Abstract
Background
The characteristics of human asthma are chronic inflammation and airway remodeling. Hyaluronan (HA), a major extracellular matrix component, accumulates during inflammatory lung diseases including asthma. Hyaluronan fragments stimulate macrophages to produce inflammatory cytokines. We hypothesized that HA and its receptors would play a role in human asthma.
Objective
To investigate the role of HA and HA binding proteins in human asthma.
Methods
Twenty-one subjects with asthma and 25 normal control subjects underwent bronchoscopy with endobronchial biopsy and bronchoalveolar lavage (BAL). Fibroblasts were cultured, HA and HA synthase expression was determined at baseline and after exposure to several mediators relevant to asthma pathobiology. The expression of HA binding proteins, CD44, TLR2 and TLR4 on BAL macrophages was determined by flow cytometry. IL-8 production by macrophages in response to HA fragment stimulation was compared.
Results
Airway fibroblasts from asthma patients produced significantly increased concentrations of lower molecular weight HA compared to those of normal fibroblasts. Hyaluronan synthase 2 mRNA was markedly increased in asthmatic fibroblasts. Asthmatic macrophages showed a decrease in cell surface CD44 expression and an increase in TLR2 and TLR4 expression. Macrophages from asthmatic subjects showed an increase in responsiveness to low molecular weight HA stimulation, as demonstrated by increased IL-8 production.
Conclusions
HA homeostasis is deranged in asthma with increased production by fibroblasts and decreased CD44 expression on alveolar macrophages. Upregulation of TLR2 and TLR4 on macrophages with increased sensitivity to HA fragments suggests a novel pro-inflammatory mechanism by which persistence of HA fragments could contribute to chronic inflammation and airway remodeling in asthma.
Keywords: Asthma, Hyaluronan, Cytokines, Fibroblasts, Macrophages
INTRODUCTION
Chronic inflammation and airway remodeling are important characteristics of human asthma. These include the infiltration of inflammatory cells and an abnormal accumulation of extracellular matrix (ECM) in the subepithelial basement membrane region and submucosa 1, 2. Fibroblasts from patients with hyperresponsive airways have been shown to produce more total proteoglycans than cells from subjects with normoresponsive airways 3. Fibronectin matrix accumulates in asthma and may contribute to the progression of asthma by altering both the airway remodeling and the functional properties of cells of the airway wall 4. Therefore, increases in ECM degradation products may be associated with airway fibrosis, and decline in lung function 1, 2, 5. Understanding the contribution of ECM accumulation to asthma pathogenesis may lead to new therapeutics for patients with asthma.
Hyaluronan (HA) is a major component of ECM 6. It exists as a high-molecular-weight polymer under normal physiological conditions and undergoes dynamic regulation resulting in accumulation of lower molecular weight species during tissue injury and inflammation 7–10. Clearance of HA degradation products is essential for inflammatory resolution and restoration of tissue integrity. HA fragment clearance from sites of inflammation requires the major HA binding protein, CD44, to be expressed on hematopoietic cells 7.
Low molecular weight HA induces the expression of a variety of genes by inflammatory cells in vitro, including chemokines, cytokines, and growth factors 11–13. However low molecular weight HA signaling can be CD44 independent under some circumstances and utilize toll-like receptors TLR4 and TLR2 14–16. Growing evidence shows that TLR signaling plays an important role in innate immunity in asthma 17, 18 and is critical for Th2 response in murine asthma models 19–21. In addition, TLR2 gene expression was upregulated in human airway epithelial cells isolated from asthmatic subjects and infected with Mycoplasma pneumoniae 22.
Human lung fibroblasts are an important source of HA production 23–25. There are three hyaluronan synthase (HAS) enzyme isoforms, HAS1, HAS2, and HAS3 26, 27. HAS2 is the major isoform expressed in human lung fibroblasts 28. Multiple studies have shown that human fibroblasts produce HA in vitro in response to stimulation by cytokines and growth factors including IL-1β, TNFα and TGFβ 25, 28–31. HA appears in low concentrations in bronchoalveolar lavage fluid (BAL) from healthy individuals and is elevated in BAL of asthma patients 32–34. The concentration of HA in BAL was found to significantly correlate with the severity of asthma 34. However, the role of HA homeostasis in human asthma has not been thoroughly explored.
We hypothesized that increased HA accumulation in the lungs of asthmatic patients contributes to chronic inflammation and airway remodeling through processes mediated by both fibroblasts and macrophages. In the present study, we isolated airway fibroblasts and alveolar macrophages from asthmatic patients and normal subjects. We have examined HA production by airway fibroblasts, cell surface expression of HA binding proteins on alveolar macrophages, and inflammatory mediator production by alveolar macrophages in response to low molecular weight HA and lipopolysaccharide (LPS) stimulation. Samples were obtained from a total of 21 asthmatic patients and 25 normal control subjects. Our results demonstrate an increase in HA production by airway fibroblasts and an imbalance in HA binding protein expression on alveolar macrophages in asthma that favors reduced clearance of HA fragments and increased production of cytokines in response to HA fragments and LPS.
METHODS
Study population
Subjects aged 18 to 60 were recruited by advertisement. Samples used for this study are from a total of 21 asthmatic patients and 25 normal control subjects. Not all studies were performed on all subjects; numbers studied in each experiment are denoted in the results section. Subjects were of mild severity per NAEPP criteria 35 and used no controller medications. Normal subjects demonstrated normal lung function, had no history of asthma and used no medications. All the subjects are never smokers. The characteristics of subjects are shown in Table 1. The study was approved by the Duke University Institutional Review Board. All individuals gave informed consent.
Table 1.
Subject Characteristics
| Asthma (21) | Controls (25) | p Value | |
|---|---|---|---|
| Gender (F/M) | 12/9 | 18/7 | 0.29 |
| Age | 27 ± 2 | 31 ± 2 | 0.17 |
| Ethnicity* | 1A:7AA:12W | 1A:13AA:11W | 0.52 |
| FEV1 (L) | 3.69 ± 0.2 | 3.28 ± 0.2 | 0.06 |
| FEV1 (% pred.) | 83 ± 4 | 103 ± 4 | 0.0005 |
| FVC (L) | 4.4 ± 0.2 | 3.9 ± 0.2 | 0.10 |
| FEV1/FVC | 83 ± 2 | 86 ± 1 | 0.23 |
| PC20 (mg/mL)† | 0.46 ± 0.10 | >16 | 0.0001 |
A: Asian; AA: African-American; W: White
The provocative concentration of methacholine resulting in a 20% fall in FEV1.
Bronchoscopy
Asthma subjects and healthy controls underwent bronchoscopy with bronchoalveolar lavage (BAL) as previously described 36. Three hundred ml of warm, sterile saline in 60 ml aliquots were used for the BAL. Cells derived from the BAL fluid were pelleted by centrifugation at 1000 rpm for 10 minutes. BAL cells were cultured for cytokine production analysis or fixed in 3.7% formaldehyde for flow cytometry.
