Abstract
Objective
To explore prognostication of incident radiographic knee osteoarthritis (OA) with OA-related biomarkers in a large sample of African-American and Caucasian men and women.
Methods
Baseline serum cartilage oligomeric matrix protein (COMP), hyaluronan (HA), high-sensitivity C-reactive protein (hsCRP), and keratan sulfate (KS) and baseline and follow-up radiographs were available for 353 knees without baseline osteophyte formation (OST), and 446 knees without baseline joint space narrowing (JSN). Cox models estimated the hazard ratio (HR) for incident knee OA for a 1-unit increase in the natural log (ln) of each biomarker, adjusting for age, race, gender, body mass index, and knee OA of the contralateral limb. Report of chronic knee symptoms was explored as a modifier of the association.
Results
The hazard of incident knee OST (HR= 2.16, 95% CI =1.39-3.37) and incident JSN (HR=1.82, 95% CI = 1.15-2.89) increased with higher baseline lnCOMP levels. The hazard of incident knee JSN increased with higher lnHA levels (HR=1.46, 95% CI= 1.14-1.87). Baseline lnhsCRP and lnKS did not predict incident knee outcomes. HRs per unit increase in lnCOMP, lnHA, and lnKS were higher among knees with chronic symptoms than those without symptoms.
Conclusion
Higher baseline lnCOMP and lnHA levels were associated with incident knee OA over an average follow-up period of 6.3 years. These results represent detection of a molecular stage of OA prior to radiographic manifestations. Chronic knee symptoms as a modifier of the biomarker-incident knee OA association requires further exploration.
Osteoarthritis (OA) is one of the most common causes of disability in the United States (1), but effective interventions for this disease are limited. One difficulty that hinders the development of successful treatments is the lack of sensitive measures of incident or progressive OA. Radiographic measures that are used include the Kellgren-Lawrence (K-L) score (2), which provides a global measure of the presence of radiographic features of OA, and radiographic features of osteophytes (OST) or joint space narrowing (JSN) that can be examined separately and graded with a standardized atlas (3, 4). However, these radiographic measures are late stage indicators of disease, and studies using these outcomes become lengthy and costly because they require follow-up times that extend over many years (5, 6). Magnetic resonance imaging (MRI) is more capable of detecting multiple bone and soft tissue structural features of OA and changes in the disease over time, but its use in clinical and observational trials and the clinic is limited due to a current lack of accepted and widely validated scoring systems, long examination and interpretation times, and cost factors (7, 8). Biochemical markers (biomarkers) of joint metabolism require only blood or urine collection, and thus, can be evaluated more quickly and at a lower cost than radiographic or MRI markers. Additionally, biomarkers may be more sensitive for detecting the development and progression of OA than current imaging methods (9), particularly at early “molecular” stages of the disease (10).
Although several biomarkers have been associated with progressive OA (11-13), currently no studies have identified prognostic biomarkers of incident knee OA in men, and there has been only one positive study in women (14). Among likely candidates for biomarkers of incident knee OA in men and women are serum cartilage oligomeric matrix protein (COMP) (15, 16), (17), serum hyaluronan (HA) (18), serum high-sensitivity C-reactive protein (hsCRP) (14, 19), and serum keratan sulfate (KS) (20). COMP is present in serum, articular cartilage, synovium, ligaments, and tendons (21), and elevated levels of COMP in joint fluid and serum may indicate a release of fragments of this protein and synovial inflammation (22). We have previously shown that levels of serum COMP are higher in persons with no radiographic knee or hip OA with hip-related symptoms but not knee-related symptoms (23). HA is a glycosaminoglycan found in all connective tissues and synovial fluids, and it may be a marker of localized joint inflammation (24). Serum hsCRP is a protein associated with systemic inflammation, and KS is a glycosaminoglycan associated with cartilage proteoglycan degradation.
In addition to biomarker levels, the presence of joint symptoms of pain, aching, or stiffness may be helpful for identifying individuals at risk of developing radiographic OA (25), including potential participants for clinical prevention trials in OA. Joint symptoms may be early indicators of OA prior to the appearance of radiographic evidence of the disease (25). Previous studies have examined the levels of biomarkers in radiographic knee OA in cross-sectional analyses or longitudinally in small samples (22, 26), but have not assessed whether specific biomarkers may predict the development of OA in longitudinal cohorts.
Previous analyses of data from this project demonstrated cross-sectional associations between OA and COMP (11), HA (24), and hsCRP (27); racial and gender differences in biomarker levels of COMP (23, 28), HA (24), and hsCRP (27); and confounding by obesity, comorbid conditions, and medication in the association of hsCRP and OA (27). The purpose of this paper was to determine the hazard of incident radiographic knee OA based on three measures (the global K-L score) and radiographic features of OST and JSN and four biomarkers (COMP, HA, hsCRP, and KS) measured 3-10 years previously in a large bi-racial sample derived from the community-based sample of the Johnston County Osteoarthritis (JoCo OA) Project. We hypothesized that higher levels of each biomarker would be associated with incident radiographic knee OA outcomes (K-L score, OST, and JSN). A secondary objective was to explore whether these associations were modified by chronic knee symptoms. We hypothesized that biomarker-OA outcome associations would be stronger among those with chronic knee symptoms at baseline compared to those without baseline knee symptoms.
