Abstract
Objective
To evaluate the association of exercise and knee-bending activities with magnetic resonance (MR)-based knee cartilage T2 relaxation times and morphologic abnormalities in asymptomatic subjects with and without osteoarthritis (OA) risk factors from the Osteoarthritis Initiative.
Methods
We studied 128 subjects with knee OA risk factors and 33 normal controls aged 45–55 years, with a body mass index of 18–27 kg/m2, and no knee pain. Subjects were categorized by exercise level, using the leisure activity component of the Physical Activity Scale for the Elderly, and by self-reported frequent knee-bending activities. Two radiologists graded the cartilage using the Whole Organ MR Imaging Score (WORMS) of right knee MR images. Cartilage was segmented and compartment specific T2 values were calculated. Statistical significance between the exercise groups and knee-bending groups was determined using multiple linear and logistic regression models.
Results
Among subjects with risk factors, light exercisers had lower T2 measurements than sedentary and moderate-strenuous exercisers (p=0.001); in women, moderate-strenuous exercise was associated with higher T2 values (p=0.001). Subjects without risk factors displayed no significant differences in T2 values by exercise. However, frequent knee-bending activities were associated with higher T2 values in both groups (p<0.02) and more severe cartilage lesions in the group with risk factors (p<0.001).
Conclusions
In subjects at risk for OA, light exercise was associated with low T2 measurements, whereas moderate-strenuous exercise in women was associated with high T2 values. Higher T2 values and WORMS grades were also found in frequent knee-benders and suggest greater cartilage degeneration in these individuals.
Introduction
As knee osteoarthritis (OA) is progressive and no effective therapies exist to regenerate cartilage (1), the identification of modifiable risk factors and protective factors is crucial in the development of preventive strategies. Physical activity is one such factor, but its association with the onset of OA remains unclear as some studies suggest a detrimental effect on articular cartilage (2,3) while others suggest either no effect (4) or a beneficial one (5,6).
Loading is necessary for healthy cartilage to develop and function properly (7,8), but excessive loading may lead to cartilage deterioration over time. In addition, physical activity may affect knee articular cartilage of asymptomatic individuals with OA risk factors – genetic predisposition, previous knee surgery or injury (9,10) – differently from those without risk factors. Subjects with and without risk factors have been analyzed together in a number of the previous studies evaluating the association of physical activity and OA development, which could, in part, explain the conflicting findings (2,5). There is a paucity of data investigating how different levels of habitual physical activity affect knee articular cartilage in asymptomatic subjects with OA risk factors versus those without them.
Magnetic resonance imaging (MRI) is the best imaging technique to non-invasively visualize articular cartilage (11); therefore, it is frequently used to assess focal abnormalities of the cartilage (12) and to evaluate cartilage volume and thickness (13,14). MRI also provides a method to non-invasively detect and quantify the cartilage biochemical changes that precede morphological deterioration (15–17). These early changes, which are quantifiable through T2 relaxation time mapping, include increase of water content and alterations in the collagen content and architecture (16,18). As T2 measurements detect the early biochemical shifts in the cartilage matrix prior to irreversible morphologic changes, it is a modality that may be well suited to evaluate the effects of risk factors, such as physical activity, on cartilage health.
Using the publicly accessible data and images from the Osteoarthritis Initiative, a large NIH-funded multicenter longitudinal cohort study of risk factors and biomarkers for the onset and progression of knee OA, the goal of our study was to assess the cross-sectional association of (i) exercise and (ii) knee-bending activities with knee cartilage T2 relaxation times and morphologic abnormalities determined from knee MRIs of middle-aged, asymptomatic subjects with and without risk factors for knee OA.
Materials and Methods
Subjects
The data used in the preparation of this article was obtained from the Osteoarthritis Initiative (OAI) database, which is available for public access at http://www.oai.ucsf.edu/. In this cross-sectional study, we analyzed subsets of subjects from the large incidence cohort and the small normal control cohort. All subjects signed informed consents approved by the local institutional review boards. Baseline clinical and image datasets 0.2.2 and 0.E.1 were used in this study.
Subjects in the OAI incidence cohort were asymptomatic at baseline; symptomatic knee OA was defined as frequent knee symptoms and tibiofemoral osteophytes in the same knee. But these subjects had one or more knee OA risk factors, which included: previous knee injury or surgery, family history of total knee replacement, Heberden’s nodes, and occasional knee symptoms (pain, aching, or stiffness in or around the joint in the past 12 months, but not on most days for at least one month). Although malalignment is also a known risk factor, it was not included because the baseline data collected by the OAI only included goniometer alignment readings, which have been found to be inaccurate (19). Subjects in the normal cohort were asymptomatic, had no tibiofemoral osteophytes or joint space narrowing, and did not have OA risk factors. We selected subjects from both cohorts with (a) body mass index (BMI) of 18–27 kg/m2, (b) baseline Western Ontario and McMaster University (WOMAC) Osteoarthritis Index pain score of zero for both knees, (c) age range of 45–55 years, and (d) Kellgren-Lawrence grade ≤1. Exclusion criteria included rheumatoid arthritis, severe knee joint space narrowing, and incomplete dataset from the OAI database.
