Summary
Neurons are exquisitely polarized cells that extend intricate axonal and dendritic arbors. Developmental cues guide axons and dendrites into circuits by inducing rapid changes in local protein expression and cytoskeleton structure. Neurons can transduce these signals through local mRNA regulation. Here, we review the latest insights regarding post-transcriptional control of gene expression through mRNA transport and local protein synthesis in developing neurons. We focus on local mRNA regulation during axon growth and guidance, dendrite morphogenesis, and synapse formation and refinement. Dysregulated mRNA transport and translation in neurological disorders are also discussed. The collection of molecules and mechanisms reviewed includes sequence-specific RNA binding proteins, microtubule motors and adaptors, microRNAs, translation initiation factors, and the receptor-mediated signaling that modulates these molecules.
Introduction
Neural circuits shape the physical and behavioral development of organisms. During circuit formation, neurons extend elaborate axonal and dendritic arbors that encounter scores of molecular cues. Precise temporal and spatial integration of these cues is vital for the patterning of synaptic connections. Although the cellular processes involved in circuit development are varied, one common element is the requirement for tightly controlled gene expression. In this review, we focus on the post-transcriptional control of gene expression through mRNA localization and local protein synthesis in developing neurons.
A navigating growth cone, a branching dendrite, and an expanding presynaptic terminal each have specific molecular demands that change rapidly during development. Localized mRNA translation is an efficient mechanism to adjust protein levels in these distinct subcellular domains. Miscues in local mRNA regulation have been linked to neurological disorders characterized by intellectual disabilities, brain hyperexcitability, and neurodegeneration [1]. Here, we highlight recent progress toward understanding how local protein synthesis regulates axon guidance and growth, dendrite morphogenesis, and synapse formation and refinement.
Axon growth and guidance
The axonal growth cone is a highly motile structure that drives axon elongation and pathfinding. Extracellular cues direct growth cones by inducing rapid changes in local protein expression, and developing axons contain the necessary translational machinery and specific mRNAs for local protein synthesis [2]. Several studies with Xenopus retinal ganglion cells (RGCs) and dorsal root ganglion neurons (DRGs) specify a role for local protein synthesis in cue-induced axon guidance; such cues include netrin-1, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), Slit, and semaphorin-3a [2]. In mouse cortical neurons, netrin-1-induced growth cone turning also requires local protein synthesis, which indicates that mRNA localization and local protein synthesis have conserved functions in the mammalian central nervous system (K Welshhans et al., unpublished).
Recently, three genome-wide analyses have described the developmental regulation of axonal mRNA localization [3–5]. Early in development, RGC growth cones contain primarily mRNAs encoding translation machinery and cytoskeleton elements. In later stages, growth cones harbor a more complex set of transcripts including mRNAs encoding synaptogenesis-related proteins. For example, Eph receptor B4 mRNA is only localized to older growth cones even though its transcription is not altered during this period [3]. Likewise, divergent subsets of mRNAs are targeted to embryonic and adult DRG axons as well as immature and mature cortical neuron axons in vitro. The total level of mRNA and translational machinery is reduced as these axons mature [5]. The developmental switches that alter mRNA targeting and translational capacity in maturing axons are unknown. Mature neurons can restore axonal translation in response to injury; this might involve mechanisms used in development or signals specific to mature neurons [6].
B-actin mRNA has been a well-studied axonal transcript since its discovery in growth cones [7]. Netrin-1 is a classic guidance cue with well-defined functions in vivo. By integrating studies on both molecules, an archetypical mechanism for RNA binding protein (RBP)-mediated mRNA transport and local translation emerges. Zipcode binding protein 1 (ZBP1) binds the 3’ untranslated region (3’ UTR) of β–actin mRNA, thereby repressing translation and mediating axonal transport [2]. At the growth cone, extracellular netrin-1 binds to DCC (deleted in colorectal cancer), which activates Src kinases. Src phosphorylates ZBP1 resulting in dissociation of ZBP1 from β–actin mRNA, ribosome recruitment to β–actin mRNA, and local β– actin synthesis [8,9]. ZBP1 phosphorylation and localized β–actin synthesis are necessary for cue-induced growth cone turning ([8] and K Welshhans et al., unpublished). Moreover, cortical neurons from ZBP1-deficient mice do not show netrin-1-induced turning or local β–actin synthesis (K Welshhans et al., unpublished). In addition, DCC interacts with ribosome subunits, translation initiation factors, and monosomes. Netrin-1 binding to DCC leads to dissociation of the translational machinery and increased protein synthesis [10]. ZBP1 likely regulates specific mRNAs; whereas, the dissociation of translational machinery from DCC could regulate any localized mRNA. As of now, it is unknown whether these mechanisms converge to regulate netrin-1-induced synthesis of specific proteins.
