Fallon WM, Riggs BL. Fractures after rheumatoid arthritis. A population-based study. Arthritis Rheum. 1984 Dec;27(12):1353–1361. doi: 10.1002/art.1780271205. [DOI] [PubMed] [Google Scholar]
- 10.Huusko TM, Korpela M, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Threefold increased risk of hip fractures with rheumatoid arthritis in Central Finland. Ann Rheum Dis. 2001 May;60(5):521–522. doi: 10.1136/ard.60.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008 Jan;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 12.Cumming RG, Klineberg RJ. Epidemiological study of the relation between arthritis of the hip and hip fractures. Ann Rheum Dis. 1993 Oct;52(10):707–710. doi: 10.1136/ard.52.10.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dequeker J, Johnell O. Osteoarthritis protects against femoral neck fracture: the MEDOS study experience. Bone. 1993;14 Suppl 1:S51–S56. doi: 10.1016/8756-3282(93)90350-j. [DOI] [PubMed] [Google Scholar]
- 14.Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, et al. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999;9(1):45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- 15.Jones G, Nguyen T, Sambrook PN, Lord SR, Kelly PJ, Eisman JA. Osteoarthritis, bone density, postural stability, and osteoporotic fractures: a population based study. J Rheumatol. 1995 May;22(5):921–925. [PubMed] [Google Scholar]
- 16.Arden NK, Nevitt MC, Lane NE, Gore LR, Hochberg MC, Scott JC, et al. Osteoarthritis and risk of falls, rates of bone loss, and osteoporotic fractures. Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1999 Jul;42(7):1378–1385. doi: 10.1002/1529-0131(199907)42:7<1378::AID-ANR11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.Bergink AP, van der Klift M, Hofman A, Verhaar JA, van Leeuwen JP, Uitterlinden AG, et al. Osteoarthritis of the knee is associated with vertebral and nonvertebral fractures in the elderly: the Rotterdam Study. Arthritis Rheum. 2003 Oct 15;49(5):648–657. doi: 10.1002/art.11380. [DOI] [PubMed] [Google Scholar]
- 18.Arden NK, Crozier S, Smith H, Anderson F, Edwards C, Raphael H, et al. Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis Rheum. 2006 Aug 15;55(4):610–615. doi: 10.1002/art.22088. [DOI] [PubMed] [Google Scholar]
- 19.National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions--United States, 2003. MMWR Morb Mortal Wkly Rep. 2007 Jan 12;56(1):4–7. [PubMed] [Google Scholar]
- 20.Projected state-specific increases in self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitations--United States, 2005–2030. MMWR Morb Mortal Wkly Rep. 2007 May 4;56(17):423–425. [PubMed] [Google Scholar]
- 21.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998 Feb;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 22.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003 Oct;13(9) Suppl:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 23.Wright NC, Riggs GK, Lisse JR, Chen Z. Self-reported osteoarthritis, ethnicity, body mass index, and other associated risk factors in postmenopausal women-results from the Women's Health Initiative. J Am Geriatr Soc. 2008 Sep;56(9):1736–1743. doi: 10.1111/j.1532-5415.2008.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walitt BT, Constantinescu F, Katz JD, Weinstein A, Wang H, Hernandez RK, et al. Validation of self-report of rheumatoid arthritis and systemic lupus erythematosus: The Women's Health Initiative. J Rheumatol. 2008 May;35(5):811–818. [PMC free article] [PubMed] [Google Scholar]
- 25.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003 Oct;13(9) Suppl:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 26.Spector TD, Hall GM, McCloskey EV, Kanis JA. Risk of vertebral fracture in women with rheumatoid arthritis. Bmj. 1993 Feb 27;306(6877):558. doi: 10.1136/bmj.306.6877.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coulson KA, Reed G, Gilliam BE, Kremer JM, Pepmueller PH. Factors influencing fracture risk, T score, and management of osteoporosis in patients with rheumatoid arthritis in the Consortium of Rheumatology Researchers of North America (CORRONA) registry. J Clin Rheumatol. 2009 Jun;15(4):155–160. doi: 10.1097/RHU.0b013e3181a5679d. [DOI] [PubMed] [Google Scholar]
