Abstract
Objectives
To assess the prevalence of neurocognitive impairment (NCI) in childhood-onset systemic lupus erythematosus (cSLE) comparing published classification criteria, and to examine associations between NCI, disease characteristics, psychosocial-well being and intelligence.
Methods
cSLE patients and ethnicity- and age-matched healthy controls completed a neuropsychological research battery, and results were categorized by three different NCI classification criteria with different cutoff scores (e.g., >2, 1.5, or 1 SD below mean) and number of required abnormal tests or domains.
Results
Forty-one cSLE subjects and 22 controls were included. Subjects were predominantly female (70%) and Hispanic (70%). Executive functioning, psychomotor and fine-motor speed were most commonly affected. Method 1 classified 34.1% of cSLE subjects with NCI, compared to Method 2 (14.6% with decline and 7.3 % with NCI) and Method 3 (63.4% with NCI). Prevalence of NCI was not significantly different between controls and patients using any of the categorization methods. NCI was not associated with SLE disease activity or characteristics, or with depression. Using Method 3, patients in the cognitive impairment group reported significantly lower quality of life estimates (69.7 vs 79.3, p=0.03). Below average intellectual functioning (IQ < 90) differentiated the number of test scores >1 and >1.5 SD, but not >2 SD below the mean.
Conclusions
NCI was prevalent in cSLE, but varied according to chosen categorization method. A similar proportion of cSLE patients and controls had NCI, reinforcing the importance of studying an appropriate control group. Categorical classification (i.e. impaired/nonimpaired) may oversimplify the commonly observed deficits in cSLE.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with diverse manifestations involving multiple organ systems. Neurocognitive impairment is one of the 19 distinct neuropsychiatric syndromes of SLE (NPSLE) defined by the American College of Rheumatology,1 and is associated with considerable morbidity among both adults and children with SLE.1–4 Since 15 to 20 percent of all patients with SLE have the onset of disease in childhood, cognitive impairment may have a substantial impact on learning, academic and vocational success. However, a major roadblock to accurate prevalence estimates and to our understanding of neuropsychological functioning among patients with childhood-onset SLE (cSLE) can be attributed to the lack of a standard criteria for neurocognitive impairment (NCI).2
The American College of Rheumatology (ACR) defines cognitive impairment as a deficit in at least one of the following seven broad cognitive domains: 1) simple or complex attention, 2) memory, 3) visual-spatial processing, 4) language, 5) reasoning and problem solving, 6) psychomotor speed and 7) executive functioning.1 Cognitive impairments may be mild to severe, represent a decline from previous functioning, and are associated with an impact on social, educational and/or occupational functioning. The ACR definition also stipulates that neuropsychological testing is mandatory for NCI diagnosis.
Neuropsychological batteries, by definition, employ several tests to assess each of the neurocognitive domains. In research, test and/or domain aggregate scores are then used to stratify individuals into a dichotomous category of neurocognitive impairment (present/absent) using varying criteria as to how far scores (test and/or domain) depart from test standardization sample expectations (e.g., 1, 1.5 or 2 standard deviations below normative sample averages). Given this variation in NCI criteria cutoffs, it is not surprising that prior studies report a prevalence of NCI in cSLE that ranges between 20 and 95 percent.5–14 This broad range may also be attributed to the different number of tests and/or domains required for diagnosis of “impairment”. Furthermore, interpretation of prevalence estimates is complicated by varying study designs and sample characteristics (i.e., patients referred for clinical assessment versus prospective research study, and the inclusion of control samples).
Three recent studies with the goal of determining NCI in SLE used clearly defined, but varying classification criteria methodologies.5–7 In the first method, Brunner et. al. employed a NCI cutoff of more than 2 standard deviations below the standardized mean in 1 domain, or scores between 1 and 2 standard deviations below the mean in 2 or more domains.5 In their study, 4 domains (memory, psychomotor speed, visual construction processing and attention-executive functioning) were assessed using 11 tests; and 59% of the sample (research recruitment) met NCI criteria. A second method outlined (but not tested) by expert consensus methodology assigns “impairment” status to individuals who score more than 2 standard deviations below the normative mean in 1 or more domains and an intermediate category of “cognitive decline” to individuals achieving scores between 1.5 and 1.9 standard deviations below published norms in 1 or more domains.6 This publication also discussed focal impairment (impairment in one domain) and multifocal impairment (impairment in more than 1 domain). Most recently, a third method outlined by Muscal et. al. operationalized NCI as 2 or more single test scores more than 1.5 standard deviations below normative means spanning two cognitive domains.7 Seven domains (as recommended by ACR: memory, psychomotor speed, visual construction processing and attention, executive functioning, intelligence, and academics) were assessed using 19 individual tests, and the prevalence of NCI ranged between 47 and 71% depending on cohort (prospective versus. retrospective).
These studies offer formal NCI criteria for cSLE; however their different definitions and sample characteristics as well as lack of healthy control comparisons preclude consistent estimates of the prevalence of NCI in cSLE. This reduces the sensitivity to successfully identify NCI in cSLE and subsequently hinders appropriate intervention for children with persistent cognitive challenges. Therefore, the primary objectives of our study were to a) to assess how use of these different NCI criteria changes the prevalence rates in a sample of predominantly urban, non-white cSLE patients, and b) to determine whether cSLE patients are significantly more likely to be categorized as having NCI than a matched control group using these differential NCI criteria methods.5–7 Our secondary objectives were to determine the associations between NCI, disease characteristics, psychosocial-well being and intellectual level in this cohort.
Patients and Methods
Participants
Individuals with cSLE followed at the Lupus Clinic at the Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center were eligible for recruitment as part of a prospective longitudinal study of neurocognitive function between June 2006 and August 2009. The data presented here represents the baseline neuropsychological assessment. Inclusion criteria for all participants were: 1) age 10 – 21 years, 2) English-speaking or fluently bilingual, attending school in English for at least two years, and ability to complete traditional neuropsychological testing in English, and 3) absence of a co-morbid condition affecting cognitive functioning (e.g., cerebral palsy, Down’s syndrome). Consecutive eligible patients attending Lupus Clinic were approached until the recruitment goal of 50 subjects was attained. Five eligible patients declined participation. A control sample of age, socioeconomic (SES) and ethnicity-matched individuals was recruited. Control subjects were predominantly friends of the cSLE subjects; however, a small number of healthy siblings of the cSLE subject with no history of SLE or other autoimmune disease and healthy neighborhood controls were also recruited. The study was approved by the Institutional Research Board at Columbia University Medical Center.
