Abstract
Objective
Vaccination against common pathogens, such as influenza, is recommended for SLE patients to decrease infections and improve health. However, most vaccination response reports are limited to evaluation of SLE patients with quiescent disease. This study focuses on understanding the clinical, serological, therapeutic, and demographic factors which influence the response to influenza vaccination in patients with a range of disease activities.
Methods
Blood specimens and disease activity information were collected from seventy-two SLE patients at baseline and 2, 6 and 12 weeks after influenza vaccination. Influenza-specific antibody responses were assessed for antibody concentration (Bmax), relative affinity (Ka), and hemagglutination inhibition (HAI). Using a cumulative score, the subjects were evenly divided into high and low responders. Autoantibody levels were evaluated at each time-point by immunofluorescence and standard ELISAs.
Results
Low responders to the vaccine were more likely to have hematologic criteria (p=0.009), exhibit more ACR criteria (p=0.05), and be on concurrent prednisone treatment (p=0.04). Interestingly, European American patients were more likely to be low responders than African Americans (p = 0.03). Following vaccination, low responders were more likely to experience disease flares (p=0.01) and to have increased ANA titers (p = 0.04). Baseline serum interferon alpha activity was significantly higher in patients that experienced a flare after vaccination compared to a matched group of patients that did not flare (p= 0.04).
Conclusions
Ancestral background, prednisone treatment, hematological criteria and evidence of increased disease flares were associated with low antibody responses to influenza vaccination in SLE patients.
Systemic lupus erythematosus (SLE) is a prototypic systemic autoimmune disease characterized by the presence of autoantibodies and multiple organ involvement. Infectious diseases are one of the leading causes of morbidity and mortality in SLE patients, accounting for 11–23% of all hospitalizations and 20–55% of all deaths (1, 2). This increased susceptibility to infection is likely due to immunosuppressive therapy and intrinsic immune defects. Indeed, corticosteroid use equivalent to ≥20 mg/daily of prednisone has been shown to increase susceptibility to infection (1). Additionally, SLE patients display immune abnormalities, such as decreased antigen presentation and disrupted T and B cell interactions, which could decrease immune responses to pathogens (3–5). This increased risk of infection in SLE patients has led to an emphasis on vaccination in this at-risk population.
Influenza infection is a major cause of morbidity and mortality in the United States with over 225,000 hospitalizations (6) and 36,000 deaths (7) annually. Immunocompromised individuals, such as SLE patients, are at high risk for all of the reasons discussed above. Therefore, vaccination of SLE patients with the influenza vaccine has become part of the standard of care. However, several reports have shown that SLE patients make lower responses to vaccinations than healthy controls (8–10). Four studies performed in the 1970s assessed the anti-influenza response in SLE patients vaccinated against the circulating H1N1. Two of the reports documented low seroconversion rates, determined by serum antibody titer and hemagglutination inhibition (HAI), in SLE patients (47–48%) as compared to healthy controls (62–94%) (11, 12). However, other studies reported no significant differences between the serum antibody or HAI titer of patients and controls (13, 14). This issue remains controversial in more recent studies as several groups have shown significantly lower HAI titers in SLE patients compared to controls (15, 16), while others have shown that patients have equivalent HAI titers compared to controls (17, 18).
Previous findings are also contradictory regarding the impact of vaccination upon autoantibody production and clinical disease (9, 10, 15, 16, 18–20). Several groups have shown that vaccination is associated with increased autoantibody levels in SLE patients (8, 13, 19) and healthy individuals (20). Application of these results to patients in general clinical practice has been limited due to the small number of unique individuals studied, the limited ethnic groups evaluated, and the selection of lupus patients with low disease activity or quiescent disease. Thus it remains unclear whether individuals with more active disease would be capable of mounting an effective immune response to influenza following vaccination.
Our objective was to evaluate the association between demographic, therapeutic, disease activity, and clinical features with influenza vaccine responsiveness in SLE patients from various ethnicities and a range of disease activities. A secondary objective was to monitor autoantibody production and disease activity following vaccination to determine if vaccination resulted in increased humoral autoimmunity or disease flares. We hypothesized that select disease activity criteria would correlate with reduced responsiveness to the vaccine and that in some patients vaccination would result in increased autoantibody production.
Methods
Study population
Seventy-two unique patients who met four or more ACR SLE classification criteria (21) were recruited from local rheumatology clinics and provided informed consent and demographic information (gender, age, and race). Seventy-two matched healthy controls were also recruited via patient friend referrals and local advertising, enrolled and followed. Exclusion criteria included severe anemia (hemoglobin <8.5), egg allergies, and pregnancy. Clinical information was extracted using the Lupus Family Registry and Repository (22) collection tool for ACR classification criteria, age at diagnosis, and medication usage. Peripheral blood was collected before vaccination, and at 2, 6, and 12 weeks post vaccination. Institutional Review Board approval was obtained from the Oklahoma Medical Research Foundation (OMRF) and Oklahoma University Health Sciences Center (OUHSC).
Disease Activity Evaluation
Disease activity was measured by SLE Disease Activity Index (SLEDAI) and Physician’s Global Assessment (PGA) (23) before vaccination and at 6 and 12 weeks post vaccination. Data on medications and hospitalizations were also gathered. Using these data, the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)-SLEDAI flare composite score was computed (24, 25) and used to classify flares as mild/moderate or severe.
Trivalent Influenza Vaccine
All patients received the subunit influenza vaccine approved for use in the United States. The 2005–2006 vaccine consisted of an A/New Caledonia/20/99 (H1N1)-like, an A/California/7/2004 (H3N2)-like, and a B/Shanghia/361/2002-like virus (Aventis Pasteur, Swiftwater, PA). The 2006–2007 vaccine consisted of an A/New Caledonia/20/99 (H1N1)-like, an A/Wisconsin/67/2005 (H3N2)-like, and a B/Malaysia/2506/2004-like virus (Chiron Vaccines Limited, Liverpool, UK). The 2007–2008 vaccine consisted of an A/Solomon Islands/3/2006 (H1N1)-like, and A/Wisconsin/67/2005 (H3N2)-like, and a B/Malaysia/2506/2004-like virus (Novartis, Emeryville, CA).
