Abstract
Mammals have two principal types of fat. White adipose tissue (WAT) primarily serves to store extra energy as triglycerides, while brown adipose tissue (BAT) is specialized to burn lipids for heat generation and energy expenditure as a defense against cold and obesity 1, 2. Recent studies demonstrate that brown adipocytes arise in vivo from a Myf5-positive, myoblastic progenitor by the action of Prdm16 (PR domain containing 16). Here, we identified a brown fat-enriched miRNA cluster, miR-193b-365, as a key regulator of brown fat development. Blocking miR-193b and/or miR-365 in primary brown preadipocytes dramatically impaired brown adipocyte adipogenesis by enhancing Runx1t1 (runt-related transcription factor 1; translocated to, 1) expression whereas myogenic markers were significantly induced. Forced expression of miR-193b and/or miR-365 in C2C12 myoblasts blocked the entire program of myogenesis, and, in adipogenic condition, miR-193b induced myoblasts to differentiate into brown adipocytes. MiR-193b-365 was upregulated by Prdm16 partially through Pparα. Our results demonstrate that miR-193b-365 serves as an essential regulator for brown fat differentiation, in part by repressing myogenesis.
Keywords: miR-193, miR-365, miR-193b-365, microRNA, brown fat, brown adipocyte, lineage determination, adipogenesis, myogenesis, Prdm16
Although the amount of BAT in human adults was previously thought to be minimal, recent studies demonstrated that adult humans have substantial amounts of functioning BAT 3–5, which inversely correlates with BMI and positively correlates with resting metabolic rate 3. Loss of BAT activity may contribute to obesity and development of insulin resistance. When BAT is genetically or surgically ablated, mice develop hyperphagia and obesity 6–8. Mice deficient of Ucp1 (Uncoupling Protein 1), the hallmark of brown fat, are more susceptible to diet-induced obesity and develop obesity at thermoneutrality even when they are fed a control diet 9. Conversely, expression of Ucp1 in white adipocytes promotes energy expenditure and prevents the development of dietary and genetic obesity 10, 11. In addition, Bartelt. et al. demonstrated that brown fat is a major organ for triglyceride clearance 12. Therefore, understanding the mechanisms controlling the development of BAT may provide new therapeutic strategies for obesity and related disorders. Although, for decades, it was believed that brown adipocytes and white adipocytes share a common progenitor, a lineage tracing study demonstrated that brown adipocytes arise from a Myf5-positive, myoblastic lineage 13. Prdm16 is a key regulator that controls the switch between brown fat and muscle lineage. Ectopic expression of Prdm16 together with CEBPβ can induce a functional BAT program in myoblasts and skin fibroblasts 14. Knockdown of Prdm16 in brown preadipocytes causes an induction of skeletal myogenesis 13. However, other factors that regulate the switch between BAT and skeletal muscle remain unknown. Here, we show that the miR-193b-365 cluster is important for lineage determination of brown adipocytes.
To uncover miRNAs that are important for brown fat lineage determination, we first identified lineage-enriched miRNAs by comparing the genome-wide miRNA expression patterns of mouse epididymal WAT, interscapular BAT and skeletal muscle using miRNA microarrays. Based on the criteria described in Methods, 91 miRNAs were expressed in at least one sample and differentially expressed between the three tissues (Fig. 1a and Fig. S1a). Among BAT-enriched miRNAs, miR-193b and miR-365 were particularly interesting, since they are co-located on chromosome 16 as a ~5kb cluster (Fig. S1b), suggesting that they are a bicistronic transcript.
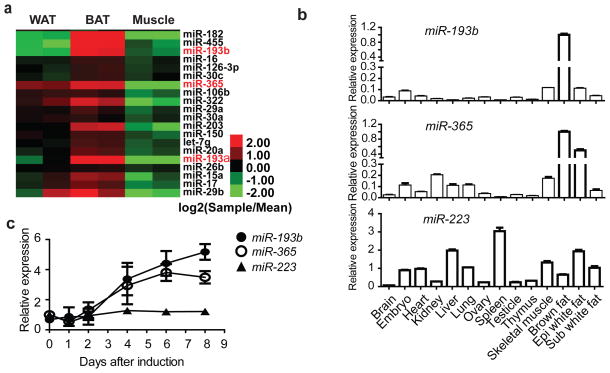
Figure 1. miR-193b-365 is enriched in BAT.
(a), Heat map showing the expression of miRNAs that are enriched in BAT compared to epididymal WAT and skeletal muscle. Red denotes higher and green denotes lower relative to the mean of the six samples for each miRNA. (P < 0.1, ANOVA). (b), Real-time PCR analysis of miR-193b, miR-365, and a control miRNA, miR-223 expression levels in BAT relative to other adult mouse tissues. n=3. (c), Real-time PCR analysis of miR-193b and miR-365 expression levels during adipogenesis of primary brown adipocyte cultures. n=3. Means ± SEM.
Cap-analysis gene expression (CAGE) Basic and Analysis Databases store original results produced by CAGE-seq which measures expression levels of transcription starting sites by sequencing large numbers of 5′ transcript ends, termed CAGE tags 15, 16. We examined the distribution of CAGE tags surrounding the miR-193b and miR-365-1 genes, and found that there was no CAGE tag between miR-193b and miR-365-1 in the direction of transcription (Fig. S1b). Furthermore, we designed 14 pairs of primers across the genomic region of miR-193b-365, and performed RT-PCR to detect the primary transcripts of overlapping segments of the miR-193b-365 gene (Supplementary Fig. S1c). The amplified fragments covered the entire region between miR-193b and miR-365. Together, these data strongly suggest that miR-193b-365 is a co-transcribed miRNA cluster.
We performed real-time PCR to examine the expression of miR-193b and miR-365 in 14 adult mouse tissues (Fig. 1b); both miRNAs were enriched in BAT. We measured their expression levels at different time points during brown adipocyte differentiation of stromal-vascular fraction (SVF) cells from interscapular brown fat (Fig. 1c). Both miRNAs showed significant up-regulation during adipogenesis, ~5-fold for miR-193b and ~4-fold for miR-365. Furthermore, the levels of these two miRNAs were reduced by ~30% in brown fat of ob/ob mice, animals in which brown fat activity was impaired (Fig. S1d) 17. Their levels in BAT were not changed in animals that were exposed to cold temperature (Fig. S1e) or in cell cultures that were treated with cAMP to induce the thermogenesis program (Fig. S1f).