Procedures
Demographic data collected included age, gender, zip code and family income. Screening psychosocial measures included the Beck Depression Inventory (BDI)15 and the standard Pediatric Quality of Life Inventory (PedsQL).16 For the cSLE subjects, disease information collected included date of diagnosis, current medications, disease activity and damage and any documented history of NPSLE as defined by the ACR case definitions.1 No subjects had previously completed a neuropsychological assessment. Disease activity was evaluated using the Systemic Lupus Erythematosus Disease Activity Index, (SLEDAI, score range 0 – 105)17 and disease-specific damage scores using the Systemic Lupus International Collaborative Clinics damage index score (SDI, score range 0–47)18 at the rheumatology visit preceding their neuropsychological evaluation. Recent laboratory results (dsDNA, complements, complete blood count, urinalysis) were collected in order to complete the disease activity measures. The presence of antiphopholipid antibodies (anticardiolipin antibodies and/or lupus anticoagulant) since cSLE diagnosis was documented.
Neuropsychological functioning was evaluated using a research battery of traditional neuropsychological tests adapted and expanded from the recommended ACR battery for assessment of adults with SLE1 in order to be suitable for evaluation of a cSLE population. The battery assessed seven overall cognitive domains: (1) memory, (2) language and verbal reasoning, (3) visual-spatial reasoning, (4) executive functioning, (5) psychomotor speed, (6) fine motor dexterity and (7) academics). This is representative of the seven domains recommended by the ACR, but differentiates fine motor control from higher level psychomotor processes and adds assessment of academic functioning, relevant to the pediatric population. Neuropsychological testing was performed by a trained psychometrist and supervised by a pediatric neuropsychologist using multiple measures as detailed below and in Table 2. Assessments typically took 3 to 4 hours to complete. Twenty-three tests were administered to evaluate the seven domains. Individual tests were included from the following nine test batteries: (1) Weschler Abbreviated Scale of Intelligence (WASI) for problem solving and reasoning skills, comprised of four subtests;19 (2) Wide Range Assessment of Memory and Learning (WRAML-2) for immediate learning and memory, comprised of four subtests;20 (3) Letter Fluency from the Delis Kaplan Executive Functioning System (DKEFS);21 (4) Comprehensive Trail Making Test Parts 1 and 5;22 (5) Stroop Color and Word Test Children’s Version (four subtests);23,24 (6) Wechsler Intellectual Scales, Adult and Child Versions (WAIS III /WISC IV) Subtests of Letter-Number Sequencing and Digit Coding;25,26 (7) Purdue Pegboard (three subtests);27 and (8) Wide Range Achievement Test - Third Edition (WRAT-III).28 In analyses examining how other neurocognitive domains varied by intellectual functioning, full scale intellectual scores were used to compare the remaining five domains: (1) executive functioning, (2) memory, (3) psychomotor speed, (4) fine motor dexterity and (5) academics. Z-scores (representing standard deviations from the test’s standardization sample mean) were generated for each test. Overall mean z-scores were then determined for each subject in each of the seven cognitive domains. The number of individual tests and domain scores falling below three different cutoff impairment levels were also computed (e.g., >1, >1.5 and >2 standard deviations (SD) below the test mean).
Table 2.
Neuropsychological Tests Mean Z Scores
| Domain and Tests | cSLE Subjects Z ± SD |
Control Subjectsb Z ± SD |
|
|---|---|---|---|
| LANGUAGE AND VERBAL REASONING | |||
| WASI Vocabulary | −0.49 ± 0.96 | −0.66 ± 0.86 | |
| WASI Similarities | −0.12 ± 0.86 | 0.04 ± 0.74 | |
| Overall Domain Score | −0.21 ± 0.82 | −0.13 ± 0.73 | |
| VISUAL SPATIAL PROCESSING | |||
| WASI Block Design | −0.06 ± 0.82 | −0.27 ± 1.11 | |
| WASI Matrix Reasoning | −0.07 ± 0.73 | 0.06 ± 0.89 | |
| Overall Domain Score | −0.14 ± 0.51 | −0.10 ± 0.76 | |
| MEMORY | |||
| Story Memory (Immediate) | −0.19 ± 0.94 | −0.26 ± 1.08 | |
| List Learning | −0.21 ± 0.96 | −0.25 ± 0.79 | |
| Design Memory | −0.09 ± 0.95 | −0.48 ± 0.93 | |
| Picture Memory | −0.01 ± 0.78 | −0.20 ± 0.74 | |
| Overall Domain Score | −0.12 ± 0.64 | −0.30 ± 0.67 | |
| EXECUTIVE FUNCTIONING | |||
| FAS (DKEF) | −0.53 ± 1.10 | −0.20 ± 1.39 | |
| CTMT 5 (Trails B) | −0.75 ± 0.92a | −0.99 ± 1.11 | |
| Stroop Color Word | −0.10 ± 0.90 | 0.23 ± 0.82 | |
| Stroop Inhibition | 0.26 ± 0.70 | 0.44 ± 0.66 | |
| WISC Letter Number | 0.27 ± 1.04 | 0.52 ± 1.02 | |
| Overall Domain Score | −0.17 ± 0.55 | 0.00 ± 0.62 | |
| PSYCHOMOTOR SPEED | |||
| Trails (CTMT1) | −1.61 ± 1.06a | −1.31 ± 1.22 | |
| WISC Coding | −0.05 ± 0.91 | −0.19 ± 0.73 | |
| StroopWord Reading | −0.18 ± 0.98 | −0.11 ± 1.02 | |
| Stroop Color Naming | −0.36 ± 0.90 | −0.44 ± 0.80 | |
| Overall Domain Score | −0.55 ± 0.63b | −0.53 ± 0.67 | |
| FINE MOTOR SPEED AND DEXTERITY | |||