The subjects’ physical activity levels were determined using a modified version of the Physical Activity Scale for the Elderly (PASE). It is a well-established, reliable, validated questionnaire that has been used to measure physical activity in individuals of similar age to those investigated in the current study (20–22). Subjects completed the questionnaire the same day as their MRI scan. PASE addresses 3 domains of physical activity over the previous seven days, including leisure, household, and occupational activities. In order to more thoroughly investigate the relationship between exercise and cartilage degeneration, we evaluated the leisure activity component of the PASE score, which assesses the amount of sitting, walking, light, moderate, and strenuous sport/recreation, and muscle strength training each subject performs. Prior to our analysis, a multidisciplinary team (musculoskeletal radiologist, Ph.D. physical therapist, and registered nurse) derived the exercise level classification defined in Table 1. Exercise is thought to have a potentially negative effect on knee cartilage primarily because of increased joint forces. Therefore, we sought to compare subjects who engaged in frequent higher impact exercise (such as running), frequent lower impact exercise (such as walking), and sedentary individuals in order to evaluate the association of exercise and cartilage degeneration. In order to obtain a clear depiction of this association, we intentionally excluded individuals whose exercise levels were between these definitions, such as those who engaged in seldom high impact exercise. Therefore, our exercise categories included E1: sedentary individuals, E2: light exercisers, and E3: moderate-strenuous exercisers.
Table 1.
Exercise Level Classification
| Exercise Level | Frequency of Activities | Examples of Activities | |
|---|---|---|---|
| E1 | Sedentary | Sitting activities and walk ≤2 days/wk for <2 hrs/day | Watch TV, read books, play on computer, play chess |
| E2 | Light Exercisers | Walk ≥3 days/wk for <2 hrs/day, or walk this amount and do light sport/recreation for <2 hrs/day on any given day | Walking, darts, table tennis, catch, fishing, golf w/a cart, frisbee, bowling |
| E3 | Moderate - Strenuous Exercisers | Moderate or strenuous sport/recreation ≥3 days/wk for >1 hr/day | Running, basketball, tennis, soccer, skiing, cycling, surfing, squash |
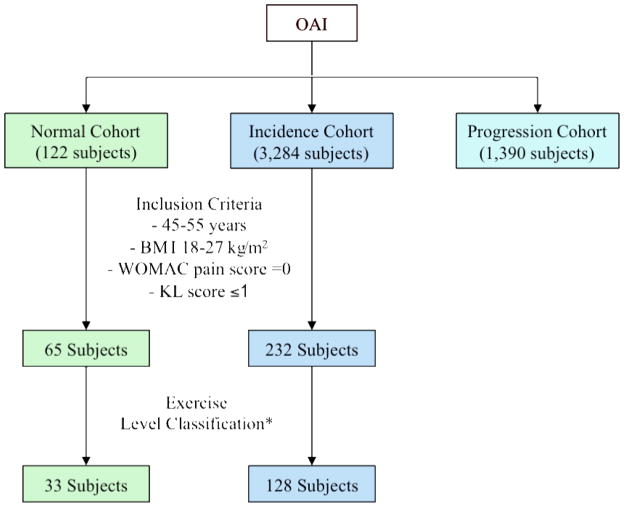
After the inclusion and exclusion criteria were applied, there were 234 incidence cohort subjects and 65 normal cohort subjects. Applying the exercise level classification, defined in Table 1, eliminated 104 incidence and 32 normal cohort subjects. Therefore, the final study population consisted of 128 incidence cohort subjects and 33 normal cohort subjects (Figure 1).
Figure 1.
The diagram illustrates how the subcohorts of our study were created. Based on our inclusion criteria and exercise level classification, 128 incidence cohort subjects and 33 normal cohort subjects were included. *The exercise level classification, which is outlined in table 1, was formulated prior to analysis with the goal of comparing frequent higher impact exercisers, frequent lower impact exercisers, and sedentary individuals.
In addition, as part of the eligibility interview conducted approximately one month prior to the MRI exam, subjects were asked whether they engaged in frequent knee-bending activities on most days during the previous 30 days, either at work or outside of work. Frequent knee-bending activities included: 1) climbing up ≥10 flights of stairs per day, 2) kneeling for ≥30 minutes per day, 3) squatting or deep knee bending for ≥30 minutes per day, and 4) lifting or moving objects weighing ≥25 pounds. Subjects who performed at least one of these activities were classified as frequent knee-benders, and the rest were not frequent knee-benders.
Bilateral Radiographs
Bilateral standing posteroanterior (PA) “fixed flexion” knee radiographs were obtained using a plexiglass frame (SynaFlexerTM). The knees had 20°–30° of flexion and the feet had 10° of internal rotation. A focus-to-film distance of 72 inches was used. Baseline knee radiographs were evaluated by two radiologists in consensus and graded using the Kellgren-Lawrence (KL) grading scale (23).
