Abstract
Purpose
We investigated the antitumor reactivity of adoptively transferred effector B cells and the mechanisms by which they may mediate tumor regression in a spontaneous metastases model.
Experimental Design
4T1 breast cancer cells were inoculated into the flanks of syngeneic Balb/C mice to prime draining lymph nodes. Tumor-draining lymph nodes (TDLN) were harvested and B cells activated ex vivo with LPS and anti-CD40 mAb. These activated B cells were adoptively transferred into mice inoculated with 4T1 tumor in the mammary fat pad. The induction of host T cell immunity was evaluated.
Results
Activated 4T1 TDLN B cells secreted IgG in response to tumor cells which was immunologically specific. These activated B cells were capable of mediating specific lysis of tumor cells in vitro. Transfer of these activated B cells alone mediated the inhibition of spontaneous metastases to the lung. Examination of the host revealed that the transfer of these B cells resulted in the induction of tumor specific T cell immunity as measured by cytotoxicity and cytokine (IFNγ and GM-CSF) production. The combined transfer of activated T and B cells from TDLN resulted in tumor regression, which was greater than either cell population alone, with host B cells capable of producing IgG that mediated lysis of tumor in the presence of complement.
Conclusions
We have found that appropriately primed B cells can mediate tumor regression by itself and confers host T cell antitumor immunity. Furthermore, effector B cells can serve as a useful adjunct in adoptive T cell therapy.
Keywords: B Cells, T Cells, Spontaneous metastasis, CTL, Cancer Immunotherapy
Introduction
The role played by B cells in the host immune response to cancer is complex and controversial. Depending upon their state of activation, B cells have had divergent roles on T cell differentiation and effector function. In tumor models, resting B cells have been reported to suppress T cell-mediated antitumor immunity 1–4. As opposed to resting B cells, several reports have indicated the efficacy of activated B cells in cellular immunotherapy of malignancies 5–11. Some of these reports have been focused on how activated B cells can be used as effective antigen presenting cells for T cell sensitization 5–9, 12, 13. It was reported that CD4 T cell therapy of cancer did not work effectively in B−/− mice 14, and that T cells by themselves were not enough in suppressing tumor growth in cancer patients 15. It was also found that B cells were required for optimal CD4+ and CD8+ T cell anti-tumor immunity, and depletion of therapeutic B cells enhanced tumor growth in mice 10. In addition, B cells have recently been found to play an important role in resisting infectious pathogens 16, 17.
We hypothesize that any successful cancer treatment strategy will have to appropriately stimulate both humoral and cellular immune responses. To date, the predominant investigative focus of adoptive immunotherapy for cancer has been understanding the mechanisms involved in the induction, activation, proliferation, and trafficking of effector T cells. We have previously shown that approximately 60% of tumor-draining lymph node (TDLN) cells are CD3+ T cells. In vitro activation of TDLN cells with anti-CD3/anti-CD28 mAbs results in the generation of therapeutic effector T cells (>90% CD3+ cells) 18–21. These effector T cells require in vivo priming with tumor, since normal lymph nodes or splenocytes from non-tumor bearing mice cannot be secondarily activated to differentiate into tumor reactive cells.22 We have also reported that simultaneous targeting of CD3 on TDLN T cells and CD40 on TDLN B cells, which comprise > 30% of the lymph node cells, augments the antitumor reactivity of tumor-primed LN cells 23. These studies established a role for engaging CD40 on TDLN B cells as APCs in the generation of effector T cells. We have also found that IL-21 augments the efficacy of T cell therapy by eliciting concurrent cellular and humoral responses 24. This study confirmed an interactive role between tumor-specific humoral responses related to IL-21 administration and adoptively transferred effector T cells that resulted in more effective tumor eradication. More recently, we reported that TDLN B cells can be therapeutic effector cells in cancer adoptive immunotherapy of either pulmonary metastases or sc tumors using two experimentally induced histologically distinct tumor models (B16-D5 and MCA 205) in B6 hosts 25. However, whether or not the adoptively transferred therapeutic B cells could induce host T cell immunity was not examined.
Metastasis represents one of the characteristics of cancer and has remained a major reason for the ineffectiveness of therapy. In this report, we investigated the ability of inhibiting cancer spontaneous metastases with B effector cells alone and in combination with T cells. We used the murine 4T1 breast cancer model. Inoculating 4T1 cells into the mammary fat pad results in the development of spontaneous pulmonary metastases. Using this model, we have documented the therapeutic efficacy of in vivo tumor-primed and in vitro activated B cells, as well as B cell therapy-conferred systemic T cell antitumor immunity in this study.
Materials and Methods
Mice
Female Balb/c mice were purchased from the Jackson Laboratories, Bar Harbor, ME. They were maintained in a pathogen-free environment and used at age 8 weeks or older. Principles of laboratory animal care (NIH publication No. 85-23, revised 1985) were followed. The University of Michigan Laboratory of Animal Medicine approved all the animal protocols.
Murine tumor cells
4T1 is a mammary carcinoma syngeneic to Balb/c mice (kindly provided by Dr. M. Sabel, University of Michigan). Inoculating 4T1 cells into the mammary fat pad results in the development of spontaneous pulmonary metastases. MT-901 (a subline of dimethylbenzantracene-induced mammary carcinoma); Renca (a kidney cancer cell line) and TSA (a highly aggressive mammary adenocarcinoma) are all syngeneic to Balb/c mice and used as specificity controls. Tumor cells were maintained in vitro in complete medium (CM).
Tumor Draining Lymph Nodes (TDLN)
To induce TDLN, 1×106 4T1 tumor cells in 0.1 ml PBS were injected subcutaneously (s.c) into the lower flanks of normal Balb/c mice. The draining inguinal lymph nodes were collected 9 days later and processed using mechanical dissociation. Multiple inguinal TDLNs were pooled from groups of mice for lymphoid cell suspension preparation. CD3+ T cells and CD19+ B cells were purified from TDLN cells or splenocytes by using antibody-coupled Microbeads and MACS separator (Miltenyi Biotec. Inc., Sunnyvale, CA).