One important function of axonal mRNA translation is to regulate growth cone architecture through local synthesis of cytoskeleton components and regulators such as β-actin, cofilin, rhoA, and Map1b [11]. Similarly, local synthesis of the polarity complex protein Par3, a regulator of cytoskeleton dynamics, is required for NGF-induced axon outgrowth [12]. A novel function for axonal protein synthesis during development is retrograde signaling that regulates transcription. NGF-induced axonal synthesis of the transcription factor CREB (cAMP response element binding protein) is necessary for subsequent CRE-dependent transcription and NGF-mediated neuronal survival [13]. This discovery indicates that distal cue-induced protein synthesis can signal to the nucleus to, perhaps, affect cell-wide gene expression. A role for axonal protein synthesis in neuron survival has implications for neuropathies, motor neuron diseases, and neurodegenerative diseases.
Spinal muscular atrophy (SMA), a degenerative disease resulting in motor neuron death and muscle atrophy, is caused by mutations in survival of motor neuron protein (SMN). SMN has a canonical role in snRNP assembly in the nucleus; however, SMN is also localized to axons and has a proposed role in mRNA transport. Indeed, SMN interacts with axonal RBPs, namely HuD and hnRNP-R [14,15]. In cultured motor neurons, SMN knockdown reduces the axonal targeting of HuD and mRNA granules [14]. hnRNP-R depletion reduces axonal β-actin mRNA levels, stunts axon growth, and decreases growth cone size, which is a recapitulation of the SMN-deficient phenotype [15]. In a mouse model of SMA, motor neuron death was prevented by reducing expression of PTEN (phosphatase and tensor homolog), a negative regulator of translation that is localized to axons [16]. Together, these studies support a role for SMN in axonal mRNA regulation.
Dendrite and spine morphogenesis
The role for dendritic mRNA regulation in synaptic plasticity has been well-studied, but remarkably less is known of its role in development. Interestingly, the rate of dendritic protein synthesis peaks during synaptogenesis and steadily declines into adulthood [17]. Some mechanisms underlying development and plasticity might be shared, but there are likely critical differences in local mRNA regulation. Recent findings underscore the impact of localized RBPs, specifically FMRP (fragile X mental retardation protein), CPEB (cytoplasmic polyadenylation element binding protein), and Pumilio2 (Pum2), on dendrite and spine development. Current research has also focused on the emerging role for localized microRNAs (miRNAs) in spine development.
FMRP regulates dendritic mRNA transport and local translation. The loss of FMRP causes fragile X syndrome (FXS), a developmental disorder associated with physical, intellectual, and behavioral abnormalities. Metabotropic glutamate receptor (mGlu) signaling is a major regulator of FMRP-mediated local mRNA translation, which has been extensively studied [18]. mGlu activation also localizes FMRP-associated mRNA-protein complexes to dendrites. FMRP interacts with kinesin light chain (KLC), a microtubule motor protein. Disrupting the FMRP:KLC interaction occludes mGlu-induced transport of FMRP and increases dendritic filopodia density and length, a phenotype consistent with FXS [19]. In drosophila, Bicaudal-D facilitates dFMRP motility by linking it to dynein motors, and this interaction is necessary for proper dendrite patterning [20]. These studies emphasize the important role of FMRP in mRNA transport, a process that is not as well understood compared to its role in mRNA translation.
Many FMRP targets are essential postsynaptic and presynaptic proteins involved in development, including scaffolds, neurotransmitter receptors, and signaling molecules. For example, FMRP associates with α-calcium/calmodulin-dependent kinase II (αCaMKII) mRNA and regulates its translation at synapses in mouse brain[18]. In the drosophila model, calcium signaling and the mRNA levels of calmodulin and calbindin (calcium binding proteins) are altered; calmodulin and calbindin mRNAs are putative dendritic and/or axonal mRNAs [3–5,21,22]. Whether FMRP regulates these mRNAs is unclear; but, given the vital role of calcium signaling in synapse formation and circuit development, it is an interesting prospect.
CPEB is another established dendritic RBP that regulates mRNA transport and translation. CPEB has a well-defined role in development and an emerging role in synaptic plasticity [23]. A pair of elegant studies has identified a role for CPEB in neural circuit formation in vivo [24,25]. Bestman and Cline used dominant-negative strategies to isolate the mRNA transport and translation functions of CPEB. Blocking CPEB-mediated transport slows dendrite development and disrupts activity-induced dendrite patterning. CPEB-mediated mRNA translation is critical for constitutive dendrite development, activity-induced dendrite growth, synapse maturation, and visual circuit formation [24]. Recognized CPEB targets, such as BDNF or (CaMKII), could mediate these effects, but another potential player is Dscam (Down syndrome cell adhesion molecule). Dscam mRNA is bound by CPEB and localized to dendrites [26]. Moreover, Dscam is critical for dendrite patterning, and it is overexpressed in the brains of patients with Down syndrome [27]. In a mouse model of Down syndrome, dendritic Dscam protein levels are increased, and GluN-induced synthesis of Dscam protein is absent [26]. This potential connection between CPEB and Dscam suggests a novel role for CPEB in neural development and, perhaps, neurological disease.