Materials and Methods
Study Participants
The JoCo OA Project is an ongoing, community-based study of knee and hip OA in African American and Caucasian residents in a rural county in North Carolina. Details of this study have been reported previously (29). Briefly, this study involved civilian, non-institutionalized adults aged 45 years and older who resided in six townships in Johnston County. Participants were recruited by probability sampling, with over-sampling of African Americans. A total of 3,187 individuals completed a baseline clinical evaluation from 19911997, and follow-up assessments were completed from 1999-2003. Serum was collected for all participants at baseline, and based on available resources, baseline biomarker assessments were completed for 800 of the participants. To allow for analyses of biomarkers levels by race, gender, OA presence, and age, the 800 participants were selected with complete radiographic data at baseline to represent roughly equal proportions of African Americans, and whites, women and men, and those with and without radiographic knee OA, representing a range of ages (categorized as 45–54 years, 55–64 years, and 65 years and older). These participants were selected without regard to return for follow-up visit.
Individuals having radiographic evidence of rheumatoid arthritis or other inflammatory arthropathies in the knees or hips were not included in the subsample. Nearly 47% (N=377) of the 800 participants did not return at follow-up. Reasons that participants were not eligible or available included emigration from study area (N=34), refusal (N=114), inability to participate due to physical or mental conditions (N=57), death (N=146), and inability to contact or find (N=26).
Baseline Biomarker Assessment
Baseline serum samples for COMP, HA, hsCRP, and KS were collected, spun, and stored at -86°C. Serum COMP was measured using an in-house sandwich enzyme linked immunosorbent assay (ELISA)(30). The reported precision is 5.8-6.6% intra-assay variability and between 8.7-9.7% inter-assay variability. Serum HA was measured with the Hylauronic Acid Test kit (Corgenix, Westimister, CO). The reported precision is 3.6-4.7% intra-assay variability and 5.7-7.0% inter-assay variability. Serum hsCRP was measured with the UBI Magiwel Enzyme Immunoassay (United Biotech Inc. Mountain View, CA). The minimum detectable concentration for this assay is 0.35 nanograms/milliliter (ng/ml), and the inter-assay precision was 7.4% (N=20) at a concentration of 3 ng/ml in the JoCo OA project biomarker substudy (27). Serum KS was measured using an in-house competitive ELISA as previously described (31). The inter-assay precision was 14.4%, and the intra-assay precision was 3.2% (31).
Radiographic Assessment of Incident Knee OA
At baseline and follow-up, participants completed bilateral anteroposterior weight-bearing radiography of the knee with foot mat placement (32). Radiographs were read paired (baseline and follow-up images read together) without knowledge of participant clinical status and blinded to time sequence by a single musculoskeletal radiologist (JBR) (32) for the overall K-L knee radiographic grade (score 0-4) (2) and for OST formation (score 0-3) and JSN (score 0-3) in the medial and lateral knee compartments based on the standardized Burnett atlas (3). Inter-rater reliability (comparison of radiograph readings between JBR and another radiologist) and intra-rater reliability (comparison of radiograph readings completed by JBR at two separate times) for the radiologist were high (weighted kappa for inter-rater reliability 0.9; kappa for intra-rater reliability 0.9) (29). Radiographs without the features of OA were defined as K-L grade of 0 (normal findings). A minute radiographic osteophyte of doubtful pathologic significance was assigned a K-L grade of 1 (questionable). Radiographs showing an osteophyte without joint space narrowing were assigned a K-L grade of 2 (mild). A moderate decrease of the joint space was assigned a K-L grade of 3 (moderate). K-L grade 4 (severe) was defined as severe joint space narrowing with subchondral bone sclerosis (2). Incident radiographic OA was defined as a K-L grade ≥2 at follow-up among participants with K-L grade <2 at baseline. Incident OST and JSN were defined as joints with grade=0 at baseline and ≥1 at follow-up.
Figure 1 details the selection of knee joints for analyses. Knees with missing follow-up data were excluded from analyses. Of the possible 1600 knees from the 800 participants with biomarker data at baseline, 728 knees (364 participants) had paired baseline and follow-up knee radiograph assessments with available K-L grade data; OST and JSN readings were completed for 670 knees (335 participants (Figure 1). Three separate risk subgroups were created based on knee radiographic OA status at baseline: 542 knees at risk for incident radiographic knee OA (baseline radiographic K-L grade <2), 349 at risk for incident OST (baseline OST grade = 0), and 440 at risk for incident JSN (baseline JSN grade =0). Among the knees at risk for incident knee OA, two knees had a total knee arthroplasty by the follow-up visit. Both knee replacement surgeries were indicated because of knee OA (per participant self-report), and thus, these knees were considered incident knee OA and included in analyses.
Figure 1.