| Purdue Pegs Dominant Hand | 0.05 ± 1.07 | −0.15 ± 0.99 | |
| Purdue Pegs Non-Dominant Hand | −0.38 ± 1.31 | −0.77 ± 1.30 | |
| Purdue Pegs Both Hands | −0.99 ± 1.11a | −1.19 ± 1.60 | |
| Overall Domain Score | −0.44 ± 1.01 | −0.70 ± 1.14 | |
| ACADEMICS | |||
| Spelling | −0.04 ± 0.91 | 0.30 ± 1.16 | |
| Math | −0.43 ± 0.85 | −0.35 ± 1.25 | |
| Reading | 0.03 ± 0.88 | 0.21 ±1.18 | |
| Overall Domain Score | −0.15 ± 0.76 | 0.05 ± 1.05 | |
significant difference for comparison of mean individual test scores to published age-matched normative scores, with p-value <0.001 (Bonferroni corrected for multiple (23) tests);
significant difference for comparison of mean domain score to published age-matched normative scores, p-value <0.001 (Bonferroni corrected for multiple tests);
no significant differences between cSLE subjects and control subjects for all individual tests and domains scores
NCI Classification Criteria
Neurocognitive impairment was operationally defined using the three recently published diagnostic methods as previously outlined.5–7
Statistical Analysis
Demographic and clinical characteristics were summarized with descriptive statistics including mean (standard deviation) for continuous variables, and frequencies for categorical variables. One-way analysis of variation (ANOVA) compared patient and control groups on continuous demographic variables (i.e., age) as well as test- and domain-based standardized neurocognitive scores, using a Bonferonni corrected alpha level (p < .002-individual test scores; p<.007 – domain scores). Pearson’s chi-squares for parametric data results were determined for comparisons of categorical variables (i.e., impairment, intellectual categories). As there were small numbers of subjects enrolled, we utilized parametric and nonparametric models in the analyses. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 19.
Results
Demographics – Patient sample
Fifty cSLE patients provided informed consent and 44 completed testing. Six patients either failed to show up for multiple appointments, or voluntarily withdrew consent because of lack of time required to complete the testing. Data from three individuals were eliminated from the final sample due to disclosure of pre-existing co-morbidities (e.g., cerebral palsy, longstanding learning issues) after testing was complete. Table 1 outlines ethnic and socioeconomic status of the participants. Subjects ranged in age from 10 to 21 years, the majority were female, and of Hispanic ethnicity. The cSLE patients reported significantly lower quality of life as measured by the PedsQL, and a trend towards more depressed symptoms than the control subjects. Mean full scale estimated intelligent quotient (IQ) scores were within the average range for both patient and control groups.
Table 1.
Baseline Demographics
| cSLE Subjects N=41 |
Control Subjects N=22 |
p-value | ||
|---|---|---|---|---|
| Age in years (mean ± SD) Range |
16.4 ± 2.5 (10.3 – 21.8) |
17.7 ± 3.0 (10.5 – 21.7) |
.06 | |
| Gender (N, %) | 30 F (73) | 14 F (64) | ns | |
| Ethnicity (N, %) | ||||
| Hispanic | 28 (68) | 16 (73) | ns | |
| African-American | 5 (12) | 3 (14) | ||
| Asian | 4 (10) | 2 (9) | ||
| Caucasian | 3 (7) | 1 (5) | ||
| Other (mixed race/ethnicity) | 1 (3) | 1 (5) | ||
| Yearly Household Income (N, %) | ||||
| <$25,000 | 18 (44) | 13 (59) | ns | |
| $25,000 – $49,999 | 12 (29) | 4 (18) | ||
| $50,000 – $99,999 | 5 (12) | 2 (9) | ||
| $100,000 – $150,000 | 1 (3) | 0 | ||
| >$150,000 | 5 (12) | 3 (14) | ||
| Disease Duration in Yrs (mean ± SD) (Range) |
3.6 ± 2.4 (0.4 – 12.4) |
N/A | ||
| Neuropsychiatric Issuesa (N, %) | ||||
| Headache | 13 (32) | N/A | ||
| Depression | 2 (5) | |||
| Psychosis | 1 (3) | |||
| Seizures | 1 (3) | |||
| Medicationsa | ||||
| Hydroxychloroquine | 40 (98) | N/A | ||
| Prednisone (N, %) | 25 (61) | |||
| Pred dose (mg/kg/d) (mean, range) | 0.23 (0.03 – 0.7) | |||
| Azathioprine | 5 (12) | |||
| Cyclophosphamide | 1 (2) | |||
| Mycophenolate Mofetil | 14 (34) | |||
| Methotrexate | 3 (7) | |||
| BDI Score (Mean ± SD) (Test Range 0 – 63) |
7.5 ± 6.0 (0 – 23) |
4.4 ± 6.6 (0 – 30) |
.07 | |
| PedsQL Score (Mean ± SD) (Test Range 0 – 100) |
73.3 ± 13.7 (46 – 100) |
88.7 ± 9.3 (62 – 100) |
<.001 | |
| Mean WASI FSIQ (Mean ± SD) (Range) |
96.8 ± 10.3 (70 – 119) |
96.7 ± 11.1 (65 – 116) |
ns | |
At time of neuropsychological testing; BDI – Beck Depression Inventory; PedsQL – Pediatric Quality of Life Scale; WASI – Weschler Abbreviated Intelligence Score; FSIQ – Full Scale IQ score
Comparison to Normative Test Standardization Means
As shown in Table 2, mean standardized test scores for the cSLE subjects were lower than standardized normative values (Z = 0; SD = 1) for three individual tests (CTMT 1 and 5, Purdue Pegs – bilateral hand co-ordination) and one domain (psychomotor speed), p<0.001 for each. In comparison to normative estimates of low scores, our sample had a significantly higher proportion of individuals meeting score cutoffs of more than 1 SD (56.1% versus 16%, p <.0001), 1.5 SD (14.6 % vs 7%, p < .01), and 2 SD (7.3% versus 2%, p <.01).
