Abstract
Objective
To investigate if enhancement of joint lymphangiogenesis by injecting VEGF-C adeno-associated virus (AAV) into joints has therapeutic efficacy in chronic inflammatory arthritis in mice.
Methods
TNF transgenic (TNF-Tg) mice were used as a model of chronic inflammatory arthritis. Human VEGF-C was cloned into an AAV expression vector to generate AAV-VEGF-C. AAV-VEGF-C or control AAV-Luc was injected into joints of TNF-Tg mice. MRI and lymphatic imaging were used during the 4-months following injection to assess changes in synovial volume and lymph flow from joint tissues to local draining lymph nodes. Joint inflammation, bone erosion and cartilage loss were examined by histologic analyses. Lymphatic vessel formation was assessed using immunohistochemistry.
Results
Intra-articular administration of AAV-VEGF-C virus significantly attenuated the increase in synovial volume and increased lymphatic vessel number in joint sections compared to AAV-Luc virus during the 4-month-period. This accompanied by reduced inflammation area, bone erosion, cartilage loss, and osteoclast numbers. Lymph flow from joints to local draining lymph nodes was slower in TNF-Tg mice than in wild-type littermates and was significantly improved with AAV-VEGF-C treatment.
Conclusion
Intra-articular injection of AAV-VEGF-C increased lymphangiogenesis and improved lymphatic drainage from inflamed joints, resulting in attenuation of joint tissue damage. Thus, improvement of joint lymphatic function by local administration of lymphatic growth factors represents a new therapeutic approach for chronic inflammatory arthritis.
Keywords: VEGF-C, lymphatic system, lymphangiogenesis, inflammation, arthritis
Introduction
Lymphatic system is important for maintenance of tissue fluid homeostasis. It removes fluid, proteins and other particles from tissue spaces and returns them to the bloodstream and thus plays an essential role in the clearance of excess interstitial fluid. Impaired lymph flow is a major rate-limiting influence in the resolution of edema, a concept that has been examined recently in animal models of acute inflammation. For example, immuno-staining with antibodies that specifically recognize lymphatic endothelium revealed increased lymphatic vasculature in inflamed tissues (1, 2), while inhibition of lymphatic vessel formation prolonged the duration of inflammation in these models (3, 4). Other studies of lymphatics in superficial tissues, such as the ear, skin, intestine and lung in models of acute inflammation (5–7) have confirmed the importance of the lymphatic system in the resolution of edema during acute inflammation. However, little is known of the role of lymphatic flow in deep tissues during chronic inflammatory processes.
Rheumatoid arthritis (RA) is a chronic inflammatory erosive disease that affects joints and the soft tissues around them. Inflamed synovium in RA recruits inflammatory cells, which produce factors that increase vascular formation (8, 9), lymphatic vessel numbers and lymph flow (10, 11). However, it is not known if these increased lymphatic vessels have normal function or if the number of inflammation-induced lymphatic vessels is sufficient to remove excess lymph from inflamed synovium. Using contrast-enhanced MRI (CE-MRI) and indocyanine green near-infrared (ICG-NIR) lymphatic imaging in a mouse model of RA, we demonstrated a dramatically increased lymphatic network around affected ankle joints during the chronic phase of inflammation (12, 13). However, the lymph flow in the same area did not increase (12), suggesting that increased lymphatic vessels in RA joints may not function well. Impaired draining function of inflammation-induced lymphangiogenesis has been reported in other animal models. For example, in UVB radiation-induced skin inflammation, lymphatic drainage is slow, despite the presence of numerous lymphatic vessels. Insufficient lymph flow is accompanied by infiltration of high numbers of macrophage (1). Thus, an important unaddressed issue regarding lymphatics and arthritis is whether further enhancement of lymphangiogenesis and/or lymphatic drainage can reduce the severity of joint inflammation and serve as a new therapeutic approach for RA and perhaps other chronic inflammatory disorders.
Vascular endothelial growth factors (VEGFs) are the main mitogenic and survival factors for vascular endothelial cells. In mammals, there are 5 known VEGFs: VEGF-A, -B, -C, -D, and placental growth factor. Of these, VEGF-C and VEGF-D primarily activate lymphatic endothelial cells, which express VEGF receptor (VEGFR)-3 (14). We showed previously that blockage of lymphatic development by a neutralizing VEGFR-3 antibody during the early stages of arthritis in TNF-transgenic (TNF-Tg) mice, a murine model of RA, increases the severity of joint inflammation (15). This loss of function study suggests that adequate lymphatic flow limits the pace at which arthritis develops in this model and that inhibition of it accelerates the progression of joint damage.
In the present study, we used a gain of function approach to induce lymphangiogenesis in TNF-Tg mice by over-expressing VEGF-C in their joints and examined the long-term effects of local VEGF-C adeno-associated virus (AAV) treatment on synovial inflammation and vascularity, bone and cartilage erosion, and lymphatic draining function from the joint area to local draining lymph nodes. We found that exogenous administration of VEGF-C into the joints reduced the severity of joint lesions by enhancing lymphatic vessel formation and lymphatic drainage. Our results demonstrate that enhancement of lymphatic function could represent a new therapeutic approach in chronic inflammatory arthritis.
Materials and methods
Animals and intra-articular injections
All animal studies were conducted using procedures approved by the University of Rochester Committee for Animal Resources. TNF transgenic (TNF-Tg) mice in a C57BL/6 background (line 3647) were obtained originally from Dr. G. Kollias. This line of TNF-Tg mice carries one copy of the human TNF transgene and develops chronic arthritis relatively slowly, as we characterized previously (15, 16). For intra-articular injection, 1010 particles of AAV were injected into the ankle (10 µl) joints using a 28 gauge needle, as we described previously (17). Initially, we injected control and AAV-hVEGF-C virus into both legs of 8 TNF-Tg mice (N=4 mice, 8 legs/group). One mouse receiving control virus died during the experiment. RT-PCR of joint tissues confirmed the expression of hVEGF-C in 5 of 8 legs that received AAV-hVEGF-C injection. Thus, data from 3 mice (6 legs) that received control virus and 4 mice (5 legs) that received AAV-VEGF-C virus were used in the data analyses.