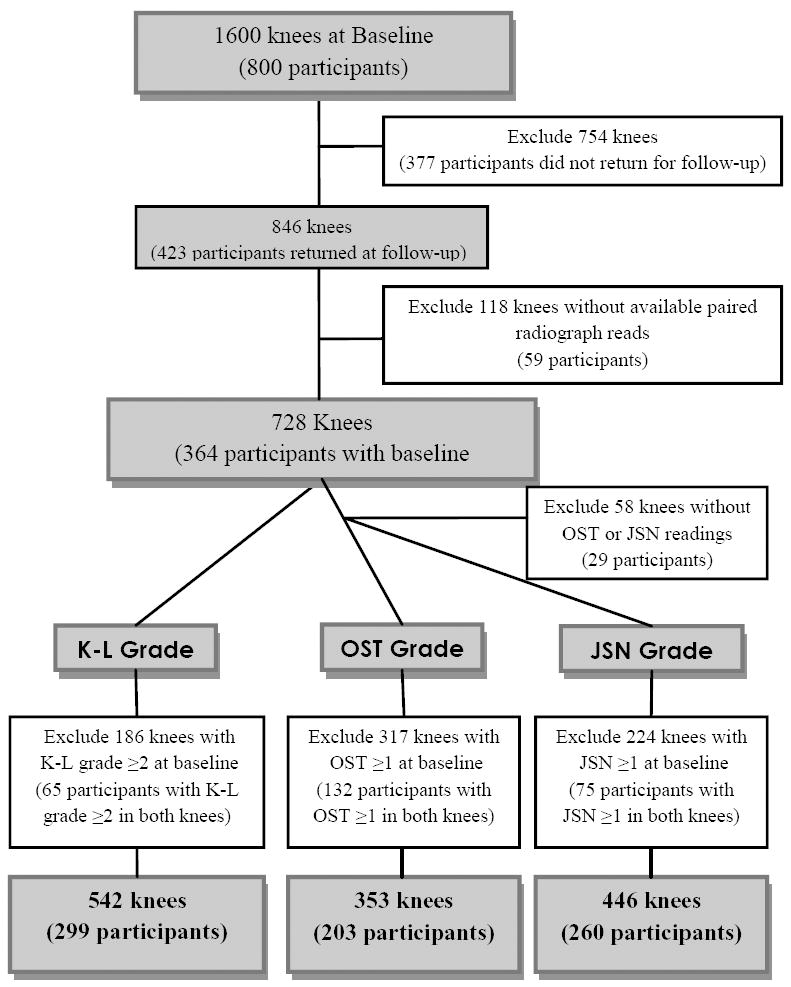
Description of knees from N= 800 participants with baseline biomarker data.
Baseline Chronic Knee Symptoms
Participants completed an interviewer-administered questionnaire in which they answered “Yes” or “No,” separately for left and right knees, to the question: “On most days do you have pain, aching or stiffness in your [left/right] knee?
Baseline Demographic and Clinical Characteristics
The following participant characteristics were examined as potential covariates in our analyses because they have been associated with biomarkers of OA and incident knee radiographic OA: gender; self-reported race (African American or Caucasian); age (continuous variable in years); body mass index at baseline (BMI: continuous variable calculated as weight in kilograms/height in meters squared). Height without shoes was measured in centimeters and weight was measured in kilograms using a balance beam scale.
Statistical Analysis
Descriptive statistics were calculated for race, gender, age, BMI, and chronic knee symptoms (for the total sample with biomarker data and baseline and follow-up paired knee radiographs and for each subgroup of incident radiographic outcome) and compared by follow-up status (chi-square statistics for categorical variables and t-test statistics for continuous variables). The natural logarithm transformation was used to produce nearly normal distributions for COMP (lnCOMP), HA (lnHA), hsCRP (lnhsCRP), and KS (lnKS) in analyses. Because of the wide range of follow-up times for participants (mean = 6.3 years; standard deviation ± 1.4 years; range= 3.0 – 10.2 years) and interval censoring (i.e., the exact time of the occurrence of the outcome is unknown), separate multivariable Cox models (with the midpoint of each individual’s follow-up period as the approximated endpoint) were used to estimate the hazard ratio (HR) for a 1-unit increase in the natural log of each biomarker with each radiographic knee outcome. We accounted for bilateral clustering of knees using robust variance estimates (33). Covariates included in adjusted models were baseline race, gender, age, BMI, and presence of contralateral knee OA (K-L grade ≥ 2), which were identified by their association with both the biomarkers and radiographic OA (K-L grade or radiographic features), either based on statistical significance in bivariate associations or on prior published knowledge of each covariate. The models used were:
where log hi(t) = hazard of radiographic OA outcome (K-L grade, OST, or JSN, respectively)
If there were notable associations between any of the biomarkers and OST, we examined this association among those with no baseline JSN to determine independence of the association. Similarly, associations between biomarkers and JSN were examined among those with no baseline OST. Multiplicative interaction terms of each biomarker (lnCOMP, lnHA, lnhsCRP, lnKS) with each covariate (race, gender, age, BMI, chronic knee symptoms) were examined. If the interaction term was statistically significant (p<0.05), then it was included in models. Additionally, the associations of each biomarker and outcome were stratified by chronic knee symptoms, to explore patterns of potential modification of the association by either of these covariates. Cox models were fit using SAS Version 9.1 software (SAS Institute, Cary, NC).