Comparison to Matched Control Sample
There were no significant differences between cSLE patients and the control sample on any of the mean standardized individual test scores or the mean aggregate domain scores (Table 2).
Differential Criteria for NCI
As shown in Table 3, 34.1% of cSLE subjects were classified as cognitively impaired according to Method 1 criteria. Using Method 2, 14.6% fulfilled criteria for cognitive decline, and 7.3% fulfilled criteria for cognitive impairment. Finally, using Method 3, 63.4% of cSLE subjects were categorized as cognitively impaired. There were no significant differences in the proportion of impaired subjects in the cSLE and control groups using any of the three methods. Overall, Method 3 categorized significantly more participants as being cognitively impaired than Methods 1 and 2 (p < .0001).
Table 3.
Cognitive Impairment Comparison by Different Categorization Methods
| Method 1a | Method 2a - Declineb |
Method 2b - Impairedc |
Method 3d | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Patients | 14 (34.1) | 6 (14.6) | 3 (7.3) | 26 (63.4) |
| Control | 8 (36.4) | 6 (27.3) | 2 (9.1) | 14 (63.6) |
1 domain score < 2 SD below mean OR 2 or more domain scores between 1 and 2 SD below mean;
1 domain score < 1.5 SD below mean;
1 domain score < 2 SD below mean
2 or more tests which span two cognitive domains with scores < 1.5 SD below mean
NCI Classification and Disease Indices
We observed no significant differences in disease duration, disease activity (SLEDAI) or damage (SDI) scores between impaired and non-impaired groups, as shown in Table 4. However, disease activity was generally higher in the NCI group, but this difference did not reach statistical significance. The proportion of all patients with positive lupus anticoagulant (LAC, 15%), anti-cardiolipin (ACL, 42%) antibody status or renal disease (49%) also did not differ by NCI categorization methods.
Table 4.
Disease Indicators by Neurocognitive Impairment
| Disease Duration (mean ± SD) |
SLEDAI score (mean ± SD) |
SLICC/SDI score (mean ± SD) |
LAC positive (N, %) |
ACL Positive (N, %) |
Renal Disease (N, %) |
||
|---|---|---|---|---|---|---|---|
| Method 1 | |||||||
| No impairment | 4.1 ± 2.5 | 3.8 ± 3.7 | 0.4 ± 0.7 | 5 (19) | 10 (37) | 12 (44) | |
| Cognitive impairmenta | 3.0 ± 1.7 | 5.6 ± 5.6 | 0.6 ± 0.9 | 1 (7) | 7 (50) | 7 (50) | |
| Method 2 | |||||||
| No impairment | 3.9 ± 2.4 | 4.5 ± 3.8 | 0.5 ± 0.7 | 5 (16) | 14 (44) | 15 (47) | |
| Decline onlyb | 2.6 ± 1.2 | 5.7 ± 7.5 | 0.5 ± 0.8 | 1 (17) | 3 (50) | 4 (67) | |
| Cognitive impairmentc | 3.7 ± 2.6 | 0.7 ± 1.2 | 1.0 ± 1.0 | 0 | 0 | 0 | |
| Method 3 | |||||||
| No impairment | 4.3 ± 2.9 | 3.6 ± 3.4 | 0.5 ± 0.7 | 4 (27) | 6 (40) | 5 (33) | |
| Cognitive impairmentd | 3.3 ± 1.8 | 4.9 ± 5.0 | 0.5 ± 0.8 | 2 (8) | 11 (42) | 14 (54) | |
1 domain score < 2 SD below mean OR 2 or more domain scores between 1 and 2 SD below mean
1 domain score < 1.5 SD below mean
1 domain score < 2 SD below mean
2 or more tests which span two cognitive domains with scores < 1.5 SD below mean
NCI and Psychosocial Indices
A self-administered depression index (BDI) and quality of life index (PedsQL) failed to differentiate between the NCI and unimpaired groups using Method 1 or 2. Using Method 3 criteria, patients in the NCI group reported significantly lower estimates of quality of life compared to the unimpaired group (Table 5). NCI was not associated with household income using any of the categorization methods (data not shown).
Table 5.
Depression and Quality of Life by Cognitive impairment
| BDI score (mean ± SD) |
PedsQL score (mean ± SD) |
Depressione (N, %) |
||
|---|---|---|---|---|
| Method 1 | ||||
| No impairment | 7.4 ± 6.1 | 75.2 ± 14.0 | 6 (23) | |
| Cognitive impairmenta | 7.6 ± 5.8 | 69.8 ± 12.9 | 4 (29) | |
| Method 2 | ||||
| No impairment | 7.5 ± 6.5 | 74.4 ± 14.2 | 8 (26) | |
| Decline onlyb | 5.8 ± 3.3 | 73.9 ± 12.1 | 1 (17) | |
| Cognitive impairmentc | 10.0 ± 4.4 | 60.8 ± 3.8 | 1 (33) | |
| Method 3 | ||||
| No impairment | 6.1 ± 6.1 | 79.3 ± 11.6 | 3 (20) | |
| Cognitive impairmentd | 8.2 ± 5.8 | 69.7 ± 13.8 | 7 (28) |
1 domain score < 2 SD below mean OR 2 or more domain scores between 1 and 2 SD below mean;
1 domain score < 1.5 SD below mean;
1 domain score < 2 SD below mean
2 or more tests which span two cognitive domains with scores < 1.5 SD below mean
Depression defined as Beck Depression Inventory Score >10
NCI and Intellectual Ability
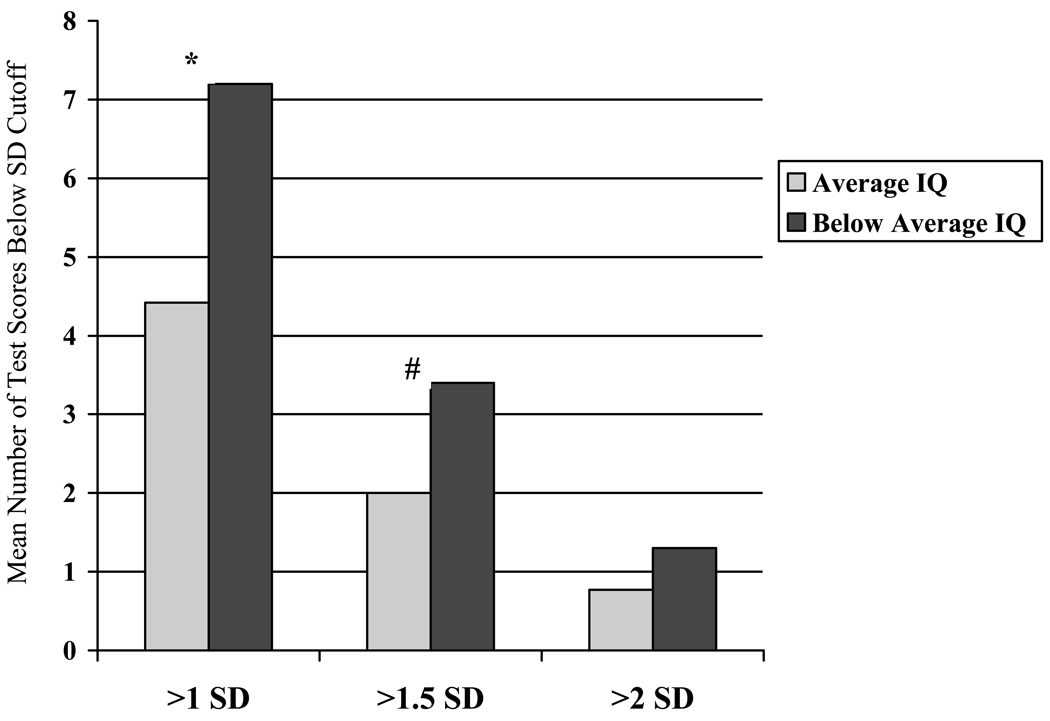
