Abstract
The main objective of the current study was to determine the sensitivity of the PET radioligand [11C]P943 to fenfluramine-induced changes in endogenous 5-HT in nonhuman primate brain. Fenfluramine-induced changes in 5-HT1B occupancy were compared to those obtained by self-block with unlabeled P943. Two baboons and 1 rhesus monkey were given preblocking or displacing doses of fenfluramine (1–5 mg/kg) or preblocking doses of unlabeled P943 (0.2 mg/kg) and imaged with [11C]P943 PET. Receptor occupancy by the low dose of fenfluramine (1 mg/kg) in the baboons was 25% and 29% and by the high dose of fenfluramine (5 mg/kg) in the rhesus macaque was 42%. Receptor occupancy by P943 (0.2 mg/kg) was 68% and 86% in the baboons. PET imaging of 5-HT1B receptors with [11C]P943 may be a useful approach for measuring changes in endogenous 5-HT in the living human brain.
Keywords: 5HT1B, serotonin, [11C]P943, nonhuman primate, PET
Introduction
The serotonergic system has been implicated in the neurochemical mechanisms underlying a variety of psychiatric disorders, especially depressive disorders. This is best highlighted by the widespread use of antidepressant drugs acting on the serotonin (5-HT) system and the association of a significant mood change with the procedure of tryptophan depletion which results in lowering of central serotonin levels (Delgado et al., 1991). Serotonin and the 5-HT1B receptor in particular are involved in a variety of physiological functions including food intake, sexual activity, and locomotion (Ruf and Bhagwagar, 2009; Sari, 2004) all of which may be disrupted in depressive disorders. Significant interest in the 5-HT1B receptor stems from the fact that these receptors occupy a key position at the nerve terminals and are responsible for synaptic 5-HT release and neuronal firing (Sari, 2004). Given this key location and function, the 5-HT1B receptor may be sensitive to endogenous levels of 5-HT as it provides a modulatory effect on the 5-HT system.
Using Positron Emission Tomography (PET), it is possible to measure the availability of 5-HT receptors and potentially changes in synaptic 5-HT levels (Paterson et al., 2010). Quantification of changes in endogenous 5-HT has been attempted using a number of 5-HT receptor related radiotracers including [18F]MPPF, [11C]WAY100635, [11C]DASB, and [11C]MDL100907, but results have been inconclusive (Huang et al., 2005; Paterson et al., 2010). A recent study using [11C]AZ10419369, a 5-HT1B ligand, showed promising results in sensitivity to changes in endogenous 5-HT (Finnema et al., 2010). [11C]P943 has recently been developed and applied to quantify 5-HT1B receptor availability in nonhuman primates (Nabulsi et al., 2010) and human subjects (Gallezot et al., 2010), and a reduction in ventral striatal 5-HT1B availability was recently demonstrated in subjects with major depressive disorder (Murrough et al., 2010). Our objective in the current study was to determine the sensitivity of [11C]P943 to fenfluramine-induced changes in endogenous 5-HT in nonhuman primate brain.
Materials and methods
Two female baboons (B1, B2, papio anubis, 9.8 and 10.4 kg) and one male rhesus monkey (M1, Macaca mulatta, 6.0 kg) served as subjects. The housing facility is fully accredited by the American Association for the Accreditation of Laboratory Animals (AAALAC) and all experiments were conducted in accordance with the Institutional Animal Care and Use Committee guidelines.
For the PET scans, fasted animals were initially anesthetized with ketamine (6–10 mg/kg, intramuscularly), and then maintained on 1.5–2.5% isoflurane. A 2 hr time period is allowed between ketamine and injection of the radiotracer. Vital signs including respiration rate, blood pressure, heart rate, and temperature were monitored continuously and recorded every 15 min and temperature was kept constant at 37 C with heated water blankets. An intravenous (IV) line was established in each leg, one for injection of fluids for hydration and injection of the radiotracer and the second for blood sampling. At equilibrium, the arterial:venous differences are expected to be small, so venous sampling was assumed to be appropriate (Carson, 2000). [11C]P943 was synthesized as previously described (Gallezot et al., 2010; Nabulsi et al., 2010) and administered as a bolus plus constant infusion, with a Kbol value (ratio of bolus dose to infusion rate) of 150 min. A study duration of 120 min for baboons and 150 min for rhesus macaque was used. Equilibrium (<5% change/hr) was established by 60 min for all regions. A total of 9 PET scans were performed in the 3 animals.