Results
Of the 800 participants with baseline biomarker data, 423 (52.9%) returned for a follow-up visit (Table 1). Those who did not return were more likely to be older, be men, be African American, or have knee OA (K-L grade ≥ 2 in at least one knee) at baseline (Table 1).
Table 1.
Characteristics of Participants with Baseline Biomarker Data by Return to Follow-Up Visit (N=800).
| Returned to Follow-Up Visit? | |||
|---|---|---|---|
| Characteristics | Yes N=423 (52.9%) | No N=377 (47.1%) | p-value* |
| Baseline Age (mean (SD)) | 60.2 (±9.6) | 64.9 (±10.9) | <0.01 |
| Baseline Body Mass Index (mean kg/m2(SD)) | 30.6 (±7.2) | 29.9 (±6.7) | 0.17 |
| Women, n/N (%) | 272/423 (64.3) | 202/377 (53.6) | <0.01 |
| African American, n/N (%) | 198/423 (46.8) | 200/377 (53.1) | 0.08 |
| Baseline Chronic Knee Symptoms, n/N (%) | 158/419 (37.7) | 146/370 (39.5) | 0.51 |
| Baseline Knee Osteoarthritis, n/N (%) | 224/423 (53.0) | 228/377 (60.5) | 0.03 |
Chi-square p-value for categorical variables and t-test p-value for continuous variables.
Kellgren-Lawrence Score ≥ 2.
The total study group for this analysis comprised 364 participants with paired knee radiographs and biomarker data (62.9% women, 42.9% African American). The mean age was 60.4 years (range 45-84 years), and on average, participants were obese at baseline with a BMI of 30.4 kg/m2 (range 16.0-58.1 kg/m2, Table 2). Nearly 50% reported chronic knee symptoms at baseline. Compared to the total study group, the subgroups at risk of incident knee outcomes (OA, OST, and JSN) were slightly younger, had a lower BMI, and had a lower proportion of women, African Americans, and those with baseline chronic knee symptoms.
Table 2.
Characteristics of Participants with Biomarker Data and Baseline and Follow-Up Paired Knee Radiographs (N=364).
| Subgroups at Risk of Incident Outcome | ||||
|---|---|---|---|---|
| Characteristics | Total (N=364, knees=728) | K-L Grade* (N=299, knees =542) | OST Grade† (N=203, knees=353) | JSN Grade‡ (N=260, knees=446) |
| Baseline Age (mean (SD)) | 60.4 (±9.6) | 58.8 (±8.8) | 58.8 (±8.6) | 59.2 (±8.9) |
| Baseline Body Mass Index (mean kg/m2(SD)) | 30.4 (±7.1) | 29.8 (±6.7) | 28.6 (±5.5) | 29.7 (±6.6) |
| Women, n/N (%) | 231/364 (62.9) | 179/299 (59.9) | 113/203 (55.7) | 153/260 (58.9) |
| African American, n/N (%) | 156/364 (42.9) | 115/299 (38.5) | 61/203 (30.5) | 99/260 (38.1) |
| Baseline Chronic Knee Symptoms, n/N (%) | 180/362 (49.7) | 127/299 (42.6) | 78/202 (38.6) | 113/259 (43.6) |
K-L risk group: K-L grade of 0 or 1 in at least one knee at baseline
OST risk group: OST grade of 0 in at least one knee at baseline
JSN risk group: JSN grade of 0 in at least one knee at baseline
Table 3 displays the hazard ratios for incident radiographic knee outcomes by an incremental increase in the natural log of each biomarker, indicating associations between higher ln biomarker levels and radiographic knee OA outcomes. In adjusted models, the hazard of incident knee OST increased with higher baseline lnCOMP levels (adjusted HR [aHR] = 2.16, 95% confidence interval [CI]) = 1.39-3.37). Among participants with no baseline JSN, the hazard of incident knee OST in adjusted models was 2.40 (95% CI = 1.44-4.01) with higher baseline lnCOMP levels. The hazard of incident knee JSN also increased with higher baseline lnCOMP levels (aHR=1.82, 95% CI= 1.15-2.89) and with higher baseline lnHA levels (aHR=1.46, 95% CI= 1.14-1.87). Among participants with no baseline OST, the hazard of incident knee JSN in adjusted models was 4.01 (95% CI = 2.08-7.72) with higher baseline lnCOMP levels and 1.26 (95% CI = 0.85-1.87) with higher baseline lnHA levels. The hazard of incident knee OA based on K-L grade also increased with higher baseline lnCOMP levels (aHR=1.39, 95% CI = 0.90-2.13) and with higher baseline lnHA levels (aHR=1.12, 95% CI = 0.90-1.40), although these associations were not statistically significant. In unadjusted models, the hazard of incident knee JSN increased with higher levels of baseline lnhsCRP (HR = 1.16, 95% CI = 1.01 – 1.34), and the hazard of incident knee OST increased with higher levels of baseline lnKS (HR = 1.89, 95% CI = 1.03-3.47). In adjusted models, higher levels of baseline lnhsCRP and lnKS did not predict the incident knee outcomes. In all models, estimated associations did not differ or differed minimally with and without adjustment for baseline contralateral knee OA. No statistically significant interactions (i.e., no evident departures from hazard ratio multiplicativity) were observed between the natural logs of each biomarker and age, BMI, race, gender, or chronic knee symptoms.
