Abstract
Purpose
Cancers overexpressing the HER2/neu gene are usually more aggressive and are associated with poor prognosis. Although trastuzumab has significantly improved the outcome, many tumors do not respond or acquire resistance to current therapies. To provide an alternative HER2-targeted therapy, we have developed and characterized a novel recombinant protein combining an HER2-specific Affibody and modified Pseudomonas aeruginosa exotoxin A (PE 38), which, after binding to HER2, is internalized and delivered to the cytosol of the tumor cell, where it blocks protein synthesis by ADP ribosylation of eEF-2.
Experimental Design
The effect of the Affitoxin on cell viability was assessed using CellTiter-Glo (Promega, Madison, WI). To assess HER2-specific efficacy, athymic nude mice bearing BT-474 breast cancer, SK-OV-3 ovarian cancers, and NCI-N87 gastric carcinoma xenografts were treated with the Affitoxin (HER2- or Tag-specific), which was injected every third day. Affitoxin immunogenicity in female BALB/c mice was investigated using standard antibody production and splenocyte proliferation assays.
Results
In vitro experiments showed that HER2-Affitoxin is a potent agent that eliminates HER2-overexpressing cells at low picomolar concentrations. Therapeutic efficacy studies showed complete eradication of relatively large BT-474 tumors and significant effects on SK-OV-3 and NCI-N87 tumors. HER2-Affitoxin cleared quickly from circulation (T1/2) < 10 minutes) and was well tolerated by mice at doses of 0.5 mg/kg and below. Immunogenicity studies indicated that HER2-Affitoxin induced antibody development after the third injected dose.
Conclusions
Our findings demonstrated that HER2-Affitoxin is an effective anticancer agent and a potential candidate for clinical studies.
Keywords: Affibody molecule, HER2, breast cancer, targeted therapy, Pseudomonas exotoxin
Introduction
HER2 is a tyrosine kinase receptor belonging to the epidermal growth factor receptor (EGFR) family (1, 2). When overexpressed in tumor cells, HER2 constitutively triggers activation of the cell signaling network, leading to upregulated transcription of genes that drive cellular proliferation, migration, differentiation, and angiogenesis, as well as apoptosis suppression/cell survival (3).
Affibody molecules are a new class of relatively small (~7 kDa) affinity proteins, that are structurally based on a 58-amino-acid scaffold derived from the Z domain of Staphylococcus aureus protein A, and are obtained by combinatorial protein engineering (4, 5). HER2-specific Affibody molecules strongly bind to their receptors (Kd = 22 pM) without changing the receptor activation status (6). It was previously reported that Affibody molecules are capable of being labeled with radionuclides, including 99mTc, 111In, 68Ga, 90Y, 125I, and 18F (7–13), optical beacons (14–16), or reporter enzymes (15). The aforementioned probes have been successfully applied to characterize HER2 expression in vitro as well as in xenografts.
Previously, we have successfully cloned, expressed, purified, and characterized Affitoxin, an HER2-specific tumoricidal agent (HER2-Affitoxin) consisting of an Affibody molecule as a targeting modality and a modified Pseudomonas exotoxin A (PE38) as an effector (17). Based on the well-described Pseudomonas aureginosa cytotoxic pathway, it is assumed that after binding to HER2, at least a fraction of the HER2-Affitoxin pool is internalized and redistributed to the cytosol, where it acts as an ADP ribosylating agent for eEF-2. This in turn blocks the enzymatic activity of eEF-2 and hinders protein synthesis on the ribosomal level (18, 19).
We have shown that HER2-Affitoxin was expressed as soluble protein and can be purified to homogeneity after 2 chromatographic steps. It binds to HER2 with high affinity (Kd ~1 nM), and the binding epitope is distinct from that being recognized by trastuzumab. HER2-Affitoxin binding is well correlated with the expression of HER2. Our previous in vitro toxicity studies confirmed that cells expressing high levels of HER2 are more sensitive to HER2-Affitoxin than cells with low receptor numbers, and the excess of Affibody molecules competing with HER2-Affitoxin for receptor binding prevents induction of cell death (17).