Following previous psychometric methodology for comparing prevalence of low scores by intellectual level,29 the cSLE subject group was stratified into individuals with average range intellectual scale scores (IQ ≥ 90 standard score, n = 31) and those with below average scores (IQ ≤ 89, n = 10). As shown in Figure 1, patients with below average intelligence had significantly more test scores more than 1 and 1.5 SD below the mean than patients with average or above intelligence (>1SD: 7.2 versus 4.4 tests, p < .01; >1.5 SD: 3.4 vs. 2.0 tests, p <.05). However, no differences were observed in the number of domain scores more than 1, 1.5 or 2 SD below the mean by using this IQ cutoff (>1SD: 0.4 versus 0.06; >1.5 SD: 0.2 versus 0.1; >2 SD: 0.90 versus 0.55, p=NS for all). Intellectual functioning did not differentiate either the number of tests or domains falling below any of the three cutoff criteria. Similar results were observed for the control sample (data not shown).
Figure 1.
Test Results by Intelligence Level
SD = standard deviation; *p<.01; #p<.05; Average IQ defined as Intelligence Quotient ≥ 90; Below Average IQ defined as Intelligence Quotient <89
Discussion
Using three different methods for categorizing NCI in cSLE, the prevalence of NCI among our sample ranged from 7.3% to 63.4%. Perhaps most importantly, test and domain scores as well as NCI prevalence estimates did not differ between cSLE patients and controls. Furthermore, these three categorization methods did not relate to measures of disease activity or damage. However, using the third method,7 NCI was associated with poorer perceived quality of life, consistent with previous research.30–34
The criteria outlined in Method 3 (two or more single tests > 1.5 SD below mean spanning two different domains) demonstrated the largest number of patients as impaired (>63%), consistent with the original authors’ findings in their cohort.7 This is not surprising, given its inclusion of single test scores in the operational definition and the psychometric reality that impaired performance on one or more tests in test batteries with greater than 20 measures occurs in the majority of cognitively healthy subjects.35 The measurement criteria outlined in Methods 1 and 2 were more moderate estimates of NCI, each categorizing impairment in approximately one quarter to one third of our sample, a lower prevalence than the original study (59%).5 Both classification methods uphold the common criterion of a domain score more than two standard deviations below the mean and also use secondary criteria to capture individuals who have less severe cognitive deficits (between 1 and 2 SD below the mean). However, we caution researchers and clinicians in using the term “cognitive decline” (as in Method 2) for studies similar to ours that assess a cross-sectional cohort and do not evaluate for an objective change in neurocognitive function over time.
Although disease activity has been linked to neurocognitive outcomes in SLE,36–40 we did not observe any association between disease duration, age at diagnosis, disease-related damage, or potential risk factors including antiphospholipid antibodies or renal disease and NCI as assessed by any of the three methods, although our small sample size may have limited our power to detect these associations. Poorer quality of life was associated with the more inclusive neurocognitive impairment defined in Method 3 and suggests the potential importance of employing greater leniency in our definitions of impairment, as well as accounting for the interrelations between mood and cognitive issues prevalent in this population.41–45
Our sample of predominantly adolescents demonstrated neurocognitive deficits in domains previously proposed to be associated with SLE – particularly in the areas of executive functioning (mental flexibility), psychomotor and fine-motor speed.42,46–50 This is consistent with proposed pathogenetic mechanisms in SLE, highlighting microstructural white matter changes, particularly in the frontal lobe, potentially explaining attentional and executive dysfunction as well as motor issues.51 Despite the salience of these deficits, cognitive interventions have rarely been evaluated in cSLE.52 In addition to educational accommodations for patients (e.g., direct instruction, modifications to environmental and task demands), specific interventions for attention problems caused by poor executive functioning are important and necessary new directions for cSLE research.
Our study also draws attention to the large number of test scores in the impaired range according to the different cutoffs, beyond those anticipated using normal curve estimates, and greater than projected base rates of abnormal scores compared to typically developing children. Although base rates of low scores are not available for this specific neuropsychological battery, one recent study demonstrated one or more scores >1 SD in 37.6% of the standardization sample for the Children’s Memory Scale, compared to 56% of our sample meeting this impairment cutoff across all our domains.29 Further consideration of the number of low scores found among healthy youth on the recommended pediatric cSLE test battery and the intercorrelations among these tests is strongly advised to guide future definitions of NCI within the cSLE population.