Table 3.
Unadjusted and Adjusted Hazard Ratios (HR) of Knee Outcomes, by Biomarker.
| Predictor | Outcome | # Knees at Risk | # Knees with Outcome | Unadjusted HR | 95% CI | Wald Chi-square P-value | Adjusted HR† | 95% CI | Wald Chi-square P-value |
|---|---|---|---|---|---|---|---|---|---|
| ln(COMP) | Knee OA (K-L)* | 542 | 110 | 1.73 | 1.18-2.53 | <0.01 | 1.39 | 0.90-2.13 | 0.13 |
| Knee OST* | 353 | 94 | 1.97 | 1.32-2.93 | <0.01 | 2.16 | 1.39-3.37 | <0.01 | |
| Knee JSN* | 446 | 102 | 2.24 | 1.46-3.45 | <0.01 | 1.82 | 1.15-2.89 | 0.01 | |
|
| |||||||||
| ln (HA) | Knee OA (K-L) | 542 | 110 | 1.33 | 1.09-1.62 | <0.01 | 1.12 | 0.90-1.40 | 0.30 |
| Knee OST | 353 | 94 | 1.20 | 0.97-1.48 | 0.09 | 0.91 | 0.70-1.19 | 0.49 | |
| Knee JSN | 446 | 102 | 1.51 | 1.22-1.87 | <0.01 | 1.46 | 1.14-1.87 | <0.01 | |
|
| |||||||||
| ln(hsCRP) | Knee OA (K-L) | 542 | 110 | 1.09 | 0.95-1.24 | 0.22 | 0.99 | 0.84-1.16 | 0.89 |
| Knee OST | 353 | 94 | 0.95 | 0.82-1.09 | 0.57 | 0.94 | 0.79-1.12 | 0.46 | |
| Knee JSN | 446 | 102 | 1.16 | 1.01-1.34 | 0.04 | 1.10 | 0.93-1.30 | 0.27 | |
|
| |||||||||
| ln (KS) | Knee OA (K-L) | 542 | 110 | 1.24 | 0.76-2.04 | 0.39 | 1.16 | 0.66-2.03 | 0.61 |
| Knee OST | 353 | 94 | 1.89 | 1.03-3.47 | 0.04 | 1.56 | 0.82-2.95 | 0.17 | |
| Knee JSN | 446 | 102 | 1.21 | 0.70-2.11 | 0.50 | 1.15 | 0.64-2.07 | 0.64 | |
OA=osteoarthritis, K-L=Kellgren-Lawrence, OST=osteophyte formation, JSN=joint space narrowing
Adjusted for age, body mass index, race, gender, and osteoarthritis in contralateral knee at baseline.
Although interactions of ln of each biomarker and knee symptoms were not statistically significant, a pattern was noted of higher adjusted HR estimates among those with chronic knee symptoms than those without for lnHA and lnKS across all three incident knee outcomes, for lnhsCRP for incident knee OST, and for lnCOMP for incident knee OA and OST. Table 4 shows the adjusted HRs for incident radiographic knee outcomes, stratified by baseline chronic knee symptoms.
Table 4.
Adjusted Hazard Ratios (HR) of Knee Outcomes, by Biomarker, Stratified by Chronic Knee Symptoms at Baseline.
| Chronic Knee Symptoms | No Chronic Knee Symptoms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Knee Outcome | # Knees at Risk | # Knees with Outcome | Adjusted HR† | 95% CI | # Knees at Risk | # Knees with Outcome | Adjusted HR† | 95% CI | P-value of Interaction‡ |
| ln (COMP) | KL score* | 166 | 46 | 2.04 | 1.05-3.99 | 374 | 64 | 1.37 | 0.77-2.45 | 0.10 |
| OST* | 97 | 33 | 4.14 | 1.84-9.30 | 254 | 61 | 1.79 | 0.99-3.20 | 0.80 | |
| JSN* | 130 | 35 | 1.99 | 0.90-4.38 | 314 | 66 | 1.90 | 1.02-3.52 | 0.55 | |
|
| ||||||||||
| ln(HA) | KL score | 166 | 46 | 1.14 | 0.79-1.66 | 374 | 64 | 1.01 | 0.76-1.33 | 0.16 |
| OST | 97 | 33 | 1.11 | 0.67-1.87 | 254 | 61 | 0.85 | 0.62-1.16 | 0.38 | |
| JSN | 130 | 35 | 1.97 | 1.27-3.05 | 314 | 66 | 1.36 | 1.00-1.83 | 0.76 | |
|
| ||||||||||
| ln(hsCRP) | KL score | 166 | 46 | 1.12 | 0.86-1.46 | 374 | 64 | 0.99 | 0.79-1.23 | 0.09 |
| OST | 97 | 33 | 1.03 | 0.76-1.41 | 254 | 61 | 0.88 | 0.71-1.09 | 0.39 | |
| JSN | 130 | 35 | 1.05 | 0.77-1.45 | 314 | 66 | 1.11 | 0.91-1.36 | 0.69 | |
|
| ||||||||||
| ln (KS) | KL score | 166 | 46 | 1.59 | 0.66-3.84 | 374 | 64 | 0.89 | 0.41-1.92 | 0.11 |
| OST | 97 | 33 | 3.47 | 1.15-10.45 | 254 | 61 | 1.19 | 0.52-2.72 | 0.43 | |
| JSN | 130 | 35 | 2.41 | 0.85-6.80 | 314 | 66 | 0.83 | 0.39-1.78 | 0.77 | |
K-L=Kellgren-Lawrence, OST=osteophyte formation, JSN=joint space narrowing
Adjusted for age, BMI, race, gender, and osteoarthritis in contralateral knee at baseline.