In this study, in vivo characterization of HER2-Affitoxin was performed, including pharmacokinetics and biodistribution analyses of the drug. This was followed by efficacy evaluation in a mouse model bearing HER2-positive breast, ovarian, or gastric cancer tumors. Finally, assuming that repeated drug administration might become inevitable during the therapy design, the ability of HER2-Affitoxin to induce immune response was evaluated.
Material and Methods
Cloning and purification of Affitoxins
HER2-Affitoxin was expressed and purified as described recently (17). Briefly, an isopropyl β-D-1-thiogalactopyranoside (IPTG)-induced culture of One ShotR BL21 Star (DE3) cells (Invitrogen Corporation, Carlsbad, CA) transformed with pAfTxKDEL31 plasmid was lysed by ultrasonication. Soluble fractions of protein were subjected to purification by the AktaPuryfier 10 chromatographic system (Amersham Bioscience, Pittsburgh, PA) using an Ni-affinity HisTrap and anion exchange HiTrapQ column (Amersham Bioscience, Pittsburgh, PA), followed by buffer exchange to PBS using sample ultrafiltration on an Amicon Ultra centrifugal filter device with a 30-kDA cut-off membrane (Millipore, Billerica, MA). The last step of purification involved endotoxin removal, which was carried out according to the provided protocol (ToxinEraser™ Endotoxin Removal Kit, GenScript, Piscataway, NJ). The endotoxin level was measured using the ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit (GenScript, Piscataway, NJ). Protein concentration was measured using the BCA™ Protein Assay Kit (Pierce, Rockford, IL) according to the provided protocol.
Non-HER2-specific Affitoxin containing an Affibody against bacterial Taq polymerase (ZTaqS1-1Affibody), called Taq-Affitoxin, was cloned according to the following procedure. Plasmid pAfTxKDEL31 was used as a template for PCR to amplify the PE38 toxin moiety using primers 60-3 and T7term70 (Supplementary Table 1). The ZTaqS1-1 moiety was PCR amplified using the pAfTxKDEL31 template and primers 5BsaI-His, 60-1c, and 60-2c (Supplementary Table 1). The resulting PCR products were gel purified and combined by megaprimer PCR using primers 5BsaI-His and T7term70. The megaprimer PCR product containing DNA coding for the ZTaqS1-1-PE38 fusion, and the pET23d+ vector (EMD Chemicals, Gibbstown, NJ) were separately digested with NcoI and HindIII restriction endonucleases (New England Biolabs, Ipswich, MA), gel purified and ligated, and then transformed into XL10-gold-competent cells (Agilent Technologies, Santa Clara, CA). The resulting pNT3 plasmid, which contains the predicted nucleotide sequence, was transformed into One ShotR BL21 Star (DE3) cells (Invitrogen Corporation, Carlsbad, CA), and the protein was processed according to the protocol used for purifying HER2-Affitoxin, as described above.
HER2-Affitoxin modification
For near-infrared (NIR) optical imaging, HER2-Affitoxin was labeled with DyLight™ 750 (Pierce, Rockford, IL) using maleimide chemistry to attach the dye to the C-terminal cysteine. First, HER2-Affitoxin was reduced by incubation in tris(2-carboxyethyl)phosphine (TCEP) for 30 minutes at 4°C, and then mixed with 4-fold excess of DyLight™ 750 maleimide derivative. The labeled HER2-Affitoxin was repurified on a HisTrap column to remove non-bound dye. After purification, the buffer was changed to PBS and, finally, the conjugate was sterilized by 0.22-μm filtration. The same protocol was applied for labeling HER2-Affibody molecules containing C-terminal cysteine.
Cell culture
Human breast cancers BT-474 and MDA-MB-468, gastric carcinoma NCI-N87, and ovarian carcinoma SK-OV-3 cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA). An SK-OV-3-luc-D3 cell line was purchased from Caliper L.S. (Caliper Life Sciences, Hopkinton, MA). The cells were grown in RPMI (BT-474, NCI-N87) or DMEM/F12 (SK-OV-3 and MDA-MB-468) culture media supplemented with 10% FBS and Pen/Strep (10,000 U penicillin, 10 mg streptomycin) at 37°C in a humidified environment with 5% CO2. A solution of 0.05% trypsin and 0.02% EDTA (Invitrogen, Carlsbad, CA) in PBS was used for cell detachment.