Hyaluronan staining and quantitation of human lung sections
Lung sections from biopsy tissues of asthma patients and normal subjects were stained with biotinylated HA binding protein (4 µg/ml) (Associates of Cape Cod Incorporated) for 1 h, then developed using a Vectastain-Elite-ABC kit (Vector Laboratories). The specificity of the staining was determined by preincubating tissue samples with 10 U/ml streptomyces hyaluronidase at 37°C for 2 h in a humidification chamber and then staining with biotinylated HA binding protein.
For each subject, photomicrographs were randomly taken at 40× magnification avoiding the regions in proximity of the edges of the biopsies. Quantitative assessment of HA staining in lung tissue was quantified using ImageJ (version 140 1.44f, National Institutes of Health). A color deconvolution module was created to automatically threshold only the tissue expressing HA. The vectors for the color deconvolution module were calculated in order to omit any marginal staining that was not directly related to HA. Epithelial cells were excluded because there was no significant staining of HA in all subjects (normal and asthma). For such calculation multiple sampling of the stained area was performed to allow consistency across photomicrographs and sections. Similarly, the total tissue area of the biopsy was calculated but using a different color deconvolution module to detect the total tissue area. Finally, the thresholded images generated by these modules were visually compared with the photomicrographs for accuracy. Separate java macro scripts were developed for HA staining and for total tissue area and applied to all images for automatic data generation. The volume percentage of positive tissue was calculated by the ratio of the value of the reactive tissue to hyaluronan and the total volume of the tissue.
Human airway fibroblast cell culture
Fibroblasts were cultured from endobronchial biopsy tissues as previously described 37. Briefly, biopsy specimens were rinsed and cut into small pieces and cultured in Dulbecco modified Eagle medium (DMEM) supplemented with FBS (10%), streptomycin (100 µg/ml), penicillin (10,000 U/ml), and gentamicin (100 µg/ml). The purity of fibroblasts was confirmed as shown previously 37. Cells from passage two to passage four were used for experiments. Airway fibroblasts were seeded in 12 well plates with 50,000 cells per well. Once the cells reached confluence, they were cultured for one additional week. The cells were then incubated in fresh serum free medium with and without 10 ng/ml IL-13 (10 ng/ml), TNFα (50 ng/ml) or TGFβ (10 ng/ml) for 48 hours. Conditioned media were collected for HA measurement and cells were harvested in Trizol reagent for RNA isolation.
HA content determination
Hyaluronan concentrations in culture medium of airway fibroblasts were measured with a competitive ELISA-like assay using biotinylated hyaluronan-binding protein (bHABP; Associates of Cape Cod Incorporated) as described previously 7. Briefly, samples and bHABP were incubated in a microtube for 1 h. The sample-bHABP mixtures were added onto hyaluronan coated microtiter CovaLink NH modules (Nunc). Bound bHABP were measured with a colorimetric reaction. Sample concentrations were calculated from a standard curve that was generated using hyaluronan standards of known concentration (ranging 0–2000 ng/ml).
mRNA analysis
RNA was extracted from airway fibroblasts using Trizol Reagent (Invitrogen) following manufacture’s instruction. For real-time PCR analysis, 0.5 µg total RNA was used for reverse transcription using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). One microliter of cDNAs were subjected to real-time PCR using Power SYBR® Green PCR Master Mix (Applied Biosystems) and the ABI Prism 7500 Detection system (Applied Biosystems). The specific primers were designed based on cDNA sequences deposited in the GenBank database. HAS1 (NM_001532) sense GAGGCCTGGTACAACCAGAA; antisense TGTACAGCCACTCACGGAAG. HAS2 (NM_005328) sense GCCTCATCTGTGGAGATGGT; antisense TCCCAGAGGTCCACTAATGC. HAS3 (AF234839) sense GGCATTATCAAGGCCACCTA; antisense GACACAGGAATGAGGCCAAT. HYAL1 (NM_007321) sense CATCCTGAACGTGACCAGTG; antisense AGCCATCTGTGCCTGATCTT. HYAL2 (NM_033158) sense ACATTGACCACCTGCAGACA, antisense GTAGCCATATTCATTGTCATA. NOX4 (NM_016931) sense AGATGTTGGGGCTAGGATTG; antisense TCTCCTGCTTGGAACCTTCT. The primers were all cDNA specific, not amplifying genomic DNA. The conditions for real-time PCR were as following: one cycle at 95°C for 10 min, 40 cycles at 95°C for 15 seconds and 60°C for 1 min, one cycle at 25°C for 2 min. The relative expression level of each gene was determined against GAPDH level in the same sample. Human GAPDH sense AATGCATCCTGCACCACCAA; antisense GTAGCCATATTCATTGTCATA.
HA size determination
Fibroblasts from asthmatic lung and normal controls were seeded in 100 mm dishes. Cells were cultured in serum free medium for one week after reaching confluence and then changed to fresh serum free medium. Forty-eight hour media were collected for experiment. Medium collected from cultured lung fibroblasts was concentrated with centrifugal filter (10,000 Da cut-off; Millipore) and then digested with protease Pronase (100 units/ml, Pronase from Streptomyces griseus; Calbiochem) at 55 °C for 2 hours, followed by inactivation of protease activity by boiling the samples at 100 °C for 10 min. Concentrated samples along with known molecular mass hyaluronan standards (1300, 790, and 132 kDa) were electrophoresed on a 0.5% agarose gel, stained with 0.005% Stain-All overnight, and then destained in water for two days and final destaining was completed by exposing the gel to amber light for 30 min. HA-agarose gels were photographed on a Geliance 600 Imaging System (PerkinElmer Life Sciences). The captured gel images were analyzed with ImageJ (National Institutes of Health, Bethesda, MD) to document hyaluronan bands on the gel. A standard curve was determined using known molecular mass hyaluronan standards. Hyaluronan peaks in the samples were calculated against the standard curve.