Each baboon was studied 4 times with [11C]P943 on 2 experimental days. On each day, a baseline measurement was followed by a preblock measurement with fenfluramine (1.0 mg/kg, IV) or unlabeled P943 (0.2 mg/kg, IV) given 5 min prior to the radiotracer injection. This resulted in 8 hrs total of anesthesia time, i.e., 2 hr set up, 2 hr baseline scan, 2 hr break, and 2 hr preblock scan. The rhesus monkey was studied once with [11C]P943 during which a displacement of a higher dose of fenfluramine (5 mg/kg, IV) was given over 5 min starting at 75 min after radiotracer injection. This resulted in 4.5 hrs total of anesthesia, i.e., 2 hr set up, and a 2.5 hr scan. In the current study, experiments in baboons were conducted with a low dose of fenfluramine (1 mg/kg) and subsequently a higher dose of fenfluramine (5 mg/kg) was used in an attempt to replicate a recent study in rhesus monkeys (Finnema et al., 2010) (Table 1). (±)-fenfluramine was dissolved in saline and the doses are expressed as relative to the free base.
Table 1.
Experimental Design
| Animal | Drug | Dose (mg/kg) | # scans/dy | Time of Drug |
|---|---|---|---|---|
| B1 | Fen | 1.0 | 2 | Preblock |
| B1 | P943 | 0.2 | 2 | Preblock |
| B2 | Fen | 1.0 | 2 | Preblock |
| B2 | P943 | 0.2 | 2 | Preblock |
| M1 | Fen | 5.0 | 1 | Displace |
There was a minimum of 14 days was allowed between experimental days. The baboon PET scans were performed on the HRRT PET camera (Siemens/CTI, Knoxville, TN, USA), which produces 207 slices with a slice separation of 1.2 mm and has a reconstructed image resolution of approximately 3 mm. The rhesus PET scan was performed on the FOCUS 220, a small animal PET scanner with a high resolution of ~1.5 mm at the center field of view. A transmission scan was acquired prior to radiotracer injection for attenuation correction. Dynamic images of radioactivity concentration were reconstructed with corrections for measured attenuation, normalization, random events, scatter, and deadtime. A magnetic resonance image (MRI) of each animal was previously obtained to perform coregistration and guide the placement of regions of interest (ROIs). The ROIs were drawn manually on each animal’s MR images and included occipital cortex, frontal cortex, cingulate cortex, globus pallidus, caudate, putamen, thalamus, insula, brainstem, and cerebellum (without the vermis).
The initial primary outcome measure examined was the equilibrium volume of distribution (VT), where VT is regional brain activity divided by metabolite-corrected plasma concentration. Parent fraction in plasma was determined by HPLC as described previously (Gallezot et al., 2010). Occupancy plots (Cunningham et al., 2009; Esterlis et al., 2010) were constructed to determine the fraction of available 5-HT1B receptor sites blocked by fenfluramine or unlabeled P943. Occupancy plots use the equation for the line [y=rx+b], where y= [VT (baseline) − VT (preblock or displacement)], and x= VT (baseline), and linear regression of the fit to the brain regions provides receptor occupancy (r) values. This approach assumes uniform receptor occupancy across all brain regions, but does not require the use of a reference region devoid of specific binding. Receptor occupancy was derived for each animal across all brain regions for each fenfluramine or unlabeled P943 preblocking (baboon) or fenfluramine displacement (rhesus macaque) scan compared to the baseline scan. In the baseline and fenfluramine and unlabeled P943 preblocking scans, data obtained at equilibrium (60–90 min) were used. In the fenfluramine displacement study, data from 60–75 min were used for baseline and values from 115–135 min were used for displacement.