In vitro efficacy of HER2-Affitoxin
To compare the toxicity of HER2- and Taq-Affitoxin, NCI-N87 cells were plated onto a 96-well plate at 1 × 104 cells/well, followed by treatment with HER2- or Taq-Affitoxin at different concentrations. The cell viability was assessed using CellTiter-Glo (Promega, Madison, WI). IC50 values were calculated using GraphPad Prism software (GraphPad Prism Software, San Diego, CA).
The minimal time necessary to affect the HER2-positive population was determined as follows: pre-plated NCI-N87 cells were treated with HER2-Affitoxin or Taq-Affitoxin for various exposure times, washed 3 times with medium, and incubated for additional time up to 72 hours. The cells’ viability (assessed as above) was expressed as the percentage of viable cells in a reference to the non-treated control group.
Animals
Female athymic nude mice (nu/nu genotype, BALB/c background) and BALB/c mice, 5 to 8 weeks old, were purchased from the Animal Production Program, (NCI-Frederick, Frederick, MD). This study was approved by the Animal Safety and Use Committee of NIH and carried out in accordance with the Department of Health and Human Services’ Guide for the Care and Use of Laboratory Animals.
Non-HER2-specific toxicity
BALB/c mice (n = 3–10 per group) were administered the indicated doses of HER2-Affitoxin by intravenous injection into the tail vein. Any reported death cases or moribund conditions that occurred within the 2-week post-injection period were taken into consideration. The LD50 value was calculated by probit analysis in Stat Plus 2009 software (AnalystSoft, Vancouver, BC).
The liver toxicity of HER2-Affitoxin was investigated in athymic mice receiving 6 injections of the drug (administered intravenously, 0.25 mg/kg each, every third day). Blood samples were obtained by submandibular bleeding and collected in heparinized tubes. Plasma samples were analyzed using a Liver Panel Test performed by the NCI Pathology/Histotechnology Laboratory (SAIC-Frederick, Inc., NCI-Frederick, Frederick, MD).
Pharmacokinetics of HER2-Affitoxin
Three athymic mice were intravenously administered 0.5 mg/kg of HER2-Affitoxin. Twenty to thirty μl of blood was withdrawn at 1, 5, 15, 30, 60, and 120 minutes post-injection. At 5 h post-injection, the mice were anesthetized and terminally bled. All samples were incubated for 15 minutes in EDTA-coated tubes and centrifuged at 2,000 × G for 3 minutes. Plasma was stored at −30°C for further analysis.
The concentration of HER2-Affitoxin in plasma was measured by cell-based ELISA. NCI-N87 cells (7.5 × 104/well) seeded on a 96-well plate were allowed to attach for 24 hours and then fixed in 4% buffered para-formaldehyde for 20 minutes at room temperature. After three washing steps (PBS + 2% FBS, 5 minutes each), the cells were exposed to 20 μl of HER2-Affitoxin (concentration range: 2 pg/ml to 1 μg/ml) or plasma samples diluted 1:50. Incubation was carried out at room temperature with intensive shaking for 1 hour. HER2-Affitoxin detection was performed using anti–Pseudomonas exotoxin A polyclonal antibody (Sigma-Aldrich, St. Louis, MO) and anti-rabbit IgG conjugated with horseradish peroxidase (Millipore, Billerica, MA). Chemiluminescent signal was recorded using a FluoStar Optima plate reader (BMG Labtech, Cary, NC) 10 minutes after incubation with LumiGLO Peroxidase Chemiluminescent Substrate (KPL, Gaithersburg, MD). The half-life and initial concentration of the drug were estimated using Graph Pad Prism Software (San Diego, CA).
In addition, the residual activity of HER2-Affitoxin present in the plasma as a function of time was tested by 72-hour in vitro exposure of NCI-N87 cells to the plasma samples obtained from mice at different time points post-injection. Following this treatment, the viability of the cells was assessed by CellTiter-Glo, (Promega, Madison, WI).