Characterization of humoral influenza responses
To quantify antibodies to native glycoproteins a sandwich ELISA was used (26) and data were subjected to a nonlinear regression model to calculate Bmax, the relative measure of total antibody concentration, and Ka(app), the apparent overall association constant of the serum antibodies (26). Hemagglutinin assays were performed using human red blood cells (28). Each of the three vaccine response measurements were ranked (including all patients and controls for a given year) and then scaled to fall between 0 and 100 for each study year. The sum of these three ranked and re-scaled measurements will fall between 0 and 300 with an average of approximately 150 (what we call sum of ranks). Therefore, high and low responders were classified by identifying a noticeable gap in the sum of ranks for each study year, resulting in equal groups of 36 high and 36 low patient responders.
Detection of ANA and dsDNA
Anti-nuclear antibodies (ANA) were detected using the NOVALite Hep-2 assay (INOVA Diagnostics, San Diego, CA, positive ≥ 1:120) (29, 30). The NOVALite Crithidia luciliae assay was used to measure anti-dsDNA (positive ≥ 1:30) (29, 31).
Autoantibody Detection
Ro, La, Sm, and nRNP (Immunovision, Springdale, AR) antibodies were tested by standard ELISA (29, 31). A sample was considered positive if the optical density (OD) was greater than the average OD plus two times the standard deviation (SD) determined for 36 healthy individuals. Antibodies against the ribosomal P antigen, the 22-amino acid carboxyl terminal peptide (R5HUP0) were detected (32) using a standard ELISA. To quantify IgG antibodies to cardiolipin antigen, Linbro/Titertek E.I.A Microtitration (ICN BioMedicals, Irvine, CA) plates were used in a modified ELISA (33, 34).
Serum IFNα activity
Cells (Wistar Institute, Susan Hayflick (WISH) cells, ATCC, Manassas, VA) were cultured with 50% patient sera and lysed (35, 36). mRNA was purified and cDNA was made from total cellular mRNA and quantified using real-time PCR (37). Forward and reverse primers for three genes known to be highly and specifically induced by IFNα (MX1, PKR and IFIT) were used and gene expression levels were normalized for GAPDH. Experimental samples were compared to the mean and standard deviation of healthy controls (n=141), and IFNα activity values reported represent the number of standard deviations above the mean of healthy donors.
Statistical analysis
Exact (permutation) Chi-square tests determined the association between vaccine response and race, age, and type of ACR criteria. Comparisons of number of ACR criteria, baseline disease activity scores and pre-vaccination to post-vaccination changes in antibody responses between SLE patients and controls were evaluated using Wilcoxon two-sample tests. Differences in the number of responders taking each medication were evaluated using a Fisher's exact test. Differences between responders in regards to changes in autoantibodies were assessed using conditional logistic regression. A one-tailed paired Student’s t test was used to evaluate the IFNα serum activity.
Results
SLE patients have reduced humoral immune responses following influenza vaccination
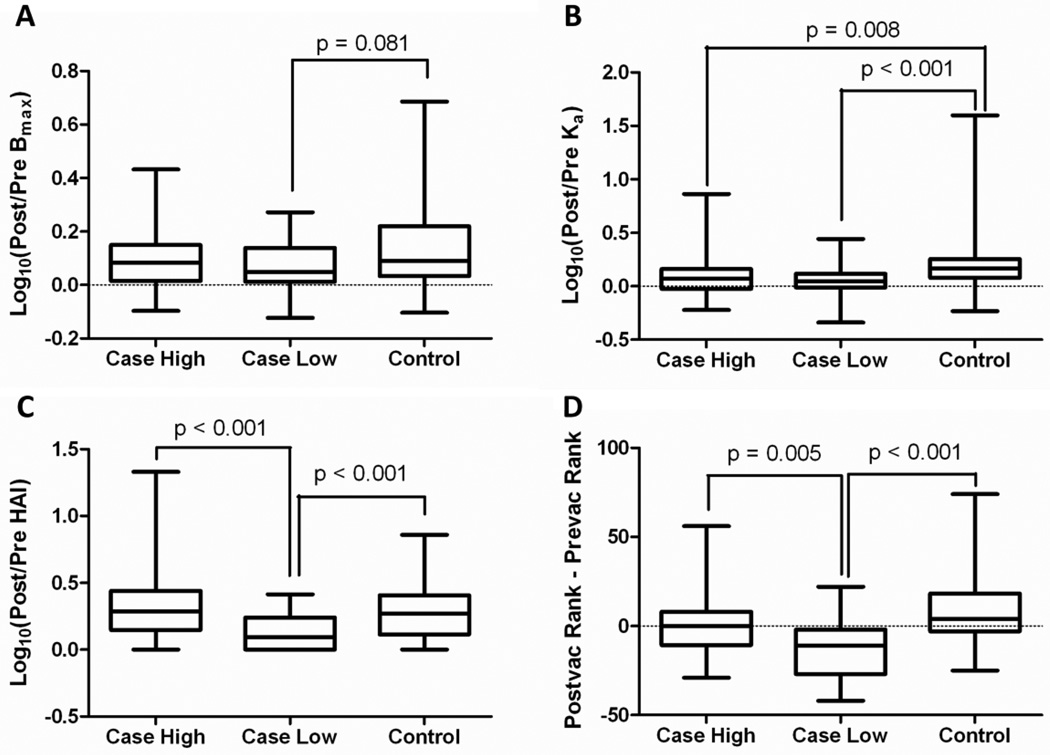
African American patients comprised 44% of this cohort and over 90% of participants were female (Table 1). As shown in Figure 1, we looked at log10 transformed ratios of post-vaccination to pre-vaccination measurements. Using the INA approach we classified patients as either high or low responders based upon their overall anti-influenza response. Although controls showed a greater increase in the total amount of native antibody after vaccination compared to all SLE patients (p = 0.035), neither the high responding patients (Bmax, Figure 1A, p = 0.254) nor the low responding patients (Bmax, Figure 1A, p = 0.081) alone showed a significantly smaller increase in total native antibody after vaccination compared to controls. Both the high (p = 0.008) and low (p < 0.001) responding patients had a significantly smaller increase in apparent affinity after vaccination compared to controls (Ka, Figure 1B). While a non-significant difference in hemagglutination inhibition was noted between SLE patients and controls (p=0.17), it should be noted that few of these individuals (patients or controls) had substantial increases in HAI titers after vaccination. Furthermore, low responding patients showed a significantly smaller increase in HAI after vaccination than either high responding patients (HAI, Figure 1C, p < 0.001) or healthy controls (HAI, Figure 1C, p < 0.001). Using the INA approach to look at differences of pre- and post-vaccination, controls showed significantly improved humoral influenza responses after vaccination than patients (p < 0.001), and this effect is driven by the poor overall antibody responses of low responding patients (Figure 1D).
