Abstract
Background
While it has been postulated that allergic disease is associated with a predominance of Th2 cells, whether IgE levels and asthma might differ in their relations with early life cytokine production is not known.
Objective
To assess relations between first year adaptive immune cytokine production with asthma and total IgE levels through age 5 years in a non-selected birth cohort.
Methods
Mitogen (ConA/PMA) stimulated IL-4, IL-5, IL-13 and IFN-γ were measured in supernatants from cord and peripheral blood mononuclear cells at birth, 3 months and 12 months. Total serum IgE and physician-diagnosed active asthma were assessed at 1, 2, 3 and 5 years. Longitudinal models that adjust for both Th1 and Th2 cytokine production were used to determine relations of outcomes.
Results
Relations of cytokines with total IgE and asthma were strikingly different. Total IgE through age 5 years was positively associated with 12-month IL-4 (p < 0.001), IL-5 (p < 0.001) and IL-13 (p = 0.02) levels when adjusted for IFN-γ, and inversely associated with 12-month IFN-γ after IL-4 adjustment (p = 0.01). Active asthma through age 5 was positively associated with 3-month IL-13 adjusted for IFN-γ (Odds ratio (OR) = 2.6, p <0.001), and inversely associated with 3-month IFN-γ adjusted for IL-13 (OR = 0.5, p = 0.001). These relations were strongest for non-atopic asthma.
Conclusion
Total IgE and active asthma through age 5 are associated with adaptive cytokine production in early life, though relations vary temporally and with regard to the relative importance of individual cytokines.
Keywords: Asthma, IgE, Cytokines, IL-4, IL-5, IL-13, IFN-γ, children
INTRODUCTION
According to the classical Th1/Th2 paradigm, a healthy immune system derives in part from a balance between cell production of Th1 and Th2 type cytokines, while manifestations of allergy including those that accompany asthma are associated with a predominance of Th2 type cytokines. Although several more recently described cell types and regulatory mechanisms indicate that additional factors may contribute toward maintaining the balance, the paradigm of a Th2 shift, per se, remains a hallmark of allergic disease (reviewed by Jutel and Adkis)1. This concept developed following the demonstration of differential cytokine production among murine derived T cell clones,2 and subsequent studies with human cells and animal models demonstrating separate roles for the cytokines produced by these cell types (reviewed by Street and Mosmann).3 Further murine and human in vitro studies of the T-helper cell paradigm have demonstrated that the Th1 cytokine IFN-γ and the Th2 cytokine IL-4 act to down-regulate one another (reviewed by Paludan),4 and thus an inverse association between Th2 and Th1 cytokine production might be expected.
However, we and others have recently shown that that mitogen stimulated production of Th1 and Th2 cytokines is strongly positively correlated for individuals in a general population sample.5, 6 This strong correlation suggests that any skewing among allergic individuals may be quite subtle. Further, associations of Th2 cytokines with allergic outcomes may either be due to high absolute amounts of a particular cytokine, or to high production of Th2 cytokines relative to production of Th1 cytokines. Thus, from a methodological standpoint, assessment of the effects of Th1 and Th2 cytokines through models adjusted for one another may provide more information than considering a single cytokine in isolation.
The close association of asthma with circulating IgE levels in humans7 together with all of the animal research implicating IgE and/or Th2 mechanisms as driving allergic inflammation in airways has produced the implicit assumption that asthma is driven, at least in part, by IgE, and that IgE is driven by Th2 cytokines. Many of the studies that are presumed to support these relations have examined relations with allergy outcomes occurring concurrently, but such studies do not provide information on temporal relations and may not capture fluctuations in symptoms or age-related effects. It is clear that Th2 cytokines are involved in immunoglobulin class switch, but in relation to asthma they may also have direct effects on the airways. Thus, cytokine production in early life may be influential, either by initiating these processes or by marking individuals in whom these processes have already been initiated, thereby having different relations with IgE levels and asthma. Unfortunately, information about relations between early cytokine production during or prior to development of disease in unselected populations is limited.
This study assessed relations between first year cytokine production and both development of asthma and total IgE levels in childhood, comparing results from single cytokine analyses to those from models that adjust for both Th1 and Th2 cytokines. We hypothesized that if elevated levels of IgE develop separately from, rather than drive, asthma (as has been suggested),8, 9 early cytokine production might be differentially related to these outcomes, either temporally or in the relative importance of individual cytokines. In consideration of previous reports of strong positive correlation, these relations might be most evident in models that adjust for both Th1 and Th2 cytokines.