Biodistribution studies
Biodistribution of DyLight™750-labeled Affitoxin was studied using a subcutaneous BT-474 tumor model, the previously described NIR fluorescence small-animal imager (20), and IVIS Lumina (Calliper Life Sciences, Hopkinton, MA).
Immunohistochemistry (IHC)
Immunohistochemistry (IHC) service was provided by the Pathology/Histotechnology Laboratory, SAIC-Frederick, Inc., National Cancer Institute at Frederick. Briefly, tissue specimens from xenografts were fixed in 10% neutral-buffered formalin. Five-μm paraffin-embedded sections were immobilized on positively charged slides and Affibody staining was performed on Leica Microsystems Bond Autostainer (Leica, Bannockburn, IL), followed by a citrate buffer antigen-retrieval step. After a blocking step in 2% normal rabbit serum (Vector Laboratories, Burlingame, CA), slides were exposed to goat anti-Affibody antibody (Abcam, Cambridge, MA) for 30 minutes at a dilution of 1:100. Signal detection was performed using a Bond Intense R Detection Kit (Leica, Bannockburn, IL) after 1-hour incubation with biotinylated rabbit anti-goat IgG (Vector Laboratories, Burlingame, CA), at a dilution of 1:100. HER2 detection was performed after immobilized slides were deparaffinized and rehydrated using a DAKO HercepTest Kit (Dako, Carpinteria, CA). Both Affibody- and HER2-stained slides were contra-stained according to Gill’s hematoxylin staining protocol.
In vivo efficacy studies
Therapeutic efficacy studies were carried out using BT-474, SK-OV-3, and NCI-N87 subcutaneous xenografts expressing high levels of HER2. The tumors were initiated by subcutaneous injection of 5 × 106 cells, which were suspended in 0.1 ml of 30% Matrigel solution (BD Biosciences, Bedford, MA), into the right forelimb of athymic mice. BT-474 growth was facilitated by implanting estrogen pellets (0.72 mg, 90-day release, Innovative Research of America, Sarasota, FL) 24 hours prior to inoculation. Tumor dimensions were measured periodically using calipers, and their volumes were calculated using the 4/3 × Π × length × width × depth/8 formula. To investigate the efficacy of HER2-Affitoxin on disseminated disease, an intraperitoneal tumor model using SK-OV-3-luc-D3 cells, expressing the firefly luciferase gene, was established by intraperitoneal injection of 5 × 106 cells suspended in 0.5 ml of PBS. Tumor growth was monitored by bioluminescent imaging using the IVIS Lumina imaging system (Caliper Life Sciences, Hopkinton, MA) 8 minutes post–intraperitoneal injection of D-Luciferin (75 mg/kg).
HER2-Affitoxin or Taq-Affitoxin, diluted in 100 μl of saline, was administered by tail vein injection. Body weight was monitored during the treatment.
Immunogenicity Studies
Antibody production
Female BALB/c mice were randomized into 3 groups receiving saline or HER2-Affitoxin (0.25 mg/kg). Four mice of each group were terminally bled by cardiac puncture 10 days after each dose injection. The development of anti-HER2-Affitoxin toxin antibodies was analyzed by ELISA using immobilized recombinant HER2-Affitoxin as an antigen. Five μg of purified recombinant protein was added to each well of a Nunc Maxi-Sorp 96-microtiter plate (eBioscience, San Diego, CA) and adhered overnight at 4°C. The unbound protein was washed away with PBS-T and blocked for 2 hours with PBS-T containing 1% bovine serum albumin (BSA). Plasma samples were serially diluted, and 100 μl of the resulting solution was added in triplicates to the appropriate wells. Following a 3-hour incubation, plates were washed 8 times with PBS-T/1% BSA, and 100 μl of a 1:2,000 dilution of peroxidase-conjugated rabbit anti-mouse IgG (Millipore, Billerica, MA) was added to each well. The plates were then incubated at room temperature for an additional 2 hours. Next, the plates were washed twice and color development was achieved using O-phenylenediamine (Sigma-Aldrich, St. Louis, MO) prepared in a phosphate-citrate buffer with 30% hydrogen peroxide (Sigma-Aldrich, St. Louis, MO). After the addition of 50 μl H2SO4 (R&D Systems, Minneapolis, MN) to each well, absorbance was measured at 490 nm. The relative generation of HER2-Affitoxin-specific antibodies over time was determined by calculating the absorbance ratio of the specific toxin divided by the absorbance from mice receiving saline alone.
