Abstract
Although many studies assert that the serotonin (5-HT) transporter (SERT) is the predominant mechanism controlling extracellular 5-HT concentrations, accumulating evidence suggests that low affinity, high capacity transport mechanisms may contribute more to 5-HT clearance than previously thought. The goal of this study was to quantify the contributions of SERT relative to other mechanisms in clearing extracellular 5-HT concentrations ranging from 50 nM to 1 μM in synaptosomes prepared from wild-type and SERT knockout mice using rotating disk electrode voltammetry. SERT inhibitors combined with decynium-22 (D-22), a blocker of several low affinity transporters, blocked all uptake of 5-HT into synaptosomes. We found that SERT is responsible for the majority of synaptosomal uptake only at relatively low 5-HT concentrations, but comprises a diminishing proportion of 5-HT clearance when extracellular 5-HT increases above 100 nM. The effect of D-22 was similar in wildtype and SERT knockout synaptosomes. Thus, there was no evidence of upregulation of low-affinity mechanisms in knockout mice across the concentrations of 5-HT tested. These are surprising results, in light of the prevailing view that SERT is the primary uptake mechanism for extracellular 5-HT at physiological concentrations. We conclude that non-SERT mediated 5-HT uptake is substantial even at modest 5-HT concentrations. These findings, in conjunction with other studies, have important implications for understanding serotonergic disorders and may explain the variable efficacy and stability of patients’ responses to antidepressants, such as the selective serotonin reuptake inhibitors.
Keywords: serotonin, transporter, SERT, uptake, knockout, voltammetry, D-22, decynium-22
INTRODUCTION
Many studies suggest that serotonin (5-HT) uptake from extracellular fluid into 5-HT terminals occurs via high affinity uptake mediated primarily by the serotonin transporter (SERT)(Blackburn et al., 1967; Blakely et al., 1991; Daws, 2009). However, recent studies highlight the roles of other proteins that possess lower affinity but have a high capacity for 5-HT uptake, including the extraneuronal monoamine transporter (EMT), the plasmalemmal monoamine transporter (PMAT) and the organic cation transporters—OCT1, OCT2, and OCT3 (Baganz et al., 2008; Feng et al., 2005; Gasser et al., 2006; Schmitt et al., 2003). These findings raise the question: what proportion of 5-HT uptake is mediated by SERT at low vs. high physiological concentrations of 5-HT? This is a crucial question given the high rate of non-response to selective serotonin reuptake inhibitor (SSRI) antidepressants for clinical depression (Warden et al., 2007). These other mechanisms of brain 5-HT clearance may contribute more than previously thought to the pathogenesis and treatment efficacy in mood disorders (Daws, 2009).
In this report we used rotating disk electrode voltammetry (RDEV) to delineate the relative proportions of clearance of high and low affinity 5-HT uptake mechanisms in whole brain synaptosomes. RDEV represents a newly validated experimental approach with superior kinetic resolution and detection limits compared with other methods of measuring 5-HT uptake (Hagan et al., 2010). The goal of these studies was to use paroxetine (a SERT blocker) and 1’-diethyl-2,2’-cyanine iodide (decynium-22; D-22), a blocker of low affinity, high capacity transporters) with whole brain synaptosomes to explore the conditions under which SERT does and does not comprise the main mechanism of uptake in clearing extracellular 5-HT concentrations within the estimated in vivo physiological range (Bunin and Wightman, 1999). These aspects of 5-HT clearance have not previously been characterized.
MATERIALS AND METHODS
Animals
The SERT knockout mice used were originally created in the laboratory of Dr. Dennis Murphy at NIMH (Bengel et al., 1998). SERT knockout mice obtained from Taconic Farms (Hudson, NY) were backcrossed to C57Bl6/J mice to generate SERT+/- mice, which were used to generate SERT wildtype and knockout mice. Male and female mice ranging in size from 25 – 30 grams were used in experiments. Mice were group housed and fed ad libitum in a temperature-controlled vivarium with a 12:12 light cycle (lights on 0600). Efforts were made to reduce the number of animals used and to minimize animal stress and discomfort during experimental procedures. Experiments were performed exactly as approved by the University of Washington Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Solutions and Chemicals
Solutions were made with water purified by a Millipore (Billerica, MA) Milli-Q ultrapurification system. The KCl and MgSO4 were purchased from Fisher Scientific (Pittsburg, PA). The NaCl, KH2PO4, NaHCO3, CaCl2, were purchased from JT Baker Chemical Co. (Philipsburg, NJ). The sucrose, glucose, HEPES, serotonin hydrochloride, paroxetine hydrochloride, GBR 12935, citalopram hydrobromide, D-22, and nisoxetine hydrochloride were purchased from Sigma (St. Louis, MO). Drugs were diluted into physiological buffer, except 5-HT and D-22. 5-HT was diluted in pH 7.2 phosphate buffered saline (PBS) to improve its stability (Huang and Kissinger, 1996). D-22 was solubilized in a 1% 1:1 dimethylformamide and methanol solution. All experiments were controlled for the effects of vehicle. For example, in the D-22 and paroxetine experiments, the vehicles for both drugs were present in all studies. The sucrose buffer contained 300 mM sucrose and 10 mM HEPES (pH 7.4). The physiological buffer contained 124 mM NaCl, 1.8 mM KCl, 1.3 mM MgSO4, 1.24 mM KH2PO4, 2.5 mM CaCl2, 26 mM NaHCO3, and 10 mM glucose (saturated with 95% O2, 5% CO2 for at least 10 minutes before use, pH 7.4). All experiments were performed in the presence of 100 nM nisoxetine and 1 μM GBR 12935 to minimize 5-HT uptake through NET and DAT, respectively.