Table 1.
Demographics of this influenza vaccination SLE patient cohort.
| All Individuals (n = 72) |
Low Responders (n = 36) |
High Responders (n= 36) |
|
|---|---|---|---|
| Average Age (SD)a | 43 (14) | 43 (13) | 43 (15) |
| African Americanb | 44% | 31% | 58%e |
| European American | 49% | 61% | 36% |
| Hispanic and Otherc | 7% | 8% | 6% |
| Female Sex | 92% | 97% | 86% |
| Median Age at Diagnosis (SD) | 31 (14) | 29 (15) | 33 (14) |
| Average number of ACR criteria (SD) | 5.8 (1.6) | 6.2 (1.4) | 5.5 (1.7) |
| Median Baseline SLEDAI (SD)d | 6.0 (3.8) | 6.0 (4.1) | 5.0(3.4)f |
| Steroid Treatment | 58% | 53% | 64% |
| Anti-Malarial Treatment | 69% | 69% | 69% |
| Combination Steroid and Anti-Malarial | 51% | 50% | 53% |
SD = standard deviation
Race was self defined
Other designation includes individuals that self identified with more than one race (n = 1)
Baseline refers to the day of vaccination
p = 0.028, by exact Chi-square test
p = 0.053, by Wilcoxon two-sample test
Figure 1. SLE patients with poor antibody responses have reduced humoral immunity compared to healthy controls following influenza vaccination.
Plasma from SLE patients (case) and normal healthy controls (control) were tested for humoral immune responses to the influenza vaccine. Cases were divided into high and low responders based upon cumulative rank as outlined in the methods section of the manuscript. Shown are the log10 transformed ratios of post-vaccination to pre-vaccination measurements for (A) antibody concentration (Bmax), (B) antibody affinity (Ka), and (C) hemagglutination inhibition (HAI). Panel D shows the overall vaccine response, expressed as the difference of the percentile ranks (postvacc minus prevacc). Shown are box and whisker plots where the middle line is the median, the box represents the middle 50% of the data, and the whiskers extend to the minimum and maximum values. P-values are derived from nonparametric Mann-Whitney tests using a Bonferroni multiple comparison correction.
Based on the INA approach used to classify patients as either high or low responders, we can see that the high responder group of patients generated an anti-influenza response which was much closer to the behavior of the controls than that of the low responding patients. For each measure of responsiveness (Bmax, Ka, HAI and overall response), the low responder group had impaired responses compared to both the high responder and control groups, particularly when evaluating HAI differences and overall differences. In the following experiments we use this classification of high and low responders to further examine correlates of poor SLE vaccine response.
African-American SLE patients are more likely to have strong influenza vaccination responses
African-American patients were more likely to have strong influenza vaccination responses than European American patients (p=0.03, Table 1). Specifically, African-American patients were 3 times more likely to be high responders than European Americans (95% CI: 1.07, 9.94). This racial association with vaccination response was not due to age since the ages between the two groups did not significantly differ (42±12.4 vs. 46±15.8, respectively). This discrepancy may be due to the impact of HLA haplotypes on vaccine responsiveness, although this requires further investigation.
Patients ranged in age from 20 to 89 years, with 42% between 29 and 45. The relationship between age and the response to vaccination was examined since older individuals may demonstrate decreased influenza antibody levels (38). Although we had only a small number of elderly individuals, we found no relationship between age and the vaccine response. Due to the predilection of SLE for females, the small number of male patients did not allow evaluation of gender differences.
Poor influenza vaccination responses are associated with hematologic manifestations and more SLE ACR classification criteria
We next analyzed age at diagnosis, number of cumulative ACR criteria, and select ACR classification criteria to determine whether these factors correlated with the subsequent response (Table 1). Low responders had more ACR criteria (median=6) than high responders (median=5, p=0.05). Indeed, 23 of the 36 (64%) low responders met six or more of the ACR SLE criteria compared to 15 of 36 (42%) high responders.
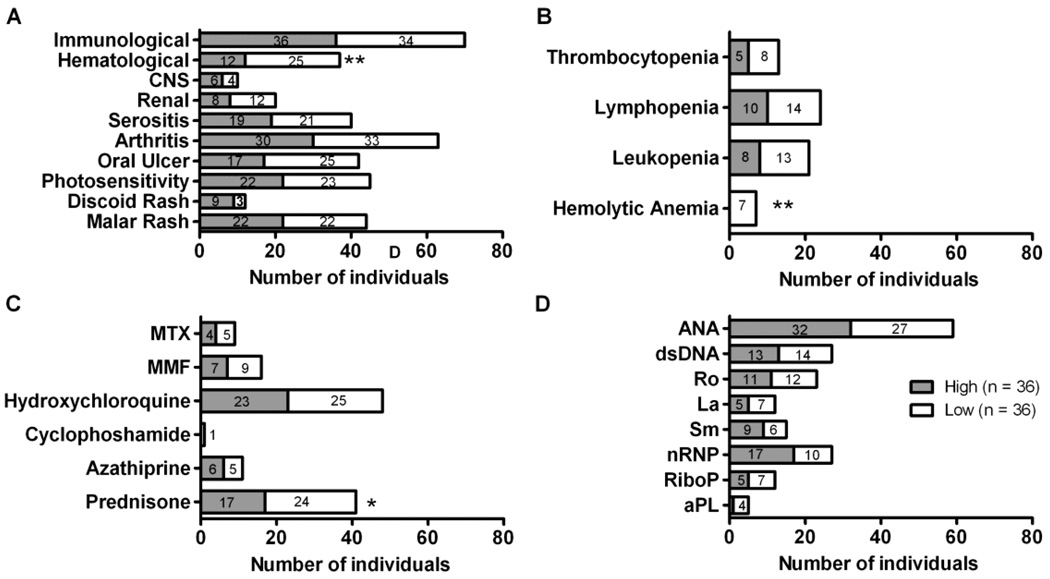
We then analyzed the types of ACR criteria found in both groups. Twenty-five out of 36 low responders (69.4%, Figure 2A) exhibited at least one hematological criterion, compared to 12 out of 36 high responders (33.3%, p=0.009). All of the individuals with hemolytic anemia were low responders (Figure 2b, p=0.01). The low responders also had a higher prevalence of thrombocytopenia (22.2% vs. 13.9%), lymphopenia (38.9% vs. 27.8%), and leucopenia (36.1% vs. 22.2%), although these were not statistically significant (Figure 2B). While not statistically significant, nine of the thirty-six (25%) high responders exhibited discoid rash compared to three of thirty-six (12.5%) low responders (p=0.1). No differences were seen in the prevalence of renal criteria or arthritis.