Dendrite and spine development are also controlled by the concerted action of miRNAs, small non-coding RNAs that silence target mRNAs, and the associated RNA-induced silencing complex (RISC). Recently, several dendritic miRNAs have been identified in hippocampal neurons including miR-134, miR-138, and miR-125a [28–30]. miR-134 limits spine growth by repressing the local synthesis of LIM domain kinase 1 (LIMK1), a regulator of actin dynamics. BDNF stimulation activates local LIMK1 translation by alleviating miR-134-mediated repression, which induces spine morphogenesis [28]. Spine growth is also limited by miR-138, which represses the local synthesis of acyl protein thioesterase 1 (APT1), a depalmitoylating enzyme. One APT1 substrate is heterotrimeric G-protein subunit alpha13 (Gα13); when palmitoylated, Gα13 activates Rho signaling, which reduces actin dynamics and restricts spine growth [31]. In addition, miRNA and RISC, specifically miR-125a and Ago2, cooperate with phosphorylated FMRP to repress local translation of PSD-95 mRNA. mGlu activation leads to FMRP de-phosphorylation, release of miR-125a-mediated repression, and PSD-95 translation. FMRP and miR-125a might regulate spine stability locally as loss of miR-125a function increases spine density and branching [30]. These findings begin to uncover functions for miRNA-mediated local translation, but how miRNAs are transported to dendrites remains entirely unknown.
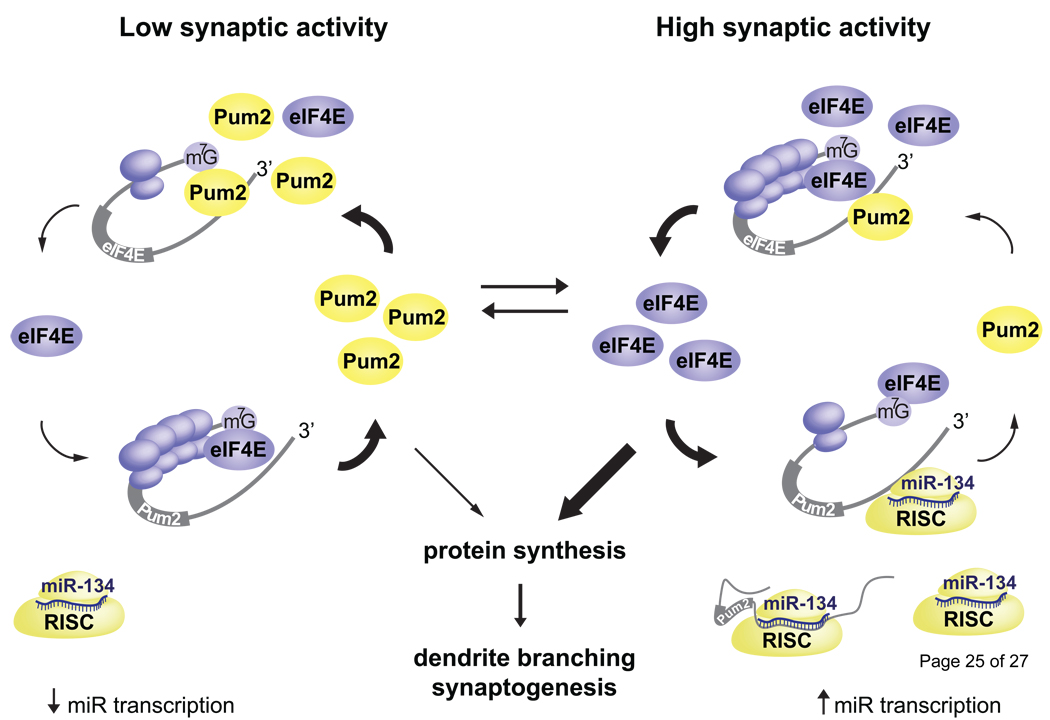
A collection of studies have uncovered an activity-dependent mechanism involving mRNA regulation by Pum2, miR-134, and translation initiation factor eIF4E (Figure 1). Pum2 binds a specific cis-element in the 3’ UTR of some mRNAs, and it inhibits translation by also binding to the 5’ cap structure, thus blocking eIF4E binding [32]. Interestingly, Pum2 binds the 3’ UTR of eIF4E mRNA and directly represses its translation. In cortical neurons, depletion of Pum2 increases dendritic branching, reduces spine number, and increases excitatory shaft synapses. Overexpression of eIF4E leads to a similar morphological phenotype [33]. Pum2 translation is repressed by miR-134, which is transcribed as part of an activity-regulated miRNA cluster [28,34]. The result is that neuronal activity increases eIF4E translation, which likely increases translation of other mRNAs and facilitates dendrite branching and synapse formation. Interestingly, Pum2 mRNA, eIF4E mRNA, and miR-134 are all localized to dendrites [22,28,35]. These mechanisms converge to balance local translation as well as activity-induced dendrite morphogenesis.
Figure 1. Balancing activity-induced dendrite morphogenesis through local mRNA regulation.