T and B cell activation and expansion
TDLN T cells and/or B cells were activated with immobilized anti-CD3 plus anti-CD28 mAbs in CM containing hrIL-2 and/or LPS (Sigma-Aldrich, St. Louis, MO) plus anti-CD40 (FGK45) mAb ascites as previously described 25. Activated and expanded TDLN T and/or B cells were used for adoptive immunotherapy, phenotype and immune function analyses. To test systemic immune responses post TDLN T and/or B cell adoptive transfer, spleens were harvested at the end of therapy. Purified splenocyte T and/or B cells were activated as described for TDLN cells.
Cytokine and antibody production assessment
Supernatants at the end of TDLN cell or splenocyte activation were collected and analyzed for IFNγ, GM-CSF, and IgM, IgG production using ELISA (BD PharMingen San Diego, CA). In addition, we tested IgG and IFNγ production in response to tumor antigen. After activation and expansion, 1×106 TDLN or splenocyte effector T and/or B cells were co-cultured with 2.5×105 irradiated (6,000 cGy) tumor stimulator cells in 24-well tissue culture plates for 24 hours. The supernatant was then collected and analyzed for the production of IgG and IFNγ in response to tumor.
Adoptive T and/or B cell therapy of spontaneous pulmonary metastasis
Healthy Balb/c mice were inoculated with 5×104 4T1 cells into the mammary fat pad to induce spontaneous pulmonary metastases. Two weeks after tumor inoculation, tumor-bearing mice were treated with tail vein i.v. injection of activated 4T1 TDLN T and/or B cells. Commencing on the day of the effector cell transfer, intraperitoneal (i.p.) injections of IL-2 (40,000 IU) were administered in 0.5 ml of PBS and continued twice daily for 8 doses. Approximately 2 weeks after T and/or B cell transfer, all mice were sacrificed, and lungs were harvested for enumeration of spontaneous pulmonary metastatic nodules.
Flow cytometry analysis
Cell surface expression of CD3, CD19 was analyzed by immunofluorescence assay. All FITC- or PE-conjugated antibodies were from BD PharMingen. Binding of IgG produced by splenocyte T+B or B cells to tumor cells was detected using FITC-anti-mouse IgG (BD Biosciences) following the incubation of tumor cells with splenocyte T+B or B cell culture supernatants.
Antibody and complement-mediated cytotoxicity
Tumor cell lysis mediated by antibodies produced by splenocytes obtained from the treated host was assessed by incubating tumor cells with splenocyte T+B or B cell culture supernatants in test tubes on ice for one hour followed by cell culture in the presence of rabbit complement (CalBiochem, Darmstadt, Germany) in a 37°C water bath for another hour. Viable cells were then counted after trypan blue staining to calculate cell lysis.
CTL cytotoxicity
CTL cytotoxicity against 4T1 tumor cells was tested using the LDH-release assay (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI) according to the manufacturer’s protocol. The assays were performed in 96-well round-bottom plates. CTLs were generated from the splenocytes and PBMCs harvested from B cell-treated tumor-bearing mice and expanded in IL-2 with anti-CD3/anti-CD28 activation. Cytolytic B cells were generated by LPS/anti-CD40 activation of in vivo-primed (TDLN) B cells. Cytotoxicity was calculated using the following formula:
Statistical Analysis
The significance of differences in numbers of metastatic nodules; the concentration of cytokine and immunoglobulins, and cell lysis was determined using one-way analysis of variance (Newman-Keuls post hoc test). P values of < 0.05 were considered statistically significant between the experiment groups.
Results
1. Tumor-primed and ex vivo activated 4T1 TDLN cells produce antibody and cytokine spontaneously and in response to 4T1 tumor
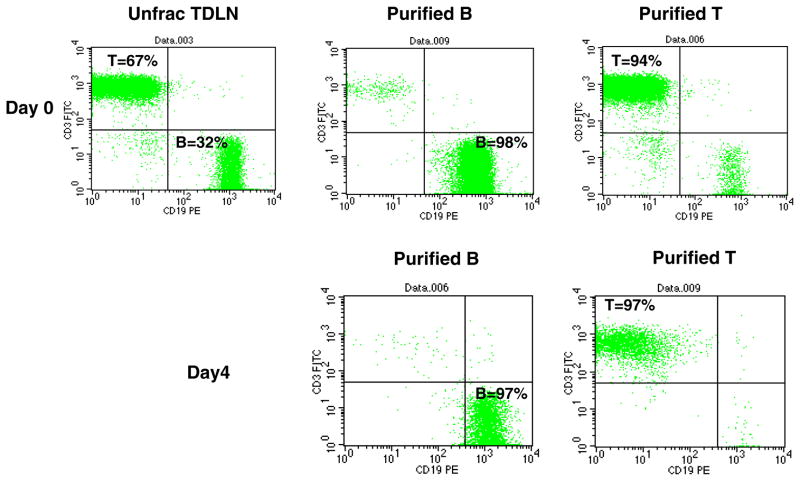
We obtained the 4T1 mammary carcinoma which when inoculated into the mammary fat pad of the Balb/c host will spontaneously metastasize to the lungs. We evaluated the ability to generate B as well as T effector cells using this model. 4T1 tumor cells were inoculated into the flanks of mice to generate tumor-draining lymph nodes (TDLN). These LNs were harvested 9-days later and T and B cells purified using immunomagnetic beads. Flow cytometry analysis indicated that in addition to the 60–70% of CD3+ T cells in the TDLN (Fig 1, Day 0), the remainder of the approximately 30–40% TDLN cells are CD19+ B cells. Enrichment using the antibody-coupled magnetic beads resulted in highly purified B cells and T cells (94%–98%). Purified TDLN B cells and T cells were activated with LPS plus anti-CD40 for B cells and anti-CD3/anti-CD28 plus IL-2 for T cells, respectively. Post in vitro activation and expansion (Fig 1, Day 4), B cells remained CD19+ (97%) while T cells remained CD3+ (97%). After exposure of unfractionated TDLN cells to LPS, the CD19+ B cell precentage increased to approximately 70%, suggesting that LPS stimulates B cell proliferation (data not shown). Activation with anti-CD40 or LPS/anti-CD40 resulted in significant decreases in CD38 and CD23 B cell markers, indicating that anti-CD40 promotes B cell differentiation (data not shown).
Fig. 1.