Although cSLE patients demonstrated difficulties on individual neurocognitive measures and domains, they did not demonstrate deficits in excess of control participants, nor were there any differences between the two groups in any of the three categorizations systems for NCI. The similarities in low scores and NCI among our patients and healthy controls suggests overall neurocognitive difficulties in this sample, and questions if poor performance can or should be attributed to SLE. These findings highlight the importance of using an appropriate control group when examining NCI in demographically diverse populations. Use of matched control groups attempts to remedy test norms that may not reflect the diversity of socioeconomic or ethnic background among medical populations such as cSLE, a disease with greater prevalence in nonwhite populations. In the past, failure to use a matched control group has led to a disproportionate number of individuals to be classified as impaired.50,53–56 However, some of the tests used in this study employ normative samples with increased representation of diverse cultures. For example, the Weschler intelligence and DKEF tests have samples that include up to 25 percent of individuals of Hispanic origins,19,21,26 suggesting that our cohort (both patients and controls) may be unique, with a higher prevalence of NCI than would be expected in a control population based on available population norms. Our sample represents a predominantly urban population of lower socioeconomic status, and as such, subjects may have had fewer educational opportunities than other cSLE samples. Overall, there were few subjects in the higher household income groups, although analysis did not show fewer cases of NCI in these higher income strata. Although recruiting friends as a comparison group controls for important sociodemographic factors including age, ethnicity, school quality, and household income, the lack of cognitive differences among our sample may reflect how individuals seek out similarly able or challenged peers for friendships. Similarities can also be attributed to our cross-sectional methodology, as previous investigators show comparability between cSLE patients and controls early in the disease, but slower acquisition of skills and increased cognitive impairment over time.57
To interpret neurocognitive performance in the context of overall level of intellectual functioning, we observed that approximately 25% of cSLE patients fell in the below average intellectual group (IQ ≤ 89). However, below average intellect did not differentiate the number of cognitive domains scoring below 2 SD of the mean, indicating how few subjects in both our patient and control sample met this level of impairment criteria. The number of domains and tests more than 1 and 1.5 SD below the mean did differ by intelligence level, again suggesting more leniencies in standard impairment criteria. Although examining different test scores by dichotomizing IQ can be controversial, given the strong correlation between IQ test scores and other neurocognitive measures, it is also important to account for differential expectations of below average scores and potential patterns of impairment that may be specific to this disease process.29,35,58 For example, a person who is lower functioning cognitively will have more low scores, and be at greater risk for misdiagnosis of NCI (i.e.,false positives), than a person who is higher functioning (and at greater risk of having a missed diagnosis, i.e.,false negatives).29 Overall it remains essential to assess the impact of disease on all neurocognitive domains, including intellectual indices and to acknowledge the additional challenges inherent in assessing NCI in cSLE patients whose cognitive skills are not yet fully developed.
Several limitations of the present study should be noted. Most saliently the small sample size limited the statistical power of our analyses. Our predominantly Hispanic sample with limited inclusion of other sociodemographic groups also restricts generalizability to other more diverse cSLE populations. As with many neurocognitive cSLE studies, this battery of neuropsychological tests was different than previous studies, yielding a different number of single test scores and reflective of slightly different domains. After the initiation of this study, a recommended test battery for cSLE was published, addressing the necessity for pediatric specific instruments, as well as sensitivity for children with multiple limitations.4 Although our study used many of the same as well as comparable tests, we recommend that future research follow published guidelines as a minimum to permit increased comparability across studies. Finally, our study does not elaborate on the causality of NCI, beyond our examination of disease characteristics, or examine any potential neuroanatomical differences among our sample.
Despite these limitations, by comparing existing criteria for NCI among cSLE patients in the same sample, our study controls for previous variability in differential neuropsychological measures and methodological differences in recruitment in assessing NCI. Our results highlight the challenges of accurately dichotomizing neurocognitive impairment generally and more specifically in a disease such as cSLE, known for its diversity in presentation and impact. Further consideration and refinement of the concept of NCI among children with SLE is needed. Future efforts are encouraged to go beyond dichotomous ranking systems and to continue to consider intelligence as well as other functional outcomes. Overall, consistent use of a single definition in research studies using similar test batteries based on these considerations will provide further data for assessment of impairment criteria. Our results suggest the importance of using domain based scores and flexibility in cutoff criteria between 1 and 1.5 SD below the mean, given how few individuals met > 2 SD thresholds. Further, addressing the cumulative number of impaired domains and addressing which neurocognitive processes these represent may be most valuable in elucidating the underlying neuropsychological networks associated with cSLE. We are currently testing this in a new multi-ethnic sample to further consider and expand upon these issues; as well as to consider potential cognitive intervention in this population. Importantly, no definition should be used in an “all or nothing” manner for decision of when to pursue educational assistance and closer follow-up. We remain optimistic that future multi-centre studies using common methodology and assessment batteries will be able to accomplish these goals and provide valuable insight into neuropsychological development and quality of life among children and adolescents with SLE.
Significance.
Although cSLE patients demonstrated difficulties on individual neurocognitive measures and domains, they did not demonstrate neuropsychological deficits in excess of control participants, highlighting the importance of using an appropriate control group when examining NCI in demographically diverse populations.
It is challenging to accurately dichotomize neurocognitive impairment in cSLE, as categorical classification (i.e. impaired/nonimpaired) oversimplifies the commonly observed deficits in childhood-onset SLE.
Innovation
cSLE patients are affected particularly in the areas of executive functioning (mental flexibility), psychomotor and fine-motor speed, suggesting future studies focus on methodologies to overcome these difficulties.