Generation and characterization of AAV-VEGF-C
The human VEGF-C cDNA was amplified from PC3 prostate cancer cells based on the published sequence (Accession # X68203) and cloned into the pAAV-BGHA vector (18, 19). AAV-VEGF-C was produced through a helper-virus-free method. AAV-luciferase (Luc), purchased from North Carolina University, was used as a control. The expression of AAV-VEGF-C was confirmed in vitro by enzyme-linked immunosorbent assay and Western blot analysis and in vivo by RT-PCR using human VEGF-C specific primers. AAV transduction efficiency was determined by bioluminescent imaging and Luc assay after intra-articular injection of AAV-Luc into joints of TNF-Tg mice (N=3 mice) over a 4-month period).
Contrast-enhanced MRI (CE-MRI) and Indocyanine green near-infrared (ICG-NIR) lymphatic imaging
CE-MRI was performed before, during and after AAV injection. Mice were positioned with a hind limb in a custom-designed murine dual RF receiver coil and scanned in a Siemens 3 Tesla clinical magnet. Analysis was performed with Amira 3.1 software as we previously described (15, 20). ICG-NIR lymphatic imaging was performed by injecting ICG intra-dermally into footpads and the dynamics of ICG fluorescence over the entire leg was visualized under an infrared laser and recorded. The images were analyzed using Image J software. Regions of interest defining popliteal lymph nodes (PLNs), draining lymphatic vessels, and the injection site were identified, yielding 4 outcome measures of lymphatic function: 1) the time it takes for the ICG to be detected in the PLN; 2) the maximum ICG signal intensity in the PLN; 3) the time it takes for a PLN to achieve maximal signal intensity; and 4) % ICG that was cleared from the PLN and footpad at 24 hours. We have described the CE-MRI and the ICG-NIR methods previously (13, 15, 20).
Histology
Joint specimens were fixed in 10% phosphate-buffered formalin, decalcified, and embedded in paraffin. Sections were immunostained with a mixture of PE-anti-CD31 or rabbit anti-LYVE-1 antibody followed by Alexa Fluor 488 goat anti-rabbit (21). The size and area of lymphatic and blood vessels were expressed per square millimeter of the synovial area, as we previously described (21) (22). The inflammatory area, expressed as a % of the total tissue area, was measured on H&E-stained sections. The data are presented as the mean from 2 representative levels cut from each joint sample.
Clinical evaluation
The severity of joint inflammation was assessed semi-quantitatively every 2 weeks using a deformation score that we developed and reflects the chronic nature of arthritis in TNF-Tg mice (23, 24). At the end of experiments, joint flexibility, defined as the range of motion, was measured using a modified clinical method (25).
RT-PCR
RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA), and cDNA synthesis was performed using GeneAmp RNA PCR core kit (Applied Biosystems, Foster City, CA). Quantitative PCR amplification was performed with gene-specific primers using an iCycler real-time PCR machine and iQ SYBR Green supermix (both from Bio-Rad Laboratories, Hercules, CA), according to the manufacturer’s instruction. The murine primer sequences are: VEGF-A forward 5’-ATTGAGACCCTGGTGGACATCTTC-3’ and reversed 5’-CTCATCTCTCCTATGTGCTGGCTT-3’; VEGF-B forward 5’-CCTGGAAGAACACAGCCAAT-3’ and reversed 5’-GGAGTGGGATGGATGATGTC-3’; VEGF-C forward 5’-GGGAAGAAGTTCCATCA-3’ and reversed 5’-ATGTGGCCTTTTCCAATACG-3’. The quantity of the target gene mRNA was obtained by division of each value by the actin value. Samples were run in triplicate. To confirm the expression of AAV-VEGF-C, total RNA was extracted from soft tissues around knee, ankle, and hand tissues and reversely transcribed to cDNA. cDNA was subjected for RT-PCR using primers specific for murine VEGF-C or human VEGF-C. The primer sequences for human VEGF-C are forward 5’-ACCAAACAAGAGCTGGATG-3’ and reversed 5’-ATTTCTGGGCAGGTTC-TTT-3’.
Statistics
Data are presented as means ± SD. Statistical analyses were performed with Stat View statistical software. Differences between 2 groups were compared using a Student t-test and among more than 2 groups using one-way ANOVA, followed by a Bonferroni/Dunnet test. p values<0.05 were considered to be statistically significant.
Results
Generation of AAV-VEGF-C and demonstration of long-term expression of intra-articular delivery of AAV-encoded genes in vivo
VEGF-C is the most commonly studied lymphatic growth factor. Previous in vivo studies mainly used adenoviral gene delivery for short-term expression of VEGF-C because the adenovirus itself induces inflammation and immune responses after 2–3 weeks of administration (27, 28). To overcome this limitation, we have used recombinant adeno-associated virus (AAV) successfully as a long-term gene delivery approach in mice without obvious adverse effects (18, 19). Here we used the same AAV expression vector to deliver VEGF-C into the joints of TNF-Tg mice (15, 16). We generated the pAAV-hVEGF-C expression vector by replacing human VEGF-C cDNA for the OPG cDNA in the pAAV-OPG that we used previously (18). The human VEGF-C was used because it is fully functional in mouse tissues (29), an ELISA kit for measuring human VEGF-C is commercially available, and the same vector could be used in human studies. The expression efficiency of AAV-VEGF-C virus was tested by infecting 293T cells with various MOIs of AAV-VEGF-C virus and control AAV-Luciferase (AAV-Luc), as described previously (18, 30). AAV-VEGF-C-infected cells expressed very high levels of VEGF-C mRNA (Figure 1A). Western blot of conditioned medium from AAV-hVEGF-C injected cells with anti-VEGF-C antibody revealed a 29 KD protein, the secreted form of VEGF-C(Figure 1B) (31, 32). We also confirmed production of VEGF-C protein by ELISA (data not shown).