Results
For the 9 PET scans, the mean (±SD) injected dose of [11C]P943 was 249±41 MBq, the mean specific radioactivity was 190.92±75.85 MBq/nmol at time of injection, and the mean injected mass was 0.105±0.046 μg/kg. Under baseline conditions, [11C]P943 uptake was high in globus pallidus, occipital cortex, thalamus and midbrain, intermediate in striatum, cingulate cortex, frontal cortex and brainstem, and low in the cerebellum.
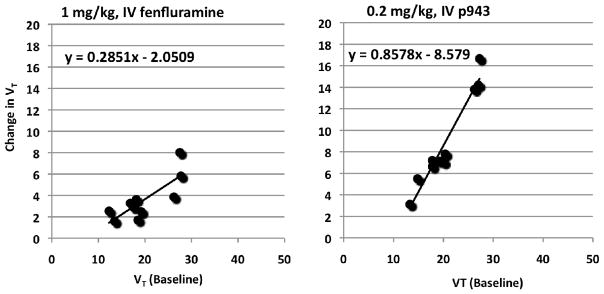
Although the cerebellum has been proposed as an appropriate reference region for 5-HT1B binding, there was a change in cerebellar [11C]P943 uptake as measured with VT between the baseline scans and fenfluramine (5% for B1; 21% for B2) or P943 (21% for B1; 23% for B2) preblock scans. Further, in the rhesus infusion study, a fenfluramine-induced displacement of [11C]P943 in the cerebellum (Figure 1) was detected and VT was reduced, with a change of 29%. Hence we calculated receptor occupancy using the occupancy plots. Receptor occupancy by the low dose of fenfluramine (1 mg/kg) in the baboons was 25% and 29% and by the high dose of fenfluramine (5 mg/kg) in the rhesus macaque was 42% (Table 1, VFigure 2). Receptor occupancy by P943 (0.2 mg/kg) was 68% and 86% in the baboons, respectively (Table 2). The nondisplaceable volume of distribution (ND) can be estimated by the intercept of the occupancy plot. In the 5 studies (3 with fenfluramine and 2 with unlabeled P943), VND was 9±1 mL/cm3.
Figure 1.
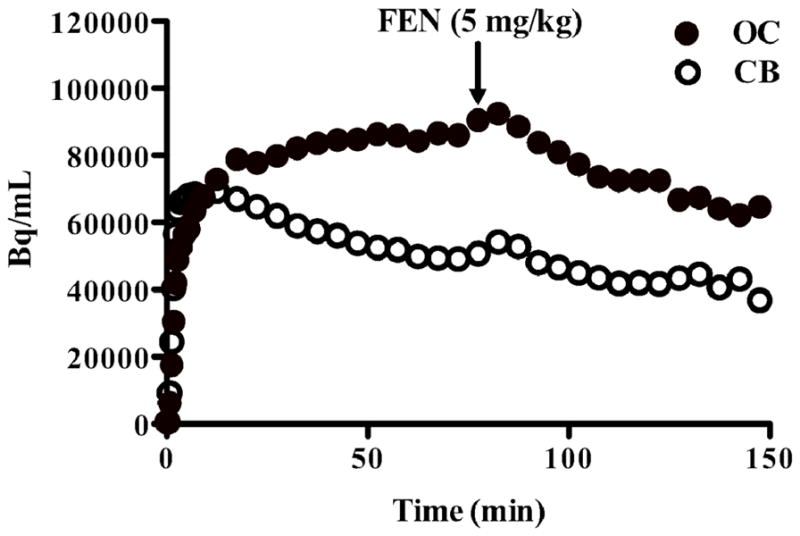
Radioactivity (Bq/mL) in the occipital cortex (closed symbols) and cerebellum (open symbols) during an infusion of [11C]P943 in rhesus monkey with a displacement of fenfluramine (5 mg/kg) given at 75 min. The “bump” in both curves following FEN is attributed to a transient increase in bioavailability of [11C]P943 due to blockade throughout the body.
Figure 2.