To determine the functions of miR-193b and miR-365 in brown adipocytes, we transfected brown fat SVF cells with locked nucleic acid (LNA) miRNA inhibitors and induced them to differentiate for 4 days. Since miR-193a shares the same seed sequence with miR-193b, the effects of a miR-193a inhibitor was also examined. For each inhibitor, RT-PCR detected a greater than 90% decrease of corresponding miRNA levels (Fig. 2a), which reflects a degradation or sequestration of miRNAs by inhibitors. Next, we performed mRNA microarray analysis to test whether knockdown of miRNAs caused global up-regulation of their targets. As predicted by TargetScanv5.118, 559 and 513 messages are predicted targets of miR-193a/b and miR-365 (context score<−0.2) respectively; 469 and 404, respectively, were expressed above background in our array data. The relative expression of each gene was calculated as a ratio of expression in knockdown vs. control cells, and the cumulative fraction was plotted as a function of the relative expression. The cumulative curve of the miRNA target genes shifted significantly to the right relative to the curve of the control genes that comprise genes without predicted miRNA target sites (Fig. 2b), indicating that miRNA targets, as a group, tended to be upregulated upon miRNA inhibitor transfection. Thus we conclude that these inhibitors functionally block downregulation of target miRNAs in cells. Note that both miR-193a and miR-193b inhibitors caused upregulation of miR-193a/b-targeted mRNAs, suggesting that either inhibitor was sufficient to suppress this miRNA family.
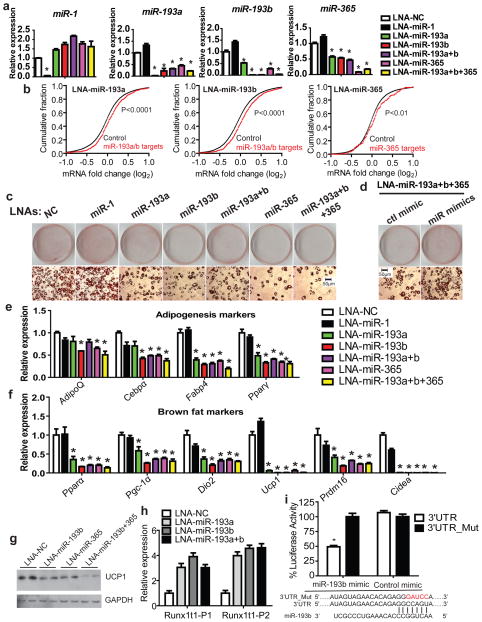
Figure 2. miR-193b-365 is required for brown adipocyte adipogenesis.
(a), SVF cells from brown fat were transfected with LNA miRNA inhibitors (100nM) one day before differentiation. RNAs were harvested at day 4. Real-time PCR was used to examine the expression of these miRNAs. n=3. (b), mRNAs from cultured primary brown adipocytes (Day 4) transfected with each inhibitor or Control inhibitor were analyzed by microarray analysis. On the x-axis is the relative expression of each gene calculated as a log2 ratio (x-axis) between its intensity in the miRNA-inhibited sample and its intensity in the control inhibitor sample. The cumulative fraction (y-axis) was plotted as a function of the relative expression (x-axis). “miRNA targets” (red line) represents the population of genes containing miRNA binding sites predicted by TargetScan, and “Control” (black line) represented all other genes lacking binding sites for the miRNA. The “targets” curve shifts to the right with a P value <0.05 as determined by the one-sided Kolmogorov-Smirnov test, indicating a trend of up-regulation of predicted targets in response to transfection of the miRNA inhibitor. (c), Oil red O staining was used to determine the accumulation of lipid droplets in brown adipocytes (Day 4). (d), SVF cells from brown fat were co-transfected with LNA miRNA inhibitors (100nM) and miRNA mimics (mimic-miR-193a 16.7nM, mimic-miR-193b 16.7nM and mimic-miR-365 16.7nM, or Control mimic 50nM) one day before differentiation. Four days after differentiation, ORO staining was used to determine lipid droplet content. (e), Real-time PCR analysis of the expression of adipogenesis markers and (f), Brown fat markers. n=3. (g), Western blot to examine the expression of Ucp1. (h), Effect of miR-193 knockdown on expression of Runx1t1. Real-time PCR was performed to examine the expression of Runx1t1. Runx1t1-P1 and Runx1t1-P2 represent two sets of PCR primers. n=3. (i), Luciferase reporter assay to examine the interactions between miR-193b and the predicted target site in Runx1t1 3′UTR. Plasmids with the Runx1t1 3′UTRs or mutated UTRs were co-transfected with miR-193b mimic or a control mimic into 293T cells. Renilla luciferase activity was measured by Dual-Glo luciferase assay system and normalized to internal control firefly luciferase activity. n=6. * P < 0.05, Student’s t-test; Means ± SEM.
Blocking miR-193a/b and/or miR-365 but not the control miRNA caused a remarkable reduction in lipid accumulation in brown adipocytes differentiated from SVF cells and brown preadipocytes (Sca-1+/CD45−/CD11b−) purified as described by Tseng laboratory 19 (Fig. 2c, Fig. S2a,b,c). A substantial fraction of transfected cells had a fibroblast-like appearance (Fig. S2d). These effects of the inhibitors could be reversed by co-transfection with miRNA mimics of miR-193a, miR-193b, and miR-365 (Fig. 2d), establishing the specificity of miRNA knockdown. As assayed by real-time PCR, cells transfected with miRNA inhibitors displayed marked down-regulation of adipogenesis markers common in brown and white fat including AdipoQ, Cebpa, Fabp4 and Pparγ (Fig. 2e, Fig. S2c). A more dramatic decrease was observed in expression of several brown fat enriched markers – Ucp1, Pparα, Pgc-1α, Dio2, Prdm16 and Cidea (Fig. 2f, g, Fig. S2c). The reduction of Ucp1 expression was confirmed by Western blotting (Fig. 2g, Fig. S2e). However, the expression of these genes was not altered in mature brown adipocytes transfected with miRNA inhibitors (Fig. S2f), suggesting that these miRNAs perform their primary functions during development and not in mature adipocytes. In addition, miR-193a/b and miR-365 were also required for white fat adipogenesis (Fig. S2h, i), indicating that they were general regulators of adipogenesis with an additional role in supporting the development of brown fat cells.