Magnetic Resonance Imaging
MR images were obtained with one of four identical 3T MRI systems (Trio; Siemens, Erlangen, Germany) using a standard knee coil. The following standard morphologic sequences and T2-mapping sequences of the right knee were analyzed: (a) coronal intermediate-weighted (IW) 2-D fast spin-echo (FSE) (repetition time/echo time (TR/TE)=3700/29 ms), (b) sagittal 3-D dual-echo in steady-state (DESS) with selective water excitation (TR/TE=16.3/4.7 ms, flip angle=25°), (c) sagittal 2-D IW FSE with fat suppression (TR/TE=3200/30 ms), and (d) sagittal T2-weighted 2-D multiecho spin-echo (SE) (TR=2700 ms, TE=10/20/30/40/50/60/70 ms), as previously described in detail (24,25).
Image Analysis
T2 Measurements
T2 maps were created using the sagittal 2-D multiecho SE images of the right knee, which were transferred to a remote workstation (SPARC; Sun Microsystems, Mountain View, CA, USA). Images were analyzed on the remote workstation using in-house spline-based software with an interactive display language (Research Systems, Boulder, CO, USA) environment. An interactive display language routine was used for segmentations of the patella, medial femoral condyle (MFC), lateral femoral condyle (LFC), medial tibia (MT), and lateral tibia (LT) to simplify the manual drawing of spines delineating cartilage areas. We segmented the whole compartment of each region on all slices with well-visualized artifact-free cartilage. The trochlea was not segmented because of the interfering flow artifacts from the popliteal artery. Tissue contrast was excellent and water-fat shift artifacts occurring at the bone-cartilage interface were well visualized on the first echo time images of the multiecho sequence, whereas fluid was well visualized on the sagittal T2 maps. To exclude both fluid and water-fat shift artifact from the regions of interest, adjustment of the splines was performed simultaneously by opening both image panels and using a synchronized cursor, section number, and zoom. An interactive display language routine was used to calculate the mean T2 values from the regions of interest created in the T2 maps. Additionally, the mean T2 value for the tibiofemoral (TF) joint was calculated by averaging the mean T2 values of the MFC, MT, LFC, and LT.
The T2 relaxation time was approximated by fitting an exponential function to the signal intensity at different echo times as follows: SI(TE) ~ exp(−TE/T2), where SI(TE) is the signal intensity as a function of echo time and T2 is the transverse relaxation time. A monoexponential decay model was used (21).
Semi-quantitative Morphologic Analysis
Two radiologists with 20 and 4 years of experience in musculoskeletal imaging separately reviewed all right knee MR images on picture archiving and communication system (PACS) workstations (Agfa, Ridgefield Park, NJ, USA). Radiologists had access to all sequences acquired and there were no time constraints during the reading sessions. Consensus readings were performed if scores were not identical. A semi-quantitative modified Whole-Organ MR Imaging Score (WORMS) was used to evaluate the cartilage (25–27). Six compartments (patella, trochlea, medial femur, lateral femur, medial tibia, and lateral tibia) were assessed rather than the original 15 WORMS regions because relatively few lesions were expected in our asymptomatic populations. Abnormalities in cartilage morphology and signal intensity were scored using an eight-point scale: 0) normal thickness and signal intensity, 1) normal thickness but increased signal intensity, 2) partial-thickness focal defect <1cm at its greatest width, 2.5) full-thickness focal defect <1cm at its greatest width, 3) multiple areas of partial-thickness loss (grade 2) or a grade 2 defect >1cm in diameter but <75% of the region, 4) diffuse (≥75% of the region) partial-thickness loss, 5) multiple areas of full-thickness loss (grade 2.5) or a grade 2.5 lesion >1cm but <75% of the region, 6) Diffuse (≥75% of the region) full-thickness loss. Reducing WORMS gradings to 6 compartments could have potentially affected the number of grade 4 or grade 6 lesions. Grade 6 lesions, however, were not expected in this cohort with early disease, and grade 4 lesions are very rare, as full-thickness lesions generally are present before >75% partial-thickness lesions in one subcompartment occur.
Based on the MRI findings, the cartilage was classified as morphologically abnormal if it received a WORMS score of >1. The maximum cartilage WORMS grades were also determined.
Reproducibility Measurements
Reproducibility of cartilage WORMS grades was determined using a sample of 20 OAI image datasets that were each assessed twice by two radiologists. The intra-observer weighted kappa values of the patella, MFC, LFC, and LT ranged from 0.816 to 1.0, with the exception of the LT of reader 1 which equaled 0.606 and the LFC of reader 2 which equaled 0.460. The inter-observer weighted kappa values were: patella=0.800, MFC=0.934, LFC=0.444, and LT=0.554. The compartments with minimal disease had somewhat lower kappa values. The weighted kappa values of the MT could not be obtained, as there were not any non-zero WORMS grades. Additionally, reproducibility of the cartilage T2 measurements was determined using eight randomly selected subjects (normal cohort: 2 men, 2 women; incidence cohort: 2 men, 2 women) that were each segmented three times by the same individual. In order to assess the level of agreement, the root mean square (RMS) errors and absolute errors were calculated for each compartment according to the calculations outlined by Gluer et al. (28). The root mean square errors were: patella=0.88%, MFC=0.74%, MT=1.51%, LFC=0.81%, and LT=1.47%. The absolute errors in milliseconds were: patella=0.39 ms, MFC=0.38, MT=0.61, LFC=0.40, and LT=0.70. Therefore, the T2 measurements were highly reproducible.