Figure 2. Patients with low influenza vaccination responses have more hematologic SLE classification criteria and more steroid use.
Subjects were divided into high (gray bar) and low (white bar) responders to influenza vaccination. Shown are the number of individuals that (A) met the indicated ACR SLE classification criteria, **p = 0.009 by exact permutation Chi-square test, (B) exhibited specific aspects of hematological disorders, *p = 0.01 by exact permutation Chi-square test, (C) taking specific medications, *p = 0.037 by Fisher’s exact test and (D) exhibiting specific autoantibodies prior to vaccination.
Poor influenza humoral immune responses are more common in lupus patients using steroids
While some report that medication has no effect on anti-influenza antibodies (11), others have shown decreases in antigen-specific responses in patients taking azathioprine or corticosteroids equivalent to ≥10 mg prednisone daily (19, 39). Twenty-four of 36 (67%) patients who were low responders took prednisone at a level equivalent to ≥10 mg/day compared to 17 of 36 patients (47%) who were high responders (p=0.04, Figure 2C). Although the sample sizes are small, higher doses of corticosteroids (≥20 mg daily) did not appear to further lessen the magnitude of the influenza immune response. No other immunomodulatory medication was associated with a low response.
Of course, the number of individuals taking these medications is relatively small. Therefore, we divided patients into those on “low immunosuppressive therapies” (no medications, hydroxychloroquine only, or hydroxychloroquine in combination with the equivalent of 7.5 mg of prednisone daily) versus those on “high immunosuppressive therapies” (methotrexate, mycophenolate mofetil, azathiprine, cyclophosphamide, greater than 7.5mg of prednisone daily, or a combination of these). Of the thirty-nine individuals on “high immunosuppressive therapies”, twenty-one were high responders (54%). Of the thirty-three individuals on “low immunosuppressive therapies”, eighteen were high responders (55%). Thus, while steroid use is associated with a lower antibody response to influenza vaccination, other immunosuppressive medications do not account for the decreased responses in some patients.
No differences were detected in baseline autoantibody specificities of patients with poor vaccination responses
To help determine what predicts a low response to vaccination in SLE patients, autoantibody levels were measured at the time of enrollment. Nearly all patients were ANA positive (Figure 2D) and high and low responders did not significantly differ in regards to ANA titer (median 1:1080 and 1:953, respectively; data not shown). No significant differences in the dsDNA antibody titer were observed between the responders (median 1:90 for each; data not shown).
While a trend toward higher cardiolipin and lower nRNP antibody frequencies was seen in the low responders, these differences were not significant, which may relate to the low number of individuals positive for these antibodies (Figure 2D). No differences were seen between the high and low responders in the proportion of individuals positive for any of the other autoantibody specificities at baseline. There was also no difference in the average number of autoantibodies in high and low responders (average 2.6 and 2.4, respectively).
Low responders have increases in autoantibody levels and new specificities after vaccination
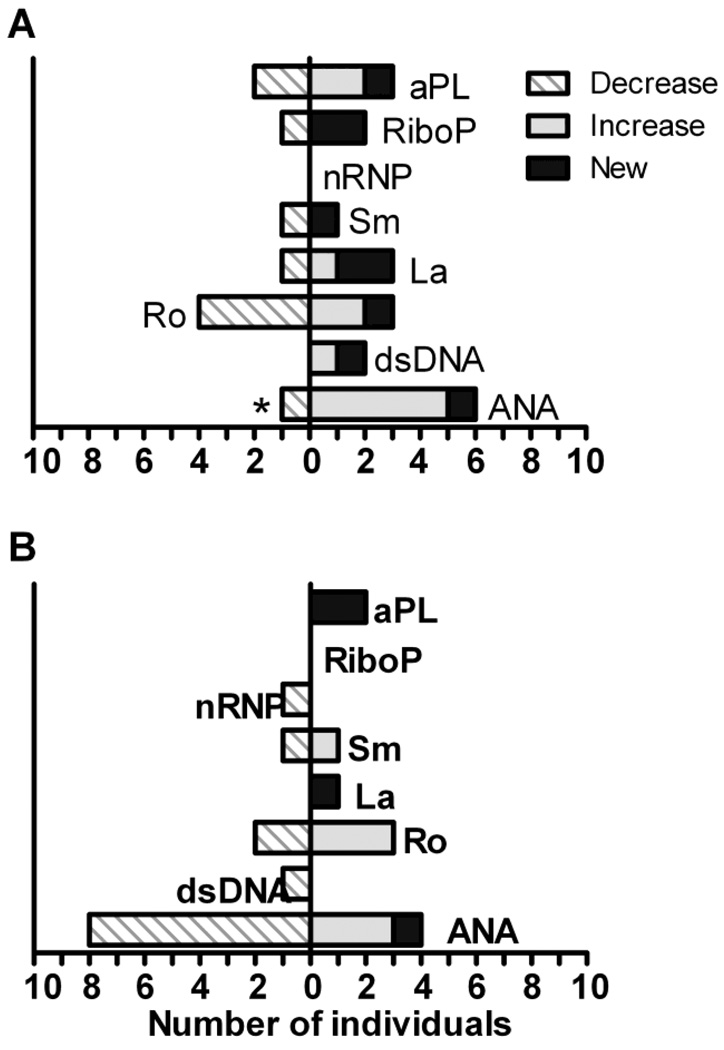
We next examined the impact of vaccination upon autoantibody specificities and levels. Figure 3 shows the number of individuals who had new onset, a two-fold or greater increase, or a two-fold or greater decrease in the specified antibody. These data show patterns of changes in certain autoantibodies, including ANA, anti-La, and anti-cardiolipin among the high responders, and ANA, anti-Ro, and anti-RiboP among the low responders. Nineteen (26%) of the patients had a change in their ANA titer at two weeks post vaccination: two with new onset, eight with increased titer, and nine with decreased titer. Low responders were more likely to have increased ANA titer than high responders (14% versus 8%, respectively), with 5 of the thirty-six low responders having an increase in ANA titer post vaccination. Eight of the high responders (22%) had decreased ANA titers compared to only 1 (3%) of the low responders. Indeed, among high responders, post-vaccination decreases in ANA were twice as common as increased or new ANA expression. Among low responders, decreases in ANA titers were one-sixth as common as increased or new ANA expression (p=0.05, Figure 3).