Purification of T, B cells from 4T1 TDLN and the phenotype of these cells post in vitro activation and expansion. Unfractionated TDLN cells prepared from freshly harvested 4T1 TDLNs and the purified TDLN B cells and T cells were double-stained with FITC-anti-CD3 and PE-anti-CD19 (Day 0). Activated and expanded cells were double stained similarly (Day 4). Data are representative of three experiments performed.
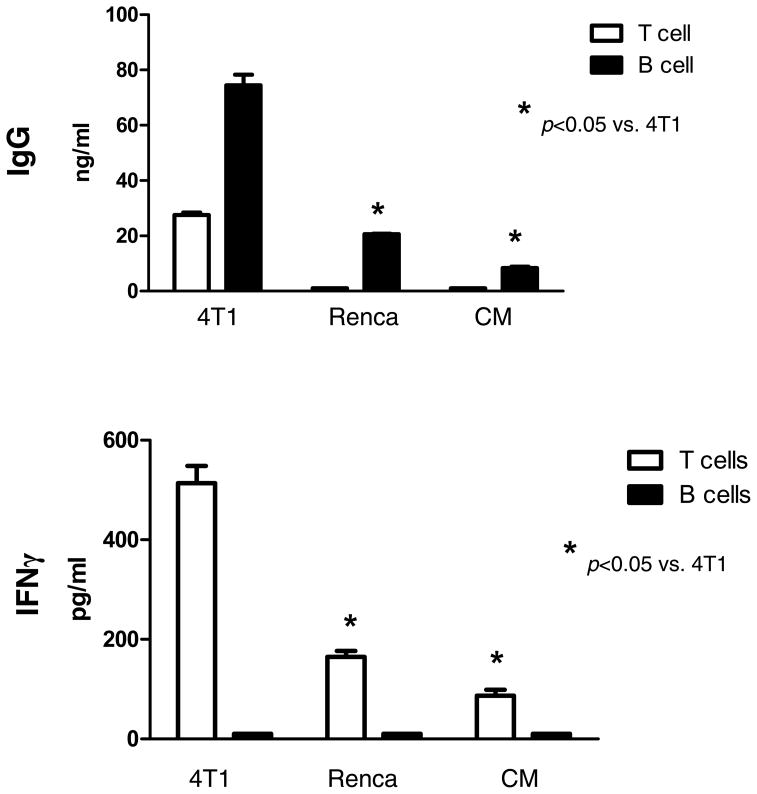
The culture supernatants were collected and assessed for cytokines and Ig production. Activated T cells secreted large amounts of IFNγ and GM-CSF as opposed to B cells which did not (data not shown). By contrast, large amounts of IgM and IgG were produced by activated B cells and not by T cells as expected. To determine if the activated 4T1 TDLN cells were reactive to tumor antigen, we performed co-culture experiments of the activated TDLN cells with irradiated tumor cells and assessed the release of IgG and IFNγ (Fig 2). Activated B cells secreted significantly large amounts of IgG in response to 4T1 tumor cells compared with their culture in CM. Importantly, this elicited IgG production was tumor antigen specific. This was revealed by the use of irrelevant Renca tumor cells in the same experiment. On the other hand, activated T cells when cultured with irradiated 4T1 tumor cells produced increased IFNγ compared to the absence of tumor cells (CM) or to the irrelevant Renca tumor cells. These experiments indicated that tumor-primed and ex vivo activated 4T1 TDLN B cells and T cells produce antibody and cytokine in response to the 4T1 tumor cells specifically.
Fig. 2.
Tumor antigen-specific IgG and IFNγ production of TDLN cells in response to tumor. B and T cells purified from 4T1 TDLNs were activated with LPS/anti-CD40 or anti-CD3/CD28/IL2 respectively. Activated 4T1 TDLN B cells and T cells (1×106) were then re-stimulated with irradiated 4T1 tumor cells. The culture supernatants were collected and analyzed for the secretion of IgG and IFNγ. Renca served as an irrelevant tumor antigen control. Data represent two experiments performed.
2. Adoptively transferred TDLN B effector cells inhibit 4T1 spontaneous pulmonary metastases by conferring host T cell anti-4T1 immunity
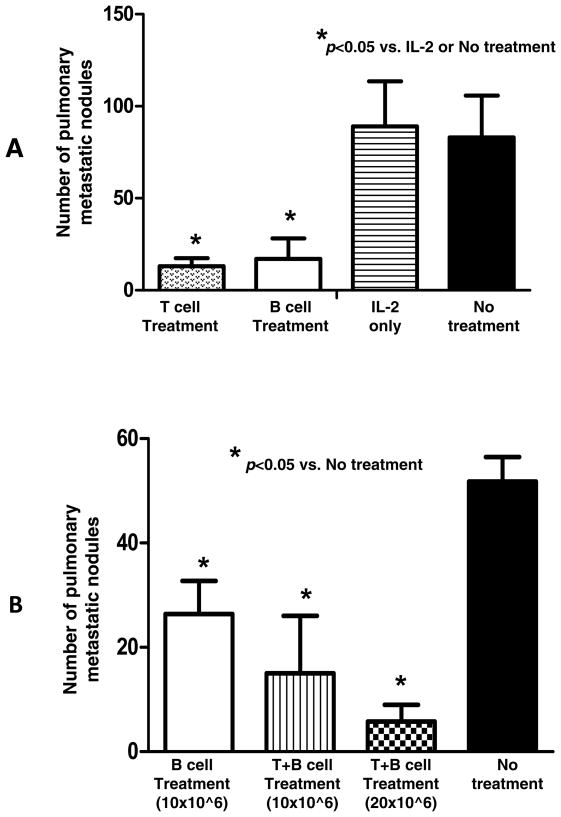
We proceeded to assess the in vivo antitumor reactivity of these B effector cells in adoptive immunotherapy experiments. Balb/c mice were inoculated with 4T1 cells in the mammary fat pad and 14 days later were treated by the adoptive transfer of activated 4T1 TDLN T or B cells i.v. Approximately 2 weeks later, mice were euthanized and the pulmonary metastases enumerated. The results of this experiment are summarized in Fig 3A. Infusion of 3×106 activated T or B cells alone each resulted in significant (p<0.05) reduction of the spontaneous 4T1 metastases. To test potential host T cell-mediated 4T1 cytotoxicity after TDLN B cell adoptive immunotherapy, we isolated PBMCs from the 4T1 tumor-bearing host subjected to TDLN B cell treatment, IL-2 treatment or no treatment. PBMCs were cultured in low concentrations of IL-2 (60 IU/ml) in the presence of anti-CD3/CD28. The resultant cells were approximately 90% T cells (data not shown) as we have achieved in our previous studies 18, 25. Such activated cells were then cultured with 4T1 tumor target cells. The lysis of the target cells was analyzed by the LDH release assay as described in Materials and Methods. As shown in Fig. 4, CTLs generated from the PBMCs harvested from hosts subjected to 4T1 TDLN B cell treatment killed 4T1 tumor cells significantly (p<0.05) more than CTLs prepared from hosts subjected to IL-2 treatment or no treatment. This CTL activity was tumor antigen specific as revealed by the decrease lysis of two irrelevant tumor targets.