The local translation of Pum2 and eIF4E balance protein synthesis and regulate dendrite and spine morphogenesis. At low activity, Pum2 represses the translation of eIF4E, thus keeping protein synthesis rates low and limiting morphogenesis and synapse formation. High neural activity induces the transcription of miR-134; this leads to repression of Pum2 translation, increased translation of eIF4E, and dendrite morphogenesis. During development, this pathway likely maintains a basal level of activation that is a by increases and decreases in synapse activity.
Synapse formation and refinement
Synapse development involves coordinated signaling between pre- and postsynaptic neurons. In Aplysia, synapse formation requires presynaptic synthesis of sensorin, a secreted neuropeptide, and axonal sensorin mRNA is redistributed to new developing terminals [36]. Moreover, local translation of sensorin mRNA occurs at only active synaptic contacts and requires calcium signaling in the postsynaptic cell [37]. These seminal studies highlight how intercellular signaling, mRNA targeting, and local translation regulate gene expression with high temporal and spatial specificity during synapse formation.
The first direct role for local protein synthesis in mammalian synapse formation was reported by Sebeo et al. in cultured hippocampal neurons [38]. Local inhibition of protein synthesis depletes the available pool of synaptic vesicles and leads to elimination of developing synaptic contacts. Vesicle recycling at these synapses depends upon constant synthesis of CaMKII; whereas, protein kinase A mediates vesicle recycling at stable synapses [38]. Although presynaptic CaMKII synthesis was not directly shown, this study indicates a conserved role for presynaptic protein synthesis in synapse formation.
BDNF signaling is essential for brain development and can trigger protein synthesis-dependent spine morphogenesis [39]. Blocking the dendritic localization of BDNF mRNA in vivo dramatically reduces dendritic BDNF protein levels, increases spine density, and decreases spine size. Therefore, local synthesis of BDNF is critical for synapse maturation and spine pruning [40]. One possible underlying mechanism is that locally synthesized and secreted BDNF binds to postsynaptic TrkB receptors and induces spine maturation, perhaps through further local synthesis of morphogenic proteins. On the other hand, postsynaptic synthesis of BDNF is necessary for presynaptic homeostatic plasticity in mature neurons [41]. Given that presynaptic activity critically regulates synapse stability, a model for newly synthesized BDNF acting upon pre- or postsynaptic TrkB receptors could be formulated.
Numerous recent studies have examined the aspects of circuit formation regulated by FMRP (see Table 1 [42–52]). A notable compilation of works from Kendal Broadie’s lab, using a drosophila model of FXS, indicate that dFMRP has specific pre- and post-synaptic functions that are critical at precise times in circuit development [43,45,46]. In specific developing circuits, FMRP forms presynaptic granules with the fragile x-related proteins 1 and 2 (FMRP homologs that also bind RNA) [53]. These granules are specifically expressed during periods of synapse formation. Fmr1 null mice have reduced granule expression, but whether these granules contain mRNAs or regulate axonal translation remains unknown [53].
Table 1. Specific roles for FMRP in neural circuit formation.
These studies demonstrate how specific neural circuits develop in FMRP-deficient organisms (dfmr1 null drosophila or Fmr1 null mice) or in wild-type organisms with dfmr1 or Fmr1 mutated in specific neuron populations.
| Organism | Circuit | Mutant phenotype | FMRP function | FMRP location | Ref. |
|---|---|---|---|---|---|
| Drosophila | NMJ | Increased connectivity; small terminal size; axon overgrowth | Restricts axon growth; presynaptic terminal growth | Axon | [43] |
| Drosophila | NMJ | Increased neurotransmission | - | Dendrite | [43] |
| Drosophila | MB | Axon overgrowth; increased connectivity | Restricts axon growth; axon pruning | Axon | [45] |
| Drosophila | CC | Axon overgrowth; increased connectivity | Synapse pruning | - | [46] |
| Mouse | SSC | Reduced connectivity; diffuse axon arbors; reduced plasticity | Activity-dependent axon refinement | - | [42] |
| Mouse | SSC | Reduced connectivity; hyperexcitability; decreased synchrony | - | - | [44] |
| Mouse | SSC | Increased spine turnover; immature spine shape | Spine stabilization; synapse maturation | - | [47] |
| Mouse | SSC | Increased spine turnover | Spine stabilization; synapse refinement | - | [48] |
| Mouse | DG | Increased connectivity; immature spine shape | Synapse maturation | - | [49] |
| Mouse* | CA1 | Increased connectivity | Synapse pruning | Dendrite | [50] |
| Mouse | SSC | Immature synapses; altered plasticity | Synapse maturation | - | [51] |
| Mouse | OBGC | Increased spines; immature spines; increased connectivity | Spine formation; synapse growth; dendrite refinement | Dendrite | [52] |
FMRP or FMRP mutant constructs were re-introduced into organotypic hippocampal slices from Fmr1 null mice. The mutant phenotypes and, when possible, the proposed function and location of FMRP are listed. NMJ: neuromuscular junction, MB: mushroom body, CC: circadian clock, SSC: somatosensory cortex, DG: dentate gyrus, CA1: hippocampus CA1 region, OBGC: olfactory bulb granule cells.