Rotating Disk Electrode Voltammetry
RDEV and synaptosome preparations were carried out as previously described (Hagan et al., 2010). Briefly, a Pine Instruments Inc. (Grove City, PA) AFMD03 glassy carbon electrode was connected to a Pine Instruments MSRX high-precision rotator and rotated at 3000 RPM in synaptosomes in a custom-made cylindrical glass chamber maintained at 37 °C by a Polyscience (Niles, IL) Series 8000 water recirculator. A constant potential of 550 mV relative to the Ag/AgCl reference electrode was applied with a Bioanalytical Systems LC-4B amperometric detector (West Lafayette, IN) with a laboratory-modified time constant (20 ms).
In all studies, drugs or vehicle and serotonin were each added in volumes of 10 μL to 480 μL of synaptosomes or buffer for a total volume of 500 μL. A constant gentle stream of 95% O2, 5% CO2 gas was directed across the top of the chamber to maintain the buffer’s oxygen saturation and pH. Drugs or vehicle (10 μl) were added following application of the potential and allowed to incubate with the tissue for 15 minutes while baseline was achieved (data sampled continuously throughout with 1000 Hz sample rate). Serotonin (10 μl) was added to the glass chamber using a Hamilton (Reno, NV) CR-700-20 constant rate syringe. All experiments were carried out with synaptosomes within four hours from the time of their suspension in buffer. Previous work has determined that tissue viability is stable and allows for consistent measurements for up to four hours (Hagan et al., 2010).
Detection currents were recorded digitally on a PC computer with an ITC-18 analog-digital converter and Ecell software from HEKA (formerly InstruTECH, Bellmore, New York). Data was acquired at a 1000 Hz sample rate with a 60 Hz digital notch filter. The data was converted to a waveform and background corrected for analysis using Igor Pro software from WaveMetrics (Portland, OR). Statistical analyses were performed with Graphpad Prism 5 (San Diego, CA).
Data Analysis
Initial velocities of 5-HT uptake were calculated from the linear slope of a tangent line of the steepest portion of a plot of [5-HT] versus time as described previously (Hagan et al., 2010). Electrode drift was subtracted and data were normalized to protein concentration, which was quantified with a bicinchoninic acid (BCA) colorimetric based assay from Pierce (Rockford, IL). Effects of paroxetine on uptake over a range of 5-HT concentrations in SERT +/+ and -/- synaptosomes were analyzed with repeated measures two-way ANOVA. A t-test was used to determine whether D-22 altered electrode response by comparing the peak currents in the presence of drug or vehicle after adding 5-HT (100 nM or 1 μM) to synaptosomes. Studies of the effects of high (up to 100 μM) concentrations of SERT inhibitors on 5-HT clearance were analyzed by one-way ANOVA and a Dunnett’s post hoc test.
Unpaired t-tests were used to compare between paroxetine-treated wildtypes and SERT knockout velocity rates to determine if non-SERT mechanisms were enhanced in SERT knockouts at each of the 5-HT concentrations tested. Neither a Michaelis–Menten curve nor a biphasic non-linear curve could be fitted to the data sets across all of the 5-HT concentrations tested. Michaelis–Menten curves were fitted from 0 – 300 nM 5-HT, the region of the data that reflects saturable processes. The Km and Vmax for SERT knockout synaptosomes and wildtype synaptosomes treated with 1 μM paroxetine were determined using curve-fitting procedures to fit velocity versus 5-HT concentration to the Michaelis–Menten equation:
| (1) |
as described by Motulsky and Christopoulos(Motulsky and Christopoulos, 2003) where v is the initial uptake velocity, Vmax the maximal uptake velocity, Km the Michaelis–Menten constant, and [5-HT] is the initial extrasynaptosomal concentration of exogenously added 5-HT. The kinetic constants were compared using the extra sum-of-squares F test. The errors reported are ± standard errors of regression (SER). A line was fitted for the data from 300 nM – 1 μM, the region of the data that reflects non-saturable clearance. The reported concentrations are initial instantaneous concentrations following addition of 5-HT to the chamber before uptake begins. Data are reported as the average ± SEM. Kinetic constants Km and Vmax are reported as the average ± SER.
In experiments comparing effects of paroxetine and D-22, Data were analyzed by a one-way ANOVA with post hoc Newman-Keuls multiple comparisons tests comparing each pair of treatments. The significance level was set at p<0.05.
Preparation of Synaptosomes
Unanesthetized mice were decapitated and brains were rapidly dissected and homogenized in 10 volumes (w/v) of ice-cold sucrose buffer with a Potter Elvehjem homogenizer rotated at 540 RPM by a 10” Ryobi drill press. The whole-brain homogenate was centrifuged (1000 × g for 10 min at 4 °C) in an Eppendorf 5804R (Westbury, NY) to pellet and remove cellular debris (P1). The resulting supernatants were centrifuged (15,900 × g for 20 min at 4 °C) to obtain the crude synaptosomal pellet (P2). P2 was rinsed twice by resuspending in 15 mL physiological buffer pre-oxygenated with 95% O2, 5% CO2 gas, and recentrifuging (15,900 × g for 5 min at 4 °C) to remove any remaining sucrose. The rinsed P2 was resuspended in 5 mL oxygenated physiological buffer and maintained with 95% O2, 5% CO2 gas in a 50 mL polypropylene conical tube on ice. The preparation was gently vortexed between assays before aliquoting tissue into the electrochemical chamber. Between assays, the glass chamber was gently wiped with a cotton-tipped applicator and rinsed with 70% ethanol followed by several (7-10) water rinses. The electrode was gently rinsed with 70% ethanol and water and rotated while in contact with a damp brown velvet electrode polishing pad from a Bioanalytical Systems electrode polishing kit (West Lafayette, IN). Each whole brain synaptosomal preparation was resuspended in 5 mL of buffer and each experiment involved a single addition of 5-HT to 500 μL mL of synaptosomes (about 3 mg/mL protein) as previously described (Hagan et al., 2010). This allowed 8 – 10 assays per animal, providing sufficient samples to allow repeated measures analysis, where indicated and described above.