METHODS
Study design and population
The Infant Immune Study (IIS) is a prospective birth cohort study of immune system maturation and its relation to the development of asthma and allergic disease in childhood. Participants were healthy children born to women who planned to obtain care for their newborns from collaborating pediatricians, as previously described.10
Cytokine measurements
Blood specimens were obtained at birth (from the umbilical cord), and by venipuncture at 3 months (2.9 ± 1.2 months; n=363) and 1 year (1.1 ± 0.1 years; n=364). Lymphocyte stimulations were performed on peripheral and cord blood mononuclear cells separated by standard density gradient centrifugation techniques as previously described.6 Cells were stimulated with 10 μg/ml Concanavalin A (ConA; Pharmacia, Piscataway, NJ) and 10 ng/ml phorbol myristate acetate (PMA; Sigma Chemical Co., St. Louis, MO). Supernatant fluids from these cultures and unstimulated controls were harvested after 18 to 24 hours and stored at −70°C for later cytokine testing. The supernatants were assayed for IL-4, IL-5, IL-13, and IFN-γ production using commercially available kits (Genzyme, Minneapolis, MN). Cytokines with more than 20% undetectable values at a given age were treated as dichotomous variables (detectable vs. undetectable). All other cytokines were treated as continuous, with undetectable values being assigned the value at the limit of detection (0.25 pg/mL for IL-4, 7.8 pg/mL for IL-5, 3.13 pg/mL for IL-13, and 15.6 pg/mL for IFN-γ).
Outcome measurements
Total and allergen specific IgE were measured from blood specimens obtained at 1 year (1.1 ± 0.1 years; n=364), 2 years (2.1 ± 0.2 years; n=310), 3 years (3.2 ± 0.3 years; n=295), and 5 years (5.1 ± 0.4 years, n=277), using the Pharmacia AutoCAP assay (Pharmacia/Upjohn, Kalamazoo, Mich) until its discontinuation in 2006 and subsequently using Immulite 2000 (Siemens Medical Solutions, Los Angeles, Calif). Samples analyzed on both instruments (n = 25) yielded a correlation coefficient of 0.995. Specific IgE levels were measured for 6 inhalant allergens (Alternaria species, Dermatophagoides farinae, Bermuda grass, careless weed, olive and mulberry tree) and 2 foods (ovalbumin and β-lactoglobulin). Each allergen-specific IgE was categorized as detectable or undetectable at each age based on a cut-off of 0.25 IU/mL, with atopy defined as detectable specific IgE for one or more allergens at any age. Children were defined as non-atopic if they had at least one negative at one or more ages, and were never positive.
Asthma was assessed by parent-completed questionnaires at ages 1 year (1.1 ± 0.1 years; n=423), 2 years (2.1 ± 0.2 years; n=362), 3 years (3.2 ± 0.3 years; n=371), and 5 years (5.1 ± 0.4 years; n=373). Specifically, parents were asked if their child ever had asthma, at what age their symptoms last occurred, and at what age they were first told by a doctor that the child had asthma. For this analysis, asthma was defined at each age as physician diagnosed asthma with reported symptoms in the last year.
Statistical analysis
Total IgE and amounts of cytokines produced were log transformed for all analyses. Log transformed cytokines were converted to z-scores for comparison of effect sizes across cytokines.
Longitudinal relations between total IgE over 1, 2, 3 and 5 years and cytokine production at each of 3 time-points (birth, 3 and 12 months) were assessed using mixed models, treating each subject as a random effect to control for within-subject correlation. In each model, age at the time of total IgE measurement was included as a categorical variable since log total IgE tends to increase with age in childhood but in a non-linear fashion. In addition, age by cytokine-level interaction terms were included to allow differing effects between cytokine levels and total IgE at each age. Regression coefficients were calculated for change in total IgE per one standard deviation in logged units of each cytokine.
Longitudinal relations between cytokine levels and physician diagnosed, active asthma were assessed using Generalized Estimating Equations (GEE) to determine odds of asthma associated with one standard deviation change in production of each cytokine. As with longitudinal models for total IgE, GEE models for asthma were run both with age-by-cytokine-level interaction terms to calculate the effect of cytokine levels at each age of asthma assessment, and without interaction terms in order to assess an overall effect of cytokine levels on asthma over ages 1 through 5. Finally, asthma-cytokine analyses were stratified by specific IgE detectability in the first 5 years in order to assess the possibility of differing relations of early cytokine production to atopic vs. non-atopic asthma.
Each of the analyses described above was performed using two approaches, designated as unadjusted and adjusted models. For unadjusted models, individual cytokines were entered into each model with no covariates other than age. Adjusted models include IFN-γ and one of 3 Th2 cytokines (IL-4, IL-5, or IL-13) together as covariates.
All analyses were conducted using Stata version 10.0. This research was approved by the Institutional Review Board of the University of Arizona and informed consent was obtained for all subjects.
RESULTS
Cytokine production from mitogen-stimulated blood mononuclear cells were measured for 450 children at least once in the first year of life (birth: n =295; 3 months: n = 323; or 1 year: n = 318). Children with cytokine data did not differ significantly from children who lacked cytokine data (n=34) with respect to gender, ethnicity, household income, day-care attendance, parental atopy or parental asthma status. Of the 450 children with cytokine data, total IgE was available at 1, 2, 3 and/or 5 years for 396 children (88.0%), and asthma data at 1, 2, 3 and/or 5 years for 425 children (94.4%).