Interaction of ln(biomarker) and knee symptoms variable.
Discussion
The hazard of incident knee radiographic OA outcomes was elevated in those with higher baseline serum lnCOMP and lnHA levels. Higher baseline serum COMP levels were independently associated with both knee OST and JSN outcomes, while higher baseline serum HA levels were associated with knee JSN outcomes. These results are consistent with prior studies that have shown an association between higher serum COMP levels and cartilage and subchondral bone turnover (34) and a correlation between serum HA and joint space narrowing (35).
The relationship between COMP and progression of knee OA has been well-documented (12, 13, 37), but no prior study has evaluated the prognostic utility of COMP for incident knee OA, although one small Swedish study (women=17, men=21) reported concurrent increase in serum COMP during the early phase of incident radiographic knee OA (38). In a study of hip OA, high levels of COMP were associated with incident radiographic disease (39). Previous studies of serum HA and knee OA demonstrated a relationship between higher HA levels and progressive disease (18) and prevalent disease(24, 40), but serum HA and incident knee OA associations have not been reported.
Baseline levels of lnhsCRP and lnKS in our study did not predict incident knee OA outcomes. Serum hsCRP is recognized as one of the most sensitive indicators of the acute phase reaction and inflammation and has been associated with incident knee OA in studies of women (14, 19). However, obesity is strongly correlated with higher hsCRP levels, and controlling for obesity has been shown to attenuate the association between hsCRP and prevalent (27) and incident knee OA (14, 41). In the present analyses, BMI was adjusted for in biomarker and radiographic knee outcome associations. In unadjusted models, a one unit increase in lnhsCRP was not a predictor of incident knee OA or OST, but the hazard of incident knee JSN was 16% higher with increasing lnhsCRP levels. Controlling for BMI and other covariates reduced the associations between hsCRP and incident knee OA and JSN, but appeared to have minimal effect on the association with knee OST. As observed by Wakitani et al (20), large variations in serum KS values among participants were observed in the present study, and this may be in part responsible for the lack of association with incident knee radiographic outcomes.
Strengths of this study are several including that it consists of African American and Caucasian men and women, includes longitudinal radiographic data and chronic symptoms data of the knee, and uses an analytical method that can accommodate the interval censoring of the study design and the bilateral clustering of knees within subjects. A novel approach to this study was the exploration of incident knee OA using three definitions of radiographic OA (K-L grade, OST formation, and JSN). Several limitations restrict interpretations of the results. The small number of knees with chronic knee symptoms limited the power for assessment of this variable as a potential modifier of the association between biomarkers and the radiographic knee outcomes. Chronic knee symptoms may be a modifier of the biomarker-incident knee OA association as indicated by an overall pattern of elevated adjusted HRs among participant with symptoms compared to those without symptoms, particularly for higher baseline levels of HA and KS, but results of testing for interactions of biomarkers and knee symptoms were not statistically significant. Another limitation of the present study is that only a single measurement of each biomarker was collected at baseline rather than repeated measures at multiple time points. Because of the size and complexity of the parent study, measures that may alter serum biomarker levels, such as diurnal variation, exercise levels, postural changes, and dietary intake, were not controlled (42). Additionally, biomarkers collected in serum may be less sensitive to changes occurring in the joint of interest and more easily influenced by systemic or metabolic effects in other joints or body systems. Synovial fluid, while more difficult to collect, enables biomarker analyses that are more proximal and likely more precise assessments of local joint tissue turnover related to early knee OA. Because of concerns about having too many covariates in models, other medical disorders were not considered as covariates, although serum HA and serum hsCRP may be elevated with systemic inflammation related to several diseases. However, individuals with rheumatoid arthritis or other inflammatory arthropathies were not included in the subsample of 800 participants with biomarker data, and age and BMI, two factors associated with greater risk of multiple diseases and inflammation, were included as covariates in analyses.