Synaptosomal 5-HT Uptake Studies
The effects of SSRIs on SERT wildtype and knockout synaptosomes was measured using 1 μM paroxetine and several different added concentrations of 5-HT (nM): 50, 100, 200, 300, 500, and 1000. Because adding similar concentrations of substrate and inhibitor may result in competitive interactions between 5-HT and SSRIs (Cheng and Prusoff, 1973), we also tested increasing concentrations of paroxetine and citalopram, alone and in combination.
D-22 in the presence of NET and DAT blockers (described above) was used to define non-SERT uptake. D-22 is a pseudoisocyanine compound that inhibits EMT (Inazu et al., 2003; Russ et al., 1993; Russ et al., 1996), the OCTs (Baganz et al., 2008; Feng et al., 2005; Gasser et al., 2006; Shang et al., 2003); and PMAT (Engel and Wang, 2005; Xia et al., 2009). The IC50 for D-22 at EMT is ~15 nM (Russ et al., 1993), the Ki at OCT3 is ~4nM (Shang et al., 2003), and the Ki at PMAT is ~0.1 μM (Engel and Wang, 2005). D-22 can also inhibit NET (Russ et al., 1993) and SERT in an intestinal epithelial cell line, albeit at very low affinity (IC50 ~10 μM) (Martel et al., 2003). D-22 (10 μM) did not interfere with the magnitude of the 5-HT signal (e.g. by electrode “fouling”) as it does in other electrochemical methods (Baganz et al., 2008). The peak current after adding 5-HT to tissue in the presence or absence of D-22 did not differ significantly at either 100 nM (control, 23 ± 1 nA vs. D-22, 23 ± 1 nA; n = 6) or 1 μM 5-HT (control, 213 ± 3 nA vs. D-22, 223 ± 4 nA; n = 6). Due to the lack of D-22’s specificity, the current study did not identify the specific transporters involved.
To determine if multiple, additive transport mechanisms were operational in taking up 5-HT at 100 nM, clearance was measured in SERT wildtype synaptosomes in the presence of vehicle, 1 μM paroxetine, 10 μM D-22, and both paroxetine and D-22. To ensure that the effect of D-22 on 5-HT uptake involved a separate (non-SERT) transport mechanism, 5-HT uptake (100 nM) was measured in SERT knockout synaptosomes in the presence of vehicle and 10 μM D-22. Both SERT wildtype and knockout synaptosomes were used to determine whether the genotypes differed with respect to the magnitude of the D-22 effect when clearing a higher value of 5-HT at 1 μM.
RESULTS
SERT-mediated uptake in SERT wildtype and knockout synaptosomes depends on substrate concentration
Figure 1 highlights several key findings: the proportion of SERT mediated uptake decreases with increasing extracellular 5-HT concentrations, and in SERT knockout synaptosomes, non-SERT mediated clearance is observable and appears not to be significantly upregulated. Paroxetine significantly slowed the initial velocity of 5-HT uptake in SERT wildtype synaptsomes at each concentration of 5-HT except the highest (1 μM). The effect of paroxetine on clearance decreased as added 5-HT increased (Figure 2A). The log concentration plot was fit by a straight line (r2=0.965, p=0.0005; Figure 2B). With a single logarithmic relationship observed, the inhibition suggested a single active binding site. In addition, no significant differences in uptake between male and female mice were observed.
Fig. 1.
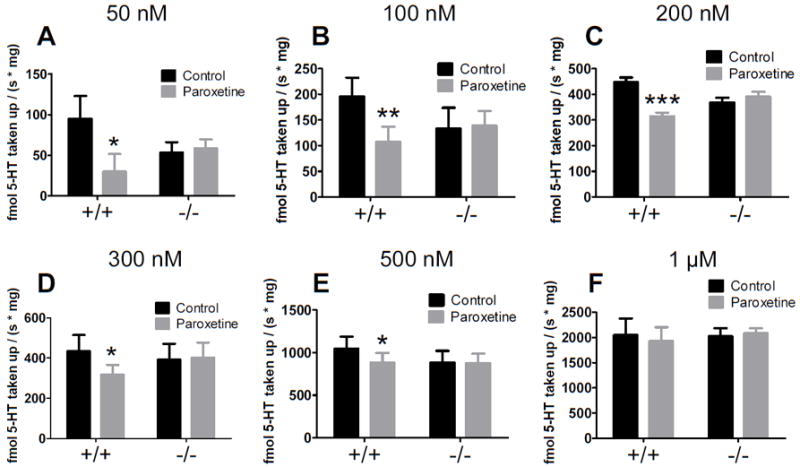
Effects of paroxetine on uptake over a range of 5-HT concentrations in SERT wildtype and knockout synaptosomes. Paroxetine decreases 5-HT clearance in SERT wildtype but not knockout mice at concentrations at 500 nM 5-HT and lower. The effect of 1 μM paroxetine was significant in SERT wildtype but not knockout synaptosomes as determined by repeated measures two-way ANOVA (p for factor paroxetine, genotype, interaction) at (A) 50 nM; p = 0.0121; 0.812; 0.005, (B) 100 nM; p = 0.003; 0.750; 0.001, (C) 200 nM; p = 0.0003; 0.957; 0.005, (D) 300 nM; p = 0.0574; 0.836; 0.028, (E) 500 nM; p = 0.0261; 0.638; 0.039, but not (F) 1 μM; p = 0.657; 0.841; 0.203 (n = 5 per [5-HT]). *p < 0.05, **p < 0.01, ***p < 0.001 as determined by post hoc Bonferroni posttests. Note that the Y-axes differ in each case so that the treatment differences are clearly represented.