Statistical Analysis
Statistical analysis was performed with JMP software version 7 (SAS Institute, Cary, NC, USA). Descriptive statistics were obtained and differences in subject characteristics were determined with ANOVA, independent t-tests, and chi-square tests. Multiple linear and logistic regression models were used to determine significant differences in T2 values and WORMS grades between the exercise and knee-bending groups and to adjust for the effects of age, sex, and BMI. A post-hoc analysis was performed with Student’s t-tests, when appropriate, to determine the pairwise significance between the exercise groups. Men and women in the incidence cohort were analyzed together and separately; there were too few subjects in the normal cohort to perform this analysis. In the incidence cohort, we also considered adjusting for the various OA risk factors to assess whether their inclusion influenced our results. But adjusting for the risk factors did not affect our conclusions qualitatively. Statistical significance was defined for all calculations as p <0.05.
Results
Subject Characteristics
The incidence and normal cohorts did not significantly differ in age, BMI, or PASE scores (p>0.05) (see Table 2); however, the normal cohort had more women than the incidence cohort (76% vs. 55%, respectively, p=0.034). The incidence cohort had significantly higher mean T2 values than the normal cohort in the tibiofemoral (p<0.001) and patellofemoral (p=0.009) joints. The sex-related differences in age, BMI, and PASE scores are displayed in Table 2, along with the breakdown of OA risk factors. We controlled for these differences during statistical analysis using multiple linear and logistic regression models. The 3 exercise groups and the 2 knee-bending groups did not significantly differ in age, BMI, or sex in either the incidence or normal cohorts.
Table 2.
Subject Characteristics†
| All | Women | Men | p value | |
|---|---|---|---|---|
| Incidence Cohort | (n=128) | (n=71) | (n=57) | t-test |
| Age (years) | 50.7 ± 2.7 | 50.8 ± 2.5 | 50.6 ± 3.0 | 0.621 |
| BMI (kg/m2) | 23.7 ± 2.0 | 23.2 ± 2.1 | 24.3 ± 1.6 | 0.003* |
| PASE | 190.8 ± 80.5 | 176.5 ± 80.8 | 208.5 ± 77.2 | 0.025* |
| Risk Factors‡ | ChiSq | |||
| History of Knee Injury | 36 (27%) | 17 (23%) | 19 (33%) | 0.264 |
| History of Knee Surgery | 12 (9%) | 2 (3%) | 10 (17%) | 0.004* |
| Heberden’s Nodes in Hands | 19 (14%) | 15 (20%) | 4 (7%) | 0.026* |
| Family History of Knee Replacement | 19 (14%) | 8 (11%) | 11 (19%) | 0.192 |
| Normal Cohort | (n=33) | (n=25) | (n=8) | t-test |
| Age (years) | 50.0 ± 2.8 | 49.8 ± 2.6 | 50.8 ± 3.7 | 0.394 |
| BMI (kg/m2) | 23.1 ± 2.2 | 22.8 ± 2.4 | 24.1 ± 1.3 | 0.133 |
| PASE | 169.5 ± 66.0 | 160.3 ± 71.0 | 196.9 ± 54.0 | 0.181 |
Values are expressed as mean ± SD unless otherwise noted
Expressed as number of subjects (percentage)
Significantly different if p<0.05
Exercise Level Classification Analyses
When T2 values were evaluated in the incidence cohort subjects categorized by exercise level (Table 3), the LT and tibiofemoral (TF) joint displayed significant differences, while the MFC was trending toward significance. Upon post-hoc analysis, the light exercisers (E2) had significantly lower mean T2 values than both the sedentary (E1) and moderate-strenuous exercisers (E3) in the LT (E2<E1: p=0.039, E2<E3: p=0.001), and had lower T2 values than the moderate-strenuous exercisers in the TF joint (E2<E3: p=0.006). When the sexes were analyzed separately, the only observed difference between men and women occurred in the MFC (women: p<0.001, men: p>0.05). Upon post-hoc analysis, the women moderate-strenuous exercisers had significantly higher T2 values than the light exercisers and sedentary individuals (E3>E1: p=0.007, E3>E2: p<0.001).
Table 3.