Cytokines
Cytokine production at birth and at 3 months in this cohort has been described in detail.6 Table I provides a summary of descriptive statistics for production of IL-4, IL-5, IL-13 and IFN-γ at birth, 3 months and 12 months. Of note, production of all cytokines increased significantly with age from two to ten-fold by one year, with the exception of IL-13, which decreased between birth and 3 months (P < 0.001), and then increased between 3 and 12 months (P < 0.001).
Table I.
Median, range, geometric mean and 95% confidence interval (in pg/mL) for cytokine production at birth, 3 and 12 months.
| Birth | 3 months | 12 months | ||||
|---|---|---|---|---|---|---|
| Median (Range) | Geometric Mean (95% CI) | Median (Range) | Geometric Mean (95% CI) | Median (Range) | Geometric Mean (95% CI) | |
| IL-4 | 0.52 (0.25, 5.07) | * | 1.00 (0.25, 19.3) | 1.01 (0.91, 1.12) | 2.30 (0.25, 30.8) | 2.00 (1.80, 2.23) |
| IL-5 | 7.8 (7.8, 460) | * | 7.8 (7.8, 545) | * | 74.2 (7.8, 962) | 68.0 (60.2, 78.8) |
| IL-13 | 154 (3.13, 1380) | 138 (123, 154) | 123 (3.13, 4210) | 116 (106, 126) | 192 (3.13, 1400) | 173 (157, 191) |
| IFN-γ | 566 (15.6, 15300) | 409 (349, 480) | 774 (15.6, 33500) | 612 (533, 703) | 1470 (15.6, 23000) | 1080 (915, 1270) |
Abbreviation: CI (Confidence Interval)
Less than 80% detectability. Treated as dichotomous (undetectable/detectable) for all analyses.
Outcomes
Geometric mean total IgE levels increased with age (1 year: 8.8 IU/mL; 2 years: 14.8 IU/mL; 3 years: 16.8 IU/mL; 5 years: 22.3 IU/mL). Similarly, prevalence of active asthma increased with age (1 year: 3.2% (13/407); 2 years: 5.2% (18/348); 3 years: 8.1% (29/358); 5 years: 12.3% (44/357)).
Relations between 1st year cytokines and outcomes
Total IgE
Table II shows regression coefficients for total IgE through age 5 years in relation to cytokine production at 3 and 12 months using longitudinal models. Three-month IL-4 levels significantly predicted subsequent IgE levels in the unadjusted model, and this relation was stronger in the IFN-γ-adjusted model. Of note, adjustment with IL-4 revealed an inverse relation between 3-month IFN-γ and total IgE that was not evident prior to adjustment.
Table II.
Regression coefficients for log total IgE from longitudinal models over 1, 2, 3 and 5 years, for individual cytokines (Unadjusted) and for Th2 cytokines adjusted for IFN-γ levels (Adjusted), at 3 and 12 months.*
| Total IgE Ages 1, 2, 3, and 5 years |
|||
|---|---|---|---|
| Coef. | P-value | ||
| 3 Month Cytokines | |||
| Unadjusted | |||
| IL-4 | 0.07 | 0.040 | |
| IL-5† | 0.10 | 0.134 | |
| IL-13 | 0.00 | 0.968 | |
| IFN-γ | −0.03 | 0.345 | |
| Adjusted | |||
| IL-4 | 0.11 | 0.005 | |
| IFN-γ | −0.08 | 0.030 | |
| IL-5† | 0.12 | 0.089 | |
| IFN-γ | −0.04 | 0.214 | |
| IL-13 | 0.03 | 0.446 | |
| IFN-γ | −0.05 | 0.191 | |
| 12 Month Cytokines | |||
| Unadjusted | |||
| IL-4 | 0.13 | <0.001 | |
| IL-5 | 0.12 | <0.001 | |
| IL-13 | 0.06 | 0.086 | |
| IFN-γ | −0.02 | 0.500 | |
| Adjusted | |||
| IL-4 | 0.17 | <0.001 | |
| IFN-γ | −0.09 | 0.010 | |
| IL-5 | 0.14 | <0.001 | |
| IFN-γ | −0.06 | 0.054 | |
| IL-13 | 0.09 | 0.016 | |
| IFN-γ | −0.07 | 0.066 | |
Abbreviation: Coef. (Coefficient)
N3 Months = 295–298; N12 Months = 309–316
Dichotomized: Detectable/Undetectable.
Production of Th2 cytokines at 12 months showed even stronger relations to total IgE through age 5 years. Total IgE through age 5 was significantly associated with IL-4 and IL-5 in unadjusted models, and with IL-13 in the IFN-γ-adjusted model. The inverse relationship between IFN-γ and total IgE was not evident in the unadjusted model, but again became significant upon adjustment with IL-4.