Fig. 2.
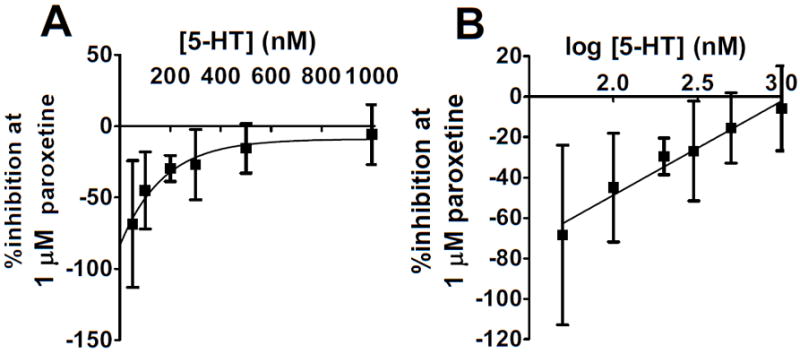
The degree of SERT-mediated clearance decreases with increasing extracellular 5-HT. (A) A plot of the % inhibition of clearance induced by 1 μM paroxetine as a function of extracellular 5-HT. (B) A plot of the % inhibition of clearance induced by 1 μM paroxetine as a function of the log [exogenously added 5-HT] reveals a logarithmic relationship: as the exogenously added 5-HT increases, the proportion of clearance mediated by SERT decreases.
Because a 1 μM concentration of 5-HT may compete with 1 μM paroxetine binding to SERT, we tested whether increasing the paroxetine dose could inhibit 5-HT uptake. However, 10 μM and 100 μM paroxetine also failed to inhibit clearance of 1 μM 5-HT significantly (p = 0.9180, n = 4 per group). Uptake rates were, in fmol / (s × mg): control, 2266 ± 217; 1 μM paroxetine, 2289 ± 535; 10 μM paroxetine, 2024 ± 234; 100 μM paroxetine, 2181 ± 311.
We then tested citalopram, another SERT blocker, at 1, 10, and 100 μM as well as a combination of paroxetine and citalopram both at 1 μM; these also had no significant effect on clearance rates of 1 μM 5-HT (p = 0.1417, n = 4 per group). Uptake rates were, in fmol / (s × mg): control, 1694 ± 166; 1 μM citalopram, 1483 ± 212; 10 μM citalopram, 1538 ± 93; 100 μM citalopram, 1438 ± 122; 1 μM citalopram plus 1 μM paroxetine, 1550 ± 133. Together, these results indicate that non-SERT clearance mechanisms predominate at 1 μM 5-HT in synaptosomes.
Non-SERT-mediated uptake in knockout synaptosomes not significantly increased relative to paroxetine-treated wildtype with one exception
Comparisons of paroxetine-treated wildtype to SERT knockout velocities (Figure 3) showed a significantly increased uptake rate in SERT knockout synaptosomes at 200 nM (n=5, p=0.0470) but there was no significant difference at any of the other concentrations (50 nM, p=0.3744; 100 nM, p=0.6220; 300 nM, p=0.4334; 500 nM, p=0.9994; 1 μM, p=0.7666; n=5 for all concentrations). Uptake velocities were increased in knockouts compared with paroxetine-treated wildtypes at concentrations of 300 nM and lower, but this was only significant at 200 nM, as described above.
Fig. 3.
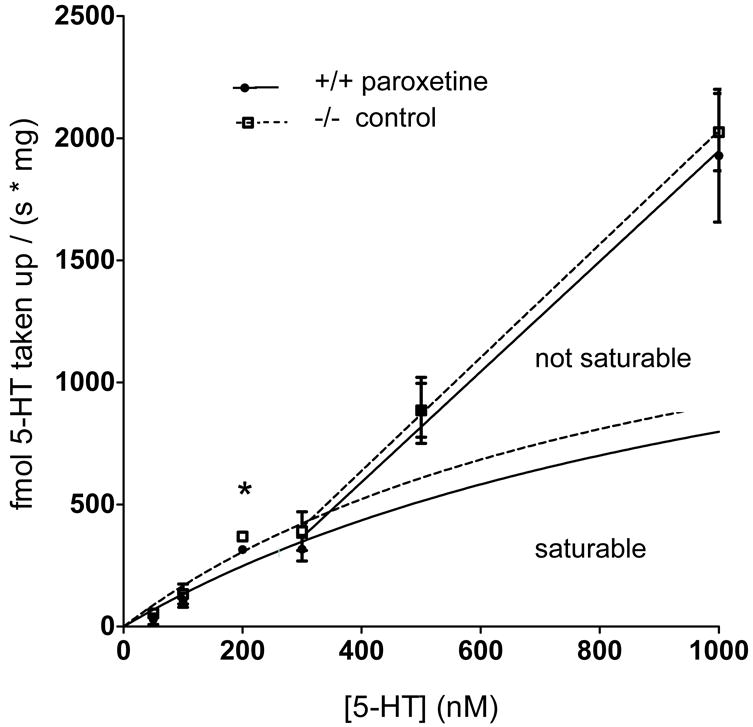
Non-SERT uptake velocities in paroxetine-treated wildtype synaptosomes (closed circle, solid lines) and SERT knockout synaptosomes (open square, dotted lines). This data is also presented in Figure 1 (A-F), but shown here as a [substrate] vs. velocity plot. Uptake was increased in SERT knockout synaptosomes at added 5-HT concentrations of 300 nM and lower, with a significantly enhanced uptake velocity when 200 nM was added (n=5, p=0.0470) as determined by t-test comparison. Error bars fall within the dimensions of the symbols at 200 nM. The Michaelis–Menten equation cannot be fitted to the entire data set. Michaelis–Menten curves were fitted from 0 – 300 nM 5-HT, the region of the data that reflects saturable processes. The following kinetic constants were calculated for paroxetine-treated wildtype synaptosomes for non-SERT uptake: Km = 1029 ± 1663 nM 5-HT; Vmax = 1530 ± 2007 fmol / (s × mg). The kinetic constants for clearance in SERT knockout synaptosomes were: Km = 1087 ± 1843 nM 5-HT; Vmax = 1962 ± 2741 fmol / (s × mg). A line was fitted for the data from 300 nM – 1 μM, the region of the data that reflects non-saturable clearance. There was no significant difference in Km or Vmax between genotypes. There was also no difference in the linear slopes between genotypes. Data are expressed as mean ± SEM, *p < 0.05. The significance level was set at p < 0.05.