Proliferation assays
Female BALB/c mice were randomized into 3 groups receiving saline and HER2-Affitoxin (1, 2, or 3 doses of 0.25 mg/kg each, 2 weeks apart). Spleens were harvested from 4 mice in each group 10 days after injecting each consecutive dose. Isolated splenocytes were resuspended in complete DMEM containing 20% FBS and were restimulated with recombinant HER2-Affitoxin in an in vitro proliferation assay to assess recall responses to the toxin. Briefly, 300,000 splenocytes were plated into a 96 u-bottom plate (100 μl/well) in triplicates. Cells were stimulated with various concentrations of HER2-Affitoxin (0–125 μg/ml). Treatment with concanavalin A (5 μg/ml), lippopolysaccaride (1 μg/ml), and PMA + ionomycin (10 ng/ml + 0.5 μM) served as positive controls for the proliferative ability of splenocytes. Wells were pulsed with 1μCi of [3H]thymidine (Amersham Bioscience, Pittsburgh, PA) during the last 16 hours of the 72-hour culture. The cells were then lysed using a Cell Harvester (Tomtech, Hamden, CT), and radioactivity was measured in a Wallac Trilux MicroBeta liquid scintillation counter (Perkin Elmer, Waltham, MA). The stimulation index (SI) for the samples was calculated by dividing the average CPM of cells + stimulation by the average CPM of unstimulated cells.
Results
Taq-Affitoxin
This off-target Affitoxin analog contains Affibody molecules directed against Taq polymerase (ZTaqS1-1 Affibody molecule) at its N-termini (21). The sequence of both Affitoxins differs by only 12 amino acids located in the binding site of Affibody molecules (Supplementary Table 2). Like HER2-Affitoxin, Taq-Affitoxin was expressed in the soluble fraction of an E. coli protein and purified almost to homogeneity after 2 chromatographic steps: nickel affinity, and ion exchange (Figure 1A). Additionally, before injection into animals, both Affitoxins were subjected to an endotoxin removal protocol. HER2-Affitoxin and Taq-Affitoxin were immunoreactive to anti-Affibody and anti–Pseudomodomas exotoxin A antibodies (Supplementary Figure 1).
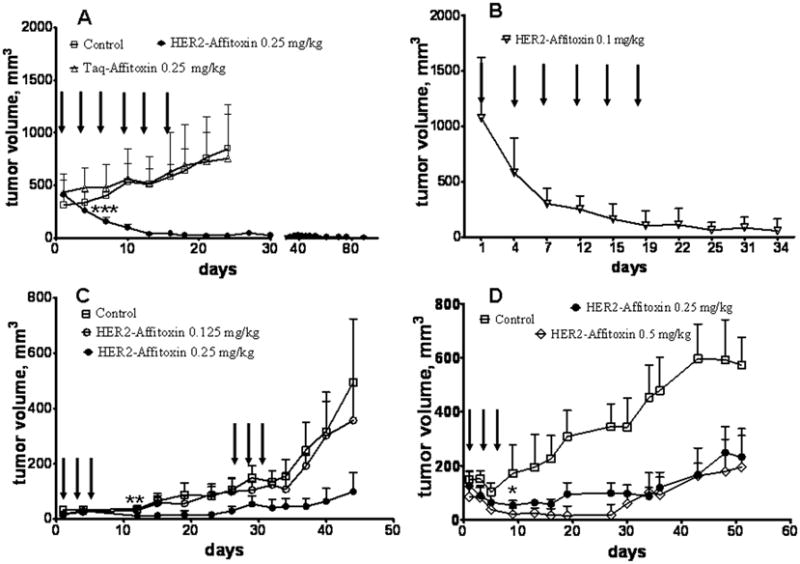
Figure 1.