Among the conserved targets predicted by TargetScanv5.1, Runx1T1 is one of the top candidates of miR-193b. Runx1t1 inhibits brown fat adipogenesis (Fig. S3a-e) and, as reported previously, inhibits white adipogenesis in 3T3-L1 cells 20–22. Upon blocking miR-193a/b, Runx1t1 was upregulated at both the messenger RNA (Fig. 2h) and the protein levels (Fig. S3f). To prove that miR-193b can directly target Runx1t1 mRNA, we cloned the 3′UTR segment of Runx1t1 containing the predicted miR-193b target site (or a mutated seed site) into the psiCHECK-2 vector. Each of the reporter constructs was co-transfected with miR-193b mimic into 293T cells. Luciferase reporter assay showed that miR-193b mimic reduced the activity of the reporter with a wild-type 3′UTR but not the one with mutations in the seed sequences (3′UTR_Mut) (Fig 2. i). This demonstrates that miR-193b directly interacts with the predicted target sites in the Runx1t1 mRNA, and the downregulation of Runx1t1 partially explains the role of miR-193b during adipogenesis.
Because previous studies have shown that brown fat and skeletal muscle share a common developmental origin 13, 14, we suspected that blockage of the brown adipocyte lineage by knockdown of miR-193b and/or miR-365 might switch the fate of brown preadipocytes to the muscle lineage. Indeed, RT-PCR analysis showed that expression of several myogenic markers (Ckm, Myf5, Myf6, Myod and Myog) was significantly induced (Fig. 3a), indicating a tendency towards myogenesis in the inhibitor-transfected brown preadipocytes.
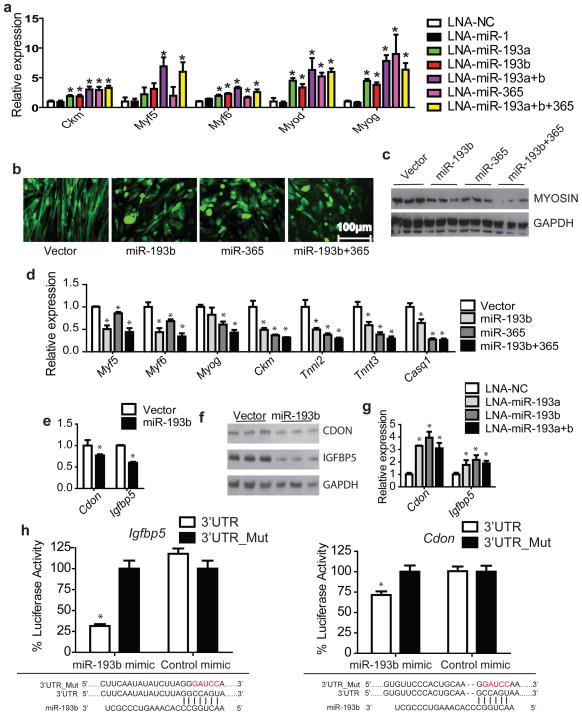
Figure 3. Ectopic expression of miR-193b and/or miR-365 inhibits C2C12 myogenic differentiation.
(a), SVF cells from brown fat were transfected with LNA miRNA inhibitors (100nM) one day before differentiation. At day 4, RNAs were extracted and Real-time PCR was performed to detect the expression of myogenic markers. n=3. (b), Representative micrographs of cells (Day 6 in 2% horse serum) differentiated from C2C12 myoblasts expressing retroviral miR-193b and/or miR-365, or control. GFP was expressed under control of an IRES downstream of the miRNA to visualize transfected cells. (c), Western blot with triplicate biological repeats to examine the expression of myosin upon ectopic expression of miR-193b and/or miR-365. (d), Real-time PCR analysis for expression of muscle markers. n=3. (e), Real-time PCR and (f), Western analysis for predicted miR-193b targets, cell adhesion molecule-related/down-regulated by oncogenes (Cdon) and insulin-like growth factor binding protein 5 (Igfbp5), in C2C12 cells expressing miR-193b or a control vector. n=3. (g), SVF cells from brown fat were transfected with LNA-miR-193a and/or LNA-miR-193b, and differentiated for 4 days. Real-time PCR was performed to examine the expression of Cdon and Igfbp5. n=6. (h), Luciferase reporter assay to examine the interactions between miR-193b and the predicted target site in Cdon and Igfbp5 3′UTR. Plasmids with the Igfbp5 or Cdon 3′UTRs or mutated UTRs were co-transfected with miR-193b mimic or a control mimic into 293T cells. Renilla luciferase activity was measured by Dual-Glo luciferase assay system, normalized to internal control firefly luciferase activity. n=6. * P < 0.05, Student’s t-test; Means ± SEM.
The induction of myogenesis in brown adipocytes could be a direct effect of blocking miR-193b and/or miR-365, or secondary to the inhibition of brown adipogenesis as muscle markers are downregulated during brown fat differentiation 23. To further distinguish among these possibilities, we used retroviral vectors to express miR-193b and/or miR-365 in C2C12 myoblasts which were subsequently induced to differentiate. At day 6, C2C12 cells expressing the control virus differentiated into elongated multinucleated myotube syncytia, whereas cells expressing miR-193b and/or miR-365 failed to differentiate (Fig. 3b, Fig. S4a). Similar results were observed when miR-193a was ectopically expressed (Fig. S4b, c, d). Real-time PCR (Fig. 3d) and Western blot (Fig. 3c, Fig. S4e) analyses confirmed that ectopic expression of either miRNA decreased the mRNA levels of the myogenic markers Myf5, Myf6, Myog, Ckm, Tnni2, Tnnt3 and Casq1, as well as the level of myosin protein. This analysis thus demonstrates that miR-193b-365 can directly repress myogenesis, thereby contributing to the regulation of brown fat versus muscle lineage determination.