Fig. 3.
A. Adoptively transferred 4T1 TDLN B cells mediated effective inhibition of the spontaneous pulmonary metastasis of 4T1 breast cancer cells injected into the fat pad. Balb/c mice were inoculated into the fat pad with 4T1 tumor cells. Two weeks later, the tumor-bearing mice were treated with tail vein injection of 4T1-primed and in vitro activated 4T1 TDLN T cells or B cells. Approximately 14 days after TDLN cell transfer, all mice were randomized and sacrificed, and lungs were harvested for enumeration of pulmonary metastatic nodules. B. Adoptively transferred 4T1 TDLN T+B cells mediated inhibition of the spontaneous pulmonary metastasis of 4T1 in a dose dependent manner. Spontaneous 4T1 pulmonary metastases were treated with 4T1-primed and in vitro activated 4T1 TDLN B cells or T + B cells.
Fig. 4.
CTL-mediated 4T1 cytotoxicity. CTLs were generated using PBMCs harvested from the 4T1 tumor-bearing hosts subjected to 4T1 TDLN B cell treatment. The effector: tumor target ratio is 25:1. CTL cytotoxicity against 4T1 tumor cells was measured using the LDH-release assays. MT-901 and TSA served as specificity control targets. Data are representative of two independent experiments conducted.
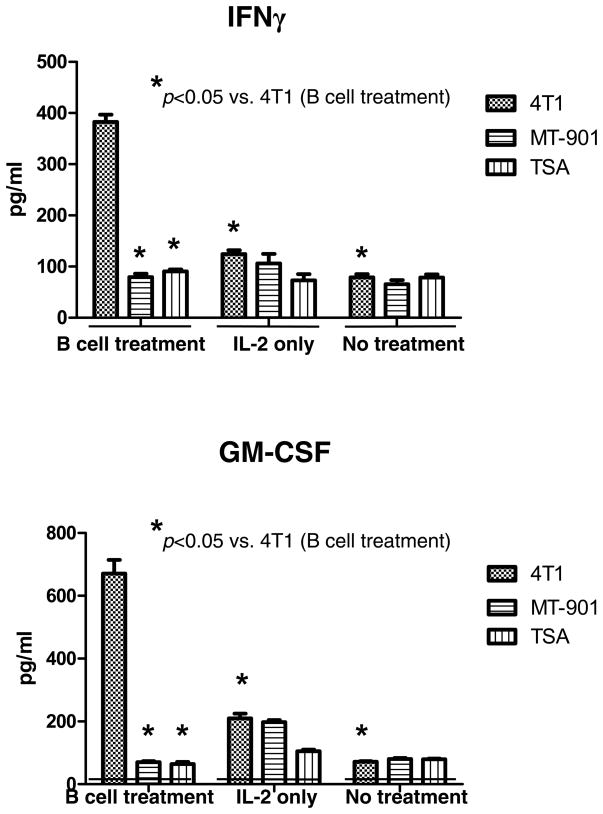
In a similar fashion, we assessed the ability of host T cells to produce IFNγ and GM-CSF in response to tumor antigen after the adoptive transfer of effector B cells. Splenocytes were harvested and activated with anti-CD3/anti-CD28 plus IL-2 as described above. After activation, the T cells were co-cultured with irradiated tumor cells and supernatants harvested 24 hrs later. As illustrated in Fig 5, activated splenocytes from hosts that received effector B cells secreted significantly (p<0.05) higher levels of IFNγ and GM-CSF in response to 4T1 tumor compared to splenocytes from mice that received IL-2 or no treatment. This cytokine release was immunologically specific. Together, these data indicate that adoptively transferred effector B cells alone can inhibit 4T1 pulmonary metastases and induce systemic host T cell-mediated anti-tumor immunity as measured by tumor lysis as well as cytokine release.
Fig. 5.
Tumor specific cytokine production by anti-CD3/anti-CD28-activated and IL-2-expanded splenocytes. Spleens were harvested from animals subjected to B cell treatment, IL-2 treatment only, or no treatment. Splenocytes were activated with anti-CD3/anti-CD28 mAb followed by IL-2 expansion. Activated and expanded splenocytes were then re-stimulated with irradiated 4T1 tumor cells or irrelevant tumor controls. The culture supernatants were collected and analyzed for the secretion of IFNγ and GM-CSF in response to irradiated tumor cells.
3. Adoptive transfer of 4T1 TDLN T+B cells confers systemic host cellular and humoral anti-tumor immunity
We proceeded by examining the antitumor reactivity of B cells in combination with T cells to treat 4T1 spontaneous metastases (Fig 3B). Mixed T and B cells purified from the 4T1 TDLN were activated with anti-CD3/anti-CD28/IL-2 + LPS/anti-CD40. While B cell transfer alone resulted in significantly (p<0.05) diminished lung tumors as observed in the previous experiment (Fig 3A), the combination of T + B cells resulted in a significantly greater (approximately 90%) reduction of lung tumors that was dose-dependent on the number of TDLN T and B effector cells given.