Patterned synaptic activity can induce long-term modifications at developing synapses. Long-term potentiation (LTP) is a classic mechanism underlying learning-related plasticity, and it also regulates synapse refinement in development. In the visual system, LTP is critical for retinotectal circuit formation, and in vivo LTP expression requires protein synthesis and GluN (NMDA receptor) activation [54]. In mature neurons, LTP induces local protein synthesis at synapses downstream of GluN activation. Although GluN activity can induce local protein synthesis at developing synapses, its role in retinotectal LTP during development is unclear [55]. LTP might appear similar in development and adult plasticity, but are the locations, identities, and functions of newly synthesized proteins the same? Or, is there a developmental switch in dendritic mRNA targeting and translational capacity as recently discovered for axons? A greater understanding of local mRNA regulation in development is needed to specify the shared versus divergent mechanisms in plasticity and synapse formation.
Conclusions
Collectively, the studies reviewed share these aims: 1) to identify when mRNA transcripts are localized, 2) to unravel cue-induced signaling pathways, and 3) to identify the roles for specific locally synthesized proteins in development (Figure 2). With the onset of growth cone profiling, we now have a better understanding of the localized mRNAs that have key roles in axon guidance [3]. It will be interesting to use similar approaches to study axonal translation during presynaptic differentiation. mRNA profiling has also identified new dendritically localized mRNAs [21], and using this approach to study dendrites before and after synapse formation could advance our understanding of dendritic translation during development.
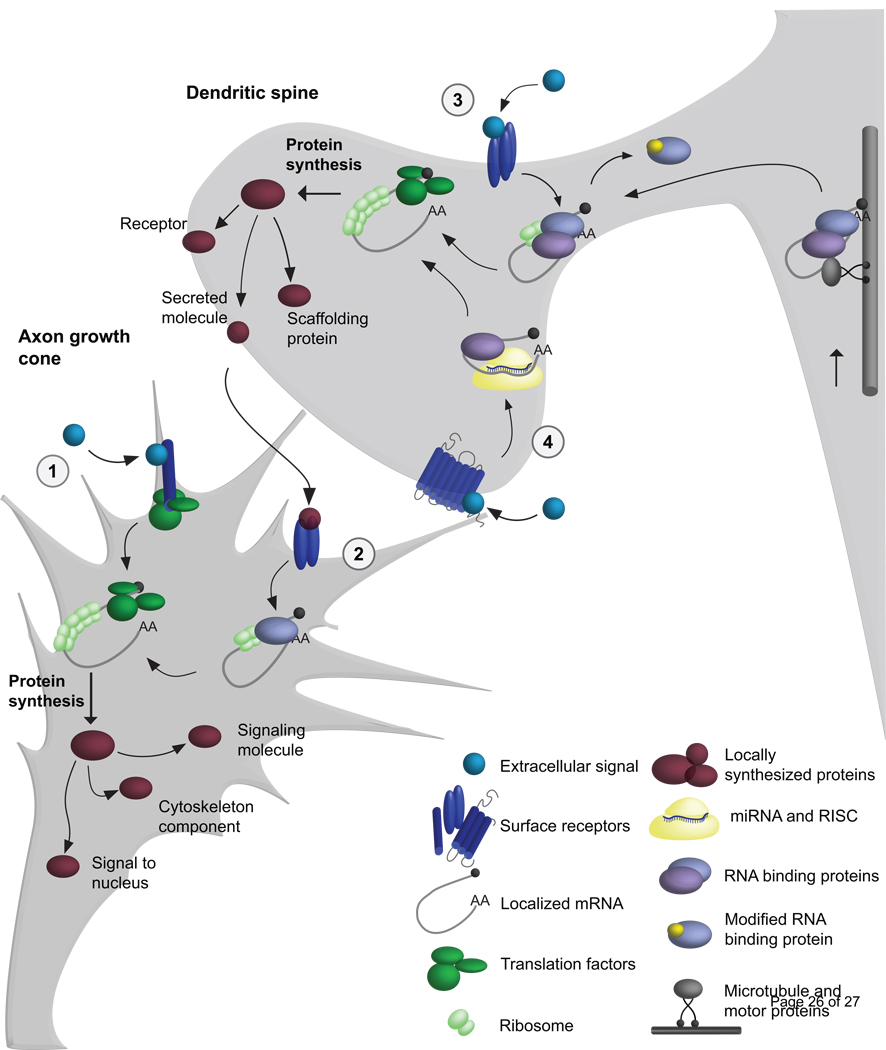
Figure 2. Local mRNA regulation in growth cone guidance and spine morphogenesis.