Flow cytometry
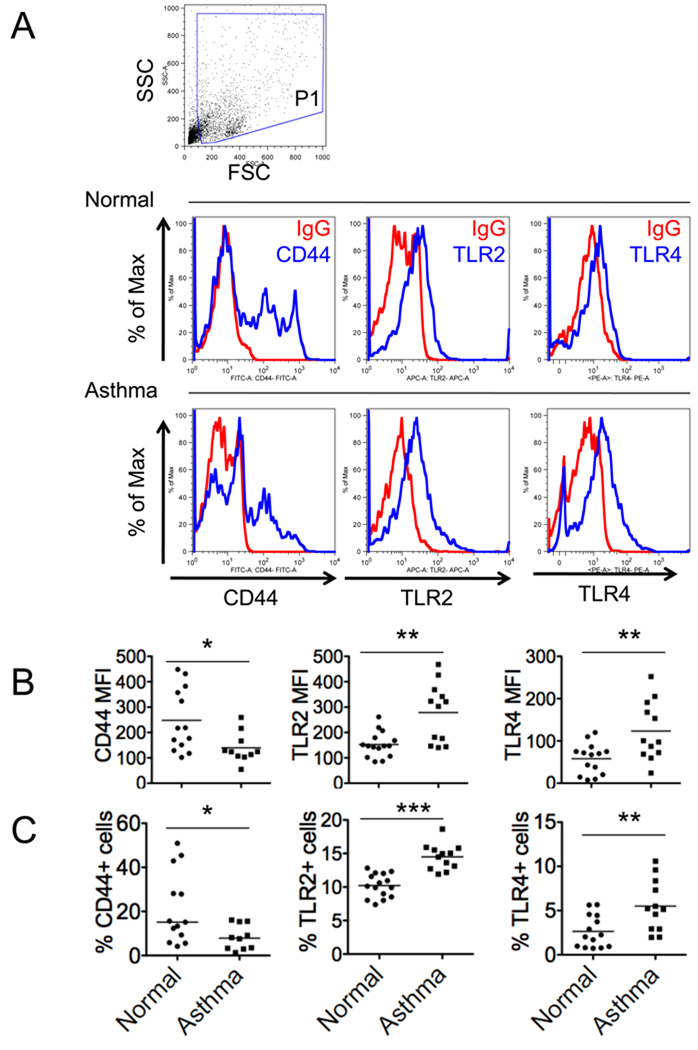
Macrophages derived from bronchoalveolar lavage fluid of either control subjects or asthma patients were fixed in 3.7% formaldehyde before staining for flow cytometry. Cells were incubated with specific anti-human CD44 (clone G44-26, BD Pharmingen), TLR2 (clone TL2.1, eBioscience), and TLR4 (clone HTA125, eBioscience) antibody in staining buffer containing 0.5% BSA and 0.02% sodium azide at 4°C for 30 minutes. Samples were washed with PBS. Flow cytometry was performed using a FACScanto II flow cytometer (BD Immunocytometry Systems) and analyzed using FlowJo 8.7 software. Macrophage population was gated based on forward and side scatter characteristics. Percentages of CD44, TLR2, and TLR4 staining positive cells were calculated by comparing to control IgG.
Macrophage culture and cytokine measurement
Macrophages derived from BAL fluid of asthma patients and normal subjects who underwent bronchoscopy with bronchoalveolar lavage were plated into 12 well plates in RPMI1640 (Invitrogen) medium containing 10% FBS with penicillin and streptomycin overnight to allow the cells attach to the plate. Cells were washed once and then treated in serum free RPMI medium containing either 100 µg/ml HA (ICN), or 100 ng/ml LPS (Sigma). Twenty-four hour conditioned media were collected and human IL-8 in the medium was measured using ELISA (R&D Systems) following the manufacturer’s instruction.
Anti-TLR neutralization of cytokine production
Macrophages derived from BAL fluid of asthma patients were incubated with anti-human TLR2 (10 µg/ml, cat # maba2-htlr2, InvivoGen) or mouse IgA2 isotype control (10 µg/ml, cat # maba2-ctrl, InvivoGen), or with anti-human TLR4 (clone HTA125, eBioscience) or mouse IgG isotype control (20 µg/ml) at 37°C for one hour. BAL macrophages were pretreated with CD44 antibody (clone 5F12, Fisher, at 20 µg/ml) and control mouse IgG on ice for 30 min. Hereafter cells were treated with low molecular HA 100 µg/ml or LPS 100 ng/ml in serum free medium. 24-hour culture media were collected for measuring human IL-8 concentration.
Statistical analysis
All statistical analyses for comparison between asthma and control subjects were performed using unpaired, 2-sided, students T-test with Prism 5 (GraphPad Software).
RESULTS
Hyaluronan accumulates in asthmatic lung tissue
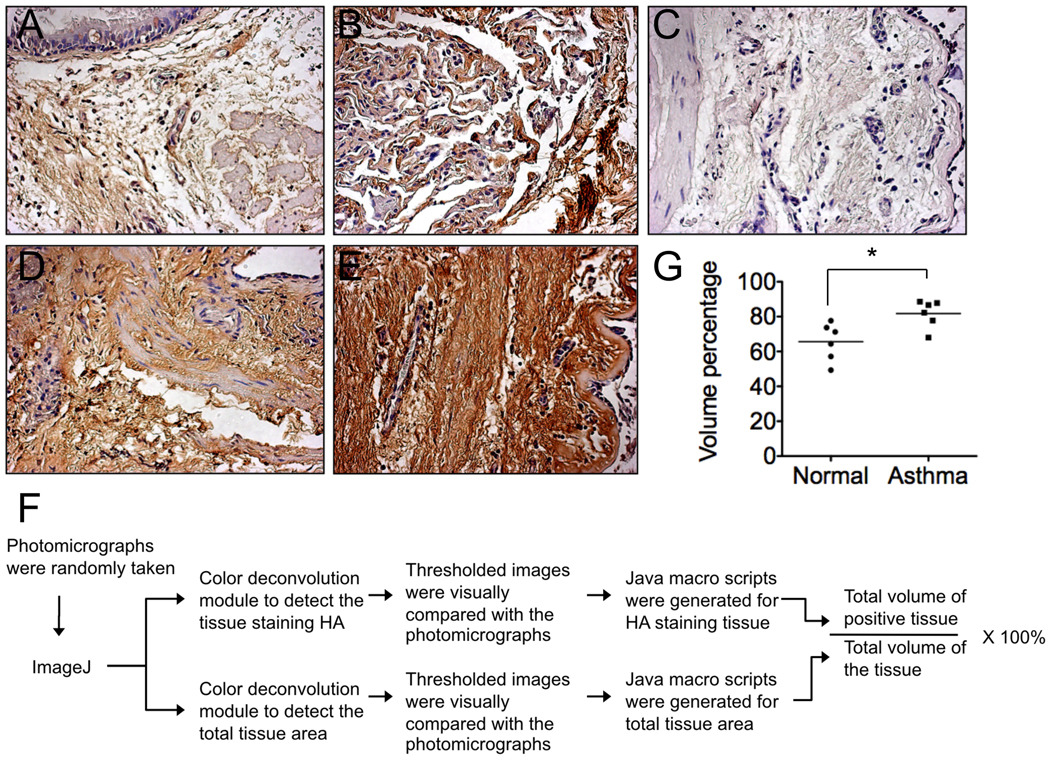
In order to determine the role of hyaluronan in human asthma, we first assessed HA accumulation in human lung tissues. Lung sections from asthmatic patients and normal control subjects were stained using a biotinylated HA binding protein-based protocol. A significant increase in HA immunohistochemical staining was observed in bronchial sections of asthma patients (Fig 1, D and E) compared to that of bronchial sections from normal subjects (Fig 1, A and B). Quantitative analysis of HA stained lung sections from multiple subjects of asthma patients and normal controls showed a significant increase in HA staining in the asthma group (Fig 1, G). These data are consistent with previous reports that HA concentration is elevated in BAL of asthma patients when compared to healthy individuals 32–34.
FIG 1.
Sections of endobronchial biopsies from asthmatic patients (FEV1: 95 ± 5% pred.; n = 6) and normal subjects (FEV1: 102 ± 5% pred.; n = 6) were stained for HA. A–B, normal. D–E, asthmatic. C, Section of an asthmatic patient was preincubated with hyaluronidase. F. Flow chart to show quantitation of HA staining. G. Quantitation of HA staining of bronchial sections (n = 6 each. *p < 0.05).