Occupancy plots are shown for baboon B2 (left panel) for change in VT between baseline and fenfluramine (1 mg/kg) scans and for baboon B2 (right panel) for change in VT between baseline and P943 (0.2 mg/kg), both as a function of baseline VT. Each data point represents a region-of-interest. The slope of these plots represents the 5-HT1B receptor occupancy.
Table 2.
Percent occupancy for each animal between baseline and drug conditions
| Animal | Fenfluramine (1–5 mg/kg) | P943 (0.2 mg/kg) |
|---|---|---|
| B1 | 25% | 68% |
| B2 | 29% | 86% |
| M1 | 42% | - |
Discussion
This study demonstrated significant occupancy of 5HT1B receptors as measured with [11C]P943 after administration of 1 and 5 mg/kg fenfluramine. Fenfluramine causes an increase in endogenous 5-HT levels by directly increasing 5-HT release and by inhibiting reuptake of 5-HT at the 5-HT transporter (Rothman and Baumann, 2002) and has been successfully used in previous studies to produce 5-HT-induced displacement of 5-HT1B (Finnema et al., 2010) and 5-HT1A (Hume et al., 2001; Zimmer et al., 2002) radioligands. A previous dual PET and microdialysis study in awake rhesus monkeys demonstrated 20-fold increases in 5-HT levels in the prefrontal cortex 15 min after administration of 5 mg/kg fenfluramine (Udo de Haes et al., 2006) illustrating that these doses of fenfluramine are sufficient to produce large increases in endogenous 5-HT. Finnema and colleagues (2010) demonstrated average regional decreases of 27% and 50% in [11C]AZ10419369 binding, a 5-HT1B radioligand, after 1 mg/kg and 5 mg/kg fenfluramine, respectively in anesthetized cynomolgus monkeys. This is highly consistent with our findings of 25–29% occupancy after 1 mg/kg and 42% occupancy after 5 mg/kg administration of fenfluramine.
Taken together, the most straightforward interpretation of these findings is that changes in 5-HT1B ligand binding are due to fenfluramine-induced increases in endogenous 5-HT, consistent with the competition model (Laruelle, 2000). Alternatively, preclinical evidence suggests that 5-HT1B receptors undergo rapid internalization upon exposure to 5-HT (Janoshazi et al., 2007), and if the radiotracer has a reduced affinity for internalized receptors as has been shown for dopamine D2 receptors (Guo et al., 2010), this would result in reduced 5-HT1B availability that is not reflective of 5-HT occupancy of the receptor. The extent to which receptor internalization contributes to the measurement of changes in synaptic 5-HT will have to be further examined.
A limitation of this radioligand is that there was a fenfluramine- and P943-induced change in cerebellar 5-HT1B receptor binding. Changes in cerebellar binding were not reported in the previous study using a similar 5-HT1B compound (Finnema et al., 2010) and were not examined in a previous study using the same compound (Nabulsi et al., 2010) since metabolite-corrected input function data were not available. It is generally accepted that the cerebellum is a good reference region for 5-HT1B radioligands as it is thought to be devoid of 5-HT1B receptors. Specifically, in a human autoradiography study no 5HT1B receptors were detected in the cerebellum (Bonaventure et al., 1997); however, rodent studies have reported moderate 5-HT1B receptor-like immunoreactivity in the deep nuclei of the cerebellum (Sari et al., 1999), and 5-HT1B receptor mRNA has been identified in the Purkinje cell layer of the cerebellum (Bruinvels et al., 1994; Maroteaux et al., 1992). Thus, in this study, we used the occupancy plot, which uses VT values, instead of binding potential values, which depend on the use of reference regions. The potential changes in cerebellar binding with this compound will need to be examined in human subjects using occupancy studies before fluctuations in endogenous 5-HT can be reliably measured in humans with reference region methods.
The occupancy plot used in this study has two major assumptions. First, it is assumed that the nondisplaceable volume of distribution (VND) is uniform across brain regions, an assumption commonly applied in receptor imaging studies. Second, the occupancy plot assumes that the fractional receptor occupancy is uniform across brain regions. This latter assumption is reasonable when using an exogenous drug, where brain concentration is assumed to be driven by plasma concentration. However, in this study, it is not at all clear that fenfluramine will produce equal concentrations of 5HT across brain regions. A previous microdialysis study suggests differential fenfluramine-induced 5-HT release between brain regions (Hume et al., 2001). Thus, potential variation in 5-HT level across brain regions may be a contributing factor to the variability found in the occupancy plots (Figure 1), where each data point represents a different brain region.