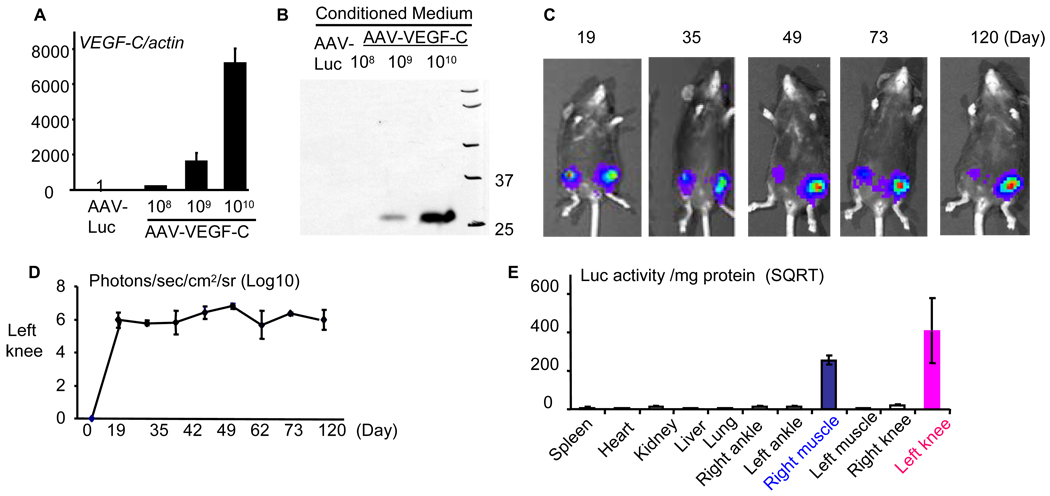
Figure 1. Generation of AAV-VEGF-C and long-term expression of an AAV-encoded gene after intra-articular injection.
293T cells were infected with various amounts of AAV-hVEGF-C or control AAV-Luc for 48 hours. (A) The expression of hVEGF-C mRNA and (B) protein in conditioned media was assessed by real time RT-PCR or Western blot analysis. 2.5-month-old TNF-Tg mice (N=3) received an intra-articular injection of 1010 particles of AAV-Luc in the left knee joint and an intramuscular injection of 5×109 particles of the same virus in the quadriceps muscle of the right leg on day 0. Bioluminescent images were taken at various times up to 120 days. (C) Bioluminescent images of a representative mouse show persistent high Luc signals at the injected sites. (D) The mean numbers of detected light units (+SD) in each image from 3 mice. (E) At the end of the experiment (day 120), various tissues were harvested and luciferase activity was measured. Values are the means ± SD of 3 mice.
We have shown previously that AAV-delivered genes can remain effective for up to 4 weeks when they were used locally in murine models of polyethylene-induced osteolysis (18) and bone allografts (19). Others have reported similar efficacy of AAV-VEGF-C in vivo for up to 4 weeks following intra-muscular injection (33). Clinically detectable arthritic symptoms develop in TNF-Tg mice when they are 10–11 weeks-old and the joint disease progresses continuously thereafter. In order to perform long-term therapy, we first determined if an AAV-encoded gene could remain active for more than 3 months in these mice. AAV-Luc was injected into the left knee joint and the right quadriceps muscle of 2.5-month-old TNF-Tg mice (N=3). Bioluminescent images were taken intermittently and these showed that the Luc signal remained strong in both sites for at least 120 days after AAV-Luc administration with no significant reduction in signal intensity during this period (Figure 1C–D). Animals were sacrificed on day 120 and luciferase activity was determined in various tissues, including spleen, liver, heart, lung, kidney, and right and left knees, ankles and quadriceps muscles. High lucifierase activity was detected in the left knee joints and the right quadriceps muscles, but all other tissues were luciferase negative (Figure 1E).
AAV-VEGF-C reduces the severity of joint lesions in TNF-Tg mice
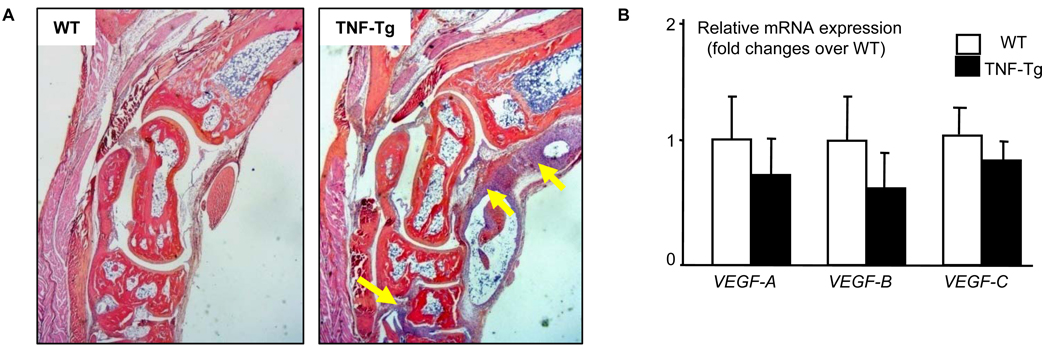
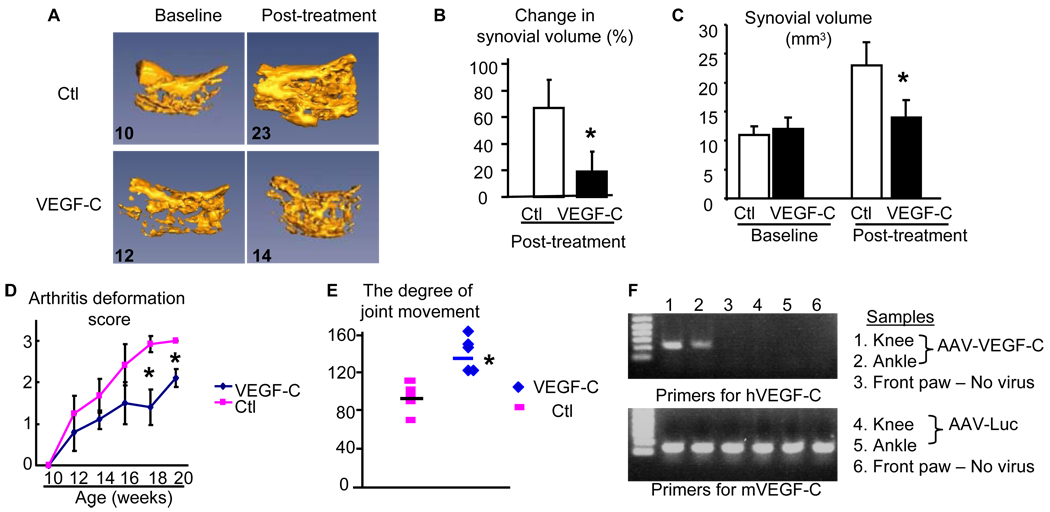
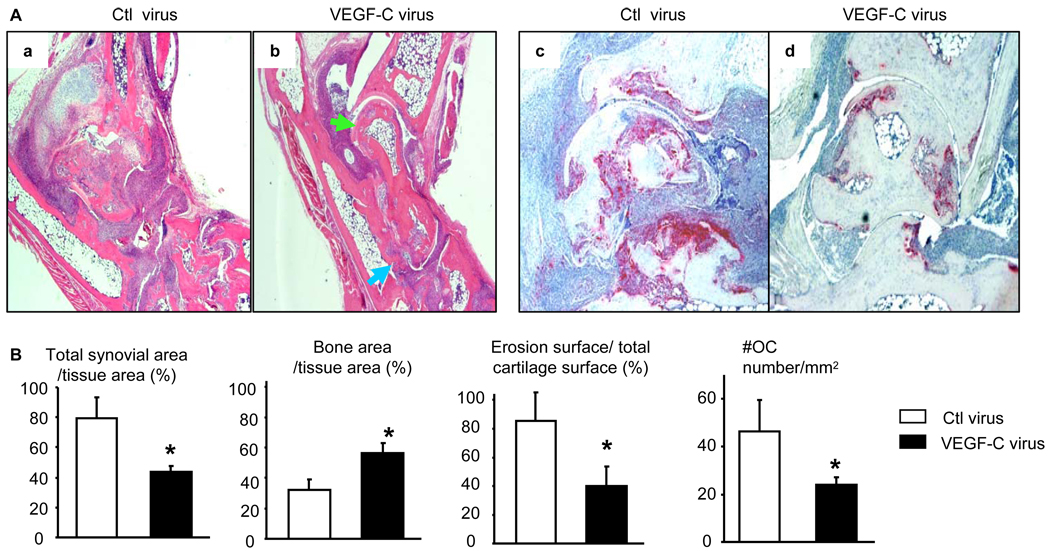
Recently, we reported that blockade of lymphangiogenesis in TNF-Tg mice with a VEGFR-3 neutralizing antibody increased the severity of joint inflammation and decreased lymphatic flow from the foot area to PLNs, the major draining LNs in mice hind limbs (15). To determine if enhancement of lymphatic function had a beneficial effect on arthritis, we injected AAV-VEGF-C or control AAV-Luc into ankle joints of 1.5-month-old TNF-Tg mice. At this age, ankle joints have developed tenosynovitis(Figure 2A), which is the earliest sign of arthritis in TNF-Tg mice (34). However the expression levels of VEGFs, including VEGF-C, in joint tissue is normal (Figure 2B). Contrast-enhanced MRI (CE-MRI) scanning revealed that mean values for synovial volume of ankle joints increased by about 60% in