Human airway fibroblasts from asthmatic patients produce increased HA relative to normal control subjects
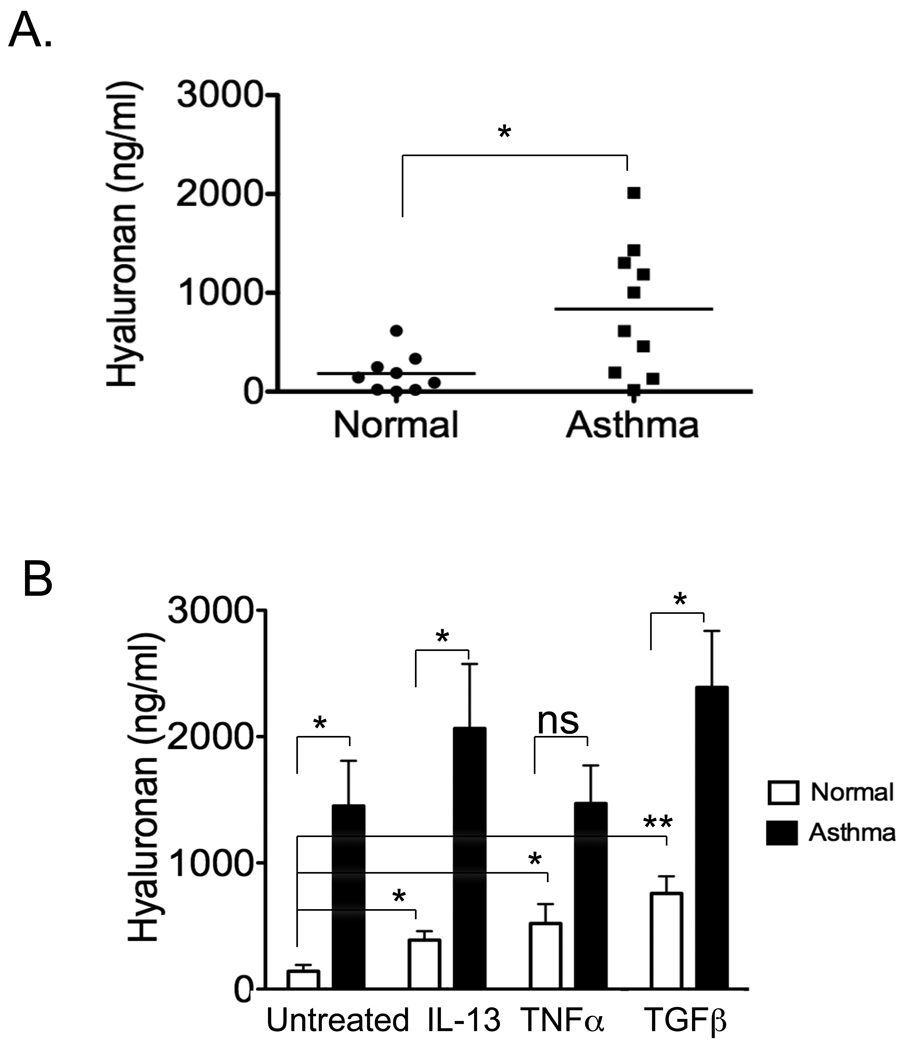
We first compared baseline HA production by airway fibroblasts cultured from endobronchial biopsies of asthmatic patients and control subjects. HA concentrations in culture medium of asthmatic fibroblasts were significantly increased compared to that of normal fibroblasts at baseline (Fig 2, A). We then sought to determine if asthmatic airway fibroblasts produced HA in response to relevant stimuli. IL-13, TNFα, or TGFβ were used to stimulate HA production. Baseline production of HA from asthmatic fibroblasts was markedly elevated and when stimulated with cytokines some further augmentation was observed; however, the fold-increase was less than that observed between asthmatic and normal fibroblasts at baseline (Fig 2, B). The key finding was the constitutive production of HA by asthmatic airway fibroblasts is increased compared to normal fibroblasts.
FIG 2.
Asthmatic fibroblasts produce increased concentrations of HA. A, HA levels in 48-h culture media of untreated airway fibroblasts from asthma patients and normal controls (n = 9/10; *p < 0.05). B, HA in 48-hour culture media of fibroblasts from asthmatic and normal controls treated with either IL-13, TNFα, or TGFβ (n = 5, *p < 0.05; **p < 0.01; ns, not significant). Within the asthma group, there was no significant difference when comparing treatment vs. no treatment.
Gene expression of hyaluronan synthase and hyaluronidase isoforms in asthmatic airway fibroblasts
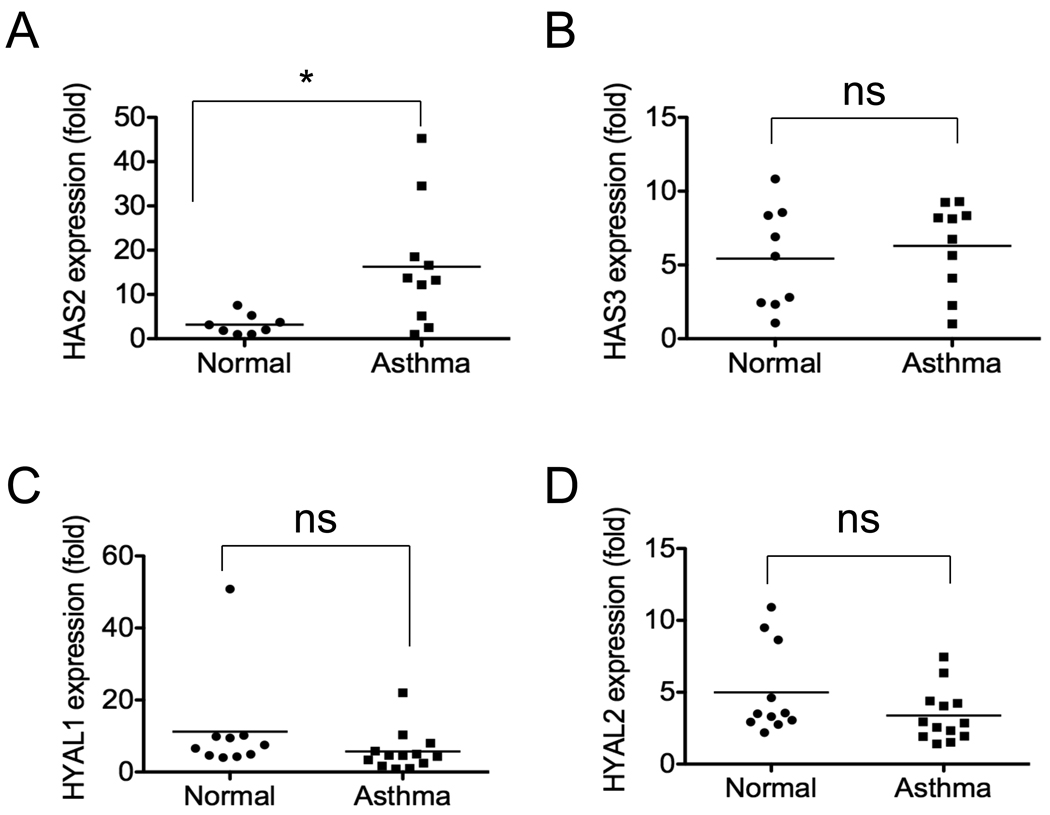
To delineate HA homeostasis in asthmatic fibroblasts we measured gene expression of HAS isoforms and hyaluronidase (HYAL) isoforms with quantitative PCR. We observed a dramatic increase in HAS2 gene expression in asthmatic fibroblasts as compared to normal fibroblasts (Fig 3, A). HAS1 was too low to be detected after 40 cycles of PCR program and showed no difference between asthmatic and normal fibroblasts after performing secondary amplification (data not shown). There were no differences in HAS3 expression between asthmatic fibroblasts and fibroblasts from control subjects (Fig 3, B). Slight reductions in HYAL1 and HYAL2 gene expression in asthmatic fibroblasts were observed, but the differences did not reach statistical significance (Fig 3, C and D). These data suggest that the accumulation of HA in asthma may be due to the elevated expression of HAS2 promoting synthesis of HA by asthmatic fibroblasts.