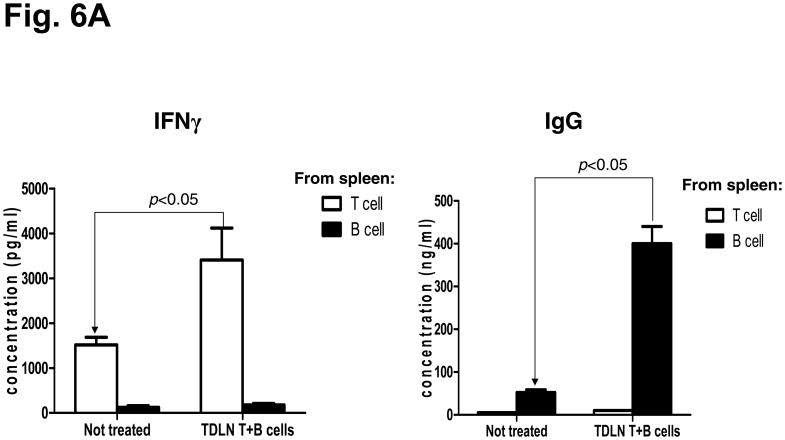
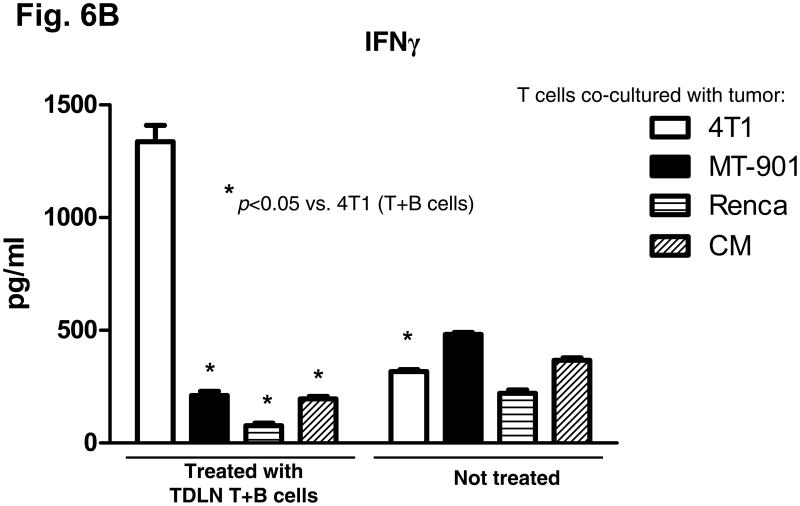
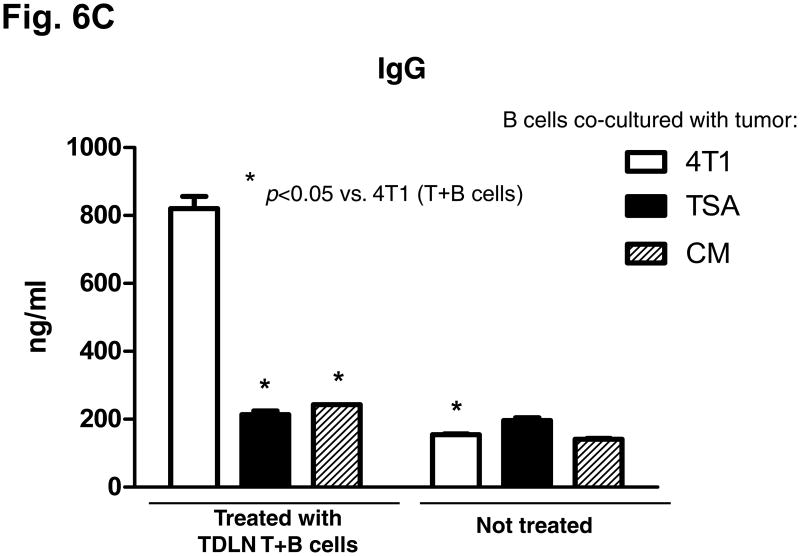
We evaluated the anti-tumor reactivity of host splenocytes after the adoptive transfer of T + B effector cells compared to no treatment. Purified T and B splenocytes were activated with anti-CD3/CD28 plus IL-2 or with LPS and anti-CD40, respectively. As illustrated in Fig 6A, supernatants from the activation culture demonstrated large quantities of IFNγ or IgG released by the activation of splenic T cells or B cells, respectively. The activated T cells or B cells were then co-cultured with irradiated tumor cells and supernatants collected 24 hrs later. As shown in Fig. 6B and 6C, the activated T and B cells isolated from the spleens of the animals subjected to TDLN T + B cell therapy produced significantly (p<0.05) more IFNγ and IgG in response to 4T1 in a tumor antigen-specific manner than T or B cells from the spleens of animals that received no treatment. Noteworthy is the observation that LPS/anti-CD40 activation of splenic B cells from non-treated 4T1-tumor bearing mice (Fig 6C) did not result in specific anti-tumor reactive B cells indicating that the in vitro activation process does not result in promiscuous B effector cells. It is only in the context of in vivo B cell priming in TDLN (Fig 2) or with the adoptive transfer of tumor reactive B cells that you can secondarily activate B lymphoid cells to differentiate into B effector cells.
Fig. 6.
A: Significantly increased IFNγ and IgG production by host T and B cells purified from the spleens of animals subjected to TDLN T + B cell therapy. Culture supernatants collected at the end of T or B splenocyte activation were assayed for IFNγ and IgG concentration using ELISA. Data are representative of two independently performed experiments.
B&C: Tumor antigen-specific IFNγ (B) and IgG (C) production by splenocytes of the host subjected to TDLN T+B cell therapy. Activated splenocyte T and B cells from the 4T1 tumor-bearing host given 4T1 TDLN T+B cell treatment were re-stimulated with irradiated 4T1 or irrelevant tumors. The culture supernatant were then collected and analyzed for the production of IFNγ and IgG. Data are representative of three independently performed experiments.