Among the conserved targets predicted by TargetScanv5.1 are Cdon (also named Cdo) and Igfbp5, both previously implicated as pro-myogenic factors. Cdon is a cell surface receptor that stimulates post-translational activation of myogenic bHLH factors and E proteins, and increases muscle-specific transcription 24, 25, 26. Knocking down Igfbp5 also impairs myogenic differentiation of C2C12 and primary skeletal muscle cells 27. Real-time PCR and Western blot analysis showed that the levels of both Cdon and Igfbp5 mRNAs and protein were significantly reduced in C2C12 cells expressing miR-193b (Fig. 3e,f, Fig. S4f). We also examined their expression in cultured brown adipocytes transfected with LNA-193a/b inhibitors, and found that their expression was upregulated (Fig. 3g). To further establish that miR-193b directly down-regulates Cdon and Igfbp5 expression, we performed luciferase reporter assay as we did for Runx1t1. As we expected, miR-193b mimic reduces the activities of both Cdon and Igfbp5 reporters with a wild-type 3′UTR but not reporters with mutations in the seed sequences in the 3′UTRs (3′UTR_Muts) (Fig 3h). This demonstrates that miR-193b directly interacts with both predicted target sites.
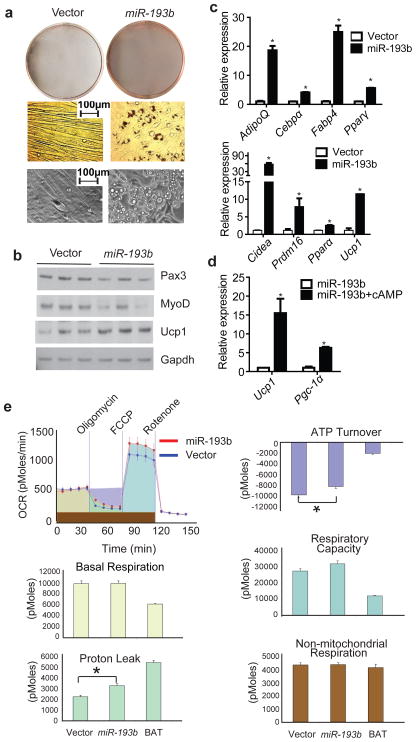
Not only did ectopic expression of miR-193b repress myogenesis by C2C12 myoblasts, it also upregulated the mRNAs encoding two master adipogenic factors Pparγ and Cebpα by ~10 fold and ~2.5 fold respectively (Fig. S5a). Furthermore, when exposed to pro-adipogenic differentiation conditions (detailed in Methods), C2C12 cells expressing miR-193b showed a round, lipid droplet-containing appearance, which resembled the morphology of adipocytes (Fig. 4a). Ectopic expression of miR-193b repressed the expression of myogenic markers Pax3 and MyoD (Fig. 4b, Fig. S5c) while it promoted a significant upregulation of the common adipogenesis markers AdipoQ, Pparγ, Cebpα and Fabp4 as well as brown fat selective markers, Ucp1, Cidea, Prdm16 and Pparα (Fig. 4b, c). Strikingly, the expression of markers such as AdipoQ, Cebpα, Pparγ and Fabp4 was comparable to their levels in cultured brown adipocytes (Fig S5b). The miR-193b-induced adipocytes also displayed an oxygen consumption rate (ORC) profile characteristic of brown adipocytes. Although ORC for basal respiration, non-mitochondrial respiration, and respiration capacity were not changed by miR-193b expression, a significantly higher ORC for proton leakage and a lower ORC for ATP turnover were observed in miR-193b-induced adipocytes (Fig. 4e). Bona fide brown adipocytes should initiate a thermogenesis program in response to beta-adrenergic stimulation, which can be mimicked by cAMP treatment. In response to cAMP treatment, the thermogenic makers, Ucp1 and Pgc-1a, were upregulated by ~15- and ~5-fold respectively in miR-193b-expressing C2C12 cells (Fig. 4d). Thus, miR-193b can induce brown fat adipogenesis in C2C12 myoblast cells.
Figure 4. Ectopic expression of miR-193b induces C2C12 to form brown adipocytes under adipogenic differentiation conditions.
(a), C2C12 cells ectopically expressing miR-193b or control were exposed to pro-adipogenic conditions (described in Methods) for 5 days. ORO staining was used to assess lipid accumulation in cells. Representative micrographs of these cells are depicted (bottom row). (b). Western blot for myogenic markers Pax3, MyoD and brown fat marker Ucp1. n=3. (c), Real-time PCR analysis for expression of common adipogenesis markers (upper row) and brown fat selective markers (bottom row). n=3. (d), C2C12 cells expressing miR-193b (day 6) were stimulated with 500uM dibutyrul cAMP for 4h, and the expression of thermogenic markers, Pgc-1α and Ucp1 was examined by real-time PCR. n=3. (e), The metabolic profile of C2C12 cells expressing miR-193b (day 6) was assessed using the Seahorse XF24 Extracellular Flux Analyzer. A representative curve of the oxygen consumption rates (OCR) of control and miR-193b expressing cells at their basal states and upon treatment with drugs used to dissect the multiple components of the respiration process is plotted in the top-left panel. The parameters analyzed are represented by different colors in the upper panel and quantitated in other panels. In vitro differentiated primary brown adipocytes (BAT) were used as a reference. n=8. * P < 0.05, Student’s t-test; Means ± SEM.
C2C12 myoblasts are known to be able to differentiate into adipocytes28. To exclude the possibility that the adipogenesis of C2C12 cells is due to effects of their transformation, and also to test whether the effects of miR-193b on myoblasts are conserved between mouse and human, we retrovirally expressed miR-193b in primary human myoblasts which were subsequently exposed to myogenic or adipogenic differentiation conditions (detailed in Methods). MiR-193b, but not miR-365 (data not shown), significantly repressed myogenesis (Fig S5d, e, f) and promoted the expression of all 5 adipogenic markers we examined (Fabp4, AipoQ, Cebpa, Lpl, Pparg) (Fig S5g). Together, our studies establish that miR-193b’s functions in repressing myogenesis and promoting adipogenesis are conserved between mouse and human.