FIG 3.
HAS and hyaluronidase mRNA expression in fibroblasts from asthmatic patients and normal controls was compared by quantitative PCR. A, HAS2 (n = 8/10, *p < 0.05); B, HAS3 (n = 9/10, p = 0.563); C, HYAL1 (n = 10 /13, p = 0.210); and D, HYAL2 (n = 11/13, P = 0.129).
Asthmatic airway fibroblasts produce lower molecular weight hyaluronan species
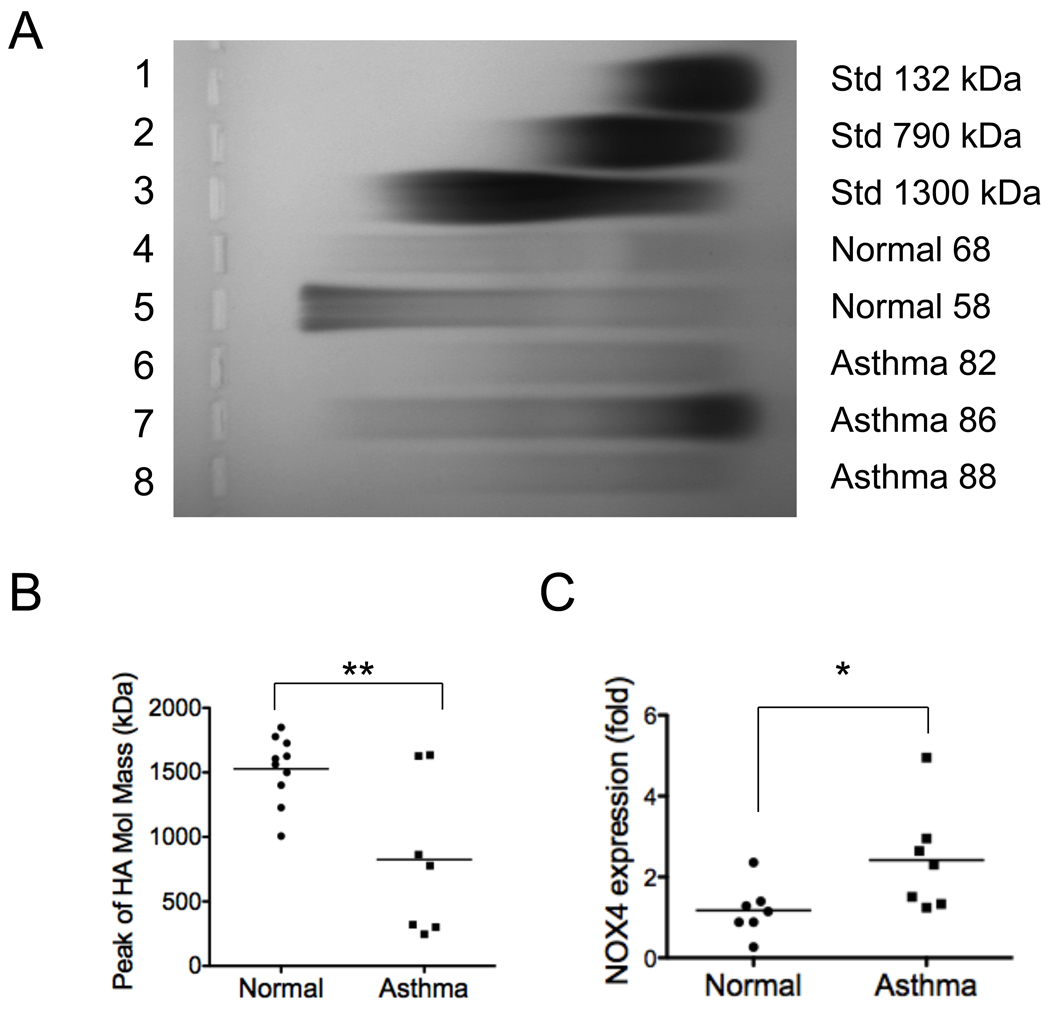
Culture media of airway fibroblasts was concentrated and electrophoresed on argarose gels to determine the molecular mass distribution of HA produced by human airway fibroblasts. We found that fibroblasts from asthmatic patients produced lower molecular weight HA fragments compared to normal human airway fibroblasts (Fig 4, A and B, and Fig E1, A–C). These data suggested that HA produced by asthmatic fibroblasts contains lower molecular weight species in the size range that has been shown to stimulate cytokine production by macrophages in vitro 12.
FIG 4.
Molecular mass of HA produced by fibroblasts from asthmatic and normal controls. A, Size of HA in conditioned media of fibroblasts from asthmatic and normal subjects. B, Graph showing HA size represents three identical experiments with total 7 samples from asthmatic fibroblasts and 10 samples from normal fibroblasts (**P <0.01). C, NOX4 mRNA expression in fibroblasts from asthmatic and normal subjects was determined with qPCR (n = 7, *p < 0.05).
Studies have shown that reactive oxygen species (ROS) induce depolymerization of hyaluronan 38 and peroxynitrate is potent in generating HA fragments from high molecular weight precursors in vitro 39, 40. HA breakdown caused by ROS in the lung further induce pathological responses 41–43. ROS are predominantly generated by the NAPDH oxidase (Nox)/Dual oxidase family of proteins 44 and NOX4 is the major isoform of NOX family that is expressed in human lung 45. We explored the possibility that smaller molecular weight HA generated by asthmatic fibroblasts was due to excessive production of reactive oxygen species. NOX4 expression in fibroblasts cultured from endobronchial biopsies of asthma patients and from normal subjects were compared with real time PCR. Our results indicated that NOX4 expression was significantly increased in asthmatic fibroblasts compared to that of fibroblasts from normal subjects (Fig 4, C).