Results from analyses assessing relations of early cytokine production to total IgE at each age by including age-by-cytokine interaction terms were not appreciably different from those reported above. These analyses do, however, show that the relations between total IgE and early production of all cytokines at 12 months tended to strengthen with age of assessment of total IgE (Table E1).
There was no association of cytokine production at birth with total IgE at 1, 2, 3 or 5 years (data not shown).
Asthma
Odds ratios for active asthma through age 5 associated with first year cytokine production are shown in Table III. Active asthma was significantly positively associated with 3-month IL-13 in the unadjusted model, and this relation was considerably stronger in the IFN-γ adjusted model. A non-significant inverse relationship between IFN-γ and asthma became slightly stronger upon adjustment for IL-4 but was markedly affected in the model that adjusted for IL-13. Unlike relations with total IgE, asthma was unrelated to 12-month cytokine production.
Table III.
Odds ratios and p-values for active asthma from longitudinal models over 1, 2, 3 and 5 years, for individual cytokines (Unadjusted) and for Th2 cytokines adjusted for IFN-γ levels (Adjusted), at 3 and 12 months, for all subjects and stratified by specific IgE detectability through age 5.*
| Active asthma, Ages 1-2-3-5 years | |||||||
|---|---|---|---|---|---|---|---|
| All subjects | No detectable specific IgE through age 5 | Any detectable specific IgE through age 5 | |||||
| OR | p-val | OR | p-val | OR | p-val | ||
| 3 Month Cytokines | |||||||
| Unadjusted | |||||||
| IL-4 | 1.3 | 0.272 | 0.8 | 0.336 | 1.4 | 0.206 | |
| IL-5** | 1.1 | 0.793 | 0.4 | 0.185 | 1.6 | 0.299 | |
| IL-13 | 1.9 | 0.004 | 2.3 | 0.008 | 1.5 | 0.153 | |
| IFN-γ | 0.8 | 0.145 | 0.7 | 0.268 | 0.7 | 0.259 | |
| Adjusted | |||||||
| IL-4 | 1.6 | 0.071 | 0.8 | 0.605 | 1.8 | 0.047 | |
| IFN-γ | 0.6 | 0.028 | 0.8 | 0.567 | 0.6 | 0.037 | |
| IL-5** | 1.2 | 0.575 | 0.4 | 0.241 | 1.7 | 0.205 | |
| IFN-γ | 0.7 | 0.127 | 0.8 | 0.373 | 0.7 | 0.201 | |
| IL-13 | 2.6 | <0.001 | 3.5 | <0.001 | 1.9 | 0.038 | |
| IFN-γ | 0.5 | 0.001 | 0.4 | 0.009 | 0.5 | 0.023 | |
| 12 Month Cytokines | |||||||
| Unadjusted | |||||||
| IL-4 | 1.2 | 0.302 | 1.1 | 0.669 | 1.2 | 0.484 | |
| IL-5 | 1.0 | 0.844 | 0.9 | 0.802 | 0.9 | 0.717 | |
| IL-13 | 1.0 | 0.884 | 1.0 | 0.998 | 1.0 | 0.966 | |
| IFN-γ | 0.9 | 0.440 | 0.7 | 0.165 | 1.1 | 0.814 | |
| Adjusted | |||||||
| IL-4 | 1.3 | 0.180 | 1.3 | 0.370 | 1.2 | 0.490 | |
| IFN-γ | 0.8 | 0.226 | 0.6 | 0.099 | 1.0 | 0.945 | |
| IL-5 | 1.0 | 0.939 | 1.0 | 0.866 | 0.9 | 0.636 | |
| IFN-γ | 0.9 | 0.445 | 0.7 | 0.170 | 1.1 | 0.676 | |
| IL-13 | 1.1 | 0.620 | 1.3 | 0.458 | 1.0 | 0.882 | |
| IFN-γ | 0.8 | 0.375 | 0.6 | 0.126 | 1.1 | 0.745 | |
All subjects: N3 Months = 307–310; N12 Months = 309–316; Subjects with no detectable specific IgE: N3 Months = 153–155; N12 Months = 154–159 Subjects with detectable specific IgE: N3 Months = 144–145; N12 Months = 155–157
Dichotomized: Detectable/Undetectable.
Odds ratios assessed by models including age by cytokine interactions, were not appreciably different from those described above. However, these results show that relations between 3-month IL-13 and active asthma tended to strengthen with age, especially over the first three years (Table E2).
As was true for IgE, there were no consistent relations between cord cytokines and asthma (data not shown).
Stratification by specific IgE detectability
To assess the possibility of differing relations for atopic and non-atopic asthma with respect to early cytokine production, we stratified asthma-cytokine analyses by atopy in the first 5 years, the prevalence of which was 51% (Table III). The relation of asthma to 3-month IL-13 was evident only among non-atopic subjects in the unadjusted model. After adjustment, IL-13 and IFN-γ relations were also stronger among non-atopics. In contrast, IL-4 was significantly positively associated with asthma only among atopic children, and only upon adjustment for IFN-γ.