In general, the two 5-HT1B receptor ligands, [11C]P943 and [11C]AZ10419369, demonstrate greater sensitivity to changes in synaptic 5-HT levels than ligands selective for the 5-HT1A (Jagoda et al., 2006; Maeda et al., 2001; Udo de Haes et al., 2002) or 5-HT2A (Meyer et al., 1999; Staley et al., 2001) receptors (and see (Paterson et al., 2010) for review). Reasons for the differences are not clear but may be related to differences in receptor subtype affinities, distribution, localization, or clearance rates of the ligands from the tissue to plasma. Also of note, the studies using 5HT1B ligands to date have used anesthetized nonhuman primates, so the results will have to be replicated in awake animals or human subjects. It may be hypothesized that if anesthesia is dampening neuronal circuits, a larger response may be evoked in conscious human or nonhuman primates. In summary, PET imaging of 5-HT1B receptors with [11C]P943 may be a useful approach for measuring changes in endogenous 5-HT in the living human brain.
Acknowledgments
We would like to acknowledge the late Dr. Julie Staley for her advice on study design and data collection.
Funding: This research was supported in part by National Institute of Health grant KO1 DA20651 (Cosgrove), and NARSAD (Cosgrove). This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, or the National Institutes of Health.
References
- Bonaventure P, Schotte A, Cras P, Leysen JE. Autoradiographic mapping of 5-HT1B- and 5-HT1D receptors in human brain using [3H]alniditan, a new radioligand. Receptors Channels. 1997;5(3–4):225–230. [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33(3–4):367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Carson RE. PET physiological measurements using constant infusion. Nucl Med Biol. 2000;27(7):657–660. doi: 10.1016/s0969-8051(00)00138-4. [DOI] [PubMed] [Google Scholar]
- Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab. 2009;30(1):46–50. doi: 10.1038/jcbfm.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado PL, Price LH, Miller HL, Salomon RM, Licinio J, Krystal JH, Heninger GR, Charney DS. Rapid serotonin depletion as a provocative challenge test for patients with major depression: relevance to antidepressant action and the neurobiology of depression. Psychopharmacol Bull. 1991;27(3):321–330. [PubMed] [Google Scholar]
- Esterlis I, Cosgrove KP, Batis JC, Bois F, Stiklus SM, Perkins E, Seibyl JP, Carson RE, Staley JK. Quantification of Smoking-Induced Occupancy of {beta}2-Nicotinic Acetylcholine Receptors: Estimation of Nondisplaceable Binding. J Nucl Med. 2010 doi: 10.2967/jnumed.109.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnema SJ, Varrone A, Hwang TJ, Gulyas B, Pierson ME, Halldin C, Farde L. Fenfluramine-induced serotonin release decreases [11C]AZ10419369 binding to 5-HT1B-receptors in the primate brain. Synapse. 2010;64(7):573–577. doi: 10.1002/syn.20780. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Nabulsi N, Neumeister A, Planeta-Wilson B, Williams WA, Singhal T, Kim S, Maguire RP, McCarthy T, Frost JJ, Huang Y, Ding YS, Carson RE. Kinetic modeling of the serotonin 5-HT(1B) receptor radioligand [(11)C]P943 in humans. J Cereb Blood Flow Metab. 2010;30(1):196–210. doi: 10.1038/jcbfm.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Guo W, Kralikova M, Jiang M, Schieren I, Narendran R, Slifstein M, Abi-Dargham A, Laruelle M, Javitch JA, Rayport S. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology. 2010;35(3):806–817. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Bae SA, Roth BL, Laruelle M. Synthesis of potent and selective serotonin 5-HT1B receptor ligands. Bioorg Med Chem Lett. 2005;15(21):4786–4789. doi: 10.1016/j.bmcl.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Hume S, Hirani E, Opacka-Juffry J, Myers R, Townsend C, Pike V, Grasby P. Effect of 5-HT on binding of [(11)C] WAY 100635 to 5-HT(IA) receptors in rat brain, assessed using in vivo microdialysis nd PET after fenfluramine. Synapse. 2001;41(2):150–159. doi: 10.1002/syn.1069. [DOI] [PubMed] [Google Scholar]