Cell surface HA binding protein expression on alveolar macrophages differs between asthmatic and normal control subjects
HA fragments stimulate macrophages to produce inflammatory cytokines through interaction with toll-like receptors as well as CD44 on macrophages 12, 14, 46. CD44 expression on macrophages is required for HA clearance from inflamed tissues and promote the resolution of inflammation in mice 7, 27. To gain further insight into the role of HA homeostasis in asthma, we compared the expression of HA binding proteins on the cell surface of alveolar macrophages derived from BAL of asthmatic patients and control subjects by flow cytometry (Fig 5, A). Interestingly, we observed a significant decrease in CD44 expression, coupled with an increase in TLR2 and TLR4 expression on asthmatic alveolar macrophages (Fig 5, B–C). This dysregulation in the expression of HA binding proteins on asthmatic alveolar macrophages has the potential to impair HA clearance and alter responsiveness to TLR ligands.
FIG 5.
Flow cytometric analysis of cell surface HA binding protein expression on alveolar macrophages from BAL of asthmatic and normal subjects. A, Live macrophage population (P1) was gated based on forward (FSC) and side scatters (SSC). Overlay of histograms of macrophages stained with specific antibodies to either CD44, TLR2, or TLR4 (blue lines) and respective control IgG (red lines) were shown. The mean fluorescence intensity (MFI) (B) and the percentages (C) of CD44, TLR2, and TLR4 staining positive cells were calculated by comparing to control IgG (CD44, n = 10/13; TLR2, n = 12/15; TLR4, n = 12/14; p < *0.05, **p < 0.01, ***p < 0.0001).
Cytokine production by asthmatic alveolar macrophages is altered in response to HA fragments and is TLR2- and TLR4-dependent
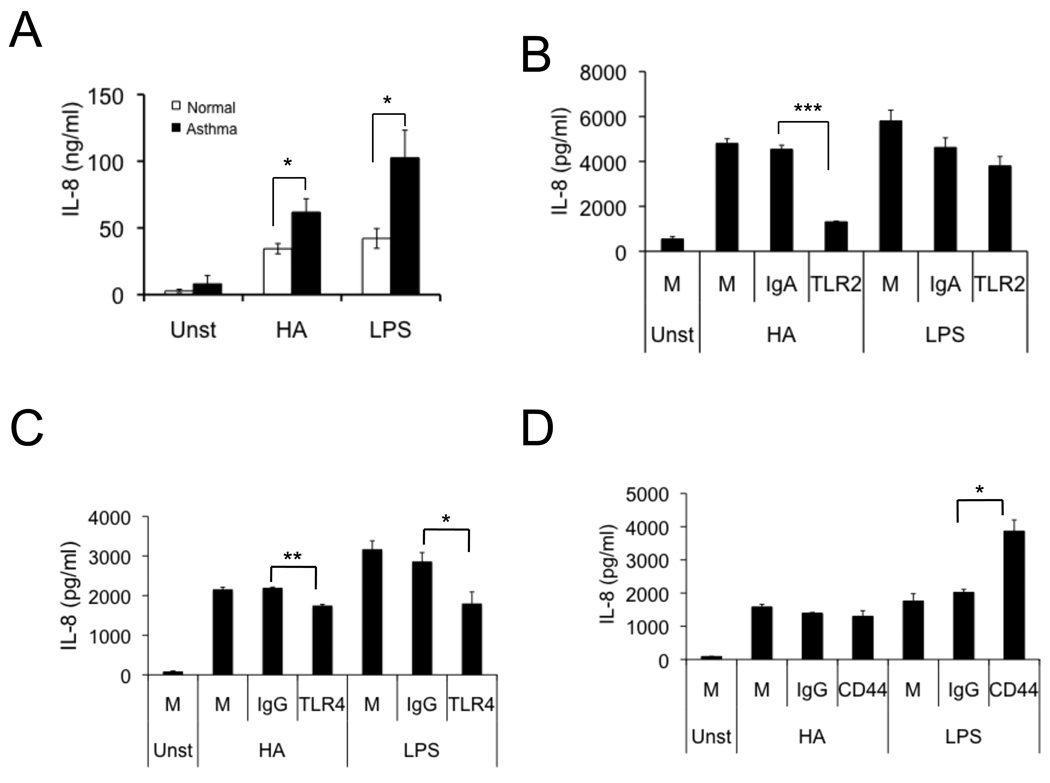
To elucidate the functional consequences of the imbalance in HA binding protein expression on alveolar macrophages from asthmatic patients, we treated macrophages from BAL of asthma patients and control subjects with low molecular weight HA and LPS and measured IL-8 production in culture medium. Untreated macrophages produced minimal cytokines. Asthmatic alveolar macrophages produced increased concentrations of IL-8 in response to HA fragment and LPS (Fig 6, A). Although HA weakly induced IL-10 release, there was no difference in IL-10 production between asthmatic and normal macrophages (Fig E2).
FIG 6.
Cytokine production by alveolar macrophages from asthmatic and normal subjects. A, IL-8 levels in 24-hour conditioned media from BAL alveolar macrophages from asthmatic and normal subjects treated with either HA or LPS (n = 8, *p < 0.05). B–D, IL-8 levels in 24-hour conditioned media from BAL alveolar macrophages from asthmatic treated with HA or LPS in the presence or absence of TLR2 (B), TLR4 (C) or CD44 (D) antibody pretreatment (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001).
Our previous study showed that HA signals through TLR2 and TLR4 in mouse macrophages 14. To determine if HA signals in the same manner in human macrophages, we measured cytokine production with anti-TLR neutralization of human monocyte/macrophage cell line U937 cells and BAL macrophages. Asthmatic alveolar macrophages were treated with HA or LPS, in the presence or absence of CD44, TLR2, or TLR4 antibodies. Our results showed that TLR2 neutralizing antibody significantly decreased HA-induced IL-8 production by BAL macrophages (Fig 6, B). TLR4 neutralizing antibody decreased IL-8 production by BAL macrophages induced by both HA and LPS (Fig 6, C). Similarly, U937 cells were pretreated with PMA and then adherent macrophages were treated with HA or LPS, in the presence or absence of TLR2 or TLR4 antibodies. Our results showed that both TLR2 and TLR4 antibodies significantly reduced HA-induced IL-8 production in U937 cells (Fig 3E). Interestingly, CD44-blockade increased LPS-induced IL-8 release by BAL macrophages (Fig 6, D). These data suggest that increased IL-8 production of asthmatic macrophages in response to HA stimulation is regulated in part by TLR signaling. The CD44 antibody data are consistent with our previous observation that alveolar macrophages from CD44 null mice produce increased amount of inflammatory chemokines in response to LPS 47.