Exercise Level vs. T2 Values in the Incidence Cohort §
| Exercise Level | T2 Values | |||||
|---|---|---|---|---|---|---|
| TF Joint | MFC | MT | LFC | LT | Patella | |
| E1 (n=25) | 46.3 ± 3.0 | 50.3 ± 3.1 | 39.1 ± 3.7 | 49.0 ± 4.2 | 39.7 ± 3.4 | 44.6 ± 4.4 |
| E2 (n=49) | 45.4 ± 2.1 | 50.0 ± 3.2 | 38.6 ± 2.5 | 48.1 ± 3.1 | 38.1 ± 3.0 | 43.1 ± 3.5 |
| E3 (n=54) | 46.3 ± 2.5 | 51.1 ± 4.0 | 39.3 ± 2.8 | 49.0 ± 3.2 | 40.0 ± 3.1 | 44.6 ± 3.7 |
| p value† | 0.021^ | 0.081 | 0.368 | 0.201 | 0.004*^ | 0.142 |
Values are the mean ± SD unless otherwise noted. T2 values are in ms.
E1 = Sedentary, E2 = Light Exercisers, E3 = Moderate-strenuous Exercisers
Multiple linear regression analysis adjusted for sex, age, and BMI
Post-hoc analysis:
E1& E2,
E2 & E3,
E1 & E3 signficanctly different: p<0.05
The mean T2 values of the normal cohort did not significantly differ in any compartment, and cartilage WORMS grades did not significantly differ between the exercise groups in either the incidence or normal cohorts.
Frequent Knee-bending Activity Analyses
Subjects engaging in frequent knee-bending activities had significantly higher T2 values in both the incidence and normal cohorts and higher WORMS grades in the incidence cohort (Table 4). Frequent knee-benders in the incidence cohort had significantly higher T2 values in the TF joint, MFC, MT, LFC, and patella. Furthermore, these subjects had more severe cartilage lesions – based on higher maximum cartilage WORMS grades and a higher percentage of subjects with WORMS grade >1. When the sexes were analyzed separately, no notable differences were observed. In the normal cohort, frequent knee-benders showed significantly elevated T2 values in the TF joint, MFC and MT, but no significant differences in WORMS grades were detected.
Table 4.
Knee-bending Activities vs. T2 Values and WORMS†
| T2 Values | Frequent Knee-bending Activities | |||||
|---|---|---|---|---|---|---|
| Incidence Cohort | Normal Cohort | |||||
| No (n=37) | Yes (n=91) | p value‡ | No (n=16) | Yes (n=17) | p value‡ | |
| TF Joint | 43.3 ± 2.7 | 44.7 ± 2.4 | 0.002* | 39.8 ± 2.0 | 41.6 ± 2.8 | 0.009* |
| MFC | 49.4 ± 3.7 | 51.0 ± 3.5 | 0.004* | 47.0 ± 3.8 | 49.9 ± 3.4 | 0.010* |
| MT | 37.8 ± 3.1 | 39.4 ± 2.6 | 0.007* | 32.5 ± 1.7 | 35.4 ± 3.8 | 0.005* |
| LFC | 47.5 ± 3.5 | 49.1 ± 3.3 | 0.005* | 44.1 ± 2.6 | 45.1 ± 3.4 | 0.360 |
| LT | 38.8 ± 3.2 | 39.4 ± 3.3 | 0.247 | 35.5 ± 3.2 | 36.1 ± 3.4 | 0.282 |
| Patella | 42.6 ± 3.5 | 44.5 ± 3.8 | 0.011* | 41.5 ± 4.5 | 42.3 ± 5.4 | 0.897 |
| WORMS | ||||||
| Cartilage Max | 1.11 ± 1.33 | 2.35 ± 1.70 | <0.0001* | 1.82 ± 1.14 | 2.00 ± 1.84 | 0.589 |
| Cartilage >1§ | 32% | 68% | <0.0001* | 64% | 65% | 0.760 |
Values are the mean ± SD unless otherwise noted. T2 values are in ms.
Multiple linear regression analysis unless otherwise noted
Logistic regression analysis
Signficantly different if p<0.05. All analyses adjusted for sex, age, and BMI.
Discussion
The results of our study showed that in asymptomatic subjects with OA risk factors, light exercise was associated with lower tibiofemoral T2 relaxation times, which suggests that lower water content and more intact collagen architecture are associated with light exercise compared to sedentary lifestyle and moderate-strenuous exercise. In women, moderate-strenuous exercise was associated with higher MFC T2 values, whereas this relationship was not observed in males. Interestingly, we did not detect any significant differences in the T2 values or WORMS grades when our normal cohort was grouped by exercise level; thus, the cartilage and meniscus of subjects without OA risk factors may not be affected in the same manner by exercise level. Finally, as frequent knee-bending activities were associated with higher tibiofemoral T2 values in all subjects, and higher patellar T2 values and WORMS grades in those with OA risk factors, such activities appear to be associated with greater cartilage degeneration.
As T2 values are a measure of early biochemical cartilage degeneration (15,20), it could be postulated that light exercise may prevent deterioration of the cartilage collagen architecture and associated increased water content in asymptomatic individuals at risk for knee OA. Therefore, it is possible that lower impact exercise has a potential protective effect on tibiofemoral knee cartilage in this patient population. As everyone in the light exercise group walked ≥3 days/week for <2 hours/day, but only some did various other light recreational activities – darts, croquet, bowling – it is possible that frequent walking is the activity that best maintains cartilage biochemical homeostasis. These findings are in agreement with previous studies that have shown that aerobic walking can decrease pain and disability in subjects with knee OA (29,30), but to the best of our knowledge, its effects have not been evaluated in asymptomatic subjects at risk for OA.