Acknowledgments
DML was supported by NIH/NIAMS grant K23AR053202, CTSA Clinical Scholars Award from Columbia University, and Pfizer Scholars Grant in Clinical Rheumatology
References
- 1.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Benseler SM, Silverman ED. Review: Neuropsychiatric involvement in pediatric systemic lupus erythematosus. Lupus. 2007;16:564–571. doi: 10.1177/0961203307078971. [DOI] [PubMed] [Google Scholar]
- 3.Kozora E, Hanly JG, Lapteva L, Filley CM. Cognitive dysfunction in systemic lupus erythematosus: past, present, and future. Arthritis Rheum. 2008;58:3286–3298. doi: 10.1002/art.23991. [DOI] [PubMed] [Google Scholar]
- 4.Levy DM, Ardoin SP, Schanberg LE. Neurocognitive impairment in children and adolescents with systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2009;5:106–114. doi: 10.1038/ncprheum0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner H, Ruth N, German A, et al. Initial validation of the Pediatric Automated Neuropsychological Assessment Metrics for childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2007;75:1174–1182. doi: 10.1002/art.23005. [DOI] [PubMed] [Google Scholar]
- 6.Mikdashi J, Esdaile J, Alarcon G, et al. Proposed response criteria for neurocognitive impairment in systemic lupus erythematosus clinical trials. Lupus. 2007;16:418–425. doi: 10.1177/0961203307079044. [DOI] [PubMed] [Google Scholar]
- 7.Muscal E, Bloom D, Hunter J, Myones B. Neurocognitive deficits and neuroimaging abnormalities are prevalent in children with lupus: clinical and research experiences at a US pediatric institution. Lupus. 2009;19:268–279. doi: 10.1177/0961203309352092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy DM, Ross GS. Cognitive and Psychiatric Manifestations of Childhood-onset Systemic Lupus Erythematosus. In: Nass R, Frank Y, editors. Cognitive and Behavioral Manifestations of Pediatric Diseases. Oxford University Press; 2010. pp. 30–46. [Google Scholar]
- 9.Sibbitt WL, Jr, Brandt JR, Johnson CR, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29:1536–1542. [PubMed] [Google Scholar]
- 10.Harel L, Sandborg C, Lee T, von Scheven E. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus and association with antiphospholipid antibodies. J Rheumatol. 2006;33:1873–1877. [PubMed] [Google Scholar]
- 11.Yancey CL, Doughty RA, Athreya BH. Central nervous system involvement in childhood systemic lupus erythematosus. Arthritis Rheum. 1981;24:1389–1395. doi: 10.1002/art.1780241109. [DOI] [PubMed] [Google Scholar]
- 12.Steinlin MI, Blaser SI, Gilday DL, et al. Neurologic manifestations of pediatric systemic lupus erythematosus. Pediatr Neurol. 1995;13:191–197. doi: 10.1016/0887-8994(95)00110-2. [DOI] [PubMed] [Google Scholar]
- 13.Loh WF, Hussain IM, Soffiah A, Lim YN. Neurological manifestations of children with systemic lupus erythematosus. Med J Malaysia. 2000;55:459–463. [PubMed] [Google Scholar]
- 14.Olfat MO, Al-Mayouf SM, Muzaffer MA. Pattern of neuropsychiatric manifestations and outcome in juvenile systemic lupus erythematosus. Clin Rheumatol. 2004;23:395–399. doi: 10.1007/s10067-004-0898-3. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, RA S. Manual for the Beck depression inventory. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- 16.Varni J, Seid M, Rode C. The PedsQL Measurement Model for the Pediatric Quality of Life Inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 18.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: Harcourt Assessment Inc; 1999. [Google Scholar]
- 20.Adams W, Sheslow D. Wide range assessment of memory and learning (WRAML), Second Edition. Wilmington: Wide range Inc; 2003. [Google Scholar]
- 21.Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System. San Antonio: Pearson, PsychCorp; 2001. [Google Scholar]
- 22.Reynolds C. Comprehensive Trail-Making Test. Austin: Pro-Ed; 2002. [Google Scholar]
- 23.Golden C, Freshwater S. Stroop Color and Word Test. Wood Dale: Stoelting Co; 2002. [Google Scholar]
- 24.Golden C. Stroop Color and Word Test Children's Version for Ages 5–14. Wood Dale: Stoelting Co; 2004. [Google Scholar]
- 25.The Psychological Corporation. WAIS-WMS-II technical manual. San Antonio: Author; 1997. [Google Scholar]
- 26.Wechsler D. Wechsler Intelligence Scale for Children (WISC)- Fourth Edition. San Antonio: Harcourt Assessment; 2004. [Google Scholar]
- 27.Tiffin J, Asher E. The Purdue Pegboard: Norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson G. Wide Range Achievement Test 3. Wilmington: Wide Range, Inc.; 1993. [Google Scholar]
- 29.Brooks BL, Iverson GL, Sherman EMS, Holdnack JA. Healthy children and adolescents obtain some low scores across a battery of memory tests. J Int Neuropsychol Soc. 2009;15:613–617. doi: 10.1017/S1355617709090651. [DOI] [PubMed] [Google Scholar]
- 30.McElhone K, Abbott J, Teh LS. A review of health related quality of life in systemic lupus erythematosus. Lupus. 2006;15:633–643. doi: 10.1177/0961203306071710. [DOI] [PubMed] [Google Scholar]
- 31.Moorthy LN, Harrison MJ, Onel KB, Lehman TJA. Relationship of quality of life and physical function measures with disease activity in children with systemic lupus erythematosus. Lupus. 2005;14:280–287. doi: 10.1191/0961203305lu2075oa. [DOI] [PubMed] [Google Scholar]
- 32.Moorthy LN, Peterson MGE, Hassett AL, et al. Relationship between health-related quality of life and SLE activity and damage in children over time. Lupus. 2009;18:622–629. doi: 10.1177/0961203308101718. [DOI] [PubMed] [Google Scholar]