DISCUSSION
In this study, we have explored the roles of HA and HA binding proteins in human asthma. HA has been shown to accumulate in the BAL of asthmatic patients and may correlate with persistence of asthma symptoms33, 34. Our data show that asthmatic airway fibroblasts produce constitutively higher concentrations of lower molecular weight HA with increased HAS2 gene expression compared to airway fibroblasts from normal controls. In addition, alveolar macrophages from asthmatic patients exhibit altered expression of HA binding proteins as compared to normal subjects, with a decrease in cell surface CD44 and increases in TLR2 and TLR4 expression. This translated into increased responsiveness of asthmatic alveolar macrophages to HA and LPS stimulation as demonstrated by increased IL-8 production. The reduction of CD44 on asthmatic alveolar macrophages could lead to impaired clearance of HA which in turn could promote persistent inflammation and potentially asthma symptoms.
Our data show that asthmatic airway fibroblasts constistutively release more HA than normal fibroblasts. In addition, asthmatic fibroblasts release relatively lower molecular weight HA than normal fibroblasts. The significant increase in HAS2 expression suggests that asthmatic fibroblasts acquired an activated phenotype with increased HA production. 48 In vitro experiments with cultured fibroblasts or COS1 cells suggested that HAS1 and HAS3 generated HA with broad size distributions (molecular masses of 2 × 105 to ~ 2 × 106 Da), whereas HAS2 generated HA with a broad but extremely large size (average molecular mass of > 2 × 106 Da) 49. Subsequent studies suggested that all three HAS enzymes drive the biosynthesis and release of high molecular mass HA (1 × 106 Da) 50. Thus, elevated expression of HAS2 may be responsible for increased HA production. HA degrading enzymes HYAL1 and HYAL2 expression was not different between asthmatic and normal fibroblasts. Hyaluronidases usually hydrolyze the hexosaminidic β(1–4) linkages in HA and release HA fragments. Although hyaluronidase from Streptococcus pneumoniae hydrolyzes HA to release disaccharide D-glucuronic acid-N-acetyl-D-glucosamine, the vertebrate hyaluronidases generate a various range of HA oligomers 51. A recent study reported that asthmatic airway smooth muscle cells showed reduced HA secretion with decreased HAS-1 and HAS2 expression and increased expression of HYAL1 52. Several reports have demonstrated that HA concentration is elevated in BAL of asthma patients 32–34 and the concentration of HA in BAL was positively correlated with the severity of asthma 34. Human lung fibroblasts are an important source of HA production 23–25, in addition to other cell types such as epithelial cells 53. Furthermore, fibroblasts isolated with our protocol are α smooth muscle actin negative 37. The reduced HA release by airway smooth muscle cells suggested that smooth muscle cells do not contribute to the HA accumulation in BAL and lung tissue in asthma patients.
Importantly, HA is susceptible to degradation by excessive reactive oxygen species during inflammation. Studies have shown that reactive oxygen species contribute to HA breakdown during lung injury 41–43 and that human lung fibroblasts express NOX4 45, 54. A recent report has shown that S-nitrosoglutathione reductase activity and inducible nitric oxide synthase were upregulated in BAL of asthma patients 36. Our results indicated that NOX4 expression was elevated in asthmatic fibroblasts and might contribute to the production of low molecular weight HA in culture medium of fibroblasts from asthma patients. To our knowledge, we are the first to demonstrate increased NOX4 expression in asthmatic fibroblasts. Collectively, these data suggest that the increase in HA fragments in asthmatic fibroblasts are likely due to enhanced NOX4 expression and oxidative depolymerization rather that digestion by hyaluronidases.
Toll-like receptors are a major component of the innate immune system and have been studied in asthma 17. Studies with murine asthma models have shown that TLR4 18, 20, 21 as well TLR2 are required for regulating Th2 immune responses in antigen-sensitized mice 55. Toll like receptors are expressed by all effector cells of innate immunity including epithelial cells, mucosal mast cells and dendritic cells 56 and are important for pathogen recognition. In this study we have found that both TLR2 and TLR4 on human alveolar macrophage from asthmatic patients are upregulated with functional consequences of enhanced cytokine production in response to ligand. Blockade of TLR4 inhibited HA fragment-stimulated expression of IL-8 in asthmatic alveolar macrophages. Future studies will examine the role of TLR2 as well as other inflammatory cells such as eosinophils. This observation has potential implications for the role of infection in the pathobiology of chronic asthma and provides some insights into the potential mechanisms to explain spirals of symptoms that develop in asthmatic patients with airway infections.
Previous work from our group has shown that CD44 is required for HA clearance and the resolution of non-infectious lung inflammation in mice 7. We have also shown that CD44 also is a negative regulator of TLR signaling. Alveolar macrophages from CD44 null mice produce increased amount of inflammatory chemokines in response to LPS 47. Loss of CD44 on alveolar macrophages from asthmatic patients may not only impair HA clearance from asthmatic lungs but also enhance TLR signaling, thus promoting persistent inflammation.
Collectively, these data suggest that HA homeostasis is deranged in asthma and may provide some insights into connections between HA accumulation, macrophage function, and fibroblast activation in asthmatic lung. Asthmatic airway fibroblasts produce increased concentrations of low molecular weight HA. Decreased CD44 expression commensurate with increases in TLR2 and TLR4 on asthmatic macrophages may enhance the response to HA and TLR ligand LPS. Impaired HA clearance by decreased CD44 expression on macrophages leading to persistent inflammation in asthmatic lung. This previously unrecognized inflammatory regulatory pathway in asthma could lead to targeted approaches in selected patients experiencing severe and persistent asthma. Targeting the pro-inflammatory cycle involving fibroblasts, macrophages and matrix turnover may present a novel therapeutic strategy in the treatment of asthma.
Supplementary Material
Acknowledgments
Supported by NIH grants AI052201, HL06539, P50-HL084917, HL77291 (PWN), and P50-HL084917 (MK).