Role of adjustment for both Th1 and Th2 cytokines
Figure 1 shows the relation of 3-month IL-13 to 3-month IFN-γ for subjects with and without asthma (dark and open circles, respectively). Lines divide the distribution for both IL-13 and IFN-γ at the medians to form 4 quadrants. This graph illustrates the value of considering the effect of IL-13 in the context of IFN-γ levels and vice-versa by showing, first, the strong correlation between 3-month IFN-γ and 3-month IL-13, and second, that high IFN-γ provides protection from asthma primarily among those with low IL-13: only 2.3% (1/44) of children in this group have active asthma at age 5 compared to 16.2% (35/216) among children from the other 3 quadrants combined (P= 0.015).
Figure 1.
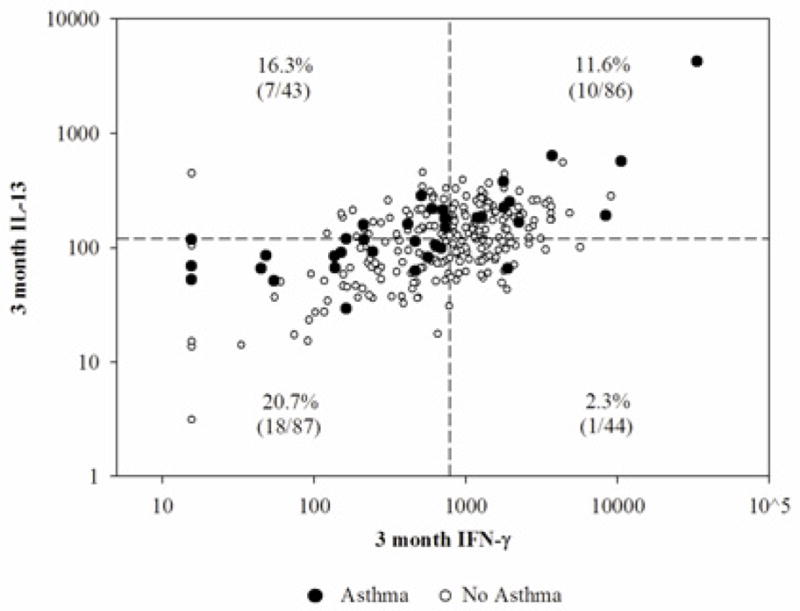
Active asthma at age 5 by 3-month IFN-γ and IL-13, with asthma prevalence shown by quadrants created by dividing IFN-γ and IL-13 values at the median.
DISCUSSION
This study found that early cytokine production was related to IgE and asthma in childhood, with positive associations for Th2 cytokines and inverse associations for IFN-γ in a non-selected population followed longitudinally from birth. However, the relations with Th1 and Th2 cytokines differed temporally and by outcome considered. Total IgE was most strongly associated with cytokine production at 12 months and showed positive relations with IL-4 and IL-5, and an inverse relation with IFN-γ. IL-13 related to IgE modestly and only after adjustment with IFN-γ. In contrast, asthma in unadjusted models was associated only with IL-13 production and only at 3-months. Adjustment for IFN-γ strengthened the relation between asthma and IL-13, and adjustment for IL-13 revealed a strong inverse association with IFN-γ. Three-month IL-4 was also positively associated with asthma, but only for atopic children. Cord cytokine levels showed no consistent association with IgE or asthma.
Many epidemiological studies have reported associations of allergic outcomes in children with increased production of Th2 cytokines11–23 and decreased production of Th1 cytokines.15, 19–22, 24–27 However, as the majority of these analyses are cross-sectional, considering concurrent relations between cytokine production and allergic outcomes, they cannot identify potential predictive relations of early cytokine production and later allergic outcomes. Further, of the few birth cohort studies, only one assessed allergic outcomes after the age of 3 years.27 Our study extends this body of literature by investigating longitudinally whether Th1 and Th2 cytokine production in early life predicts total IgE and asthma through age 5 in a non-selected population. In addition, cytokine production was measured at 3 time-points during the first year of life, which revealed that the predictive relation of cytokine production to allergic outcomes is age- as well as outcome-dependent. Finally, this study expands our previous analysis in this population pertaining to wheeze in the first year of life,24 by considering outcomes through age 5, and showing that significant cytokine-asthma relations were apparent at 3, but not 12 months.