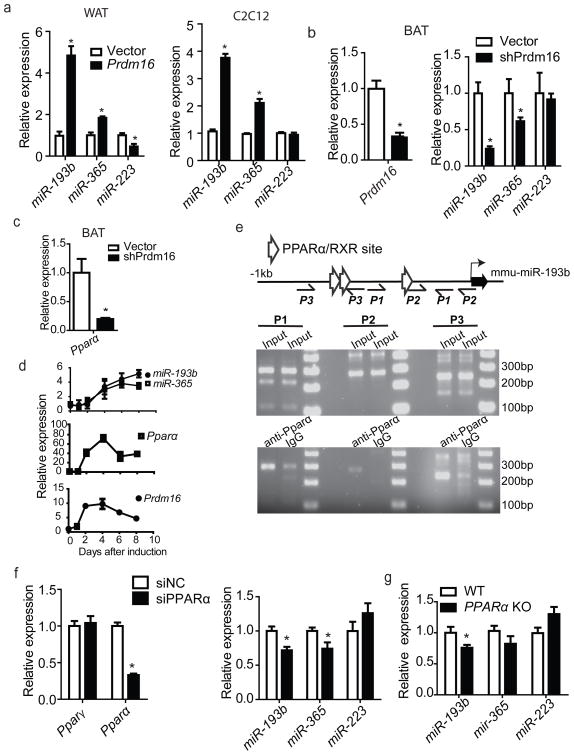
Prdm16 has been reported to be the “master” regulator of BAT lineage determination 13, 14, 29, 30. To determine whether induction of miR-193b-365 is part of the Prdm16-induced program, we used retroviral vectors to introduce Prdm16 into subcutaneous primary white preadipocytes and C2C12 myoblasts. By day 6 of differentiation, both miR-193b and miR365 were significantly upregulated in cells ectopically expressing Prdm16 (Fig. 5a, Fig. S6a, b). Conversely, retroviral shRNA knockdown of Prdm16 in brown adipocyte cultures resulted in ~75% decrease in miR-193b expression and ~50% decrease in miR-365 expression (Fig. 5b). Thus, miR-193b-365 is regulated by Prdm16.
Figure 5. miR-193b-365 is regulated by Prdm16.
(a), Subcutaneous white pre-adipocytes (left) and C2C12 myoblasts (right) were infected by retrovirus expressing Prdm16 two days before differentiation. 5 days after differentiation, real-time PCR were performed to examine the expression of miR-193b, miR-365, and as a control miR-223. n=3. (b), Primary brown preadipocytes were infected by retrovirus expressing shRNA targeting Prdm16 two days before differentiation. By D5 of differentiation, real-time PCR was used to examine the mRNA level of Prdm16 (left), and miR-193b, miR-365, and the control miR-223 (right). n=3. (c), Real-time PCR analysis for Pparα in primary brown adipocytes retrovirally expressing shRNA for Prdm16. (d), Real-time PCR analysis of miR-193b, miR-365, Prdm16 and Ppara expression levels during adipogenesis of primary brown adipocyte cultures. n=3. (e), ChIP analysis for the interaction between Ppara and the promoter region of miR-193b-365. Immortalized brown adipocyte cultures (Day 5) were fixed and sheared by ultrasonication. Immunoprecipitation was performed with anti-Pparα and control IgG. Recovered DNA was amplified by 3 pairs (P1, P2 and P3) of primers designed to span the 1Kb promoter region. Input samples are in the top panel and immunoprecipitated ones in the bottom. (f), Primary brown preadipocytes were transfected with siRNA targeting Pparα, and differentiated for 3 days. RT- PCR was performed to examine the expression of Pparα, and miR-193b and miR-365. Pparγand miR-223 were used as controls. n=3. (g), RT-PCR was performed to examine the expression of miRNAs in brown adipose tissue isolated from Pparα knockout mice (8 week old, Male). Age-matched wild- type mice were used as control. n=6. * P < 0.05, Student’s t-test; Means ± SEM.
Sequence analysis of the promoter region of miR-193b-365 by ExPlain 3.0 of BIOBASE predicted three Pparα/RXR binding sites within the 1 KB segment upstream of miR-193b (Fig. 5e). Pparα is a brown fat-selective transcriptional factor and is important for fatty acid oxidation 31, 32 and suppressing expression of muscle-associated proteins in brown adipose tissue 33. Interestingly, the expression of Pparα could be induced by ectopic expression of Prdm16 in primary white preadipocytes (Fig. S6a) and Pparγ-deficient mouse embryonic fibroblasts 29. Pparα can also directly interact with Prdm16 13. Thus, we hypothesized that Prdm16 might regulate miR-193b-365 through Pparα. To test this hypothesis, we knocked down Prdm16 in primary brown adipocyte cultures, which resulted in about 80% decrease of Pparα mRNA (Fig. 5c). Consistently, the induction of Prdm16 and Pparα was slightly prior to the induction of miR-193b-365 during brown fat adipogenesis (Fig. 5d). A direct interaction between Pparα and the promoter region of miR-193b-365 was confirmed by chromatin immunoprecipitation (ChIP) (Fig. 5e). In addition, a moderate but statistically significant down-regulation of miR-193b and miR-365 was observed in primary brown adipocyte cultures transfected with Pparα siRNA (Fig. 5f) and in brown adipose tissues from Pparα knockout compared to wild- type mice (Fig. 5g). To rigorously prove that Prdm16-Ppara regulates miR-193b-365 at the transcriptional level, we used Real-time PCR to examine the levels of pri-miR-193b-365 and found that the pri-miRNA was regulated similarly as mature miR-193b and miR-365 by Prdm16 and Ppara (Fig. S6c-h). Together, these data suggest that Prdm16 can induce miR-193b-365 expression at least partially by inducing expression of Pparα.
Since ectopic expression of miR-193b promotes the expression of Prdm16 (Fig. 4c) and since blocking miR-193b in brown preadipocytes results in a down-regulation of Prdm16 (Fig. 2f), we hypothesize a feed-forward loop that ensures differentiation of brown adipocytes from bipotential brown adipocyte/myocyte progenitors (Fig S6m). To test whether the effects of miR-193b on brown fat adipogenesis can be solely attributed to its effects on Prdm16, we blocked miR-193b-365 in brown adipocyte cultures ectopically expressing Prdm16. Overexpression of Prdm16 in brown preadipocytes was not sufficient to fully rescue the deficiency in adipogenesis due to a blockage of miR-193a/b or miR-365 (Fig. S6i-l). Thus, miR-193b likely regulates more factors additional to Prdm16 to control brown fat adipogenesis.