4. Immune host splenic B cell-produced IgG binds to 4T1 tumor cells and results in tumor cell lysis
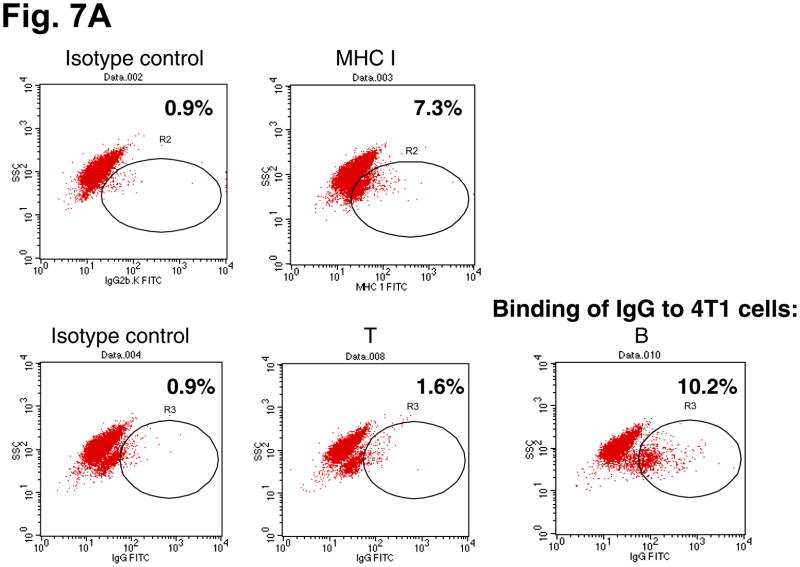
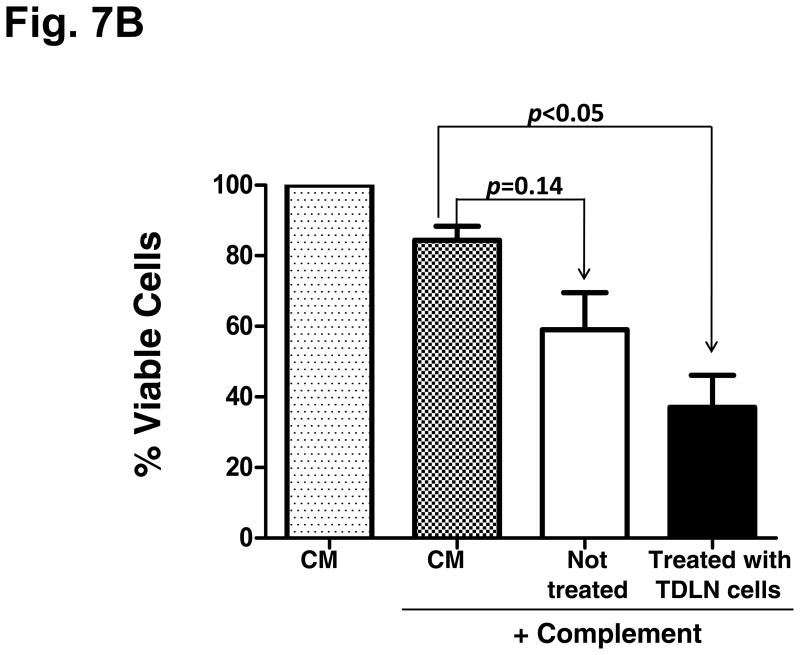
To define the immunological significance of host splenic B cell-produced IgG after adoptive transfer of TDLN effector cells, we tested the interaction of these antibodies with 4T1 tumor cells. Cell staining and flow cytometry revealed that IgG secreted by the B splenocytes obtained from the animals subjected to TDLN cell therapy were able to bind to 4T1 tumor cells (10.2%, Fig. 7A). The MHC I staining served as a positive control, and the supernatants produced by splenic T cells served as a negative control. We then tested the consequence of the binding of IgG to tumor cells by testing complement-mediated cytotoxicity. In Fig. 7B, antibody produced by splenic B cells obtained from hosts given TDLN T+B cells significantly (p<0.05) lysed 4T1 tumor cells in the presence of complement, more efficiently than antibody produced by B cells obtained from non-treated host (p=0.14 compared with complement alone).
Fig. 7.
A: Immune host B splenocyte-produced IgG binds to 4T1 cells. 4T1 cells were incubated with the culture supernatant of T and B splenocytes harvested from the 4T1 tumor-bearing mice subjected to 4T1 TDLN T + B cell treatment. Bound IgG onto the tumor cells was detected by secondary antibody FITC-anti-mouse IgG. FITC-coupled secondary antibody isotype-matching control was also used. Numbers within each immunofluorescence dot plots indicate the percentage of viable cells stained positive with the FITC-coupled antibodies. Data are representative of two independently performed experiments.
B: Antibody and complement-mediated 4T1 cytotoxicity. Viable 4T1 cells (0.5 × 106) were cultured with supernatant produced by activated B cells purified from the spleens harvested from the 4T1 tumor-bearing mice subjected to 4T1 TDLN T + B cell treatment or no treatment. CM only and complement alone served as controls. After antibody incubation, rabbit complement was added followed by incubation for another one hour. Viable cells were then counted under the microscope after trypan blue staining. Percentage (%) of viable cells was calculated by dividing the remaining viable cells with 0.5 × 106. Data are representative of two independent experiments conducted.
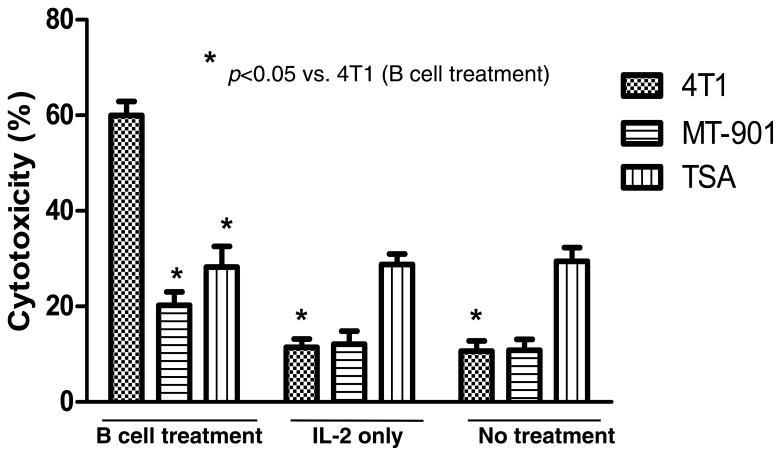
5. Activated TDLN B cells directly mediate tumor specific cytotoxicity
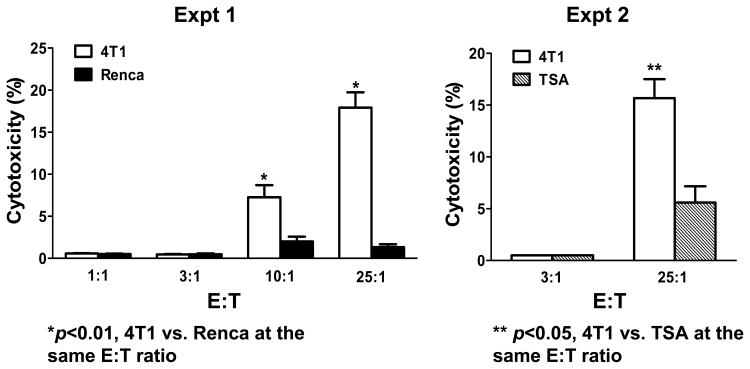
In addition to producing antibody that can kill tumor cells via complement-mediated cytotoxicity, we examined whether 4T1-activated B cells can mediate tumor lysis directly. B cells from 4T1 TDLN were isolated and activated ex vivo with LPS/anti-CD40. The activated B cells were then assessed for tumor cytotoxicity using the LDH release assay. As shown in Fig. 8, activated 4T1 TDLN B cells were able to mediate lysis of 4T1 tumor cells in vitro in 2 independent experiments. The cytotoxicity was specific when compared to the irrelevant Renca and TSA tumor targets respectively.