Our results are consistent with other epidemiological studies that have shown positive relations of total IgE with IL-4,11 IL-5,11, 17, 23 and IL-13,23 and inverse associations between IFN-γ and atopy, defined in part by elevated total IgE levels.20, 21 These findings are consistent with the mechanistic roles of these Th1 and Th2 cytokines, as IL-4, IL-5, and IL-13 promote and IFN-γ inhibits IgE synthesis.28, 29 Our finding that relations to total IgE were strengthened in adjusted models including both a Th2 and Th1 cytokine can also be interpreted mechanistically. We know that IL-4 both drives IgE synthesis by B cells,28, 29 and reduces IFN-γ production by inhibiting Th1 cell development from which IFN-γ is derived.4 IL-13 also drives30 and IL-5 enhances31 IgE synthesis. In contrast, IFN-γ inhibits both IgE class switch and Th2 cell growth (as reviewed by Robinson29), thereby potentially decreasing IL-4, IL-5 and IL-13 levels. Thus, any relation between IFN-γ production and total IgE becomes more evident if adjusted for Th2 cytokine production, and vice-versa.
With respect to asthma, this study found that IL-13 was positively and IFN-γ inversely associated with both atopic and non-atopic asthma, whereas IL-4 was only associated with asthma for atopic children. As suggested by studies relating to IL-13 variants, our finding of a strong relation of asthma to IL-13 is not surprising.32 Moreover, these findings are similar to previous epidemiological studies that have shown positive relations between IL-13 and asthma,13, 14 IL-4 and atopic asthma,22 and inverse relations between IFN-γ and asthma,15, 26 atopic asthma22 and wheeze.24, 27 However, to our knowledge, this is the first study in children that examines relations of cytokines to asthma stratified by atopic status. Our finding that IL-13 was related to both atopic and non-atopic asthma suggests that IL-13 acts independently of mechanisms involved in allergic response, a speculation supported by studies that find over-expression of IL-13 in airways of both allergic and non-allergic asthmatics.33, 34 These results emphasize the possibility that asthma may develop independently of IgE as previous epidemiologic findings have suggested.8, 9 Studies in animal models have demonstrated that IL-13 has direct effects on airway tissues, including mucus hypersecretion, airway inflammation, and subepithelial fibrosis.35 In contrast to IL-13, we found that IL-4 was only associated with asthma in the presence of specific IgE sensitization, which corresponds with earlier findings of over-expression of IL-4 among allergic, but not among non-allergic asthmatics.33, 34
We found that adjusted analyses between cytokine production and asthma followed a pattern similar to that for IgE, in that adjustment for IFN-γ strengthened the relation with IL-13 and vice versa, again emphasizing that it is the relative rather than the absolute production of Th1 and Th2 cytokines that influences risk. The value of adjustment is further demonstrated in Figure 1, which provides a graphic representation of one slice of our asthma data at age 5 in relation to 3-month IFN-γ and IL-13 production. By showing the effect of IL-13 relative to IFN-γ levels and vice-versa, we see clearly that only individuals with both low IL-13 and high IFN-γ appear to be protected from asthma, a relation that would be missed if we were to examine IL-13 and IFN-γ in isolation. Other studies have modeled the effect of Th1 cytokines relative to Th2 cytokines using ratios as single variables in analyses.15, 22 We opted a priori to use Th1-Th2 cytokine-adjusted models rather than ratios, both because of the questionable validity of results from ratio-based analyses,36 and in order to better interpret individual effects of each cytokine which might be missed if our assessed variables were collapsed to a single ratio.
We found no relation of cord blood cytokine production and either asthma or total IgE. Though at least two studies have found relations between cord blood mononuclear cell IL-13 production and subsequent atopic disease,18, 37 the direction of the relation was inconsistent for these studies. Furthermore, several larger studies corroborate our null results, reporting no associations between allergic outcomes and the cord blood cytokines that we assess in this paper.17, 23, 38 Our finding that total IgE through age 5 is associated with 12 month, and, in the case of IL-4 and IFN-γ, 3-month cytokine production but not with cord blood cytokine production, suggests that these mechanisms are not fully determined at birth, but rather develop during the first year of life. Similarly, the transient relation we find between 3-month cytokine production and later asthma, which is no longer present at 12 months, suggests there may be an early window of vulnerability that is influential in asthma, and that is marked by altered production of IL-13, IFN-γ, and in the case of atopic asthma, IL-4. The fact that these relations vary depending on the timing of the assessment underscores the value of longitudinal follow-up with repeated measures of both the predictors and the outcomes.
Our study has certain limitations. First, cytokine production from mitogen-stimulated peripheral blood mononuclear cells is likely to be, at best, a crude reflection of cytokine production within local tissue sites where T-cell stimulation occurs as a result of antigen presentation (or perhaps endogenous mitogen stimulation). Thus, the reported associations cannot necessarily be attributed to cause and effect, but may be driven by some common factor that we have not considered. More mechanistic studies are required to determine whether the relationships observed have biologic significance. Second, our follow-up of outcomes currently ends at age 5. Clearly, this age precludes a definitive assessment of asthma. Third, while we have described the cytokines produced as being Th1 and Th2, we acknowledge that there may be additional cell sources other than T helper cells, given that the cell stimulation studies utilized peripheral blood mononuclear cells. Fourth, our study is limited by its focus on Th1 and Th2 cytokines. While reflecting the state of the science in the 1990s, other more recently discovered cytokines including regulatory cytokines may also show differential relations with asthma outcomes. Finally, we acknowledge that using an 18–24 hour window for harvesting supernatant fluids may not optimally capture the capacity to produce particular cytokines. However we have no reason to suspect that outcome groups would be affected differentially.