In women with OA risk factors, moderate-strenuous exercise was associated with greater MFC cartilage degeneration at the molecular level – based on T2 relaxation times. Thus, it is possible that higher impact exercise further increases these women’s risk of developing knee OA. In contrast, MFC T2 values did not differ between exercise groups in male subjects. One possible explanation for this discrepancy between sexes is the difference in loading behaviors inherent to men and women (31). However, it is unclear if these differences during various activities are directly related to changes observed in the MFC in the current investigation. Interestingly, women do have a higher incidence of knee OA than men, and the most common location is the medial compartment (32,33). Other contributing factors could be the specific sport/recreational activities performed by each sex, the strength of knee stabilizing muscles, or the thinner cartilage and smaller joint surfaces in women (34).
In contrast to this study, Stehling et al., who evaluated a similar group of incidence cohort subjects in two previous studies, found that increased physical activity levels were correlated with higher patellar T2 values and WORMS grades (21,27). However, their subjects were grouped by total PASE scores. Total PASE scores take into account not only exercise, but also household and occupational activities, which the scoring system weighs more heavily than exercise. It is possible that an increased amount of household and occupational activities may be associated with increased knee-bending activities, which we found to have a potential negative impact on knee cartilage. This could explain our conflicting findings. Our study evaluated exercise and knee-bending activities separately. We derived an exercise classification that would allow us to compare the knee cartilage of sedentary individuals, frequent low impact exercisers, and frequent higher impact exercisers, and we analyzed T2 values in both the tibiofemoral and patellofemoral joints rather than only in the patella. From our knowledge, this is the first study investigating the association of exercise on knee health in asymptomatic subjects with risk factors for OA using quantitative and morphological MR parameters.
Similar to previous studies (4,35,36), we did not detect any differences in the cartilage of our normal cohort when analyzed by exercise levels. Recently, Chakravarty and colleagues examined 45 long distance runners and 53 controls and found that runners did not have more prevalent or severe OA when compared to controls (4). However, other studies have shown a protective (5,37,38) or detrimental (3,39) effect. It should be noted that the majority of previous studies did not consider OA risk factors in their analysis. Kujala and colleagues found that former soccer players were at increased risk of developing premature OA, but concluded that previous knee injuries contributed to this finding (39). Additionally, many studies used radiographs (4,36,38,39), which cannot depict cartilage, menisci and internal knee structures, while only a few used MRI (37,40,41). To the best of our knowledge only one other small study has examined the habitual effects of physical activity on quantitative T2 and/or T1rho values. Similar to our findings, Stahl et al. found no significant difference in T2 and T1rho values between 13 active and 7 sedentary asymptomatic healthy subjects, although they did not note whether any of the subjects had OA risk factors (41).
Frequent knee-bending activities were associated with higher tibiofemoral T2 values in both the incidence and normal cohorts, and higher patellar T2 values and more severe cartilage lesions – based on WORMS grades – in those with OA risk factors. Thus, it is possible that such activities are associated with greater cartilage degeneration in all individuals, but potentially to a greater degree in subjects already at risk for knee OA. Our data is consistent with several studies that have shown that knee-bending activities are risk factors for knee OA (42–46). During deep knee flexion, the stresses and loads in the knee dramatically increase (47). It has been reported in cadaver studies that deep knee flexion to 90 degrees can result in tibiofemoral joint stresses of 26.6 MPa, exceeding the threshold for which cartilage lesions are known to occur (47). Interestingly, the medial compartment experienced approximately 70% greater peak pressures during deep knee bending, which is consistent with the increased T2 values observed in the medial compartment in subjects who performed knee-bending activities in the current study. Additionally, with deep knee-bending activities, the patella also experiences high loads and may be subject to greater shear stresses during squatting than the femoral surfaces (48).
There are several limitations in the current study that should be noted. First, we did not have information about the specific activities performed by each subject. The PASE scale combines weight-bearing and non-weight-bearing sports into the same categories. Soccer and cycling would load the knee joint quite differently. Similarly, all knee-bending activities were grouped together. Second, given our inclusion and exclusion criteria, a relatively small sample of subjects (n=33) from the OAI database qualified as normal subjects. This limitation may have affected our ability to detect differences in the normal cohort. Third, the predictive value of T2 quantification to project future cartilage deterioration remains unclear. Fourth, our study only took into account current activity level rather than lifelong activity history. Clearly cartilage composition is influenced by recent and habitual loading behaviors.
Future studies will need to investigate which specific activities increase or decrease a person’s risk for developing knee OA. Populations with and without known OA risk factors should be evaluated separately in longitudinal studies that utilize MRI-based quantitative imaging methods (e.g. T2, T1rho, dGEMRIC), which detect cartilage damage prior to irreversible morphological changes. Knowledge of this early degeneration would allow for preventive measures to be explored. Furthermore, our results indicate that the cartilage of men and women may respond differently to physical activities; however, these findings warrant further investigation to better understand the causal relationships.