Figure 3. Patients with low responses to influenza vaccination have increases in autoantibody levels and new specificities after vaccination.
Autoantibody titers were measured in sera from the initial (pre-vaccination) and post-vaccination time-points. Shown is the number of (A) low responders or (B) high responders with a two-fold increase (solid gray bar), a two-fold decrease (hatched bar), or new onset (solid black bar) of the specified autoantibody, *p = 0.045 by conditional logistic regression.
The small number of patients exhibiting changes in antibodies to La, Ro, cardiolipin, and RiboP do not allow a definitive statistical evaluation of differences between high and low responders. However, both groups had new onset of autoantibodies directed against La and cardiolipin (Figure 3). Three low responders and one high responder had new onset/increased antibodies to the La antigen. Both the high responder group and the low responder group had two individuals with new onset/increased antibodies to cardiolipin.
Low responders to influenza vaccination are more likely to have a disease flare
We next examined disease activity following vaccination. Five individuals (6.9%), four of which were low responders, had an increase in SLEDAI of three or more points at 6 weeks post vaccination. Eight individuals (11%), four high and four low responders, had a three point SLEDAI change at 12 weeks post vaccination. Overall, no increased frequency nor severity of flares were seen in the time period immediately after vaccination (6 weeks) compared to a time period further from vaccination (12 weeks). However, this method does not capture some indications of disease flare such as medication changes or hospitalizations.
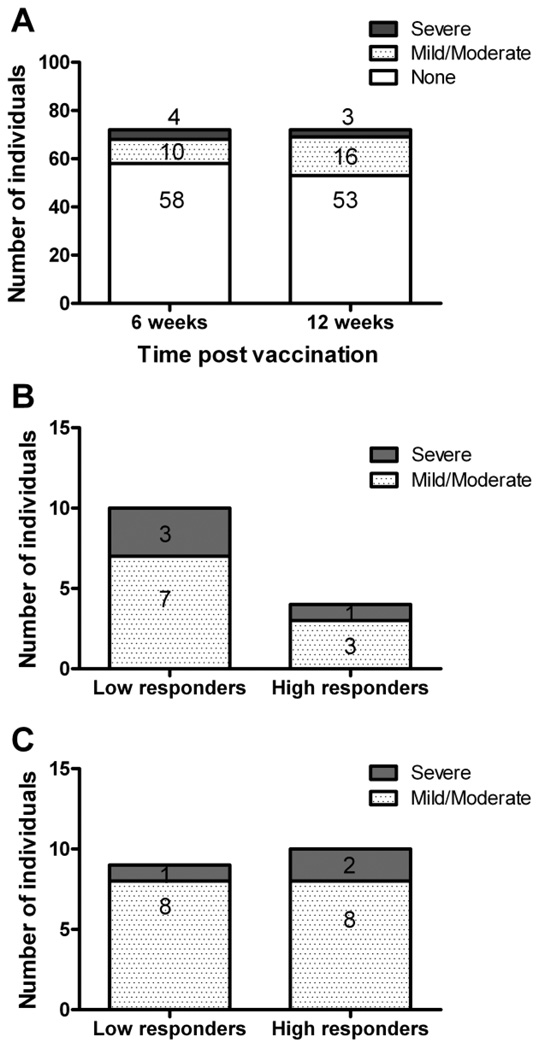
To further refine our method of determining flares, the SELENA-SLEDAI flare index was used (24, 25). Using this index, fourteen patients (19.4%) flared at six weeks post vaccination including 10 (13.8%) with mild/moderate and 4 (5.6%) with severe flares (Figure 4a). At twelve weeks there were nineteen (26.4%) flares including 16 (22.2%) mild/moderate and 3 (4.2%) severe flares (Figure 4a). Of the patients flaring at 6 weeks, 10 (71.4%) were low responders and 4 (28.6%) were high responders (Figure 4b, p = 0.01). By twelve weeks after vaccination, no significant difference was observed in the flare rate between high and low responders, 53% and 47% respectively (Figure 4c). Using data collected from previous studies where patients did not receive influenza vaccination, we observed that 1 of 8 patients (12.5% vs 19.4% in our study) had a mild/moderate flare when observed over a six to eight week time frame. Of 41 patients observed over a nine to eighteen week timeframe without vaccination, 8 (19.5% vs 26.4% in our study) had mild/moderate flares. The differences between flare rates over similar time periods in vaccinated versus non-vaccinated SLE patients were not statistically significant.
Figure 4. SLE disease flares are more frequent in low responders at 6 weeks, but not 12 weeks, after influenza vaccination.
High and low antibody responders to influenza vaccination were evaluated for the presence of flares using the SELENA-SLEDAI method (24, 25) for all individuals at six and twelve weeks post vaccination. Shown is the total number of individuals experiencing no flare (white bars), mild/moderate flare (dotted bars), or severe flares (gray bar) (A), the vaccine responsiveness (high or low) of individuals with a flare at 6 weeks post vaccination (B), and the vaccine responsiveness (high or low) of individuals with a flare at 12 weeks post vaccination (C).
Serum Interferon alpha activity associates with disease flare following vaccination
We next asked if there were characteristics that would predict which patients would flare following vaccination. To this end we compared groups of patients that flared at six weeks post vaccination (n = 14) to a group of matched patients that did not experience a flare. We found that age at diagnosis, number of SLE ACR criteria, and baseline disease activity did not correlate with a flare. Select SLE ACR criteria were more prevalent in the individuals experiencing a flare and these included renal disease (43% vs. 29%), central nervous system involvement (21% vs. 0), and hematological disorder (50% vs. 29%). In contrast, fewer individuals with serositis (29% vs. 50%) or oral ulcers (57% vs. 71%) were in the flare group. Unfortunately, the small numbers of patients in these groups do not allow a definitive statistical evaluation of the impact of these criteria upon flare.