Neither a Michaelis–Menten curve nor a biphasic non-linear curve could be fitted to the data sets across all of the 5-HT concentrations tested, due to the nonsaturable region of the data in the higher 5-HT concentrations.
The Michaelis–Menten fit of the velocities from 0 – 300 nM 5-HT (Figure 3) was used to determine the following kinetic constants in paroxetine-treated wildtype synaptosomes for non-SERT uptake: Km = 1029 ± 1663 nM 5-HT; Vmax = 1530 ± 2007 fmol / (s × mg). The kinetic constants for clearance in SERT knockout synaptosomes were: Km = 1087 ± 1843 nM 5-HT; Vmax = 1962 ± 2741 fmol / (s × mg). Neither kinetic constant significantly differed between groups as compared using the extra sum-of-squares F test (Km comparison p =0.9787; Vmax comparison p =0.8844).
Straight line fits for the data from 300 nM – 1 μM also did not significantly differ by slope or Y-intercept (Figure 3, p=0.8879 and p=0.9391, respectively). The slope for paroxetine-treated wildtype was 2.260 ± 0.3267. For SERT knockout the slope was 2.320 ± 0.256.
Clearance of low and moderate concentrations of 5-HT involves both paroxetine-sensitive and D-22-sensitive uptake mechanisms
Figure 4A shows raw data traces of uptake of 100 nM 5-HT in the presence of no drug, 1 μM paroxetine, 10 μM D-22 or both paroxetine and D-22. Initial velocities are calculated from the linear slope of the zero-order portion of the trace; however, a longer duration of uptake is shown to enable the differences among the groups to be more clearly visualized in the raw data. Both drugs blocked a component of 5-HT uptake and an additive effect of paroxetine and D-22 together on initial velocity was observed (Figure 4A, 4B). Additionally – clearance is similar between paroxetine-treated wild type (grey bar Figure 4B) and knockouts (open bar Figure 4C). D-22 completely blocks uptake of 100 nM 5-HT in SERT knockout synaptosomes (Figure 4C) and only partially inhibits uptake in wildtype synaptosomes (striped bar Figure 4B). This suggests D-22 sensitive clearance mechanisms shown in Figure 4A and 4B are SERT-independent mechanisms. Additionally, D-22 had a significant and similarly sized effect in both genotypes at 1 μM 5-HT (Figure 4D). Overall clearance rates at 1 μM 5-HT were not different between genotypes (wildtypes, n = 6, knockouts, n = 4), suggesting that D-22-sensitive mechanisms were not upregulated in SERT knockout mice.
Fig. 4.
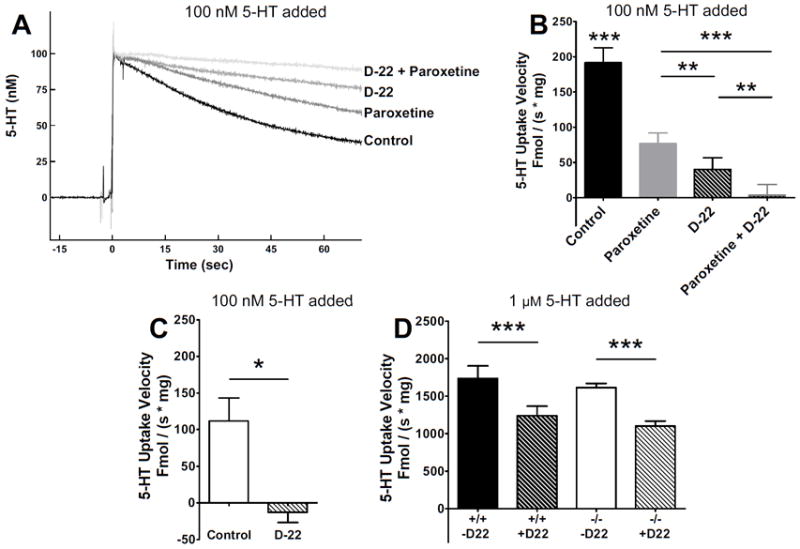
Effects of paroxetine and D-22 in SERT wildtype and knockout synaptosomes. (A) Representative oxidation currents (converted to nM values) produced by adding 100 nM 5-HT to whole-brain synaptosomes in SERT wildtype mice treated with vehicle (control), 1 μM paroxetine, 10 μM D-22, or both drugs. For continuity and for convenience, vehicle and paroxetine traces are those illustrated in (Hagan, 2010), and were taken at the same time as the other two traces in this figure. (B) Initial clearance velocity was 192 ± 21 fmol / (s × mg) in control synaptosomes (n = 6), 77 ± 15 fmol / (s × mg) in paroxetine-treated synaptosomes (n = 6), 40 ± 16 fmol / (s × mg) in D-22 treated synaptosomes (n = 6), and 3 ± 15 fmol / (s × mg) in synaptosomes treated with both paroxetine and D-22 (n = 6). Data were analyzed by a one-way ANOVA with post hoc Newman-Keuls multiple comparisons tests comparing each pair of treatments. (C) Initial clearance rate of 100 nM 5-HT in SERT knockout synaptosomes was 112 ± 31 fmol / (s × mg) (n = 8). D-22 reduced clearance to -13 ± 14 fmol / (s × mg) (n = 3). Data were analyzed by an unpaired t-test. (D) Initial velocity taking up 1 μM 5-HT in control SERT wildtype and knockout synaptosomes, 1735 ± 129 (n = 6) and 1614 ± 55 fmol / (s × mg) (n = 4), respectively, was significantly reduced with D-22 to 1240 ± 126 fmol / (s × mg) in SERT wildtype (n = 6) and 1099 ± 68 fmol / (s × mg) in SERT knockout (n = 4). A two-way ANOVA revealed no significant effect of genotype, but drug effects on clearance were significant at p < 0.001. Data are expressed as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. The significance level was set at p < 0.05.