AAV-Luc-treated legs 2 months after viral injection compared to baseline values, and these had not increased further after 4 months. In contrast, synovial volumes had increased by ~40% at 2 months in AAV-VEGF-C-treated mice compared to baseline values, but were reduced to 20% at 4 months (Figure 3A&B). The absolute value of synovial volume from CE-MRI also indicated an increased synovial volume in control virus mice at 4 months after treatment compared to baseline while VEGF-C treatment significantly reduced synovial volume (Figure 3C). Clinical evaluation showed that joint deformity and limitation of movement in the legs of AAV-VEGF-C-injected mice were significantly less than in AAV-Luc-treated mice (Figure 3D&E), complementing the CE-MRI findings. The expression of AAV-VEGF-C was confirmed by measuring human VEGF-C in soft tissues from joints by human specific primers. Human VEGF-C was identified in joints received AAV-VEGF-C injection but not in joints received AAV-Luc or no virus injection (Figure 3F). Histologic examination and histomorphometric analysis showed that ankles from AAV-Luc-treated mice had severe inflammation, massive cartilage and bone destruction, associated with numerous TRAP+ osteoclasts. All of these parameters were significantly lower in ankles of AAV-VEGF-C-injected mice (Figure 4A&B). Similar changes were observed in AAV-VEGF-C injected knee joints (Supplemental Figure 1)
Figure 2. Normal histology and expression levels of VEGFs in joints of TNF-Tg mice before AAV treatment.
Seven-weeks-old TNF-Tg and WT littermates were used (N=4/genotype). (A) H&E sections of ankle joints show the development of tenosynovitis in TNF-Tg mice (arrows). (B) The expression of VEGFs mRNA in wrist joint specimen was measured by real-time RT-PCR.
Figure 3. AAV-VEGF-C virus decreases joint inflammation and tissue damage in TNF-Tg mice.
Seven week-old TNF-Tg mice received intra-ankle injection of AAV-Luc control virus (N=3 mice and a total of 6 legs) or AAV-VEGF-C virus (N=4 mice and a total of 5 legs). CE-MRI scans were performed before, 2, and 4 months after viral injection. (A) Representative 3D MRI images at baseline and 4 months show changes in synovial volume. The numbers are the absolute synovial values (mm3) from the image. (B) Percentage changes of synovial volume at 2 and 4 months compared to baseline values in the same joints was derived from MRI images. (C) The absolute synovial values at the baseline and 4 months after viral injection. (D) The arthritis deformation score during the course of treatment. (E) The degree of joint flexibility at the end of treatment. Values are the mean ± SD of 5–6 legs per group. *p<0.05 vs control virus. (F) Expression of virus encoded human VEGF-C in joints of TNF-Tg mice 4 months after viral injection.
Figure 4. AAV-VEGF-C reduces joint tissue damage in TNF-Tg mice.
Four months after virus injection, legs were harvested and subjected to histologic analysis. (A) Representative H&E (×2) and TRAP (×4) stained sections show decreased joint tissue damage, including cartilage erosion (green arrows) and bone loss (blue arrows), and TRAP+ osteoclasts in the VEGF-C-treated joints. (B) Quantitation of synovial volume, bone area, cartilage erosion, and osteoclast numbers. Values are the mean ± SD of 5–6 legs per group. *p<0.05 vs control virus.