- 28.Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum. 2000 Mar;43(3):522–530. doi: 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Vestergaard P, Rejnmark L, Mosekilde L. Osteoarthritis and risk of fractures. Calcif Tissue Int. 2009 Apr;84(4):249–256. doi: 10.1007/s00223-009-9224-z. [DOI] [PubMed] [Google Scholar]
- 30.Burger H, van Daele PL, Odding E, Valkenburg HA, Hofman A, Grobbee DE, et al. Association of radiographically evident osteoarthritis with higher bone mineral density and increased bone loss with age. The Rotterdam Study. Arthritis Rheum. 1996 Jan;39(1):81–86. doi: 10.1002/art.1780390111. [DOI] [PubMed] [Google Scholar]
- 31.Lethbridge-Cejku M, Tobin JD, Scott WW, Jr, Reichle R, Roy TA, Plato CC, et al. Axial and hip bone mineral density and radiographic changes of osteoarthritis of the knee: data from the Baltimore Longitudinal Study of Aging. J Rheumatol. 1996 Nov;23(11):1943–1947. [PubMed] [Google Scholar]
- 32.Stewart A, Black AJ. Bone mineral density in osteoarthritis. Curr Opin Rheumatol. 2000 Sep;12(5):464–467. doi: 10.1097/00002281-200009000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Javaid MK, Lane NE, Mackey DC, Lui LY, Arden NK, Beck TJ, et al. Changes in proximal femoral mineral geometry precede the onset of radiographic hip osteoarthritis: The study of osteoporotic fractures. Arthritis Rheum. 2009 Jul;60(7):2028–2036. doi: 10.1002/art.24639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jadelis K, Miller ME, Ettinger WH, Jr, Messier SP. Strength, balance, and the modifying effects of obesity and knee pain: results from the Observational Arthritis Study in Seniors (oasis) J Am Geriatr Soc. 2001 Jul;49(7):884–891. doi: 10.1046/j.1532-5415.2001.49178.x. [DOI] [PubMed] [Google Scholar]
- 35.Leveille SG, Bean J, Bandeen-Roche K, Jones R, Hochberg M, Guralnik JM. Musculoskeletal pain and risk for falls in older disabled women living in the community. J Am Geriatr Soc. 2002 Apr;50(4):671–678. doi: 10.1046/j.1532-5415.2002.50161.x. [DOI] [PubMed] [Google Scholar]
- 36.Sturnieks DL, Tiedemann A, Chapman K, Munro B, Murray SM, Lord SR. Physiological risk factors for falls in older people with lower limb arthritis. J Rheumatol. 2004 Nov;31(11):2272–2279. [PubMed] [Google Scholar]
- 37.Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989 Jul;44(4):M112–M117. doi: 10.1093/geronj/44.4.m112. [DOI] [PubMed] [Google Scholar]
- 38.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. Jama. 1989 May 12;261(18):2663–2668. [PubMed] [Google Scholar]
- 39.Foley SJ, Lord SR, Srikanth V, Cooley H, Jones G. Falls risk is associated with pain and dysfunction but not radiographic osteoarthritis in older adults: Tasmanian Older Adult Cohort study. Osteoarthritis Cartilage. 2006 Jun;14(6):533–539. doi: 10.1016/j.joca.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Chen YT, Miller PD, Barrett-Connor E, Weiss TW, Sajjan SG, Siris ES. An approach for identifying postmenopausal women age 50–64 years at increased short-term risk for osteoporotic fracture. Osteoporos Int. 2007 Sep;18(9):1287–1296. doi: 10.1007/s00198-007-0380-6. [DOI] [PubMed] [Google Scholar]
- 41.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007 Jul;62(7):744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 42.Holmberg AH, Johnell O, Nilsson PM, Nilsson JA, Berglund G, Akesson K. Risk factors for hip fractures in a middle-aged population: a study of 33,000 men and women. Osteoporos Int. 2005 Dec;16(12):2185–2194. doi: 10.1007/s00198-005-2006-1. [DOI] [PubMed] [Google Scholar]
- 43.Kulmala J, Sihvonen S, Kallinen M, Alen M, Kiviranta I, Sipila S. Balance confidence and functional balance in relation to falls in older persons with hip fracture history. J Geriatr Phys Ther. 2007;30(3):114–120. doi: 10.1519/00139143-200712000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Rohde G, Mengshoel AM, Wahl AK, Moum T, Haugeberg G. Is health-related quality of life associated with the risk of low-energy wrist fracture: a case-control study. BMC Musculoskelet Disord. 2009;10:80. doi: 10.1186/1471-2474-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women's Health Initiative observational study and clinical trials. Menopause. 2004 May–Jun;11(3):264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008 Jan;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]