Furthermore, the sizeable loss of participants by the follow-up visit (47%) may have influenced observed associations. Among participants without follow-up data, 39% had died, 15% had a decline in their health since baseline and were unable to participate at follow-up, and nearly 1/3 refused, limiting the longitudinal analyses to those participants who were more likely to be younger and healthier at baseline. The loss of participants at follow-up along with the availability of biomarker data for only a subset of the JoCo OA cohort reduces the generalizability of the results. Additionally, the loss of participants at follow-up reduced the power available for assessment of biomarker-OA outcome associations by baseline chronic knee symptoms
In this sample, higher lnCOMP and lnHA levels predicted incident knee OST and JSN. Chronic knee symptoms as a modifier of the biomarker-incident knee OA association requires further exploration, as this variable in combination with higher biomarker levels may indicate early stages of OA. Future studies should examine biomarker-incident knee OA associations after the initial appearance of knee symptoms, tracking biomarker levels over short, regular time intervals to determine the role this potential modifier plays to help identify individuals at risk of knee OA for clinical trials and interventions.
Acknowledgments
Supported by the Centers for Disease Control and Prevention/Association of Schools of Public Health S043 and S3486, Multipurpose Arthritis and Musculoskeletal Diseases Center grant 5-P60-AR-30701 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; Arthritis and Immunology T-32 Training grant from the National Institutes of Health AR-07416; National Institute of Arthritis and Musculoskeletal and Skin Diseases /National Institute on Aging grant P30 AG028716.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention (CDC) Prevalence and most common causes of disability among adults--United States, 2005. Morb Mortal Wkly Rep. 2009;58(16):421–6. [PubMed] [Google Scholar]
- 2.Kellgren JH, Lawrence JS. The epidemiology of chronic rheumatism, atlas of standard radiographs. Oxford: Blackwell Scientific; 1963. [Google Scholar]
- 3.Burnett SJ, Hart DJ, Spector TD. A radiographic atlas of osteoarthritis. London: Springer-Verlag; 1994. [Google Scholar]
- 4.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;155(Suppl A):A1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Mazzuca SA, Brandt KD. Is knee radiography useful for studying the efficacy of a disease-modifying osteoarthritis drug in humans? Rheum Dis Clin North Am. 2003;29(4):819–30. doi: 10.1016/s0889-857x(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 6.Mazzuca SA, Brandt KD, Katz BP, Lane KA, Buckwalter KA. Comparison of quantitative and semi-quantitative indicators of joint space narrowing in subjects with knee osteoarthritis. Ann Rheum Dis. 2005;65:64–8. doi: 10.1136/ard.2005.037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckstein F, Mosher T, Hunter D. Imaging of knee osteoarthritis: data beyond the beauty. Curr Opin Rheumatol. 2007;19(5):435–43. doi: 10.1097/BOR.0b013e328248b4be. [DOI] [PubMed] [Google Scholar]
- 8.Guermazi A, Hunter DJ, Roemer FW. Plain radiography and magnetic resonance imaging diagnostics in osteoarthritis: validated staging and scoring. J Bone Joint Surg Ann. 2009;91(Suppl 1):54–62. doi: 10.2106/JBJS.H.01385. [DOI] [PubMed] [Google Scholar]
- 9.Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14(8):723–7. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Kraus VB, Burnett B, Coindreau J, Cottrell S, Eyre D, Gendreau M, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis: OARSI FDA Osteoarthritis Biomarkers Working Group. Osteoarthritis Cartilage. 2011 doi: 10.1016/j.joca.2010.08.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42(11):2356–64. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Hunter DJ, Li J, LaValley M, Bauer DC, Nevitt M, DeGroot J, et al. Cartilage markers and their association with cartilage loss on magnetic resonance imaging in knee osteoarthritis: the Boston Osteoarthritis Knee Study. Arthritis Res Ther. 2007;9(5):R108. doi: 10.1186/ar2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vilím V, Olejárová M, Machácek S, Gatterová J, Kraus VB, P K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(9):707–13. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 14.Sowers M, Jannausch M, Stein E, Jamadar D, Hochberg M, Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage. 2002;10(8):595–601. doi: 10.1053/joca.2002.0800. [DOI] [PubMed] [Google Scholar]
- 15.Kelman A, Lui L, Yao W, Krumme A, Nevitt M, Lane NE. Association of higher levels of serum cartilage oligomeric matrix protein and N-telopeptide corsslinks with the development of radiographic hip osteoarthritis in elderly women. Arthritis Rheum. 2006;54:236–43. doi: 10.1002/art.21527. [DOI] [PubMed] [Google Scholar]
- 16.Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegard D, et al. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34:306–10. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- 17.Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegard D, et al. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34:306–10. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- 18.Sharif M, George E, Shepstone L, Knudson W, Thonar EJ, Cushnaghan J, et al. Serum hyaluronic acid level as a predictor of disease progression in osteoarthritis of the knee. Arthritis Rheum. 1995;38(6):760–7. doi: 10.1002/art.1780380608. [DOI] [PubMed] [Google Scholar]