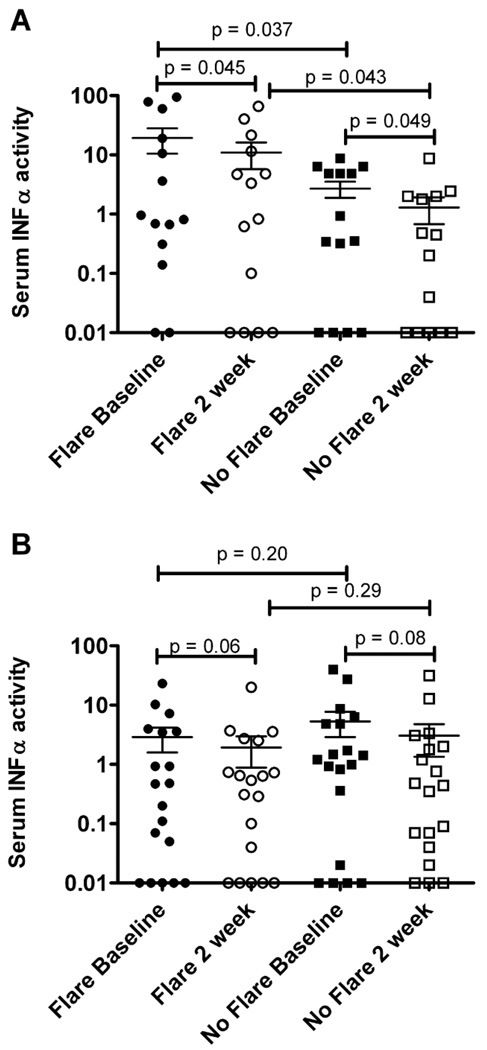
Since increased IFNα induced gene expression correlates with higher disease activity in SLE patients (37), we examined serum IFNα activity. We found that patients experiencing a flare six weeks post vaccination had higher baseline serum IFNα activity than patients that did not have a flare (mean activity 19.3 vs. 2.7, p = 0.04, Figure 5a). While patients that experienced a flare had a decrease in IFNα activity at two weeks post vaccination (mean activity of 10.95), this activity was still higher than seen in individuals that did not experience a flare (Figure 5a). This difference in serum IFNα activity was not observed in the individuals that had a flare at twelve weeks post vaccination (mean activity 2.9 vs. 5.3, p = 0.2, Figure 5b). Of note, we found no significant correlation between IFNα activity level and the overall magnitude of the influenza-specific response.
Figure 5. Patients that flare at six weeks post vaccination had higher baseline IFN alpha serum activity.
Patients were divided into individuals that had a flare (flare) and age and race matched SLE patients that did not have a flare during the course of the study (no flare). Shown is the serum IFN alpha activity at baseline and two weeks in the patients that had a flare at 6 weeks (panel A, p value determined by unpaired t test) and those with a flare at 12 weeks (panel B, no significance by unpaired t test). Each symbol represents one individual and the mean and standard error of the mean are shown.
Discussion
The goal of this study was to identify factors which correlate with the immune response to influenza vaccination in SLE patients and examine the impact of vaccination on autoimmune disease activity. We found no differences in the immune response based on age or gender; however, high responders were enriched for African American patients. Previous reports about influenza vaccination in SLE patients were performed in other geographic regions, using Israeli (8, 17, 19, 40, 41), Italian (18), Mexican (16), or Dutch subjects (15, 39, 42, 43) or were not able to evaluate race (11–14). Surprisingly, little is known about racial differences in immune responses to vaccinations. Healthy young African Americans had higher levels of neutralizing antibodies against HIV gp-120 after vaccination compared to European Americans (44), yet older African Americans had impaired T cell responses to influenza vaccination (45). Additional work is necessary to fully understand the influence of race on vaccination responses.
SLE patients with a history of hemolytic anemia were more likely to be low responders. Our initial assumption was that this association would be driven by patients with significant lymphopenia and/or leucopenia. However, no significant differences were found in the percentage of lymphocytes at baseline between the low and high responders (28.1% vs. 34.8%, respectively). In addition, severe lymphopenia (those individuals with ≤ 500/µl) was found in 14% of low responders compared to 11% of high responders, indicating that lymphopenia was not the main factor in this association. Rather, hemolytic anemia was the dominant hematologic manifestation driving the association with low vaccination response. No other reports could be found examining the response to vaccination in patients with hemolytic anemia or thrombotic thrombocytopenic pupura. Examinations of vaccination responses of larger collections of patients with hemolytic anemia are warranted to uncover mechanisms for this epidemiologic association.
Reports regarding changes in autoantibody concentration and/or specificities in SLE patients after vaccination have been conflicting (17–20). These studies have been limited by small sample sizes (n = 14 to 18) (16, 18), differences in methodologies, and limited number of autoantibodies selected for analysis (16). Abu-Shakra et al. found that influenza vaccination had no effect on anti-dsDNA, a transient effect on Sm, nRNP, Ro, and La, and a prolonged effect on anti-cardiolipin antibodies (19). These results are supported in part by our findings.
We observed two striking findings in regards to autoantibodies following vaccination. First, patients who mounted a strong response were significantly more likely to decrease their ANA titer. This suggests that some SLE patients have an immune system poised to generate antigen-specific immune responses while minimizing the stimulation, expansion, and maturation of autoreactive B cells. Second, over half of the low responders developed new autoantibodies or increased the concentration of existing autoantibodies, suggesting that these patients are more likely to have immune responses focused, at least in part, against self antigens. Further investigation into the longevity of these responses and associated long-term clinical consequences are underway. Additional evaluation of the influence of genetic, environmental and other disease-specific factors on determining whether SLE patients will focus their immune responses on antigen-specific or autoantigen-specific pathways is warranted.
Several groups have reported no differences in disease activity measures between influenza vaccinated and unvaccinated SLE patients (41, 46). However, the majority of these studies were small (n = 23–24) (41, 46). Holvast et al. (15) found that influenza vaccination did not result in significant changes to disease activity scores in 56 SLE patients. We showed no increased flare rates within six weeks post-vaccination compared to the six to twelve week post-vaccination time interval. However, patients who flared within six weeks post vaccination were predominantly low responders. Being able to identify these individuals before vaccination and modify approaches for influenza vaccination in ways that do not negatively impact their underlying disease would be clinically useful.