In conclusion, our results suggest that total IgE and active asthma through age 5 are associated with adaptive cytokine production in the first year of life but that patterns of relation differ temporally and with regard to the relative importance of individual cytokines. At 3-months, production of IL-13 and IFN-γ were positively and negatively, respectively, associated with asthma, as was IL-4 for the subset of atopic children. In contrast, cytokine production at 12 months was associated with total IgE, with IL-4 and IL-5 showing the strongest relations. These data demonstrate the complex interplay between Th1 and Th2 cytokines in the development of allergic disease.
Supplementary Material
Key Messages.
Cytokine production in the first year of life shows different relations with total IgE and with asthma through age 5.
Several cytokines at age 1 year associate with IgE levels assessed longitudinally.
Asthma relates directly to IL-13 and inversely to IFN-γ adjusted for IL-13 at 3 months but not to cytokine production later.
Acknowledgments
This work was funded by NIH grants AI 42268 and AI 61811
The authors thank the study nurses Heidi Erickson, Lydia de la Ossa, Nicole Pargas and Jody Mallie for data collection on study subjects; David Spies and Bruce Saul for database management; and all of the IIS study subjects and families for their participation.
Abbreviations
- IL
Interleukin
- IFN
Interferon
- IgE
Immunoglobulin E
- Coef
Coefficient
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jutel M, Akdis CA. T-cell subset regulation in atopy. Curr Allergy Asthma Rep. 11:139–45. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 3.Street NE, Mosmann TR. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991;5:171–7. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- 4.Paludan SR. Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scand J Immunol. 1998;48:459–68. doi: 10.1046/j.1365-3083.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 5.Gabrielsson S, Soderlund A, Nilsson C, Lilja G, Nordlund M, Troye-Blomberg M. Influence of atopic heredity on IL-4-, IL-12- and IFN-gamma-producing cells in in vitro activated cord blood mononuclear cells. Clin Exp Immunol. 2001;126:390–6. doi: 10.1046/j.1365-2249.2001.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halonen M, Lohman IC, Stern DA, Spangenberg A, Anderson D, Mobley S, et al. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J Immunol. 2009;182:3285–93. doi: 10.4049/jimmunol.0711996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 8.Burrows B, Martinez FD, Cline MG, Lebowitz MD. The relationship between parental and children’s serum IgE and asthma. Am J Respir Crit Care Med. 1995;152:1497–500. doi: 10.1164/ajrccm.152.5.7582283. [DOI] [PubMed] [Google Scholar]
- 9.Halonen M, Stern DA, Lohman C, Wright AL, Brown MA, Martinez FD. Two subphenotypes of childhood asthma that differ in maternal and paternal influences on asthma risk. Am J Respir Crit Care Med. 1999;160:564–70. doi: 10.1164/ajrccm.160.2.9809038. [DOI] [PubMed] [Google Scholar]
- 10.Su Y, Rothers J, Stern DA, Halonen M, Wright AL. Relation of early antibiotic use to childhood asthma: confounding by indication? Clin Exp Allergy. doi: 10.1111/j.1365-2222.2010.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crestani E, Lohman IC, Guerra S, Wright AL, Halonen M. Association of IL-5 cytokine production and in vivo IgE levels in infants and parents. J Allergy Clin Immunol. 2007;120:820–6. doi: 10.1016/j.jaci.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Duramad P, Harley K, Lipsett M, Bradman A, Eskenazi B, Holland NT, et al. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006;114:1916–22. doi: 10.1289/ehp.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzpatrick AM, Higgins M, Holguin F, Brown LA, Teague WG. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 125:851–7. e18. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heaton T, Rowe J, Turner S, Aalberse RC, de Klerk N, Suriyaarachchi D, et al. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365:142–9. doi: 10.1016/S0140-6736(05)17704-6. [DOI] [PubMed] [Google Scholar]
- 15.Hoekstra MO, Hoekstra Y, De Reus D, Rutgers B, Gerritsen J, Kauffman HF. Interleukin-4, interferon-gamma and interleukin-5 in peripheral blood of children with moderate atopic asthma. Clin Exp Allergy. 1997;27:1254–60. [PubMed] [Google Scholar]