Abbreviations used
- HA
Hyaluronan
- HAS
hyaluronan synthase
- HYAL
hyaluronidase
- bHABP
biotinylated hyaluronan-binding protein
- TLR
Toll-like receptor
- BAL
bronchoalveolar lavage
- ECM
extracellular matrix
- ROS
reactive oxygen species
- NOX
NAPDH oxidase
REFERENCES
- 1.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 2.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westergren-Thorsson G, Chakir J, Lafreniere-Allard MJ, Boulet LP, Tremblay GM. Correlation between airway responsiveness and proteoglycan production by bronchial fibroblasts from normal and asthmatic subjects. Int J Biochem Cell Biol. 2002;34:1256–1267. doi: 10.1016/s1357-2725(02)00058-4. [DOI] [PubMed] [Google Scholar]
- 4.Hocking DC. Fibronectin matrix deposition and cell contractility: implications for airway remodeling in asthma. Chest. 2002;122:275S–278S. doi: 10.1378/chest.122.6_suppl.275s. [DOI] [PubMed] [Google Scholar]
- 5.Beckett PA, Howarth PH. Pharmacotherapy and airway remodelling in asthma? Thorax. 2003;58:163–174. doi: 10.1136/thorax.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, et al. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 8.Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 9.Noble PW, Jiang D. Matrix regulation of lung injury, inflammation, and repair: the role of innate immunity. Proc Am Thorac Soc. 2006;3:401–404. doi: 10.1513/pats.200604-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, et al. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L789–L799. doi: 10.1152/ajplung.90306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med. 1996;183:2373–2378. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton MR, Burdick MD, Strieter RM, Bao C, Noble PW. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-gamma in mouse macrophages. J Immunol. 1998;160:3023–3030. [PubMed] [Google Scholar]
- 14.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 15.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 16.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroder NW, Maurer M. The role of innate immunity in asthma: leads and lessons from mouse models. Allergy. 2007;62:579–590. doi: 10.1111/j.1398-9995.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 18.Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, et al. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci U S A. 2006;103:2286–2291. doi: 10.1073/pnas.0510685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phipps S, Lam CE, Kaiko GE, Foo SY, Collison A, Mattes J, et al. Toll/IL-1 signaling is critical for house dust mite-specific Th1 and Th2 responses. Am J Respir Crit Care Med. 2009;179:883–893. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 20.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol. 2002;168:4524–4530. doi: 10.4049/jimmunol.168.9.4524. [DOI] [PubMed] [Google Scholar]
- 22.Kraft M, Adler KB, Ingram JL, Crews AL, Atkinson TP, Cairns CB, et al. Mycoplasma pneumoniae induces airway epithelial cell expression of MUC5AC in asthma. Eur Respir J. 2008;31:43–46. doi: 10.1183/09031936.00103307. [DOI] [PubMed] [Google Scholar]
- 23.Westergren-Thorsson G, Sarnstrand B, Fransson LA, Malmstrom A. TGF-beta enhances the production of hyaluronan in human lung but not in skin fibroblasts. Exp Cell Res. 1990;186:192–195. doi: 10.1016/0014-4827(90)90227-2. [DOI] [PubMed] [Google Scholar]
- 24.Tufvesson E, Westergren-Thorsson G. Alteration of proteoglycan synthesis in human lung fibroblasts induced by interleukin-1beta and tumor necrosis factor-alpha. J Cell Biochem. 2000;77:298–309. doi: 10.1002/(sici)1097-4644(20000501)77:2<298::aid-jcb12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 25.Sampson PM, Rochester CL, Freundlich B, Elias JA. Cytokine regulation of human lung fibroblast hyaluronan (hyaluronic acid) production. Evidence for cytokine-regulated hyaluronan (hyaluronic acid) degradation and human lung fibroblast-derived hyaluronidase. J Clin Invest. 1992;90:1492–1503. doi: 10.1172/JCI116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 27.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson TS, Potter-Perigo S, Tsoi C, Altman LC, Wight TN. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am J Respir Cell Mol Biol. 2004;31:92–99. doi: 10.1165/rcmb.2003-0380OC. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy CI, Diegelmann RF, Haynes JH, Yager DR. Proinflammatory cytokines differentially regulate hyaluronan synthase isoforms in fetal and adult fibroblasts. J Pediatr Surg. 2000;35:874–879. doi: 10.1053/jpsu.2000.6869. [DOI] [PubMed] [Google Scholar]
- 30.Stuhlmeier KM, Pollaschek C. Differential effect of transforming growth factor beta (TGF-beta) on the genes encoding hyaluronan synthases and utilization of the p38 MAPK pathway in TGF-beta-induced hyaluronan synthase 1 activation. J Biol Chem. 2004;279:8753–8760. doi: 10.1074/jbc.M303945200. [DOI] [PubMed] [Google Scholar]
- 31.Oguchi T, Ishiguro N. Differential stimulation of three forms of hyaluronan synthase by TGF-beta, IL-1beta, and TNF-alpha. Connect Tissue Res. 2004;45:197–205. doi: 10.1080/03008200490523031. [DOI] [PubMed] [Google Scholar]
- 32.Soderberg M, Bjermer L, Hallgren R, Lundgren R. Increased hyaluronan (hyaluronic acid) levels in bronchoalveolar lavage fluid after histamine inhalation. Int Arch Allergy Appl Immunol. 1989;88:373–376. doi: 10.1159/000234719. [DOI] [PubMed] [Google Scholar]
- 33.Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, et al. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med. 1998;157:403–409. doi: 10.1164/ajrccm.157.2.96-08040. [DOI] [PubMed] [Google Scholar]
- 34.Bousquet J, Chanez P, Lacoste JY, Enander I, Venge P, Peterson C, et al. Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J Allergy Clin Immunol. 1991;88:649–660. doi: 10.1016/0091-6749(91)90159-l. [DOI] [PubMed] [Google Scholar]
- 35.NAEPP. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 36.Que LG, Yang Z, Stamler JS, Lugogo NL, Kraft M. S-Nitrosoglutathione Reductase -- An Important Regulator in Human Asthma. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200901-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis CC, Chu HW, Westcott JY, Tucker A, Langmack EL, Sutherland ER, et al. Airway fibroblasts exhibit a synthetic phenotype in severe asthma. J Allergy Clin Immunol. 2005;115:534–540. doi: 10.1016/j.jaci.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 38.McNeil JD, Wiebkin OW, Betts WH, Cleland LG. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann Rheum Dis. 1985;44:780–789. doi: 10.1136/ard.44.11.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soltes L, Stankovska M, Kogan G, Gemeiner P, Stern R. Contribution of oxidative-reductive reactions to high-molecular-weight hyaluronan catabolism. Chem Biodivers. 2005;2:1242–1245. doi: 10.1002/cbdv.200590094. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Rosenfeld L, Vilar RE, Cowman MK. Degradation of hyaluronan by peroxynitrite. Arch Biochem Biophys. 1997;341:245–250. doi: 10.1006/abbi.1997.9970. [DOI] [PubMed] [Google Scholar]
- 41.Casalino-Matsuda SM, Monzon ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol. 2006;34:581–591. doi: 10.1165/rcmb.2005-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao F, Koenitzer JR, Tobolewski JM, Jiang D, Liang J, Noble PW, et al. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J Biol Chem. 2008;283:6058–6066. doi: 10.1074/jbc.M709273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao H, Arunachalam G, Hwang JW, Chung S, Sundar IK, Kinnula VL, et al. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc Natl Acad Sci U S A. 107:15571–15576. doi: 10.1073/pnas.1007625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 47.Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, et al. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178:2469–2475. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- 48.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 50.Spicer AP, Tien JY. Hyaluronan and morphogenesis. Birth Defects Res C Embryo Today. 2004;72:89–108. doi: 10.1002/bdrc.20006. [DOI] [PubMed] [Google Scholar]
- 51.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klagas I, Goulet S, Karakiulakis G, Zhong J, Baraket M, Black JL, et al. Decreased hyaluronan in airway smooth muscle cells from patients with asthma and COPD. Eur Respir J. 2009 doi: 10.1183/09031936.00070808. [DOI] [PubMed] [Google Scholar]
- 53.Johnsson H, Heldin P, Sedin G, Laurent TC. Hyaluronan production in vitro by fetal lung fibroblasts and epithelial cells exposed to surfactants of N-acetylcysteine. Ups J Med Sci. 1997;102:199–209. doi: 10.3109/03009739709178941. [DOI] [PubMed] [Google Scholar]
- 54.Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 65:733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, et al. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 56.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.