AAV-VEGF-C increases lymphatic flow and lymphangiogenesis of joints
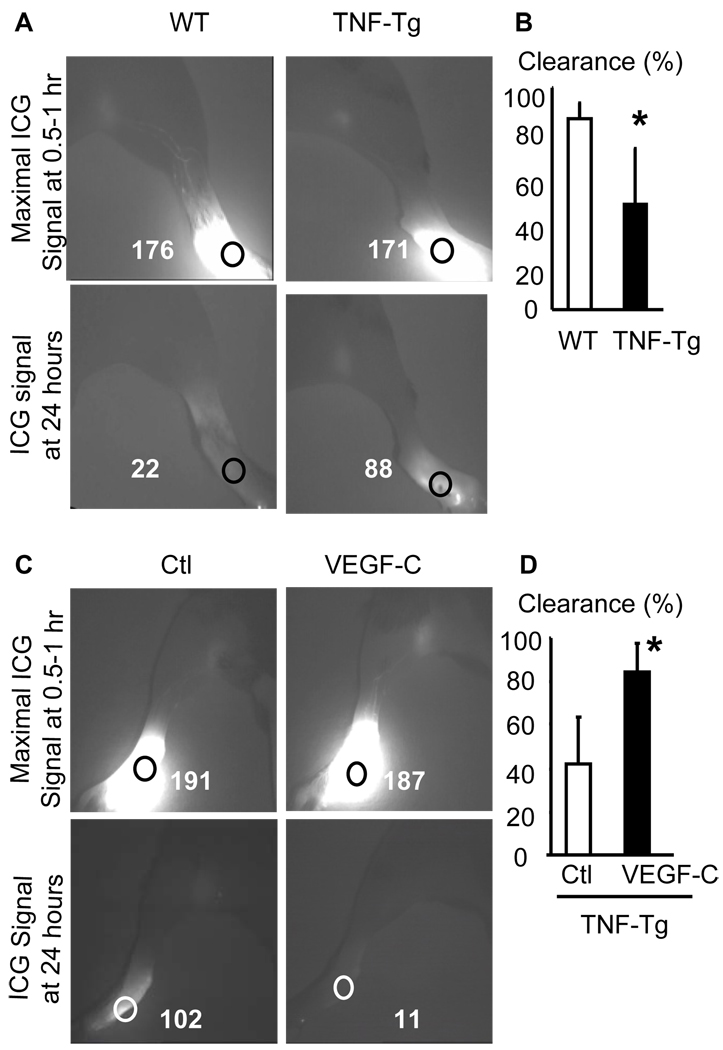
During inflammation process, edema occurs as result of the accumulation of extracellular fluid due to excessive leakage from hyper-permeable blood vessels. It is also due to a failure of lymphatic vessels to sufficiently drain fluid from the interstitial spaces. AAV-VEGF-C has been used previously in lymphedema generated in fore paws of wild-type mice by removal of draining LNs (35) or in tails by physically blocking lymphatic flow (36). In these models, AAV-VEGF-C reduced edema and tissue swelling. However, it has not been used in a disease model of chronic inflammatory arthritis. To this end, we first examined lymphatic draining function in affected joints of 2.5 and 5 month-old TNF-Tg mice and WT littermates using indocyanine green near infrared (ICG-NIR) imaging (12, 15). No clear difference in lymphatic flow from footpads to draining PLNs was observed in 2.5 month-old TNF-Tg vs WT mice (data not shown). However, the ICG signal intensity was significantly higher in the footpads of 5 month-old TNF-Tg mice than in those of WT mice 24 hours post-ICG injection, indicating that less ICG was removed through lymphatics from the paws of TNF-Tg mice (Figure 5A&B). Furthermore, the ICG signal intensity in AAV-VEGF-C-injected legs was significantly lower at 24 hours than in AAV-Luc-treated legs, suggesting that VEGF-C treatment enhanced the removal of ICG from the paws of TNF-Tg mice (Figure 5C&D) and improved local lymphatic drainage.
Figure 5. Impaired lymphatic drainage in ankles of TNF-Tg mice, which is improved by AAV-VEGF-C administration.
(A) TNF-Tg mice and WT littermates were subjected to ICG-NIR lymphatic imaging at 2.5 and 5 month-old. Representative ICG-NIR images of 5-month-old animals at 0.5–1 and 24 hours after ICG injection showing that TNF-Tg legs have higher ICG signal intensity in the ankle area than control mice at 24 hours. (B) Quantitation of lymphatic images in A. Values are the mean ± SD of 8 legs per group. *p<0.05 vs WT legs. (C) Four months after AAV injection, the animals were subjected to ICG-NIR. AAV-VEGF-C treatment decreased ICG signal intensity in the ankle area at 1 and 24 hours after ICG injection. (D) Quantitation of lymphatic images. Values are the mean ± SD of 5–6 legs per group. *p<0.05 vs control virus.