In this model, we illustrate mechanisms controlling local mRNA transport and translation in developing axons and dendrites. mRNA transport (at right): Microtubule motor proteins, adaptors, and RNA binding proteins mediate mRNA transport, while suppressing mRNA translation. 1) At the growth cone, cues signal through surface receptors to directly activate the translation machinery. 2) A secreted molecule from the post-synaptic cell can activate pre-synaptic translation by regulating mRNA binding proteins. 3) Localized mRNAs are regulated by multiple mechanisms, such as two different RNA binding proteins. Receptor-mediated signaling can lead to post-translational modification of RNA binding proteins and de-repression. 4) miRNAs and RISC suppress translation within dendrites, and post-synaptic receptor signaling can alleviate miRNA-mediated silencing and promote local mRNA translation. Locally synthesized proteins include several classes of molecules with local and distal functions.
While new insights are continually emerging, some fundamental questions remain unanswered: How are multiple post-transcriptional mechanisms integrated to regulate a single mRNA? How are mRNA decay, protein synthesis, and protein degradation balanced at synapses? Do locally synthesized proteins have a unique function compared to their distally synthesized and transported counterparts? Roles for local translation in global cellular processes, such as cell death, transcription, and paracrine signaling, speak toward the influence of local translation on neural circuits and systems. Determining how and when local mRNA regulation impacts these cellular functions will be central to understanding how miscues in local protein expression contribute to neurological disease states.
Acknowledgements
The authors would like to thank Christina Gross for helpful comments on the manuscript. Funding was provided by a NIH/NINDS F31NS063668 (SAS), and NIH (HD055835 and MH085617) and NARSAD Investigator Award (GJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang W, van Niekerk E, Willis DE, Twiss JL. RNA transport and localized protein synthesis in neurological disorders and neural repair. Dev Neurobiol. 2007;67:1166–1182. doi: 10.1002/dneu.20511. [DOI] [PubMed] [Google Scholar]
- 2.Jung H, Holt CE. Local translation of mRNAs in neural development. WIREs RNA. 2011;2:153–163. doi: 10.1002/wrna.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zivraj KH, Tung YC, Piper M, Gumy L, Fawcett JW, Yeo GS, Holt CE. Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs. J Neurosci. 2010;30:15464–15478. doi: 10.1523/JNEUROSCI.1800-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gumy LF, Yeo GS, Tung YC, Zivraj KH, Willis D, Coppola G, Lam BY, Twiss JL, Holt CE, Fawcett JW. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA. 2011;17:85–98. doi: 10.1261/rna.2386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor AM, Berchtold NC, Perreau VM, Tu CH, Li Jeon N, Cotman CW. Axonal mRNA in uninjured and regenerating cortical mammalian axons. J Neurosci. 2009;29:4697–4707. doi: 10.1523/JNEUROSCI.6130-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo S, van Niekerk EA, Merianda TT, Twiss JL. Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration. Exp Neurol. 2010;223:19–27. doi: 10.1016/j.expneurol.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassell GJ, Zhang H, Byrd AL, Femino AM, Singer RH, Taneja KL, Lifshitz LM, Herman IM, Kosik KS. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J Neurosci. 1998;18:251–265. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki Y, Welshhans K, Wen Z, Yao J, Xu M, Goshima Y, Zheng JQ, Bassell GJ. Phosphorylation of zipcode binding protein 1 is required for brain-derived neurotrophic factor signaling of local beta-actin synthesis and growth cone turning. J Neurosci. 2010;30:9349–9358. doi: 10.1523/JNEUROSCI.0499-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly CJ, Fainzilber M, Twiss JL. Subcellular communication through RNA transport and localized protein synthesis. Traffic. 2010;11:1498–1505. doi: 10.1111/j.1600-0854.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 2010;141:632–644. doi: 10.1016/j.cell.2010.04.008. The netrin-1 surface receptor DCC interacts with ribosome subunits, translation intiation factors, and monosomes, but DCC does not interact with polysomes. Netrin-1 binding to DCC dissociates the translational machinery components and promotes local protein synthesis.
- 11.Hengst U, Jaffrey SR. Function and translational regulation of mRNA in developing axons. Semin Cell Dev Biol. 2007;18:209–215. doi: 10.1016/j.semcdb.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hengst U, Deglincerti A, Kim HJ, Jeon NL, Jaffrey SR. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat Cell Biol. 2009;11:1024–1030. doi: 10.1038/ncb1916. The authors show that the polarity complex proteins Par3 and Par6 are required for NGF- and netrin-1-induced axon outgrowth. The local translation of Par3 mRNA, within axons, is required for NGF-induced axon outgrowth, but not basal axon growth.
- 13.Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol. 2008;10:149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fallini C, Zhang H, Su Y, Silani V, Singer RH, Rossoll W, Bassell GJ. The Survival of Motor Neuron (SMN) Protein Interacts with the mRNA-Binding Protein HuD and Regulates Localization of Poly(A) mRNA in Primary Motor Neuron Axons. The Journal of Neuroscience. 2011;31:3914–3925. doi: 10.1523/JNEUROSCI.3631-10.2011. SMN interacts with HuD in motor neuron axons and spinal cord tissue. SMN depletion reduces the axonal localization of HuD and poly(A) RNA granules, and a patient-derived missense mutation in SMN ablates the interaction between SMN and HuD.