DISCUSSION
In this study we quantified and characterized several aspects of SERT and non-SERT-mediated transport occurring over a physiological range of 5-HT concentrations using whole brain synaptosomes. The major findings were 1) SERT function contributes an unexpectedly decreasing and small proportion of clearance as extracellular 5-HT concentration increases and 2) SERT- and D-22-sensitive mechanisms of clearance both contribute to clearing low concentrations of 5-HT (~100 nM). These are surprising results, in light of the current literature indicating that SERT is the primary mechanism by which 5-HT is taken up. The conclusions support the growing evidence that non-SERT mediated 5-HT uptake has significant biological importance. The possibility that low-affinity uptake mechanisms may hold promise for more efficacious mood disorder treatments has been raised previously and reviewed (Daws, 2009; Millan, 2006; Schildkraut and Mooney, 2004).
RDEV is a particularly sensitive method for detecting the relative contribution of high and low affinity uptake mechanisms. RDEV initial uptake velocities of serotonin uptake are calculated from the linear slope of the initial apparent zero order portion of a plot of [5-HT] versus time. The methodology complements findings in the field using other approaches such as radioligand binding and chronoamperometry, offering some advantages. Advantages include a very low detection limit (5 nM), minimal effects of tissue and drugs in reducing electrode sensitivity (‘fouling’), and improved resolution over diffusion-dependent methods as previously described (Hagan et al., 2010). Additionally, high control over assay constituent concentrations facilitates interpretation of results. The applications of RDEV, however, are limited to in vitro assays; we previously discussed the advantages and disadvantages of RDEV vs. other uptake assays for 5-HT (Hagan et al., 2010).
Synaptic levels of 5-HT have been estimated to reach concentrations up to 6 mM during normal 5-HT release, although modeling studies suggest that these high levels will exist for no more than a few milliseconds (Bunin and Wightman, 1998). 5-HT can then diffuse more than 20 μm to the micromolar to nanomolar range (Bunin and Wightman, 1998; Bunin and Wightman, 1999; Fuxe et al., 2007). We’ve previously determined the Km of SERT in wild type mice to be about 100 nM as measured by RDEV (Hagan et al., 2010). Our finding that SERT only comprised ~50% of clearance at 100 nM 5-HT in this in vitro paradigm suggests that, while SERT could strongly influence basal levels of 5-HT, other transport mechanisms become increasingly importance as 5-HT concentrations rise. This may be especially important during acute stress exposures, when raphe neurons fire rapidly and extracellular serotonin is briefly elevated (Kirby et al., 1997; Takase et al., 2004; Valentino et al., 2010). If concentrations in the synapse are in the mM range and become diluted to the μM – nM range during volume transmission (where most estimated affinity values for receptors and transporters fall), then our data suggest SERT would not be the primary clearance mechanism for the majority of extracellular 5-HT. This interpretation is consistent with other studies suggesting that extracellular concentrations of serotonin are regulated by multiple uptake mechanisms (Baganz et al., 2010; Baganz et al., 2008; Feng et al., 2010; Feng et al., 2005; Gasser et al., 2006).
The 5-HT concentration at which low affinity mechanisms overcome high affinity mechanisms has been debated (See Daws, 2009 for review). Microdialysis studies have found differences in basal levels of 5-HT between SERT knockout and wildtype mice (Shen et al., 2004), suggesting that alternate 5-HT clearance mechanisms can be functional at low 5-HT levels—as low as 14-18 nM (Mathews et al., 2004). Our data support these studies, demonstrating that non-SERT mechanisms comprise ~30% of uptake at 50 nM, a concentration reported to be near the high end of basal extrasynaptic levels (Bunin and Wightman, 1998).
We detected active uptake in SERT knockout synaptosomes. This supports the conclusions of previous in vivo chronoamperometry studies showing 5-HT clearance in SERT knockout mice was not simply due to diffusion, metabolism, or uptake by NET or DAT. Many other studies show SERT knockout mice do have active 5-HT uptake via non-SERT mechanisms (Baganz et al., 2008; Daws, 2009; Daws et al., 2006; Daws and Toney, 2007), and 5-HT uptake by primary neuronal cultures from SERT knockout mice has also been observed (Pan et al., 2001). This evidence suggests that low affinity mechanisms are engaged and contribute to clearance at low to moderate concentrations of 5-HT.