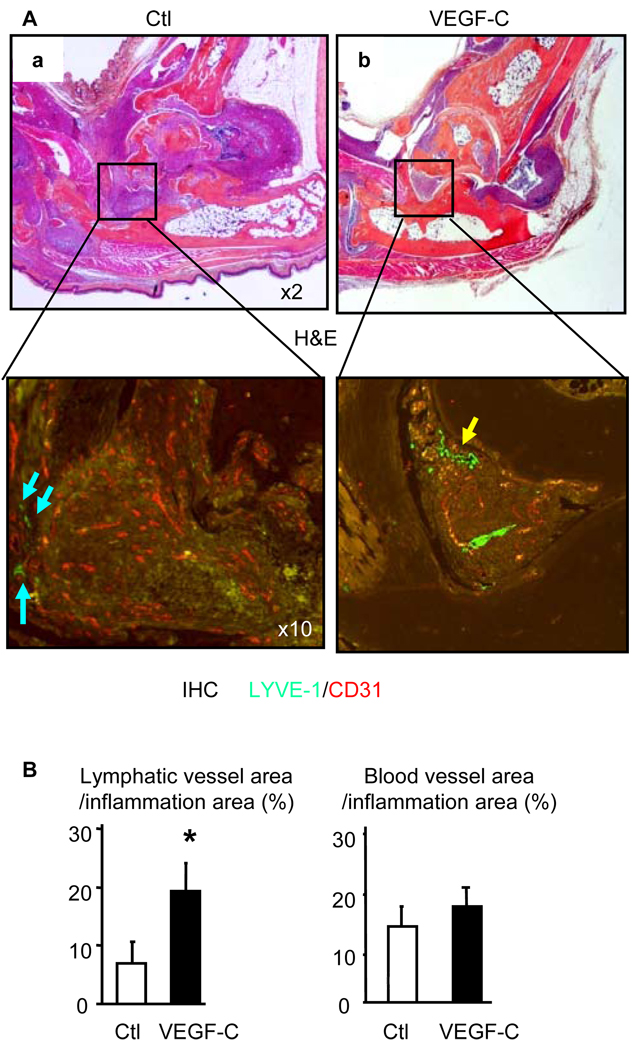
To examine the effect of VEGF-C over-expression on lymphatic and blood vessel formation of joints we used double immuno-staining with anti-LYVE-1 and anti-CD31 antibodies to visualize lymphatic and blood vessel endothelial cells, respectively. We found that lymphatic vessels were localized around the inflammatory pannus, but not in the synovial tissues in sections of AAV-Luc-treated legs (Figure 6A). Interestingly, we observed increased numbers of lymphatic vessels within the pannus of VEGF-C-treated mice (Figure 6A&B). VEGF-C has been shown to increase blood vessel growth or capillary stability in some animal models (27, 37). However, we did not detect increased CD31+ blood vessel formation in sections of legs from VEGF-C-treated mice (Figure 6 A&B), suggesting that the VEGF-C encoded by our AAV vector does not affect blood vessel formation in inflamed joints.
Figure 6. AAV-VEGF-C increases lymphangiogenesis in joints of TNF-Tg mice.
(A) Four months after AAV injection, legs were harvested and subjected to double immuno-fluorescence staining with anti-LYVE-1 and anti-CD31 antibodies. Representative H&E (×2), LYVE-1 (green-lymphatic vessels, ×10) and CD31 (orange-blood vessels, ×10) stained ankle sections show that LYVE-1+ lymphatic vessels are present around synovium (blue arrows) in control virus treated legs or within the synovium (yellow arrows) in VEGF-C-treated legs. (B) Quantitation of LYVE-1+ lymphatic and CD31+ blood vessel area inside the areas of inflammation. Values are the means ± SD of 5–6 legs per group. *p<0.05 vs. control virus.
Discussion
Tissue responses in inflammatory arthritis include a number of complex pathological changes in affected joints, including edema. During inflammatory reactions, factors that increase the drainage function of lymphatic vessels could lead to enhanced clearance of inflammatory cells and pro-inflammatory cytokines from tissues to draining LNs, resulting in less severe inflammation. Here, we report for the first time using TNF-Tg mice as a model of chronic inflammatory arthritis, that promotion of lymphangiogenesis by intra-articular injection of AAV-VEGF-C decreased the severity of the synovitis and joint destruction accompanied by increased numbers of lymphatic vessels and improved lymph flow from the affected joint area to draining lymph nodes. These findings suggest that VEGF-C treatment can both affect the severity of inflammatory process and improve the function of new lymphatics.
It has been a general accepted concept that inflammation stimulates lymphangiogenesis as a compensatory mechanism to enhance the clearance of inflammatory products. This conclusion is mainly based on immunostaining observations of inflamed tissues with antibodies specifically recognizing lymphatic endothelium cells, such as LYVE-1 (2, 21, 38, 39). Increase in lymphangiogenesis is due to inflammatory cells such as macrophages and myeloid precursors producing lymphatic growth factors (2, 21, 38, 40), leading to proliferation of lymphatic endothelium cells and perhaps remodeling of existing lymphatic networks. For instance, we and others have reported that TNF stimulates VEGF-C production by synovial fibroblasts (41) and macrophages (21). However if these newly formed lymphatic vessels in response to inflammatory stimuli fully function is not clear. Our findings suggest that increased LYVE-1+ lymphatics vessels in synovium of TNF-Tg mice are either functionally impaired or present in insufficient numbers to be fully effective and that AAV-VEGF-C can rectify these deficiencies. This finding is consistent with a recent report using keratin 14-VEGF-A and VEGF-C double transgenic mice, in which ICG lymphatic imaging indicates that chronic skin inflammation induced by VEGF-A transgene is associated with impaired lymphatic drainage from inflamed sites to local draining LNs. Over-expressing VEGF-C under the same promoter reduces the degree of inflammation and improves the lymph flow (42). Thus functional improvement of lymphatic vessels within the inflammatory sites and surrounding tissues is another therapeutic alternative to inflammation-induced edema and tissue damage.
The roles of vascular growth factors and their receptors in lymphangiogenesis in inflammatory conditions have only recently begun to be elucidated. VEGF-A binds to VEGFR-2, while VEGF-C and D bind to VEGFR-3 on lymphatic endothelium to regulate lymphatic vessel formation in physiologic conditions. However, it is not clear if one of these vascular growth factors or receptors plays a dominant role in the induction of new lymphatic vessel formation during inflammation. Most studies have focused on VEGF-A because of its clearly defined role in new blood vessel formation in many inflammatory processes. For example, in UVB-induced inflammation, UVB up-regulates epidermal VEGF-A expression resulting in hyper-permeability of cutaneous lymphatic vessels and decreased lymphatic drainage. In this model, blockade of VEGF-A reduced inflammation and the size of lymphatic vessels, while VEGF-A over-expression increased the number of lymphatic vessels at the inflammatory site as well as in draining lymph nodes (42, 43). Similarly, in TNF-Tg arthritic mice, blockade of VEGF-A signaling through a VEGFR-2 neutralizing antibody diminished synovial inflammation as well as the size of lymphatic vessels in joints and draining lymph nodes (15).