In summary, the cartilage of individuals with and without knee OA risk factors appears to respond differently to physical activity. In subjects at risk for OA, light exercise was associated with lower tibiofemoral T2 relaxation times in both sexes, whereas moderate-strenuous exercise was associated with higher T2 values in women. Therefore, if subjects have OA risk factors, light exercise – possibly frequent walking in particular – may aid in preventing biochemical cartilage degeneration, while moderate-strenuous exercise, particularly in women, may further increase their risk of developing knee OA. In our normal cohort grouped by exercise level, we did not detect any significant differences in T2 measurements or WORMS grades; however, this cohort was smaller. Finally, frequent knee-bending activities appear to be associated with greater cartilage degeneration in all individuals, but possibly to a greater degree in subjects already at risk for knee OA.
Acknowledgments
“The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. This study was also funded in part by the Intramural Research Program of the National Institute on Aging. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. In addition, the analyses performed in this study were funded through the National Institute of Arthritis and Musculoskeletal and Skin Diseases U01AR059507. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.”
References
- 1.Brandt KD, Doherty M, Lohmander LS, editors. Osteoarthritis. New York: Oxford University Press Inc; 1998. [Google Scholar]
- 2.Spector TD, Harris PA, Hart DJ, Cicuttini FM, Nandra D, Etherington J, et al. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum. 1996;39:988–95. doi: 10.1002/art.1780390616. [DOI] [PubMed] [Google Scholar]
- 3.Cheng Y, Macera CA, Davis DR, Aiinsworth BE, Troped PJ, Blair SN. Physical activity and self-reported, physician-diagnosed osteoarthritis: is physical activity a risk factor? J Clin Epidemiol. 2000;53:315–22. doi: 10.1016/s0895-4356(99)00168-7. [DOI] [PubMed] [Google Scholar]
- 4.Chakravarty EF, Hubert HB, Lingala VB, Zatarain E, Fries JF. Long distance running and knee osteoarthritis. A prospective study. Am J Prev Med. 2008;35:133–8. doi: 10.1016/j.amepre.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers LQ, Macera CA, Hootman JM, Ainsworth BE, Blairi SN. The association between joint stress from physical activity and self-reported osteoarthritis: an analysis of the Cooper Clinic data. Osteoarthritis Cartilage. 2002;10:617–22. doi: 10.1053/joca.2002.0802. [DOI] [PubMed] [Google Scholar]
- 6.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–14. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 7.Jones G, Ding C, Glisson M, Hynes K, Ma D, Cicuttini F. Knee articular cartilage development in children: a longitudinal study of the effect of sex, growth, body composition and physical activity. Pediatr Res. 2003;54:230–36. doi: 10.1203/01.PDR.0000072781.93856.E6. [DOI] [PubMed] [Google Scholar]
- 8.Vanwanseele B, Eckstein F, Knecht H, Stussi E, Spaepen A. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 2002;46:2073–78. doi: 10.1002/art.10462. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Chitnavis J, Sinsheimer JS, Clipsham K, Loughlin J, Sykes B, Burge PD, et al. Genetic influences in end-stage osteoarthritis. Sibling risk of hip and knee replacement for idiopathic osteoarthritis. J Bone Joint Surg Br. 1997;79:660–4. doi: 10.1302/0301-620x.79b4.7437. [DOI] [PubMed] [Google Scholar]
- 11.Burstein D, Gray M. New MRI techniques for imaging cartilage. J Bone Joint Surg Am. 2003;85:70–7. doi: 10.2106/00004623-200300002-00009. [DOI] [PubMed] [Google Scholar]
- 12.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226:373–81. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 13.Eckstein F, Cicuttini F, Raynauld J-P, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14:46–75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein F, Heudorfer L, Faber SC, Burgkar R, Englmeier K-H, Reiser M. Long-term and resegmentation precision of quantitative cartilage MR Imaging (qMRI) Osteoarthritis Cartilage. 2002;10:922–28. doi: 10.1053/joca.2002.0844. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Ma CB, Link TM, Castillo D-D, Blumenkrantz G, Lozano J, et al. In vivo T1ρ and T2 mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–97. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liess C, Lüsse S, Karger N, Heller M, Glüer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907–13. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 17.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–8. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dijkgraaf LC, de Bont LG, Boering G, Liem RS. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53:1182–92. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 19.Hinman RS, May RL, Crossley KM. Is there an alternative to the full-leg radiograph for determining knee joint alignment in osteoarthritis? Arthritis Rheum. 2006;55:306–13. doi: 10.1002/art.21836. [DOI] [PubMed] [Google Scholar]