In our studies, the Km and Vmax for the non-SERT saturable clearance in both paroxetine-treated wildtype and SERT knockout synaptosomes were an order of magnitude higher than our estimates for SERT in untreated wild-type synaptosomes (SERT Km =99 ± 35 nM; Vmax =181 ± 11 fmol / (s × mg protein) (Hagan et al., 2010). This order of magnitude difference supports the use of the relativistic terms ‘high’ and ‘low’ as they are often applied in literature studying ‘high-affinity’ SERT uptake and ‘low-affinity’ non-SERT uptake.
Chronoamperometric studies have found functional evidence for upregulation of D-22-sensitive non-SERT mechanisms, with some indication that these effects may be mediated by OCT3 (Baganz et al., 2008; Daws et al., 2006). This finding is supported by other evidence that OCT3 is upregulated in SERT knockout mice (Chen et al., 2001; Schmitt et al., 2003). We observed a significant enhancement in uptake velocity in SERT knockout mice compared with paroxetine-treated wildtype when 200 nM 5-HT was added. While there were no significant differences at any other added 5-HT concentration, but the overall trends in our data suggest that 5-HT was cleared more quickly in synaptosomes from SERT knockout mice relative to paroxetine-treated wildtype synaptosomes at concentrations of 300 nM and lower.
It is unclear why 200 nM added 5-HT revealed a significant detectable enhancement of non-SERT uptake mechanisms in SERT knockout synaptosomes while lower concentrations and comparisons of kinetic constants showed no difference. These lower concentrations represent 5-HT concentrations where SERT exerts moderate influence on uptake rates in whole brain synaptosomes. We hypothesized that enhancement of non-SERT mechanisms would be most detectable in this range, since multiple transport mechanisms comprise clearance in this range. Uptake velocities at 200 nM added 5-HT may either reflect a point of minimal variation related to the biology of the active transporter systems, or reflect conditions that optimize the assay for minimal noise levels. The Km and Vmax between paroxetine-treated wildtype and SERT knockout were also not significantly different. A more definitive conclusion about putative upregulation of D-22 sensitive clearance could be accomplished in future studies with a complete dose-response analysis of multiple concentrations of D-22 and multiple substrate concentrations of 5-HT, particularly in the lower range (0 – 300 nM).
Differences between our experimental approach and chronoamperometry should be considered in interpreting the discrepancies in the data. Our assay may be less sensitive than chronoamperometry to changes in OCT3 function for a couple of reasons. First, synaptosomes model synaptic physiology. The preparation leads to disruption of the intact cells as well as their local and long feedback connections. The model excludes non-synaptic transport mechanisms of intact brain. This could be relevant for studying OCT3 function, as some studies have identified the transporter in glia (Cui et al., 2009; Gasser et al., 2009). Second, whole brain preparations prohibit detection of regionally specific compensatory changes in transporters for 5-HT. In vivo, specific transport mechanisms may be locally potentiated (or reduced) based on the regional expression of various transporters, with no net change detectable in whole brain synaptosomes. Moreover, Daws and colleagues have not only shown with chronoamperometry that 5-HT clearance rates vary by brain region and SERT expression levels (Daws et al., 2005; Daws and Toney, 2007; Montanez et al., 2002), but also shown that stress exposure can also alter SERT-independent (most likely OCT3 mediated) 5-HT clearance in the hippocampus (Baganz et al., 2010). This underscores the dynamic state of these transport systems and how their relative quantity, location, and activity levels within brain microenvironments are central to defining the importance of SERT and other transporters for regulating extracellular 5-HT levels (Daws, 2009).
Our data show that D-22 doesn’t inhibit uptake completely in either wildtype or SERT knockout synaptosomes at 1 μM added 5-HT. Moreover, the Michaelis–Menten could not be fitted to the velocity data from paroxetine-treated wild-type and knockout synaptosomes shown in Figure 3 unless the linear (unsaturable) region of the data was excluded. The Michaelis–Menten curves in Figure 3 were fitted from 0 – 300 nM 5-HT, the region of the data that appeared to reflect saturable processes. The saturable processes could include the contributions of OCT3, PMAT, or other transport systems. A line was fitted for the data in Figure 3 from 300 nM – 1 μM, the region of the data that appeared to reflect non-saturable clearance. Non-saturable mechanisms, such as enzymatic metabolism by monoamine oxidase appear not to exert a significant influence at lower 5-HT concentrations, given that inhibitors completely obliterated initial rates. However, contributions of MAO could be expected to increase with increasing added concentrations of 5-HT, given the Km of monoamine oxidase A and B for 5-HT are estimated at 39 ± 7 μM and 1704 ± 122 μM, respectively (Freeman et al., 1993). While specifically identifying the non-SERT mechanisms was beyond the scope of the current study, RDEV in combination with selective inhibitors and tissue from knockout animals could a useful tool in future studies characterizing 5-HT clearance mechanisms.
In conclusion, these in vitro data strongly support an important body of growing in vivo evidence that non-SERT uptake of extracellular 5-HT plays a significantly more important role than previously thought. Together with previous results, these data suggest the possibility that non-SERT mediated 5-HT uptake mechanisms could be important therapeutic targets for treating mood and stress disorders associated with altered serotonergic function.
Acknowledgments
The authors sincerely thank Dr. Trent Volz, Dr. Paul Phillips, Dr. Ross McDevitt, Dr. Nicole Bjorklund, Dr. Michele Kelly, and Dr. Scott Ng-Evans for their feedback and technical advice, and Ms. Hannah DeMeritt for her technical assistance. We also thank Dr. Nicholas Poolos and Dr. Diane Lattemann for loaning equipment for these studies. The authors also give a special thank you to Dr. Denny Liggitt and the University of Washington’s Department of Comparative Medicine for supporting this work. This work was supported by NIH grants R01 MH63303, T32 RR07019, K01 RR024471, and the State of Washington.