Fig 8.
Activated 4T1 TDLN B cells mediate direct and specific cytotoxicity of 4T1 tumor cells. An LDH cytotoxicity release assay demonstrates the ability to activated 4T1 TDLN B cells to directly lyse 4T1 tumor cells and not the irrelevant Renca or TSA tumor targets as shown in two independent experiments.
Discussion
This report documents the effectiveness of tumor specific B effector cells after adoptive transfer in suppressing the development of spontaneous metastatic carcinoma. More importantly, we provide experimental evidence in this report that adoptively transferred B effector cells induce host T cell antitumor immunity. Antigen-specific systemic T cell immunity was documented in the recipient hosts after adoptive transfer of activated TDLN B cells. This was evident by the presence of host CTL activity and tumor-specific T cell production of cytokines against 4T1 tumor. The modulation of host T cell antitumor immunity by adoptively transferred effector B cells in this setting represents a new finding and was shown to significantly augment the efficacy of adoptive T cell therapy.
In contrast to resting B cells, several reports have described the application of activated B cells in the immunotherapy of cancer. The majority of these reports have indicated how activated B cells can be used as effective antigen presenting cells (APCs) for in vitro T cell sensitization 5–8, 12. In another setting, activated B cells have been reported to enhance the ability to generate tumor-infiltrating lymphocytes ex vivo in a culture system involving anti-CD3 activation and IL-2 expansion 26. To our knowledge, there is only one report that has demonstrated the utility of activated B cells to mediate regression of solid tumor after adoptive transfer. Harada et al. reported that LPS-activated B cells bound to anti-CD3 resulted in a modest increase in survival in mice bearing B16 lung metastases after adoptive transfer 27. The authors postulated that the activated B cells provided additional co-stimulatory signals to a T cell response of the host. Besides antigen-presentation or co-stimulation, activated B cells may mediate direct tumor cell death. Kemp et al. reported that CpG-containing oligodeoxynucleotide stimulation of B cells results in the induction of TRAIL/Apo-2 ligand expression that can mediate tumor cell killing 28. In our study, we have demonstrated that LPS/anti-CD40-activated B cells can directly mediate lysis of tumor cells in standard in vitro cytotoxiciy assays. This may represent one mechanism by which adoptively transferred effector B cells mediate tumor regression. We plan to further investigate the mechanisms by which in vivo tumor-primed and in vitro LPS/anti-CD40-activated B cells directly kill tumor cells.
We have begun to evaluate methods to enhance the antitumor efficacy of adoptively transferred B effector cells. In a previous report, we found that lymphodepletion prior to B cell transfer in tumor-bearing mice significantly augmented the antitumor effects mediated by B cells25. The enhanced antitumor reactivity of activated B cells in the lymphodepleted host may be analogous to what was observed with the adoptive transfer of immune T cells which has been reported to be due to depletion of suppressor cells such as Tregs or the elaboration of homeostatic cytokines that expand adoptively transferred lymphoid cells 29–31. Recently, there have been several reports documenting the presence of regulatory B cells that can suppress antitumor responses. Yanaba et al. has reported a subset of B cells that secrete IL-10 that can have adverse effects on immune T cells 32. In preliminary studies, we have documented the presence of such cells in our activated B cell effector population. Depletion of these cells either in the activation cultures or in the tumor-bearing host may potentially result in enhanced antitumor responses. Another approach in modulating the effectiveness of adoptive B cell therapy may be the exogenous administration of biologic agents that could enhance the persistence or survival of the adoptively transferred B cells. Zhang et al. reported that anti-CD137 mAb enhanced the proliferation, survival and function of activated B cells in vitro 33. It would seem logical that the in vivo administration of anti-CD137 concomitantly with effector B cell transfer could enhance their antitumor efficacy in a similar fashion to adoptive T cell therapy which we have reported 34.
The use of LPS plus anti-CD40 as TDLN B cell stimuli in this report may represent a combined stimulation of innate immunity (by LPS) and adaptive immunity (by anti-CD40 to mimic the CD40L signaling from T helper cells). Evaluation of additional B cell activation pathways, such as CD137 33 and the engagement of the B cell antigen receptor (BCR) by using anti-Ig in the form of F(ab′)2 may lead to further B cell activation in vitro 35. Furthermore, studies of the trafficking and accumulation of infused B cells in tumor would increase our understanding of the events behind the observed therapeutic efficacy of adoptively transferred effector B cells.
In spite of the findings that activated B cells can mediate tumor regression in adoptive immunotherapy, we do not envision this as a monotherapy. Rather, we view this as an adjunct to T cell therapy. The collaborative interactions between activated T and B cells are currently unknown as are the interactions with host immune cells. Our laboratory is currently investigating this area to optimize this approach. Collectively, the findings we report herein may provide alternative strategies in developing more effective adoptive cellular therapies.
Statement of Translational Relevance.
This manuscript describes pre-clincal evidence regarding the utility of adoptively transferred tumor-primed B cells in modulating host antitumor T cell immunity. This is the first report indicating that tumor-primed B cells can inhibit the growth of spontaneous metastases. The administration of effector B cells in combination with effector T cells was therapeutically more efficacious that either cell population alone. The use of activated B cells may be a useful adjunct in the cellular therapy of malignancy.