- 33.Dobkin PL, Da Costa D, Drista M, et al. Quality of Life in Systemic Lupus Erythematosus Patients During More and Less Active Disease States: Differential Contributors to Mental and Physical Health. Arthritis Care Res. 1999;12:401–410. doi: 10.1002/1529-0131(199912)12:6<401::aid-art8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Brunner HI, Higgins GC, Wiers K, et al. Health-related Quality of Life and Its Relationship to Patient Disease Course in Childhood-onset Systemic Lupus Erythematosus. J Rheumatol. 2009;36:1536–1545. doi: 10.3899/jrheum.081164. [DOI] [PubMed] [Google Scholar]
- 35.Binder LM, Iverson GL, Brooks BL. To err is human: “Abnormal” neuropsychological scores and variability are common in healthy adults. Arch Clin Neuropsychol. 2009;24:31–46. doi: 10.1093/arclin/acn001. [DOI] [PubMed] [Google Scholar]
- 36.Denburg SD, Carbotte RM, Ginsberg JS, Denburg JA. The relationship of antiphospholipid antibodies to cognitive function in patients with systemic lupus erythematosus. J Int Neuropsychol Soc. 1997;3:377–386. [PubMed] [Google Scholar]
- 37.Afeltra A, Garzia P, Mitterhofer AP, et al. Neuropsychiatric lupus syndromes: relationship with antiphospholipid antibodies. Neurology. 2003;61:108–110. doi: 10.1212/01.wnl.0000058904.94330.a7. [DOI] [PubMed] [Google Scholar]
- 38.Alarcon-Segovia D, Estanol B, Garcia-Ramos G, Villa AR. Antiphospholipid antibodies and the antiphospholipid syndrome. Clinical relevance in neuropsychiatric systemic lupus erythematosus. Ann N Y Acad Sci. 1997;823:279–288. doi: 10.1111/j.1749-6632.1997.tb48401.x. [DOI] [PubMed] [Google Scholar]
- 39.Emori E, Matsushima E, Aihara O, et al. Cognitive dysfunction in systemic lupus erythematosus. Psychiatry Clin Neurosci. 2005;59:584–589. doi: 10.1111/j.1440-1819.2005.01418.x. [DOI] [PubMed] [Google Scholar]
- 40.Denburg SD, Denburg JA. Cognitive dysfunction and antiphospholipid antibodies in systemic lupus erythematosus. Lupus. 2003;12:883–890. doi: 10.1191/0961203303lu497oa. [DOI] [PubMed] [Google Scholar]
- 41.Lapteva L, Nowak M, Yarboro CH, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2505–2514. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- 42.Monastero R, Bettini P, Del Zotto E, et al. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. J Neurol Sci. 2001;184:33–39. doi: 10.1016/s0022-510x(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 43.Denburg SD, Carbotte RM, Denburg JA. Cognitive impairment in systemic lupus erythematosus: a neuropsychological study of individual and group deficits. J Clin Exp Neuropsychol. 1987;9:323–339. doi: 10.1080/01688638708405054. [DOI] [PubMed] [Google Scholar]
- 44.Sonies B, Klippel J, Gerber R, Gerber C. Cognitive performance in systemic lupus erythematosus, abstracted. Arthritis Rheum. 1982;25 Suppl.:S80. [Google Scholar]
- 45.Kozora E, Ellison M, West S. Depression, fatigue and pain in systemic lupus erythematosus (SLE): Relationsihp to the American College of Rheumatology SLE neuropsychological battery. Arthritis Rheum. 2006;55:628–635. doi: 10.1002/art.22101. [DOI] [PubMed] [Google Scholar]
- 46.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 47.DiFrancesco M, Holland S, Ris M, et al. Functional magnetic resonance imaging assessment of cognitive function in childhood onset systemic lupus eryhematosus: a pilot study. Arthritis Rheum. 2007;56:4151–4163. doi: 10.1002/art.23132. [DOI] [PubMed] [Google Scholar]
- 48.Hanly JG, Walsh NM, Sangalang V. Brain pathology in systemic lupus erythematosus. J Rheumatol. 1992;19:732–741. [PubMed] [Google Scholar]
- 49.Fisk JD, Eastwood B, Sherwood G, Hanly JG. Patterns of cognitive impairment in patients with systemic lupus erythematosus. Br J Rheumatol. 1993;32:458–462. doi: 10.1093/rheumatology/32.6.458. [DOI] [PubMed] [Google Scholar]
- 50.Standards for Educational and Psychological Testing. New York: American Psychological Association; 1999. [Google Scholar]
- 51.Filley CM. White matter: Organization and functional relevance. Neuropsychol Rev. 2010;20:158–173. doi: 10.1007/s11065-010-9127-9. [DOI] [PubMed] [Google Scholar]
- 52.Harrison MJ, Morris KA, Horton R, et al. Results of intervention for lupus patients with self-perceived cognitive difficulties. Neurology. 2005;65:1325–1327. doi: 10.1212/01.wnl.0000180938.69146.5e. [DOI] [PubMed] [Google Scholar]
- 53.Ardila A, Rosselli M, Puente M. Neuropsychological evaluation of the Spanish speaker. New York: Plenum Press; 1994. [DOI] [PubMed] [Google Scholar]
- 54.Ferraro F. Minority and cross-cultural aspects of neuropsychological assessments. Herreweg, Lisse, The Netherlands: Swets & Zeitlinger; 2001. [Google Scholar]
- 55.Figueroa R. Psychological testing of linguististic-minority students: Knowledge gaps and regulations. Except Child. 1989;56:145–152. doi: 10.1177/001440298905600206. [DOI] [PubMed] [Google Scholar]
- 56.Duran R. Assessment and instruction of at-risk Hispanic students. Except Child. 1989;56:154–158. doi: 10.1177/001440298905600207. [DOI] [PubMed] [Google Scholar]
- 57.Klein-Gitelman MS, Zelko F, Kress A, Hunter S, Wagner-Weiner L. Comparison of Neuro-Cognitive Function in Children with Pediatric Systemic Lupus Erythematosus (pSLE) and Their Peers - Second Year Follow Up. Arthritis Rheum. 2002;46:S216. [Google Scholar]
- 58.Brooks BL, Strauss E, Sherman EMS, Iverson GL, Slick DJ. Developments in neuropsychological assessment: Refining psychometric and clinical interpretive methods. Can Psychol. 2009;50:196–209. [Google Scholar]