- 16.Macaubas C, Sly PD, Burton P, Tiller K, Yabuhara A, Holt BJ, et al. Regulation of T-helper cell responses to inhalant allergen during early childhood. Clin Exp Allergy. 1999;29:1223–31. doi: 10.1046/j.1365-2222.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 17.Neaville WA, Tisler C, Bhattacharya A, Anklam K, Gilbertson-White S, Hamilton R, et al. Developmental cytokine response profiles and the clinical and immunologic expression of atopy during the first year of life. J Allergy Clin Immunol. 2003;112:740–6. doi: 10.1016/s0091-6749(03)01868-2. [DOI] [PubMed] [Google Scholar]
- 18.Ohshima Y, Yasutomi M, Omata N, Yamada A, Fujisawa K, Kasuga K, et al. Dysregulation of IL-13 production by cord blood CD4+ T cells is associated with the subsequent development of atopic disease in infants. Pediatr Res. 2002;51:195–200. doi: 10.1203/00006450-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Rowe J, Kusel M, Holt BJ, Suriyaarachchi D, Serralha M, Hollams E, et al. Prenatal versus postnatal sensitization to environmental allergens in a high-risk birth cohort. J Allergy Clin Immunol. 2007;119:1164–73. doi: 10.1016/j.jaci.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Smart JM, Kemp AS. Increased Th1 and Th2 allergen-induced cytokine responses in children with atopic disease. Clin Exp Allergy. 2002;32:796–802. doi: 10.1046/j.1365-2222.2002.01391.x. [DOI] [PubMed] [Google Scholar]
- 21.Smart JM, Tang ML, Kemp AS. Polyclonal and allergen-induced cytokine responses in children with elevated immunoglobulin E but no atopic disease. Clin Exp Allergy. 2002;32:1552–7. doi: 10.1046/j.1365-2222.2002.01532.x. [DOI] [PubMed] [Google Scholar]
- 22.Tang ML, Coleman J, Kemp AS. Interleukin-4 and interferon-gamma production in atopic and non-atopic children with asthma. Clin Exp Allergy. 1995;25:515–21. doi: 10.1111/j.1365-2222.1995.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 23.Uekert SJ, Akan G, Evns MD, Li Z, Roberg K, Tisler C, et al. Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol. 2006;118:1375–81. doi: 10.1016/j.jaci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Guerra S, Lohman IC, Halonen M, Martinez FD, Wright AL. Reduced interferon gamma production and soluble CD14 levels in early life predict recurrent wheezing by 1 year of age. Am J Respir Crit Care Med. 2004;169:70–6. doi: 10.1164/rccm.200304-499OC. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Kim KH, Woo HY, Shim JY. Maternal cytokine production during pregnancy and the development of childhood wheezing and allergic disease in offspring three years of age. J Asthma. 2008;45:948–52. doi: 10.1080/02770900802419676. [DOI] [PubMed] [Google Scholar]
- 26.Nurse B, Haus M, Puterman AS, Weinberg EG, Potter PC. Reduced interferon-gamma but normal IL-4 and IL-5 release by peripheral blood mononuclear cells from Xhosa children with atopic asthma. J Allergy Clin Immunol. 1997;100:662–8. doi: 10.1016/s0091-6749(97)70171-4. [DOI] [PubMed] [Google Scholar]
- 27.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–41. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 28.Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med. 2007;39:440–56. doi: 10.1080/07853890701449354. [DOI] [PubMed] [Google Scholar]
- 29.Robinson DS, Durham SR, Kay AB. Cytokines. 3. Cytokines in asthma. Thorax. 1993;48:845–53. doi: 10.1136/thx.48.8.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vercelli D, Jabara HH, Arai K, Yokota T, Geha RS. Endogenous interleukin 6 plays an obligatory role in interleukin 4-dependent human IgE synthesis. Eur J Immunol. 1989;19:1419–24. doi: 10.1002/eji.1830190811. [DOI] [PubMed] [Google Scholar]
- 32.Vercelli D. Genetics of IL-13 and functional relevance of IL-13 variants. Curr Opin Allergy Clin Immunol. 2002;2:389–93. doi: 10.1097/00130832-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Humbert M, Durham SR, Kimmitt P, Powell N, Assoufi B, Pfister R, et al. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucosa of atopic and nonatopic subjects with asthma. J Allergy Clin Immunol. 1997;99:657–65. doi: 10.1016/s0091-6749(97)70028-9. [DOI] [PubMed] [Google Scholar]
- 34.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–15. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. Journal of the Royal Statistical Society, Series A. 1993;156:379–92. [Google Scholar]
- 37.Williams TJ, Jones CA, Miles EA, Warner JO, Warner JA. Fetal and neonatal IL-13 production during pregnancy and at birth and subsequent development of atopic symptoms. J Allergy Clin Immunol. 2000;105:951–9. doi: 10.1067/mai.2000.106211. [DOI] [PubMed] [Google Scholar]
- 38.Tadaki H, Arakawa H, Sugiyama M, Ozawa K, Mizuno T, Mochizuki H, et al. Association of cord blood cytokine levels with wheezy infants in the first year of life. Pediatr Allergy Immunol. 2009;20:227–33. doi: 10.1111/j.1399-3038.2008.00783.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