The role of VEGF-C in inflammation has not been clear to date. In chronic airway inflammation induced by Mycoplasma pulmonis infection (38) or in chronic arthritis induced by TNF over-expression (15), systemic blockade of VEGFR-3 signaling caused exaggerated tissue edema and damage. These findings suggest that VEGF-C-mediated activation of the VEGFR-3 pathway in lymphatic endothelium might possibly play an important role in counteracting the pro-inflammatory effects of VEGF-A that are mediated by VEGFR-2. To support this hypothesis, dermal administration of VEGF-C that specifically activates VEGFR-3 but not VEGFR-2 reduces inflammation-induced skin edema (1). In current study, we demonstrated that intra-articular injection of VEGF-C reduces the degree of tissue damage and bone destruction and improves lymph flow in joints, highlighting the importance of lymphatic flow. However, does VEGF-C only act on lymphangiogenesis or does it possibly have chemotactic or other immunomodulatory effects? VEGF-C increases survival of lymphatic endothelium, sprouting of lymphatic vessels, chemotactis of monocytes, and migration and invasion of cancer cells. A recent study indicated that VEGF-C blockade reduces CD4+ T cell infiltration in a mouse cardiac allografts model (44) due to inhibition of lymphatic vessel formation. However, there is no report describing that VEGF-C has immunomodulatory effects. Thus if VEGF-C works through other mechanisms to reduce joint inflammation apart from improvement of lymphatic drainage should be investigated in the future, such as whether VEGF-C stimulates its targeting cells to produce inflammatory inhibitors.
Currently, we do not fully understand the molecular mechanisms mediating lymphangiogenesis in RA joints, especially why newly formed lymphatic vessels are not fully functional. We speculate that observed lymphatic vessels in the tissue sections of RA synovium are lymphatic capillaries, which can be identified by LYVE-1. However, these IHC results cannot detect mature lymphatic vessels, which are LYVE-1 negative, have valves and execute the lymph transport function. Development of tools to identify these matures lymphatic vessels morphologically in joint synovium are essential for assessing changes of lymphatics during RA development and progression. Finally, although locally administered VEGF-C reduced the severity of inflammation and joint destruction and improved lymphatic drainage, the effects were incomplete, suggesting that either the concentration of VEGF-C attained locally was insufficient or more likely that other factors contribute to the inflammation, joint destruction and lymphatic drainage. Thus it may be necessary to use combinations of therapies to achieve optimal responses.
In summary, our study and others using different models of inflammation clearly demonstrate that lymphangiogenesis developing in response to inflammation has a limited compensatory effect overall on resolution of the process (2, 15, 38, 45). These inflammation-induced lymphatic vessels are not sufficiently functional, but their function can be improved through the administration of exogenous VEGF-C or other lymphatic factors. Further studies will be required to determine the precise nature of the defect in inflammation-induced lymphatic vessels, if this is cell-mediated and if factors produced during inflammation inhibit lymphatic function.
Supplementary Material
Acknowledgements
The authors thank Ms. Yanyun Li for technical assistance with the histology. This work was supported by research grants from the National Institutes of Health PHS awards (AR48697 and AR53586 to LX, AR 43510 to BFB, DE17096 and AR54041 to ES). Part of Dr. Zhou’s salary was supported by grants from the National Basic Research Program (2010CB530400 to YJW) and from Shanghai Science Technology Qimingxing Program (09QA1405600 to QZ).
Footnotes
Author contributions
Dr. Lianping Xing has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Drs. Zhou, Guo, Liang, Wood, Wang, Boyce, Schwarz, and Xing.
Acquisition of data. Drs. Zhou, Guo, Liang, Wood, and Xing.
Analysis and interpretation of data. Drs. Zhou, Wood, Guo, Liang, Wang, Boyce, Schwarz, Xing.
Manuscript preparation. Drs. Zhou, Guo, Liang, Wood, Wang, Boyce, Schwarz, and Xing.
Statistical analysis. Drs. Zhou, Guo, Liang,
References
- 1.Kajiya K, Sawane M, Huggenberger R, Detmar M. Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced edema formation and skin inflammation by promoting lymphangiogenesis. J Invest Dermatol. 2009;129(5):1292–1298. doi: 10.1038/jid.2008.351. [DOI] [PubMed] [Google Scholar]
- 2.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113(22):5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 3.Xu H, Edwards J, Banerji S, Prevo R, Jackson DG, Athanasou NA. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann Rheum Dis. 2003;62(12):1227–1229. doi: 10.1136/ard.2003.005876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paavonen K, Mandelin J, Partanen T, Jussila L, Li TF, Ristimaki A, et al. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J Rheumatol. 2002;29(1):39–45. [PubMed] [Google Scholar]
- 5.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, et al. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119(10):2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uzarski J, Drelles MB, Gibbs SE, Ongstad EL, Goral JC, McKeown KK, et al. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol. 2008;294(3):H1326–H1334. doi: 10.1152/ajpheart.00900.2007. [DOI] [PubMed] [Google Scholar]
- 7.Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann N Y Acad Sci. 2008;1131:89–99. doi: 10.1196/annals.1413.008. [DOI] [PubMed] [Google Scholar]
- 8.Schett G. Review: Immune cells and mediators of inflammatory arthritis. Autoimmunity. 2008;41(3):224–229. doi: 10.1080/08916930701694717. [DOI] [PubMed] [Google Scholar]
- 9.Haynes DR. Inflammatory cells and bone loss in rheumatoid arthritis. Arthritis Res Ther. 2007;9(3):104. doi: 10.1186/ar2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wauke K, Nagashima M, Ishiwata T, Asano G, Yoshino S. Expression and localization of vascular endothelial growth factor-C in rheumatoid arthritis synovial tissue. J Rheumatol. 2002;29(1):34–38. [PubMed] [Google Scholar]
- 11.Olszewski WL, Pazdur J, Kubasiewicz E, Zaleska M, Cooke CJ, Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44(3):541–549. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Wood R, Schwarz EM, Wang YJ, Xing L. Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis Rheum. 2010;62(7):1881–1889. doi: 10.1002/art.27464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proulx ST, Kwok E, You Z, Beck CA, Shealy DJ, Ritchlin CT, et al. MRI and quantification of draining lymph node function in inflammatory arthritis. Ann N Y Acad Sci. 2007;1117:106–123. doi: 10.1196/annals.1402.016. [DOI] [PubMed] [Google Scholar]