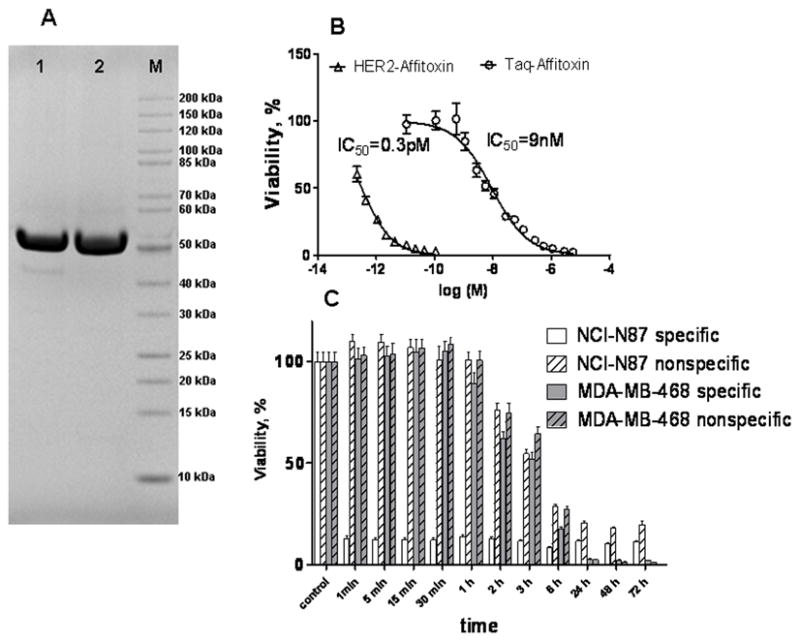
Electropherogram of purified Taq-Affitoxin and HER2-Affitoxin (A) and their efficacy in eliminating NCI-N87 cells (B and C).
A, 10 μg of Taq-Affitoxin (lane 1) and HER2-Affitoxin (lane 2) were resolved after reduction and denaturation steps in 4–12% polyacrylamide gel. After electrophoresis, the gel was stained with MicrowaveBlue (Protiga, Frederick, MD). B, NCI-N87 cells (1 × 104) were plated in a 96-well flat-bottom plate and subjected to indicated concentrations of toxins for 72 hours. C, Alternatively, NCI-N87 (1 × 104) and MDA-MB-468 (1 × 104) cells were allowed to attach to 96- well plate and then exposed to HER2- or Taq-Affitoxin at 100 nM for indicated periods of time. Next, cells were rinsed, and incubated for additional 72 hours. Cell viability was assessed using CellTiter-Glo proliferation assay (Promega, Madison, WI). IC50 values were calculated using GraphPad Prism software.
In vitro studies
Data obtained from a toxicity assay using NCI-N87 cells, which expressed a high level of HER2, showed that HER2-Affitoxin was significantly better at killing NCI-N87 cells compared to the off-target toxin. The IC50 values obtained from measurements of residual ATP levels following exposure to increasing concentrations of either HER2-Affitoxin or Taq-Affitoxin indicated that the former was 30,000 times more potent in inducing cell death than its off-target analog (Figure 1B).
Next, we tested the minimal exposure time for the toxin that is sufficient to eliminate HER2-expressing cells. Exposure to HER2-Affitoxin for as short as 1 minute, followed by drug removal and an additional 72-hour incubation period, resulted in the inactivation of nearly 90% of HER2-overexpressing NCI-N87 cells. In contrast, the whole population of cells treated with the Taq-Affitoxin remained fully viable after 30–60 minutes of exposure, and more than 6 hours of exposure was needed to obtain a cell death rate similar to that resulting from 1-minute exposure to HER2-Affitoxin. A similar toxicity pattern was observed for HER2-negative cells, MDA-MB-468, treated both with HER2-specific or -non-specific Affitoxins (Figure 1C).
Pharmacokinetics of HER2-Affitoxin and acute toxicity
Pharmacokinetics data obtained by cell-based ELISA indicated that HER2-Affitoxin’s half-life in the bloodstream is 8.69± 1.31 minutes (Supplementary Figure 2) and 5.5 ± 0.53 minutes, as estimated by residual plasma toxicity (Supplementary Figure 3). The initial concentration of HER2-Affitoxin in the plasma was 6.87± 0.52 μg/ml, which corresponded to the injected dose of the drug.