- 19.Spector TD, Hart DJ, Nandra D, Doyle DV, Mackillop N, Gallimore JR, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40:723–7. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 20.Wakitani S, Nawata M, Kawaguchi A, Okabe T, Takaoka K, Tsuchiya T, et al. Serum keratan sulfate is a promising marker of early articular cartilage breakdown. Rheumatology (Oxford) 2007;11:1652–1656. doi: 10.1093/rheumatology/kem220. [DOI] [PubMed] [Google Scholar]
- 21.Williams FM, TD S. Biomarkers in osteoarthritis. Arthritis Res Ther. 2008;10(1):101. doi: 10.1186/ar2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohmander LS, Saxne T, Heinegard DK. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann Rheum Dis. 1994;53(1):8–13. doi: 10.1136/ard.53.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dragomir AD, Kraus VB, Renner JB, Lutas G, Clark A, Vilim V, et al. Serum cartilage oligomeric matrix protein and clinical signs and symptoms of potential pre-radiographic hip and knee pathology. Osteoarthritis Cartilage. 2002;10(9):687–91. doi: 10.1053/joca.2002.0816. [DOI] [PubMed] [Google Scholar]
- 24.Elliott AL, Kraus VB, Luta G, Stabler T, Renner JB, Woodard J, et al. Serum hyaluronan levels and radiographic knee and hip osteoarthritis in African Americans and Caucasians in the Johnston County Osteoarthritis Project. Arthritis Rheum. 2005;52(1):105–11. doi: 10.1002/art.20724. [DOI] [PubMed] [Google Scholar]
- 25.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Lohmander LS, Ionescu M, Jugessur H, Poole AR. Changes in joint cartilage aggrecan after knee injury and in osteoarthritis. Arthritis Rheum. 1999;42(3):534–44. doi: 10.1002/1529-0131(199904)42:3<534::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Kraus VB, Stabler TV, Luta G, Renner JB, Dragomir AD, Jordan JM. Interpretation of serum C-reactive protein (CRP) levels for cardiovascular disease risk is complicated by race, pulmonary disease, body mass index, gender, and osteoarthritis. Osteoarthritis Cartilage. 2007;15(8):966–71. doi: 10.1016/j.joca.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, et al. Ethnic and sex differences in serum levels of cartilage oligometric matrix protein. Arthritis Rheum. 2003;48(3):675–81. doi: 10.1002/art.10822. [DOI] [PubMed] [Google Scholar]
- 29.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis Care Res. 1995;8:242–50. doi: 10.1002/art.1790080407. [DOI] [PubMed] [Google Scholar]
- 30.Vilím V, Vobůrka Z, Vytásek V, Senolt L, Tchetverikov I, Kraus VB, et al. Monoclonal antibodies to human cartilage oligomeric matrix protein: epitope mapping and characterization of sandwich ELISA. Clin Chim Acta. 2003;328(1-2):59–69. doi: 10.1016/s0009-8981(02)00375-3. [DOI] [PubMed] [Google Scholar]
- 31.Lindhorst E, Vail TP, Guilak F, Wang H, Setton LA, Vilim V, et al. Longitudinal characterization of synovial fluid biomarkers in the canine meniscectomy model of osteoarthritis. J Orthop Res. 2000;18(2):269–80. doi: 10.1002/jor.1100180216. [DOI] [PubMed] [Google Scholar]
- 32.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomier AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172–80. [PubMed] [Google Scholar]
- 33.Lin DY, Wei LJ. The robust inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 34.Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegard D, et al. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34(4):306–10. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- 35.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41(7):1233–40. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Cibere J, Zhang H, Garnero P, Poole AR, Lobanok T, Saxne T, et al. Association of biomarkers with pre-radiographically defined and radiographically defined knee osteoarthritis in a population-based study. Arthritis Rheum. 2009;60(5):1372–80. doi: 10.1002/art.24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharif M, Kirwan JR, Elson CJ, Granell R, Clarke S. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50(8):2479–88. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]
- 38.Petersson IF, Boegard T, Svensson B, Heinegard D, Saxne T. Changes in cartilage and bone metabolism identified by serum markers in early osteoarthritis of the knee joint. Br J Rheumatol. 1998;37(1):46–50. doi: 10.1093/rheumatology/37.1.46. [DOI] [PubMed] [Google Scholar]
- 39.Kelman A, Lui L, Yao W, Krumme A, Nevitt M, Lane NE. Association of higher levels of serum cartilage oligomeric matrix protein and N-telopeptide crosslinks with the development of radiographic hips osteoarthritis in elderly women. Arthritis Rheum. 2006;54:236–43. doi: 10.1002/art.21527. [DOI] [PubMed] [Google Scholar]
- 40.Turan Y, Bal S, Gurgan A, Topac H, Koseoglu M. Serum hyaluronan levels in patients with knee osteoarthritis. Clin Rheumatol. 2007;26:1293–8. doi: 10.1007/s10067-006-0499-4. [DOI] [PubMed] [Google Scholar]
- 41.Engstrom G, GerhardssondeVerdier M, Rollof RJ, Nilsson PM, Lohmander LS. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis: A population-based cohort study. Osteoarthritis Cartilage. 2009;17(2):168–73. doi: 10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Kraus VB. Do biochemical markers have a role in osteoarthritis diagnosis and treatment? Best Pract Res Clin Rheumatol. 2006;20(1):69–80. doi: 10.1016/j.berh.2005.09.001. [DOI] [PubMed] [Google Scholar]