Several differences between the published studies and our work may influence the flare rates and disease activity changes detected. First, in previous studies, a flare was defined as an increase of three or more points as measured by SLEDAI. Our study calculated flares using the SELENA-SLEDAI index which also takes into account hospitalization and medication changes (24, 25). Second, the studies cited above enrolled patients with quiescent disease (SLEDAI ≤ 4) whereas our patient population was composed of individuals with both quiescent and active disease. Indeed, 41 of our 72 patients (57%) had a SLEDAI of 6 or more at baseline. Lastly, as mentioned earlier, our study was composed of roughly equal numbers of European Americans and African American SLE patients whereas the majority of the previously reported studies were composed primarily of patients of European descent.
When we examined patients that flared following vaccination, we found that select ACR criteria were either over or under-represented. Renal disease, central nervous system involvement, and hematological disorder were more prevalent at baseline in the patients that flared. Additionally, patients that flared at six weeks post vaccination had higher baseline serum IFNα activity. Others have shown that IFNα activity correlates with SLE disease criteria and that an elevated IFNα score is associated with severe manifestations of SLE (47, 48). The relationships between interferon activity, vaccination responses and subsequent disease flares need to be confirmed in a larger cohort and the biological significance examined. This information is especially important to investigate since several groups have advocated using IFNα as an adjuvant for influenza vaccination (48, 49).
Our data indicate that some SLE patients, especially those with a history of hematologic disorder or individuals taking prednisone, mount weak responses to the influenza vaccine. Furthermore, low responders are more likely than high responders to increase autoantibody production and experience disease flares following vaccination. Current studies are underway to explore biomarkers which might better predict which patients are likely to flare following vaccination. Coupled with select serologic biomarkers in patients who are anticipated to have low responses, this information would help identify the subset of patients who may need to have their underlying disease more aggressively treated before receiving influenza or other vaccination.
Acknowledgements
We would like to thank all of the study participants for their time and commitment to this work as well as the referring physicians: Drs. Craig Carson, John Harley, Ana Ahluwahlia Kumar, Linda Zacharias and physician assistants Teresa Aberle and Jama Kendall Shoemaker. We also thank our clinical coordinator, Virginia Roberts, as well as Jourdan Anderson, Wendy Klein, Susan Macwana, Wade DeJager, Phillip McGhee, Gabriel Vidal, Jeremy Levin, and John Johnson for technical assistance. Finally, we thank Dr. J. Donald Capra for critically reading the manuscript.
Supported by the National Institutes of Health (NIAID: HHSN266200500026C, AR058554, RR015577, AI082714 and AR053483), Kirkland Foundation Scholar Support and the Lou C. Kerr Chair in Biomedical Research (JAJ).
Footnotes
Author contributions
All authors were involved in drafting the article or critically revising it.
Study conception and design: James, L. Thompson, Air, Crowe
Acquisition of data: Crowe, Vista, Dedeke, James, Franek, Niewold
Analysis and interpretation of data: Crowe, Vista, James, L. Thompson, Air, Merrill, D. Thompson, Niewold, Guthridge, Stewart
References
- 1.Fessler BJ. Infectious diseases in systemic lupus erythematosus: risk factors, management and prophylaxis. Best Pract Res Clin Rheumatol. 2002;16(2):281–291. doi: 10.1053/berh.2001.0226. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore) 1999;78(3):167–175. doi: 10.1097/00005792-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Colonna L, Dinnall JA, Shivers DK, Frisoni L, Caricchio R, Gallucci S. Abnormal costimulatory phenotype and function of dendritic cells before and after the onset of severe murine lupus. Arthritis Res Ther. 2006;8(2):R49. doi: 10.1186/ar1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsokos GC, Liossis SN. Immune cell signaling defects in lupus: activation, anergy and death. Immunol Today. 1999;20(3):119–124. doi: 10.1016/s0167-5699(98)01395-4. [DOI] [PubMed] [Google Scholar]
- 5.Via CS, Tsokos GC, Bermas B, Clerici M, Shearer GM. T cell-antigen-presenting cell interactions in human systemic lupus erythematosus. Evidence for heterogeneous expression of multiple defects. J Immunol. 1993;151(7):3914–3922. [PubMed] [Google Scholar]
- 6.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 7.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Shakra M, Press J, Buskila D, Sukenik S. Influenza vaccination of patients with systemic lupus erythematosus: safety and immunogenecity issues. Autoimmun Rev. 2007;6(8):543–546. doi: 10.1016/j.autrev.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Battafarano DF, Battafarano NJ, Larsen L, Dyer PD, Older SA, Muehlbauer S, et al. Antigen-specific antibody responses in lupus patients following immunization. Arthritis Rheum. 1998;41(10):1828–1834. doi: 10.1002/1529-0131(199810)41:10<1828::AID-ART15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Elkayam O, Paran D, Caspi D, Litinsky I, Yaron M, Charboneau D, et al. Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus. Clin Infect Dis. 2002;34(2):147–153. doi: 10.1086/338043. [DOI] [PubMed] [Google Scholar]
- 11.Brodman R, Gilfillan R, Glass D, Schur PH. Influenzal vaccine response in systemic lupus erythematosus. Ann Intern Med. 1978;88(6):735–740. doi: 10.7326/0003-4819-88-6-735. [DOI] [PubMed] [Google Scholar]
- 12.Williams GW, Steinberg AD, Reinertsen JL, Klassen LW, Decker JL, Dolin R. Influenza immunization in systemic lupus eruthematosus. A double-blind trial. Ann Intern Med. 1978;88(6):729–734. doi: 10.7326/0003-4819-88-6-729. [DOI] [PubMed] [Google Scholar]
- 13.Louie JS, Nies KM, Shoji KT, Fraback RC, Abrass C, Border W, et al. Clinical and antibody responses after influenza immunization in systemic lupus erythematosus. Ann Intern Med. 1978;88(6):790–792. doi: 10.7326/0003-4819-88-6-790. [DOI] [PubMed] [Google Scholar]
- 14.Ristow SC, Douglas RG, Jr, Condemi JJ. Influenza vaccination of patients with systemic lupus erythematosus. Ann Intern Med. 1978;88(6):786–789. doi: 10.7326/0003-4819-88-6-786. [DOI] [PubMed] [Google Scholar]