To the best of our knowledge, the present work is the first study to investigate the functions of miRNA in brown fat specification, and our results have revealed another layer of regulation of brown fat lineage determination. Further functional characterization of miR-193b and miR-365 in animal models and better understanding of the mechanisms regulating brown fat development may lead to identification of novel therapeutic targets and strategies against obesity and related metabolic disorders.
METHODS
Cell cultures
Primary brown adipocytes and SVF cells were fractionated and cultured according to published methods with few modifications 34, 35. Briefly, 2 week old C57BL/6 mice were sacrificed. Interscapular BAT were harvested and digested with collagenase (0.2%). SVF cells were collected by centrifugation, red blood cells were lysated with NH4Cl and then SVF were filtered through 40uM. Primary white SVF cells and adipocytes from subcutaneous fat were isolated and fractionated according to published methods 36. Primary SVF cells were cultured to confluence in DMEM with 10% New Born Calf Serum (Invitrogen) and induced to differentiate for 2 days with DMEM containing 10%FBS DMEM, Insulin 850nM (Sigma), Dexamethasone 0.5uM (Sigma), IBMX 250uM (Sigma), Rosiglitazone 1uM (Cayman Chemical), T3 1nM (Sigma), and Indomethacin 125nM (Sigma). The induction medium was replaced with DMEM containing 10% FBS and 160nM insulin for 2 day. Then cells were incubated in DMEM with 10% FBS.
The immortalized brown adipocyte cell line used for ChIP was a generous gift from the Dr. Ronald Kahn lab. Cells were cultured to confluence then exposed to differentiation media: 10%FBS DMEM, Insulin 20nM (Sigma), Dexamethasone 0.5uM (Sigma), IBMX 250uM (Sigma), T3 1nM (Sigma), and Indomethacin 125nM (Sigma) for 2 days. Cells were then switched to media containing 10%FBS, T3 (1nM) and Insulin (20nM).
C2C12 and HEK 293Tcells from the American Type Culture Collection (ATCC) were cultured or differentiated according to manufacturer’s instructions.
Primary human myoblasts were purchased from ZenBio. Cells were cultured and differentiated according to manufacturer’s instructions.
Animal studies
Male C57BL/6J mice, Pparα knockout mice (B6:129S4) and ob/ob mice (000632) from the Jackson Laboratory were maintained at the animal facility of the Whitehead Institute for Biomedical Research. All animal experiments were performed with the approval of the Massachusetts Institute of Technology Committee on Animal Care. Epididymal WAT, interscapular BAT and skeletal muscle from 12 week old mice were harvested after perfusion for miRNA expression profiling and RT-PCR. Tissues from 4–5 mice were pooled for each RNA sample preparation. Brown fat of Pparα knockout mice was harvested from 8 weeks old mice (male), and age-matched wild-type mice were used as a control. 8 week old male C57BL/6 mice were kept in 4oC for 24 hours to stimulate brown fat thermogenesis.
Plasmids and Viral Vectors
Authentic miRNA stem-loop and ~ 220 nucleotide flanking sequences on the 5′ and 3′ side of the mmu-miR-193a/b and mmu-miR-365-1 gene were amplified from normal mouse genomic DNA (Clontech) and cloned into a retroviral vector containing IRES-GFP as described previously 37 for retrovirus production. The Prdm16 retroviral construct (15504), Prdm16-shRNA retroviral construct (15505), and Pparg2 retroviral construct (8859) were purchased from Addgene. Runx1t1 and its GFP vector control constructs are generous gift from Rochford’s lab. Retroviral package vectors pcl-ECO and pcl-10A1 are from IMGENEX.
To generate reporter constructs for luciferase assays, about 400 bp segments containing predicted miRNA target sites in the 3′UTRs of Runx1t1, Cdon and Igfbp5 were cloned into the psiCHECK-2 vector (Promega) between the XhoI and NotI sites immediately downstream of the Renilla luciferase gene. To generate reporters with mutant 3′UTRs, five nucleotides (CCAGT) in the target site complementary to the position 2–6 of miR-193b seed region were mutated to GATCC by a QuikChange Site-Directed Mutagenesis kit according to the manufacturer’s protocol (Stratagene).
Induction of brown fat adipogenesis in myoblasts
To induce miR-193b-expressing myoblasts for adipogenesis, C2C12 myoblasts or primary human myoblasts were infected as described below, cultured to confluence, and then exposed to brown adipocyte differentiation conditions: 10%FBS DMEM, Insulin 850nM (Sigma), Dexamethasone 0.5uM (Sigma), IBMX 250uM (Sigma), Rosiglitazone 1uM (Cayman Chemical), T3 1nM (Sigma), and Indomethacin 125nM (Sigma). After 2 days, cells were incubated in culture medium containing insulin 160nM and Rosiglitazone 1uM for another 2 days, and then were switched to 10% FBS DMEM. To stimulate thermogenesis, cells were incubated with dibutyryl cAMP 0.5mM (Sigma) for 4 h.
FACS sorting
Brown fat SVF cells were isolated as described above. SVF cells were stained with a FITC-conjugated CD45 antibody (Ebioscience), APC-conjugated CD11b (Ebioscience) antibody and PE-conjugated Sca1 antibody (Ebioscience) for 15 mins on ice. Cells were washed twice and then resuspended in PBS (2% FBS, PI). FACS sorting was performed at Whitehead Institute core facility to enriched CD45− CD11b−Sca1+ preadipocytes.
Retrovirus production and Infection
Empty vector or expression plasmid (8 μg) was co-transfected with retrovirus packaging vector pCL-Eco (4 μg) into 80% confluent 293T cells (100mm plate) using FuGene 6 according to the manufacturer’s protocol (Roche). Virus supernatant was collected 2 days after transfection.
Primary white and brown fat SVF cells and C2C12 myoblasts were cultured to 30–50% confluence and infected with retrovirus in the presence of 4 μg/ml polybrene (Sigma). Infection efficiency was generally higher than 80%, as judged by GFP expression when viewed under the microscope or by FACS analysis.
Luciferase Reporter assay
293T cells were seeded in 96-well white assay plates (Corning) at a density of 30,000 cells per well one day before transfection. Ten nanograms of each reporter construct were co-transfected with miR-193b mimic or a mimic control (Dharmacon) at final concentration 50nM into 293T cells using Lipofectamine 2000 according to the manufacturer’s protocol (Invitrogen). After 48 hours, firefly and Renilla luciferase activities were measured with the Dual-Glo luciferase assay system according to the manufacturer’s instructions (Promega).