- 15.Glinka M, Herrmann T, Funk N, Havlicek S, Rossoll W, Winkler C, Sendtner M. The heterogeneous nuclear ribonucleoprotein-R is necessary for axonal beta-actin mRNA translocation in spinal motor neurons. Hum Mol Genet. 2010;19:1951–1966. doi: 10.1093/hmg/ddq073. [DOI] [PubMed] [Google Scholar]
- 16.Ning K, Drepper C, Valori CF, Ahsan M, Wyles M, Higginbottom A, Herrmann T, Shaw P, Azzouz M, Sendtner M. PTEN depletion rescues axonal growth defect and improves survival in SMN-deficient motor neurons. Hum Mol Genet. 2010;19:3159–3168. doi: 10.1093/hmg/ddq226. [DOI] [PubMed] [Google Scholar]
- 17.Phillips LL, Pollack AE, Steward O. Protein synthesis in the neuropil of the rat dentate gyrus during synapse development. J Neurosci Res. 1990;26:474–482. doi: 10.1002/jnr.490260410. [DOI] [PubMed] [Google Scholar]
- 18.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianco A, Dienstbier M, Salter HK, Gatto G, Bullock SL. Bicaudal-D regulates fragile X mental retardation protein levels, motility, and function during neuronal morphogenesis. Curr Biol. 2010;20:1487–1492. doi: 10.1016/j.cub.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J Neurosci. 2006;26:13390–13399. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong J, Zhang T, Bloch LM. Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci. 2006;7:17. doi: 10.1186/1471-2202-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Bestman JE, Cline HT. The RNA binding protein CPEB regulates dendrite morphogenesis and neuronal circuit assembly in vivo. Proc Natl Acad Sci U S A. 2008;105:20494–20499. doi: 10.1073/pnas.0806296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bestman JE, Cline HT. The Relationship between Dendritic Branch Dynamics and CPEB-Labeled RNP Granules Captured in Vivo. Front Neural Circuits. 2009;3:10. doi: 10.3389/neuro.04.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alves-Sampaio A, Troca-Marin JA, Montesinos ML. NMDA-mediated regulation of DSCAM dendritic local translation is lost in a mouse model of Down's syndrome. J Neurosci. 2010;30:13537–13548. doi: 10.1523/JNEUROSCI.3457-10.2010. Dscam mRNA is bound by the RNA binding protein CPEB, and the dendritic localization of Dscam mRNA is regulated by CPE elements in its 3’ UTR. DSCAM is over-expressed in a mouse model of Down syndrome, and the NMDA-induced synthesis of DSCAM is absent in hippocampal neurons from this mouse model.
- 27.Saito Y, Oka A, Mizuguchi M, Motonaga K, Mori Y, Becker LE, Arima K, Miyauchi J, Takashima S. The developmental and aging changes of Down's syndrome cell adhesion molecule expression in normal and Down's syndrome brains. Acta Neuropathol. 2000;100:654–664. doi: 10.1007/s004010000230. [DOI] [PubMed] [Google Scholar]
- 28.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 29.Saba R, Schratt GM. MicroRNAs in neuronal development, function and dysfunction. Brain Res. 2010;1338:3–13. doi: 10.1016/j.brainres.2010.03.107. [DOI] [PubMed] [Google Scholar]
- 30. Muddashetty RS, Nalavadi VC, Gross C, Yao X, Xing L, Laur O, Warren ST, Bassell GJ. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation and mGluR signaling. Mol Cell. doi: 10.1016/j.molcel.2011.05.006. in press. This study reveals that FMRP phosphorylation promotes the formation of an AGO2-miR-125a inhibitory complex on PSD-95 mRNA, whereas mGlu signaling of translation requires FMRP de-phosphorylation and release of AGO2 from the mRNA. FMRP and miR-125a-mediated inhibition of PSD-95 mRNA translation constrains dendritic spine formation and branching.
- 31. Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, Khudayberdiev S, Leuschner PF, Busch CJ, Kane C, et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 2009;11:705–716. doi: 10.1038/ncb1876. The authors show that synaptic microRNA-138 limits spine growth through suppressing APT1. By suppressing APT1 synthesis, the level of activated (palmitoylated) G-protein subunit alpha is increased, which leads to increased activation of Rho signaling and thus inhibited spine growth. The loss of miR-138 function leads to enlarged spine, which is rescued by expression of the palmitoylated G-protein or APT1.
- 32.Cao Q, Padmanabhan K, Richter JD. Pumilio 2 controls translation by competing with eIF4E for 7-methyl guanosine cap recognition. RNA. 2010;16:221–227. doi: 10.1261/rna.1884610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vessey JP, Schoderboeck L, Gingl E, Luzi E, Riefler J, Di Leva F, Karra D, Thomas S, Kiebler MA, Macchi P. Mammalian Pumilio 2 regulates dendrite morphogenesis and synaptic function. Proc Natl Acad Sci U S A. 2010;107:3222–3227. doi: 10.1073/pnas.0907128107. The authors show that the depletion of the RNA binding protein Pumilio2 increases excitatroy synapses and the frequency of basal neurotransmission. Pumilio2 binds the mRNAs for a Scn1a and eIF4E, and was shown to regulate the translation of eIF4E mRNA.