References
- Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci. 2010;30(45):15185–15195. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105(48):18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53(4):649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Blackburn KJ, French PC, Merrills RJ. 5-Hydroxytryptamine uptake by rat brain in vitro. Life Sci. 1967;6:1653–1663. doi: 10.1016/0024-3205(67)90176-2. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354(6348):66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci. 1998;18(13):4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Paracrine neurotransmission in the CNS: involvement of 5-HT. Trends Neurosci. 1999;22(9):377–382. doi: 10.1016/s0166-2236(99)01410-1. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21(16):6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A. 2009;106(19):8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121(1):89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26(24):6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Owens WA, Gould GG, Frazer A, Toney GM, Gerhardt GA. Transport mechanisms governing serotonin clearance in vivo revealed by high-speed chronoamperometry. J Neurosci Methods. 2005;143(1):49–62. doi: 10.1016/j.jneumeth.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Daws LC, Toney GM. High-speed chronoamperometry to study kinetics and mechanisms for serotonin clearance in vivo. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton, FL: CRC Press; 2007. pp. 63–81. [PubMed] [Google Scholar]
- Engel K, Wang J. Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol. 2005;68(5):1397–1407. doi: 10.1124/mol.105.016832. [DOI] [PubMed] [Google Scholar]
- Feng N, Lowry CA, Lukkes JL, Orchinik M, Forster GL, Renner KJ. Organic cation transporter inhibition increases medial hypothalamic serotonin under basal conditions and during mild restraint. Brain Res. 2010;1326:105–113. doi: 10.1016/j.brainres.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Mo B, Johnson PL, Orchinik M, Lowry CA, Renner KJ. Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus. Brain Res. 2005;1063(1):69–76. doi: 10.1016/j.brainres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Bulawa MC, Zeng Q, Blank CL. Rapid and simultaneous determination of monoamine oxidase A and monoamine oxidase B activities in mouse brain homogenates by liquid chromatography with electrochemical detection. Anal Biochem. 1993;208(1):182–196. doi: 10.1006/abio.1993.1026. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlstrom A, Hoistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, Leo G, Staines W, Guidolin D, Kehr J, Genedani S, Belluardo N, Agnati LF. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55(1):17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26(34):8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512(4):529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- Hagan CE, Neumaier JF, Schenk JO. Rotating disk electrode voltammetric measurements of serotonin transporter kinetics in synaptosomes. J Neurosci Methods. 2010;193(1):29–38. doi: 10.1016/j.jneumeth.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Kissinger PT. Liquid chromatographic determination of serotonin in homogenized dog intestine and rat brain tissue using a 2 mm i.d. PEEK Column. Current Separations. 1996;14(3/4):114–119. [Google Scholar]
- Inazu M, Takeda H, Matsumiya T. Expression and functional characterization of the extraneuronal monoamine transporter in normal human astrocytes. J Neurochem. 2003;84(1):43–52. doi: 10.1046/j.1471-4159.2003.01566.x. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760(1-2):218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT) J Pharmacol Exp Ther. 2003;306(1):355–362. doi: 10.1124/jpet.103.049668. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140(1-2):169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Multi-target strategies for the improved treatment of depressive states: Conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther. 2006;110(2):135–370. doi: 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Montanez S, Daws LC, Gould GG, Gerhardt GA, Frazer A. Differential in vivo clearance of serotonin in rat dorsal raphe nucleus and CA3 region. Brain Res. 2002;955(1-2):236–244. doi: 10.1016/s0006-8993(02)03470-4. [DOI] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A. Fitting Models to Biological Data using Linear and Nonlinear. Regression: a practical guide to curve fitting. 2003:245–251. [Google Scholar]
- Pan Y, Gembom E, Peng W, Lesch KP, Mossner R, Simantov R. Plasticity in serotonin uptake in primary neuronal cultures of serotonin transporter knockout mice. Brain Res Dev Brain Res. 2001;126(1):125–129. doi: 10.1016/s0165-3806(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Russ H, Sonna K, Keppler S, Baunach S, Schomig E. Cyanine-related compounds: a novel class of potent inhibitors of extraneuronal noradrenaline transport. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:458–465. doi: 10.1007/BF00173203. [DOI] [PubMed] [Google Scholar]
- Russ H, Staust K, Martel F, Gliese M, Schomig E. The extraneuronal transporter for monoamine transmitters exists in cells derived from human central nervous system glia. Eur J Neurosci. 1996;8(6):1256–1264. doi: 10.1111/j.1460-9568.1996.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ, Mooney JJ. Toward a rapidly acting antidepressant: the normetanephrine and extraneuronal monoamine transporter (uptake 2) hypothesis. Am J Psychiatry. 2004;161(5):909–911. doi: 10.1176/appi.ajp.161.5.909. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Mossner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, Koepsell H, Lesch KP. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res. 2003;71(5):701–709. doi: 10.1002/jnr.10521. [DOI] [PubMed] [Google Scholar]
- Shang T, Uihlein AV, Van Asten J, Kalyanaraman B, Hillard CJ. 1-Methyl-4-phenylpyridinium accumulates in cerebellar granule neurons via organic cation transporter 3. J Neurochem. 2003;85(2):358–367. doi: 10.1046/j.1471-4159.2003.01686.x. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI, Baratta M, Bland ST, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Inescapable shock activates serotonergic neurons in all raphe nuclei of rat. Behav Brain Res. 2004;153(1):233–239. doi: 10.1016/j.bbr.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- Xia L, Zhou M, Kalhorn TF, Ho HT, Wang J. Podocyte-specific expression of organic cation transporter PMAT: implication in puromycin aminonucleoside nephrotoxicity. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.00046.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