- 20.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 21.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254:509–20. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein MG, Pinto BM, Marcus BH, Lynn H, Jette AM, Rakowski W, et al. Physician-based physical activity counseling for middle-aged and older adults: a randomized trial. Ann Behav Med. 1999;21:40–7. doi: 10.1007/BF02895032. [DOI] [PubMed] [Google Scholar]
- 23.Kellgren J, Lawrence J. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–501. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Peterfy CG, Gold G, Eckstein F, Cicuttini F, Dardzinski B, Stevens R. MRI protocols for whole-organ assessment of the knee in osteoarthritis. Osteoarthritis Cartilage. 2006;14:A95–111. doi: 10.1016/j.joca.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18:776–86. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5:262–70. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger WH, Jr, Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST) JAMA. 1997;277:25–31. [PubMed] [Google Scholar]
- 30.Roddy E, Zhang W, Doherty M. Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann Rheum Dis. 2005;64:544–8. doi: 10.1136/ard.2004.028746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigward SM, Powers CM. The influence of gender on knee kinematics, kinetics and muscle activation patterns during side-step cutting. Clin Biomech. 2006;21:41–8. doi: 10.1016/j.clinbiomech.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Iorio R, Healy W. Unicompartmental arthritis of the knee. J Bone Joint Surg. 2003;85:1351–64. doi: 10.2106/00004623-200307000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Otterness IG, Eckstein F. Women have thinner cartilage and smaller joint surfaces than men after adjustment for body height and weight. Osteoarthritis Cartilage. 2007;15:666–72. doi: 10.1016/j.joca.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Hootman JM, Macera CA, Helmick CG, Blair SN. Influence of physical activity-related joint stress on the risk of self-reported hip/knee osteoarthritis: a new method to quantify physical activity. Prev Med. 2003;36:636–44. doi: 10.1016/s0091-7435(03)00018-5. [DOI] [PubMed] [Google Scholar]
- 36.Felson DT, Niu J, Clancy M, Sack B, Aliabadi P, Zhang Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham study. Arthritis Rheum. 2007;57:6–12. doi: 10.1002/art.22464. [DOI] [PubMed] [Google Scholar]
- 37.Teichtahl AJ, Wluka AE, Forbes A, Wang Y, English DR, Giles GG, et al. Longitudinal effect of vigorous physical activity on patella cartilage morphology in people without clinical knee disease. Arthritis Rheum. 2009;61:1095–102. doi: 10.1002/art.24840. [DOI] [PubMed] [Google Scholar]
- 38.White JA, Wright V, Hudson AM. Relationships between habitual physical activity and osteoarthrosis in ageing women. Public Health. 1993;107:459–70. doi: 10.1016/s0033-3506(05)80172-6. [DOI] [PubMed] [Google Scholar]
- 39.Kujala UM, Kettunen J, Paananen H, Aalto T, Battié MC, Impivaara O, et al. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38:539–46. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 40.Krampla WW, Newrkla SP, Kroener AH, Hruby WF. Changes on magnetic resonance tomography in the knee joints of marathon runners: a 10-year longitudinal study. Skeletal Radiol. 2008;37:619–26. doi: 10.1007/s00256-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 41.Stahl R, Luke A, Li X, Carballido-Gamio J, Ma CB, Majumdar S, et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients--a 3.0-Tesla MRI study. Eur Radiol. 2009;19:132–43. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- 42.Jensen LK. Knee osteoarthritis: influence of work involving heavy lifting, kneeling, climbing stairs or ladders, or kneeling/squatting combined with heavy lifting. Occup Environ Med. 2008;65:72–89. doi: 10.1136/oem.2007.032466. [DOI] [PubMed] [Google Scholar]
- 43.Coggon D, Croft P, Kellingray S, Barrett D, McLaren M, Cooper C. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000;43:1443–9. doi: 10.1002/1529-0131(200007)43:7<1443::AID-ANR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Dahaghin S, Tehrani-Banihashemi SA, Faezi ST, Jamshidi AR, Davatchi F. Squatting, sitting on the floor, or cycling: are life-long daily activities risk factors for clinical knee osteoarthritis? Stage III results of a community-based study. Arthritis Rheum. 2009;61:1337–42. doi: 10.1002/art.24737. [DOI] [PubMed] [Google Scholar]
- 45.Amin S, Goggins J, Niu J, Guermazi A, Grigoryan M, Hunter DJ, et al. Occupation-related squatting, kneeling, and heavy lifting and the knee joint: a magnetic resonance imaging-based study in men. J Rheumatol. 2008;35:1645–9. [PMC free article] [PubMed] [Google Scholar]
- 46.Rytter S, Jensen LK, Bonde JP, Jurik AG, Egund N. Occupational kneeling and meniscal tears: a magnetic resonance imaging study in floor layers. J Rheumatol. 2009;36:1512–9. doi: 10.3899/jrheum.081150. [DOI] [PubMed] [Google Scholar]
- 47.Thambyah A, Goh JC, De SD. Contact stresses in the knee joint in deep flexion. Med Eng Phys. 2005;27:329–35. doi: 10.1016/j.medengphy.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Besier TF, Gold GE, Beaupré GS, Delp SL. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med Sci Sports Exerc. 2005;37:1924–30. doi: 10.1249/01.mss.0000176686.18683.64. [DOI] [PubMed] [Google Scholar]