- 34.Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell SW, Kim TK, Greenberg ME, Schratt G. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28:697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon IS, Cho SJ, Seog DH, Walikonis R. Neuronal activation increases the density of eukaryotic translation initiation factor 4E mRNA clusters in dendrites of cultured hippocampal neurons. Exp Mol Med. 2009;41:601–610. doi: 10.3858/emm.2009.41.8.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyles V, Zhao Y, Martin KC. Synapse formation and mRNA localization in cultured Aplysia neurons. Neuron. 2006;49:349–356. doi: 10.1016/j.neuron.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 37. Wang DO, Kim SM, Zhao Y, Hwang H, Miura SK, Sossin WS, Martin KC. Synapse- and stimulus-specific local translation during long-term neuronal plasticity. Science. 2009;324:1536–1540. doi: 10.1126/science.1173205. The authors show that presynaptic sensorin mRNA is translated specifically in response to long-term facilitation, but not long-term depression in Aplysia neurons. Sensorin mRNA is only translated at active synaptic contacts, and this local protein synthesis requires calcium signaling in the postsynaptic neuron.
- 38. Sebeo J, Hsiao K, Bozdagi O, Dumitriu D, Ge Y, Zhou Q, Benson DL. Requirement for protein synthesis at developing synapses. J Neurosci. 2009;29:9778–9793. doi: 10.1523/JNEUROSCI.2613-09.2009. The authors demonstrate that local protein synthesis is necessary for presynaptic activity and vesicle recycling in developing hippocampal neurons. When local protein synthesis is inhibited, small, developing synapses are eliminated, whereas large mature synapses remain stable. This effect is, at least in part, due to the developmental switch from CaMKII to PKA as a necessary regulator of vesicle recycling.
- 39.Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, et al. Distinct role of long 3' UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, Carruthers CJ, Sutton MA. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143–1158. doi: 10.1016/j.neuron.2010.11.034. The authors demonstrate that locally synthesized BDNF is necessary for homeostatic scaling of neurotransmission. After long-term GluA receptor blockade, BDNF is synthesized in the postsynaptic compartment, secreted, and acts on the presynaptic cell. The presynaptic changes require postsynaptic BDNF synthesis, whereas the postsynaptic changes do not.
- 42.Bureau I, Shepherd GM, Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J Neurosci. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatto CL, Broadie K. Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development. 2008;135:2637–2648. doi: 10.1242/dev.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–1557. doi: 10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatto CL, Broadie K. Temporal requirements of the fragile x mental retardation protein in modulating circadian clock circuit synaptic architecture. Front Neural Circuits. 2009;3:8. doi: 10.3389/neuro.04.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruz-Martin A, Crespo M, Portera-Cailliau C. Delayed stabilization of dendritic spines in fragile X mice. J Neurosci. 2010;30:7793–7803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:17768–17773. doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grossman AW, Aldridge GM, Lee KJ, Zeman MK, Jun CS, Azam HS, Arii T, Imoto K, Greenough WT, Rhyu IJ. Developmental characteristics of dendritic spines in the dentate gyrus of Fmr1 knockout mice. Brain Res. 2010;1355:221–227. doi: 10.1016/j.brainres.2010.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pfeiffer BE, Zang T, Wilkerson JR, Taniguchi M, Maksimova MA, Smith LN, Cowan CW, Huber KM. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron. 2010;66:191–197. doi: 10.1016/j.neuron.2010.03.017. Activity-induced synapse elimination is regulation by Mef2 transcription factors. This function for Mef2 is absent in hippocampal neurons from Fmr1 KO mice, and postsynaptic expression of wild type FMRP rescues Mef2-mediated synapse elimination, but expression of mutant FMRP that lacks mRNA binding does not.
- 51.Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scotto-Lomassese S, Nissant A, Mota T, Neant-Fery M, Oostra BA, Greer CA, Lledo PM, Trembleau A, Caille I. Fragile X Mental Retardation Protein Regulates New Neuron Differentiation in the Adult Olfactory Bulb. J Neurosci. 2011;31:2205–2215. doi: 10.1523/JNEUROSCI.5514-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Christie SB, Akins MR, Schwob JE, Fallon JR. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci. 2009;29:1514–1524. doi: 10.1523/JNEUROSCI.3937-08.2009. FMRP forms presynaptic granules along with FXR1P and FXR2P that are expressed in specific circuits during period of synaptogenesis. FXR2P is required for the expression of these axonal granules, and the loss of FMRP significantly reduces granule expression.
- 54.Gong LQ, He LJ, Dong ZY, Lu XH, Poo MM, Zhang XH. Postinduction Requirement of NMDA Receptor Activation for Late-Phase Long-Term Potentiation of Developing Retinotectal Synapses In Vivo. J Neurosci. 2011;31:3328–3335. doi: 10.1523/JNEUROSCI.5936-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]