Abbreviations used in this paper
- TDLN
tumor-draining lymph node
- CM
complete medium
- s.c.
subcutaneous
- i.v.
intravenously
- i.p.
intraperitoneal
- APC
antigen-presenting cells
Footnotes
This work was supported in part by NIH grant CA82529 (AEC) and the Gillson Longenbaugh Foundation.
References
- 1.Perricone MA, Smith KA, Claussen KA, et al. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother. 2004;27:273–81. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Shah S, Divekar AA, Hilchey SP, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–86. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 3.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 4.Watt V, Ronchese F, Ritchie D. Resting B cells suppress tumor immunity via an MHC class-II dependent mechanism. J Immunother. 2007;30:323–32. doi: 10.1097/CJI.0b013e31802bd9c8. [DOI] [PubMed] [Google Scholar]
- 5.Schultze JL, Michalak S, Seamon MJ, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–25. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 7.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–43. [PubMed] [Google Scholar]
- 8.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 9.Chung Y, Kim BS, Kim YJ, et al. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res. 2006;66:6843–50. doi: 10.1158/0008-5472.CAN-06-0889. [DOI] [PubMed] [Google Scholar]
- 10.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 184:4006–16. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 185:4977–82. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 12.Kondo E, Topp MS, Kiem HP, et al. Efficient generation of antigen-specific cytotoxic T cells using retrovirally transduced CD40-activated B cells. J Immunol. 2002;169:2164–71. doi: 10.4049/jimmunol.169.4.2164. [DOI] [PubMed] [Google Scholar]
- 13.Van den Bosch GA, Ponsaerts P, Nijs G, et al. Ex vivo induction of viral antigen-specific CD8 T cell responses using mRNA-electroporated CD40-activated B cells. Clin Exp Immunol. 2005;139:458–67. doi: 10.1111/j.1365-2249.2005.02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Noh HS, Chen J, Kim JH, Falo LD, Jr, You Z. Potent tumor-specific protection ignited by adoptively transferred CD4+ T cells. J Immunol. 2008;181:4363–70. doi: 10.4049/jimmunol.181.6.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manjili MH, Kmieciak M, Keeler J. Comment on “Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma”. J Immunol. 2006;176:4511. doi: 10.4049/jimmunol.176.8.4511. author reply -2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanden Bush TJ, Buchta CM, Claudio J, Bishop GA. Cutting Edge: Importance of IL-6 and cooperation between innate and adaptive immune receptors in cellular vaccination with B lymphocytes. J Immunol. 2009;183:4833–7. doi: 10.4049/jimmunol.0900968. [DOI] [PubMed] [Google Scholar]
- 17.Ronet C, Hauyon-La Torre Y, Revaz-Breton M, et al. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 184:886–94. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Yu B, Grover AC, Zeng X, Chang AE. Therapeutic effects of tumor reactive CD4+ cells generated from tumor-primed lymph nodes using anti-CD3/anti-CD28 monoclonal antibodies. J Immunother (1997) 2002;25:304–13. doi: 10.1097/00002371-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Carr A, Ito F, Teitz-Tennenbaum S, Chang AE. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res. 2003;63:2546–52. [PubMed] [Google Scholar]
- 20.Li Q, Carr AL, Donald EJ, et al. Synergistic effects of IL-12 and IL-18 in skewing tumor-reactive T-cell responses towards a type 1 pattern. Cancer Res. 2005;65:1063–70. [PubMed] [Google Scholar]
- 21.Tanigawa K, Takeshita N, Craig RA, et al. Tumor-specific responses in lymph nodes draining murine sarcomas are concentrated in cells expressing P-selectin binding sites. J Immunol. 2001;167:3089–98. doi: 10.4049/jimmunol.167.6.3089. [DOI] [PubMed] [Google Scholar]
- 22.Ito F, Carr A, Svensson H, Yu J, Chang AE, Li Q. Antitumor reactivity of anti-CD3/anti-CD28 bead-activated lymphoid cells: implications for cell therapy in a murine model. J Immunother (1997) 2003;26:222–33. doi: 10.1097/00002371-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Grover AC, Donald EJ, et al. Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells. J Immunol. 2005;175:1424–32. doi: 10.4049/jimmunol.175.3.1424. [DOI] [PubMed] [Google Scholar]
- 24.Iuchi T, Teitz-Tennenbaum S, Huang J, et al. Interleukin-21 augments the efficacy of T-cell therapy by eliciting concurrent cellular and humoral responses. Cancer Res. 2008;68:4431–41. doi: 10.1158/0008-5472.CAN-07-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009;183:3195–203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamada K, Harada M, Okamoto T, et al. Specific antitumor activity of tumor-infiltrating lymphocytes expanded first in a culture with both anti-CD3 monoclonal antibody and activated B cells and then in a culture with interleukin-2. Cancer Immunol Immunother. 1995;41:339–47. doi: 10.1007/BF01526553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harada M, Okamoto T, Kurosawa S, et al. The antitumor activity induced by the in vivo administration of activated B cells bound to anti-CD3 monoclonal antibody. Cell Immunol. 1995;161:132–7. doi: 10.1006/cimm.1995.1017. [DOI] [PubMed] [Google Scholar]
- 28.Kemp TJ, Moore JM, Griffith TS. Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeoxynucleotide stimulation. J Immunol. 2004;173:892–9. doi: 10.4049/jimmunol.173.2.892. [DOI] [PubMed] [Google Scholar]
- 29.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–9. [PubMed] [Google Scholar]
- 31.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor induced suppressor T cells. J Exp Med. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–72. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Voskens CJ, Sallin M, et al. CD137 promotes proliferation and survival of human B cells. J Immunol. 184:787–95. doi: 10.4049/jimmunol.0901619. [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Iuchi T, Jure-Kunkel MN, Chang AE. Adjuvant effect of anti-4-1BB mAb administration in adoptive T cell therapy of cancer. Int J Biol Sci. 2007;3:455–62. doi: 10.7150/ijbs.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poovassery JS, Vanden Bush TJ, Bishop GA. Antigen receptor signals rescue B cells from TLR tolerance. J Immunol. 2009;183:2974–83. doi: 10.4049/jimmunol.0900495. [DOI] [PMC free article] [PubMed] [Google Scholar]