Transfection of LNA miRNA inhibitor and siRNAs
When cultured SVF cells or sorted preadipocytes were grown to 70–80% confluence, Locked Nucleic Acids (LNAs) miRNA inhibitors (100nM) or anti-Pparα siRNA (200nM) were transfected by DharmaFect 2 (6ul/ml) according to the manufacturer’s instruction. 24 hrs after transfection, cells were recovered in full culture media and grown to confluence for differentiation. LNAs inhibitors were from Exiqon, LNA-193b (139676-00), LNA-365 (139215-00), LNA-193 (139481-00) and LNA-Ctl (199002-08). siRNAs for Pparα were from Invitrogen, MSS207857 and negative control (12935300)
miRNA microarray and data analysis
miRNA microarray and analysis were performed as previously described 38. Specifically, microarrays with miRNA content corresponding to miRBase v10.1 (six probes for each miRNA on one chip) were used. After global normalization, miRNAs with an intensity more than 500 arbitrary units were considered as expressed. Differentially regulated miRNAs were identified by one-way ANOVA analysis (P <0.1). 91 miRNAs expressed in at least one sample and differentially expressed between three tissues were selected for clustering analysis. Unsupervised hierarchical clustering was performed with average linkage and uncentered correlation as the similarity metric using Cluster3.0 39. The heat map was generated in Java Treeview. Accession number: E-MEXP-2553 (ArrayExpress).
mRNA microarray and data analysis
Total mRNA was obtained from primary brown adipocytes transfected with miRNA inhibitor or Control inhibitor. Affymetrix arrays were performed by the Genome Technology Core at Whitehead Institute. Microarray data were preprocessed with RMA from the Affymetrix package in Bioconductor using custom probeset definitions for NCBI Entrez Genes derived from previous method40. To reduce data noise, genes with intensities below threshold (log2(Value)<3, arbitrary units) were removed from further analysis. TargetScan v5.1 18 was used for predicting miRNA targets. Relative expression of each gene was calculated as the log2 ratio between the intensity of the mRNA in the miRNA inhibitor sample relative to the intensity of the control inhibitor sample. The cumulative fraction was plotted as a function of the relative expression. The cumulative curves for both miRNA targets and the genes without miRNA binding sites were plotted, and one-sided Kolmogorov-Smirnov test was performed as the statistical test. mRNA microarray data have been uploaded to GEO GSE27614.
Quantitative real-time PCR assay and Western blot
RT PCR for miRNA and mRNA was performed and analyzed as previously described 38. Primer sequences are listed in Supplementary Table SI. 18S was used as an internal control.
For Western blot analyses, cells or tissues were lysed in RIPA buffer (0.5% NP-40, 0.1% SDS, 150 m M NaCl, 50 mM Tris-Cl (pH 7.5)). Proteins were separated by SDS–PAGE, transferred to Nitrocellulose membrane (Millipore) and probed with anti-Cdon (AF2429, R&D system), anti-Igfbp5 (AF578, R&D system), Anti-Ucp1 (ab10983, Abcam), anti-MyoD (SC-760, Santa Cruz), anti-PAX3 (ab15717, Abcam), anti-Runx1t1 (SC-9737, Santa Cruz),and anti-Myosin (MF20, Developmental Studies Hybridoma Bank) antibodies.
Chromatin immunoprecipitation
Immortalized brown fat cell culture cells (3×10^7 cells per sample) were fixed with formaldehyde at 1% final concentration for 20 minutes. The cross-linking was quenched with ice-cold 2.5M glycine, and the cells were rinsed twice with 1x PBS. Cross-linked cells were resuspended, then lysed with detergent to separate the nuclear pellet which was resuspended in 3ml sonication buffer (50mM Tris HCl, 140mM NaCl, 1mM EDTA, 1% Triton-X 100, 0.1% Na-deoxycholate, 0.1% SDS), and then sonicated to shear the cross-linked DNA to an optimal fragment size ranging from 200 to 500 bp. Sonication was performed using a Branson sonifier (at Power 4.5 for 24W at maximum power) for nine 20-second pulses (with a 60-second pause between pulses). Simultaneously, magnetic protein-G beads (Dynal) were incubated with 20ug polyclonal antibody against Pparα (SC-9000X, Santa Cruz) or 20ug IgG control (rat IgG #012-000-003 from Jackson) for 6 hours on ice. An aliquot (1/600) of the sonicated nuclear pellet was set aside as the whole cell extract control (WCE) and then taken through the rest of the protocol exactly as the immunoprecipitated (IP) fraction. The remainder (about 3ml) of the sonication sample was combined with the preincubated beads and this immunoprecipitation was run overnight at 4 degrees C. The beads were then collected with a magnet and washed with RIPA buffer 6 times. Bound DNA-protein complexes were eluted from the beads by reversal of cross-linking through heating to 65 degrees C overnight. The enriched IP and unenriched WCE DNA fragments were purified using RNAse A and proteinase K incubation, phenol-chloroform extraction, and then ethanol precipitation. The resulting DNA was then used for gene-specific PCR.
Measurements of oxygen consumption and extracellular acidification rates
C2C12 cells were seeded (5,000 cells/well) into gelatin-coated XF24 V7 cell culture microplates (Seahorse Bioscience) and cultured overnight at 37°C with 5% CO2. The next day, cells were transduced with retroviruses expressing miR-193b or empty vector in the presence of polybrene, cultured to confluence, and induced to differentiate with a brown adipocyte cocktail. As a control, primary brown preadipocytes were isolated, seeded into gelatin-coated XF24V7 cell culture microplates, and differentiated into mature brown adipocytes. At day 6 of differentiation, the media was replaced with prewarmed XF24 assay media for 1 h; this unbuffered media consisted of DMEM (Sigma), 1 mM Glutamax-1 (Invitrogen), 2 mM pyruvate, 141 mM NaCl, and 25 mM glucose. Using the Seahorse Bioscience XF24-3 Extracellular Flux Analyzer, theO2 tension immediately around the cells was measured by optical fluorescent biosensors embedded in a sterile disposable cartridge placed into the wells of the microplate during the assay. O2 consumption rate (OCR) was calculated by plotting the O2 tension of the media in the microenvironment above the cells as a function of time (pmoles/min). OCR was measured during 4 min cycles repeated four times at 10-min intervals. Firstly, basal respiration was assessed in untreated cells. Secondly, ATP turnover was calculated in response to oligomycin (Calbiochem) treatment (10 μM). Maximum respiratory capacity was assessed after FCCP (Sigma) stimulation (1 μM). Finally, mitochondrial respiration was blocked by Rotenone (Sigma) (1 μM) and the residual OCR was considered non-mitochondrial respiration. Proton leak was calculated by subtracting the ATP turnover and the non-mitochondrial respiration components of basal respiration.