- 15.Holvast A, Huckriede A, Wilschut J, Horst G, De Vries JJ, Benne CA, et al. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann Rheum Dis. 2006;65(7):913–918. doi: 10.1136/ard.2005.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercado U, Acosta H, Avendano L. Influenza vaccination of patients with systemic lupus erythematosus. Rev Invest Clin. 2004;56(1):16–20. [PubMed] [Google Scholar]
- 17.Abu-Shakra M, Press J, Varsano N, Levy V, Mendelson E, Sukenik S, et al. Specific antibody response after influenza immunization in systemic lupus erythematosus. J Rheumatol. 2002;29(12):2555–2557. [PubMed] [Google Scholar]
- 18.Del Porto F, Lagana B, Biselli R, Donatelli I, Campitelli L, Nisini R, et al. Influenza vaccine administration in patients with systemic lupus erythematosus and rheumatoid arthritis. Safety and immunogenicity. Vaccine. 2006;24(16):3217–3223. doi: 10.1016/j.vaccine.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Shakra M, Press J, Sukenik S, Buskila D. Influenza virus vaccination of patients with SLE: effects on generation of autoantibodies. Clin Rheumatol. 2002;21(5):369–372. doi: 10.1007/s100670200099. [DOI] [PubMed] [Google Scholar]
- 20.Toplak N, Kveder T, Trampus-Bakija A, Subelj V, Cucnik S, Avcin T. Autoimmune response following annual influenza vaccination in 92 apparently healthy adults. Autoimmun Rev. 2008;8(2):134–138. doi: 10.1016/j.autrev.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Malik S, Bruner GR, Williams-Weese C, Feo L, Scofield RH, Reichlin M, et al. Presence of anti-La autoantibody is associated with a lower risk of nephritis and seizures in lupus patients. Lupus. 2007;16(11):863–866. doi: 10.1177/0961203307083365. [DOI] [PubMed] [Google Scholar]
- 23.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 24.Petri M. Disease activity assessment in SLE: do we have the right instruments? Ann Rheum Dis. 2007;66 Suppl 3:iii61–iii64. doi: 10.1136/ard.2007.078477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 26.Feng J, Gulati U, Zhang X, Keitel WA, Thompson DM, James JA, et al. Antibody quantity versus quality after influenza vaccination. Vaccine. 2009;27(45):6358–6362. doi: 10.1016/j.vaccine.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008 doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulati U, Kumari K, Wu W, Keitel WA, Air GM. Amount and avidity of serum antibodies against native glycoproteins and denatured virus after repeated influenza whole-virus vaccination. Vaccine. 2005;23(11):1414–1425. doi: 10.1016/j.vaccine.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 29.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 30.Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC, Harley JB, et al. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. 2007;56(7):2344–2351. doi: 10.1002/art.22665. [DOI] [PubMed] [Google Scholar]
- 31.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11(1):85–89. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 32.Bruner BF, Wynn DM, Reichlin M, Harley JB, James JA. Humoral antigenic targets of the ribosomal P0 lupus autoantigen are not limited to the carboxyl region. Ann N Y Acad Sci. 2005;1051:390–403. doi: 10.1196/annals.1361.081. [DOI] [PubMed] [Google Scholar]
- 33.Harris EN, Hughes GR, Gharavi AE. Anti-cardiolipin antibodies and the lupus anticoagulant. Clin Exp Rheumatol. 1986;4(2):187–190. [PubMed] [Google Scholar]
- 34.McClain MT, Arbuckle MR, Heinlen LD, Dennis GJ, Roebuck J, Rubertone MV, et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 2004;50(4):1226–1232. doi: 10.1002/art.20120. [DOI] [PubMed] [Google Scholar]
- 35.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54(6):1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 36.Niewold TB, Adler JE, Glenn SB, Lehman TJ, Harley JB, Crow MK. Age- and sex-related patterns of serum interferon-alpha activity in lupus families. Arthritis Rheum. 2008;58(7):2113–2119. doi: 10.1002/art.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8(6):492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulati U, Keitel WA, Air GM. Increased antibodies against unfolded viral antigens in the elderly after influenza vaccination. Influenza Other Respi Viruses. 2007;1(4):147–156. doi: 10.1111/j.1750-2659.2007.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holvast A, van Assen S, de Haan A, Huckriede A, Benne CA, Westra J, et al. Studies of cell-mediated immune responses to influenza vaccination in systemic lupus erythematosus. Arthritis Rheum. 2009;60(8):2438–2447. doi: 10.1002/art.24679. [DOI] [PubMed] [Google Scholar]
- 40.Abu-Shakra M, Shoenfeld Y. Azathioprine therapy for patients with systemic lupus erythematosus. Lupus. 2001;10(3):152–153. doi: 10.1191/096120301676669495. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Shakra M, Zalmanson S, Neumann L, Flusser D, Sukenik S, Buskila D. Influenza virus vaccination of patients with systemic lupus erythematosus: effects on disease activity. J Rheumatol. 2000;27(7):1681–1685. [PubMed] [Google Scholar]
- 42.Holvast A, van Assen S, de Haan A, Huckriede A, Benne CA, Westra J, et al. Effect of a second, booster, influenza vaccination on antibody responses in quiescent systemic lupus erythematosus: an open, prospective, controlled study. Rheumatology (Oxford) 2009 doi: 10.1093/rheumatology/kep200. [DOI] [PubMed] [Google Scholar]
- 43.Holvast B, Huckriede A, Kallenberg CG, Bijl M. Influenza vaccination in systemic lupus erythematosus: safe and protective? Autoimmun Rev. 2007;6(5):300–305. doi: 10.1016/j.autrev.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Montefiori DC, Metch B, McElrath MJ, Self S, Weinhold KJ, Corey L. Demographic factors that influence the neutralizing antibody response in recipients of recombinant HIV-1 gp120 vaccines. J Infect Dis. 2004;190(11):1962–1969. doi: 10.1086/425518. [DOI] [PubMed] [Google Scholar]
- 45.Gardner EM, Gonzalez EW, Nogusa S, Murasko DM. Age-related changes in the immune response to influenza vaccination in a racially diverse, healthy elderly population. Vaccine. 2006;24(10):1609–1614. doi: 10.1016/j.vaccine.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 46.Stojanovich L. Influenza vaccination of patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) Clin Dev Immunol. 2006;13(2–4):373–375. doi: 10.1080/17402520600800820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 49.Blazevic V, Trubey CM, Shearer GM. Comparison of in vitro immunostimulatory potential of live and inactivated influenza viruses. Hum Immunol. 2000;61(9):845–849. doi: 10.1016/s0198-8859(00)00170-1. [DOI] [PubMed] [Google Scholar]