- Jagoda EM, Lang L, Tokugawa J, Simmons A, Ma Y, Contoreggi C, Kiesewetter D, Eckelman WC. Development of 5-HT1A receptor radioligands to determine receptor density and changes in endogenous 5-HT. Synapse. 2006;59(6):330–341. doi: 10.1002/syn.20246. [DOI] [PubMed] [Google Scholar]
- Janoshazi A, Deraet M, Callebert J, Setola V, Guenther S, Saubamea B, Manivet P, Launay JM, Maroteaux L. Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors. Mol Pharmacol. 2007;71(6):1463–1474. doi: 10.1124/mol.106.032656. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20(3):423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Ogawa M, Okauchi T, Kawabe K, Zhang MR, Semba J, Suzuki K. In vivo binding properties of [carbonyl-11C]WAY-100635: effect of endogenous serotonin. Synapse. 2001;40(2):122–129. doi: 10.1002/syn.1033. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci U S A. 1992;89(7):3020–3024. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Cho R, Kennedy S, Kapur S. The effects of single dose nefazodone and paroxetine upon 5-HT2A binding potential in humans using [18F]-setoperone PET. Psychopharmacology (Berl) 1999;144(3):279–281. doi: 10.1007/s002130051004. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, Neumeister A. Reduced ventral striatal/ventral pallidal serotonin(1B) receptor binding potential in major depressive disorder. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabulsi N, Huang Y, Weinzimmer D, Ropchan J, Frost JJ, McCarthy T, Carson RE, Ding YS. High-resolution imaging of brain 5-HT 1B receptors in the rhesus monkey using [11C]P943. Nucl Med Biol. 2010;37(2):205–214. doi: 10.1016/j.nucmedbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson LM, Tyacke RJ, Nutt DJ, Knudsen GM. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J Cereb Blood Flow Metab. 2010;30(10):1682–1706. doi: 10.1038/jcbfm.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacol Biochem Behav. 2002;71(4):825–836. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- Ruf BM, Bhagwagar Z. The 5-HT1B receptor: a novel target for the pathophysiology of depression. Curr Drug Targets. 2009;10(11):1118–1138. doi: 10.2174/138945009789735192. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28(6):565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Verge D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88(3):899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Staley JK, Van Dyck CH, Tan PZ, Al Tikriti M, Ramsby Q, Klump H, Ng C, Garg P, Soufer R, Baldwin RM, Innis RB. Comparison of [(18)F]altanserin and [(18)F]deuteroaltanserin for PET imaging of serotonin(2A) receptors in baboon brain: pharmacological studies. Nucl Med Biol. 2001;28(3):271–279. doi: 10.1016/s0969-8051(00)00212-2. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Bosker FJ, Van Waarde A, Pruim J, Willemsen AT, Vaalburg W, Den Boer JA. 5-HT(1A) receptor imaging in the human brain: effect of tryptophan depletion and infusion on [(18)F]MPPF binding. Synapse. 2002;46(2):108–115. doi: 10.1002/syn.10134. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Harada N, Elsinga PH, Maguire RP, Tsukada H. Effect of fenfluramine-induced increases in serotonin release on [18F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse. 2006;59(1):18–26. doi: 10.1002/syn.20209. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Mauger G, Le Bars D, Bonmarchand G, Luxen A, Pujol JF. Effect of endogenous serotonin on the binding of the 5-hT1A PET ligand 18F-MPPF in the rat hippocampus: kinetic beta measurements combined with microdialysis. J Neurochem. 2002;80(2):278–286. doi: 10.1046/j.0022-3042.2001.00696.x. [DOI] [PubMed] [Google Scholar]