Statistical analysis
Data are expressed as means ± SE unless otherwise indicated. Student’s t test (unpaired, two-tailed) was used to compare the two groups, and the P value was calculated in Excel (Microsoft) or GraphPad Prism 5 (GraphPad Software). P < 0.05 was considered as statistically significant.
Supplementary Material
Acknowledgments
This work is supported by NIH grants DK047618, DK 068348, DK076848, and 5P01 HL066105, grant C-382-641-001-091 from the Singapore-MIT Alliance (SMA) and a graduate fellowship from SMA. Thanks for intellectual support, materials, and advice from members of the laboratories of Drs. Patrick Seale, David Bartel, Charles Emerson, and from all members of the Lodish laboratory. Thanks for Rochford’s lab for their generous gifts of Runx1t1 plasmid.
Footnotes
AUTHOR CONTRIBUTIONS
L.S, H.X and H.F.L conceived the project and designed the experiments. L.S, H.X, M.A.M, R.A, B.Y, S.M.H and Q.L performed the experiments. All authors analyzed data. L.S, H.X, M.A.M, H.F.L wrote the manuscript. C.R.K and H.F.L supervised the project.
COMPETING GINANCIAL INTERESTS
The authors declare no financial conflict of interest.
Accession number:
E-MEXP-2553; GSE27614
References
- 1.Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes Dev. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 5.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 6.Connolly E, Morrisey RD, Carnie JA. The effect of interscapular brown adipose tissue removal on body-weight and cold response in the mouse. Br J Nutr. 1982;47:653–658. doi: 10.1079/bjn19820077. [DOI] [PubMed] [Google Scholar]
- 7.Lowell BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 8.Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology. 1996;137:21–29. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- 9.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. Ucp1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopecky J, et al. Reduction of dietary obesity in aP2-Ucp transgenic mice: mechanism and adipose tissue morphology. Am J Physiol. 1996;270:E776–786. doi: 10.1152/ajpendo.1996.270.5.E776. [DOI] [PubMed] [Google Scholar]
- 12.Bartelt A, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 13.Seale P, et al. Prdm16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajimura S, et al. Initiation of myoblast to brown fat switch by a Prdm16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaji H, et al. CAGE Basic/Analysis Databases: the CAGE resource for comprehensive promoter analysis. Nucleic Acids Res. 2006;34:D632–636. doi: 10.1093/nar/gkj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carninci P, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 17.Hogan S, Himms-Hagen J. Abnormal brown adipose tissue in obese (ob/ob) mice: response to acclimation to cold. Am J Physiol. 1980;239:E301–E309. doi: 10.1152/ajpendo.1980.239.4.E301. [DOI] [PubMed] [Google Scholar]
- 18.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz TJ, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2010;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne VA, et al. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem J. 2009;425:215–223. doi: 10.1042/BJ20091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumberg JM, et al. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol Chem. 2006;281:11205–11213. doi: 10.1074/jbc.M510343200. [DOI] [PubMed] [Google Scholar]
- 22.Rochford JJ, et al. ETO/MTG8 is an inhibitor of C/EBPbeta activity and a regulator of early adipogenesis. Mol Cell Biol. 2004;24:9863–9872. doi: 10.1128/MCB.24.22.9863-9872.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmons JA, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole F, Zhang W, Geyra A, Kang JS, Krauss RS. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev Cell. 2004;7:843–854. doi: 10.1016/j.devcel.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Kang JS, Mulieri PJ, Miller C, Sassoon DA, Krauss RS. CDO, a robo-related cell surface protein that mediates myogenic differentiation. J Cell Biol. 1998;143:403–413. doi: 10.1083/jcb.143.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang JS, et al. A Cdo-Bnip-2-Cdc42 signaling pathway regulates p38alpha/beta MAPK activity and myogenic differentiation. J Cell Biol. 2008;182:497–507. doi: 10.1083/jcb.200801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren H, Yin P, Duan C. IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J Cell Biol. 2008;182:979–991. doi: 10.1083/jcb.200712110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teboul L, et al. Thiazolidinediones and fatty acids convert myogenic cells into adipose-like cells. J Biol Chem. 1995;270:28183–28187. doi: 10.1074/jbc.270.47.28183. [DOI] [PubMed] [Google Scholar]
- 29.Seale P, et al. Transcriptional control of brown fat determination by Prdm16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kajimura S, et al. Regulation of the brown and white fat gene programs through a Prdm16/CtBP transcriptional complex. Genes Dev. 2008;22:1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbera MJ, et al. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276:1486–1493. doi: 10.1074/jbc.M006246200. [DOI] [PubMed] [Google Scholar]
- 32.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 33.Tong Y, et al. Suppression of expression of muscle-associated proteins by PPARalpha in brown adipose tissue. Biochem Biophys Res Commun. 2005;336:76–83. doi: 10.1016/j.bbrc.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 34.Cannon B, Nedergaard J. Cultures of adipose precursor cells from brown adipose tissue and of clonal brown-adipocyte-like cell lines. Methods Mol Biol. 2001;155:213–224. doi: 10.1385/1-59259-231-7:213. [DOI] [PubMed] [Google Scholar]
- 35.Tseng YH, Ueki K, Kriauciunas KM, Kahn CR. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J Biol Chem. 2002;277:31601–31611. doi: 10.1074/jbc.M202932200. [DOI] [PubMed] [Google Scholar]
- 36.Rodbell M. Metabolism of Isolated Fat Cells. I. Effects of Hormones on Glucose Metabolism and Lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 37.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 38.Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes. 2009;58:1050–1057. doi: 10.2337/db08-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 40.Dai M, et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.