- 14.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21(2):154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Guo R, Zhou Q, Proulx ST, Wood R, Ji RC, Ritchlin CT, et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009;60(9):2666–2676. doi: 10.1002/art.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, Schwarz EM, O'Keefe RJ, Ma L, Looney RJ, Ritchlin CT, et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50(1):265–276. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 17.Yin G, Liu W, An P, Li P, Ding I, Planelles V, et al. Endostatin gene transfer inhibits joint angiogenesis and pannus formation in inflammatory arthritis. Mol Ther. 2002;5(5 Pt 1):547–554. doi: 10.1006/mthe.2002.0590. [DOI] [PubMed] [Google Scholar]
- 18.Yang SY, Mayton L, Wu B, Goater JJ, Schwarz EM, Wooley PH. Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis Rheum. 2002;46(9):2514–2523. doi: 10.1002/art.10527. [DOI] [PubMed] [Google Scholar]
- 19.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11(3):291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proulx ST, Kwok E, You Z, Papuga MO, Beck CA, Shealy DJ, et al. Longitudinal assessment of synovial, lymph node, and bone volumes in inflammatory arthritis in mice by in vivo magnetic resonance imaging and microfocal computed tomography. Arthritis Rheum. 2007;56(12):4024–4037. doi: 10.1002/art.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Lu Y, Proulx ST, Guo R, Yao Z, Schwarz EM, et al. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res Ther. 2007;9(6):R118. doi: 10.1186/ar2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyce BF. Bone biopsy and histomorphometry in metabolic bone disease. In: Stevenson JC, editor. New techniques in metabolic bone disease. London: Butterworths; 1990. pp. 110–131. [Google Scholar]
- 23.Yao Z, Xing L, Boyce BF. NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest. 2009;119(10):3024–3034. doi: 10.1172/JCI38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Z, Li P, Zhang Q, Guo R, Schwarz EM, Boyce BF, et al. Disruption of Rankl/Rank Signaling Reduces TNF-Induced Joint Inflammation In Vivo. The Open Arthritis Journal. 2009;2:7–13. [Google Scholar]
- 25.Rothstein JM, Miller PJ, Roettger RF. Goniometric reliability in a clinical setting. Elbow and knee measurements. Phys Ther. 1983;63(10):1611–1615. doi: 10.1093/ptj/63.10.1611. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MR, Wang K, Smith JB, Heslin MJ, Diasio RB. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal Biochem. 2000;278(2):175–184. doi: 10.1006/abio.1999.4461. [DOI] [PubMed] [Google Scholar]
- 27.Saaristo A, Veikkola T, Enholm B, Hytonen M, Arola J, Pajusola K, et al. Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. Faseb J. 2002;16(9):1041–1049. doi: 10.1096/fj.01-1042com. [DOI] [PubMed] [Google Scholar]
- 28.Bhardwaj S, Roy H, Gruchala M, Viita H, Kholova I, Kokina I, et al. Angiogenic responses of vascular endothelial growth factors in periadventitial tissue. Hum Gene Ther. 2003;14(15):1451–1462. doi: 10.1089/104303403769211664. [DOI] [PubMed] [Google Scholar]
- Goldman J, Le TX, Skobe M, Swartz MA. Overexpression of VEGF-C Causes Transient Lymphatic Hyperplasia but Not Increased Lymphangiogenesis in Regenerating Skin. Circ Res. 2005;96(11):1193–1199. doi: 10.1161/01.RES.0000168918.27576.78. [DOI] [PubMed] [Google Scholar]
- 30.Ulrich-Vinther M, Carmody EE, Goater JJ, Goater JJ, Sb K, O'Keefe RJ, Schwarz EM. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Joint Surg Am. 2002;84-A(8):1405–1412. doi: 10.2106/00004623-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. Embo J. 1997;16(13):3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274(45):32127–32136. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- 33.Anisimov A, Alitalo A, Korpisalo P, Soronen J, Kaijalainen S, Leppanen VM, et al. Activated forms of VEGF-C and VEGF-D provide improved vascular function in skeletal muscle. Circ Res. 2009;104(11):1302–1312. doi: 10.1161/CIRCRESAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayer S, Redlich K, Korb A, Hermann S, Smolen J, Schett G. Tenosynovitis and osteoclast formation as the initial preclinical changes in a murine model of inflammatory arthritis. Arthritis Rheum. 2007;56(1):79–88. doi: 10.1002/art.22313. [DOI] [PubMed] [Google Scholar]
- 35.Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13(12):1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 36.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006;72(3):161–171. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onimaru M, Yonemitsu Y, Fujii T, Tanii M, Nakano T, Nakagawa K, et al. VEGF-C regulates lymphangiogenesis and capillary stability by regulation of PDGF-B. Am J Physiol Heart Circ Physiol. 2009;297(5):H1685–H1696. doi: 10.1152/ajpheart.00015.2009. [DOI] [PubMed] [Google Scholar]
- 38.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115(2):247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, et al. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood. 2010;115(2):418–429. doi: 10.1182/blood-2008-12-196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proulx ST, Luciani P, Derzsi S, Rinderknecht M, Mumprecht V, Leroux JC, et al. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 2010;70(18):7053–7062. doi: 10.1158/0008-5472.CAN-10-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cha HS, Bae EK, Koh JH, Chai JY, Jeon CH, Ahn KS, et al. Tumor necrosis factor-alpha induces vascular endothelial growth factor-C expression in rheumatoid synoviocytes. J Rheumatol. 2007;34(1):16–19. [PubMed] [Google Scholar]
- 42.Huggenberger R, Ullmann S, Proulx ST, Pytowski B, Alitalo K, Detmar M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med. 2010;207(10):2255–2269. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajiya K, Hirakawa S, Detmar M. Vascular Endothelial Growth Factor-A Mediates Ultraviolet B-Induced Impairment of Lymphatic Vessel Function. Am J Pathol. 2006;169(4):1496–1503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nykanen AI, Sandelin H, Krebs R, Keranen MA, Tuuminen R, Karpanen T, et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010;121(12):1413–1422. doi: 10.1161/CIRCULATIONAHA.109.910703. [DOI] [PubMed] [Google Scholar]
- 45.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–1191. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohela M, Helotera H, Haiko P, Dumont DJ, Alitalo K. Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am J Pathol. 2008;173(6):1891–1901. doi: 10.2353/ajpath.2008.080378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.