To assess its toxicity in BALB/c mice, HER2-Affitoxin was administered by bolus intravenous injection into the tail vein. As listed in Supplementary Table 3, a 100% mortality rate was recorded for mice injected with 4, 2, and 1 mg/kg of the drug. One out of 6 mice survived the treatment with 0.625 mg/kg. All death cases were reported within 72 hour post-injection. No mortality was reported in groups treated with 0.5 mg/kg or 0.25 mg/kg. The calculated LD50 value was 0.572 ± 0.051 mg/kg.
Biodistribution of HER2-Affitoxin
Images were taken 2 hours post-injection of fluorescently-labeled HER2-Affitoxin into mice bearing BT-474 tumors and showed an accumulation of fluorescence in the kidneys and the liver. A significantly lower but still over-the-background signal was detected in the tumors (Figure 2A and C). All animals showed similar accumulation patterns, confirmed by postmortem fluorescence analysis of dissected organs (Figure 2B). Tumor-to-muscle ratio was approximately 4 for all tested mice (Figure 2D). Similar biodistribution patterns were observed using the IVIS Lumina imaging system (Supplementary Figure 4). Affitoxin accumulation in the tumor was further confirmed by immunostaining BT-474 tumor sections extracted 2 hours post-HER2-Affitoxin injection (Figure 2E), while no signal was detected in saline-injected animals (Supplementary figure 5A). Both samples showed positive staining for HER2 receptors (Figure 2F and Supplementary Figure 5B).
Figure 2.
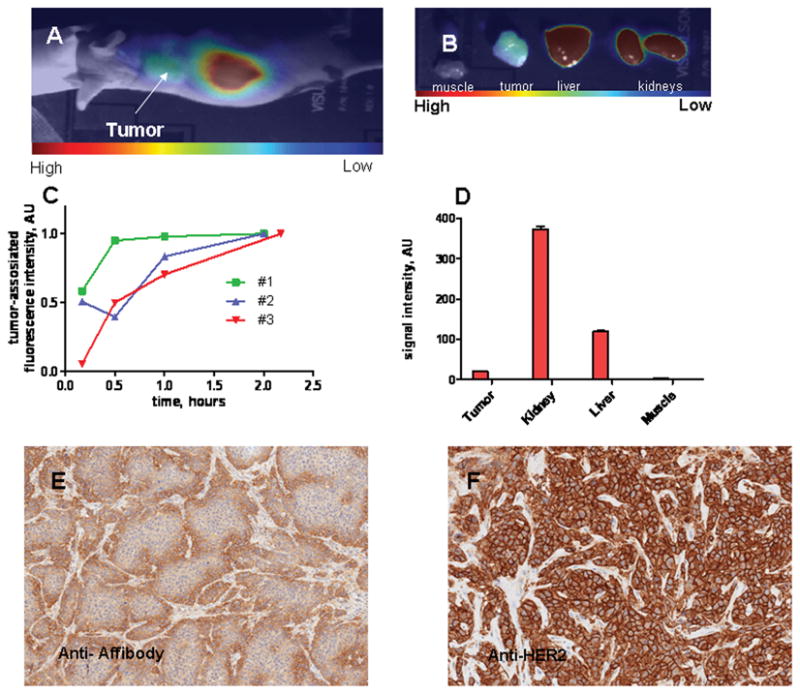
Near-infrared imaging of a BT-474 xenograft-bearing mouse injected with DyLight™750-labeled HER2-Affitoxin.
Three mice bearing BT-474 subcutaneous xenografts received 5 mg/kg of HER2-Affitoxin by tail vein injection, followed by imaging performed 10, 30, 60, and ~120 minutes post-injection. For the same time points, tumor and contra-lateral sites were scanned, as described in the Material and Methods section. A, A representative CCD camera image of mice 2 hours post-HER2-Affitoxin injection. B, CCD camera image of organs 2 hours post-HER2-Affitoxin injection. C, Quantification of tumor-associated fluorescence. The tumor-originated signal, after background subtraction (the signal from the contra-lateral site), was normalized to the maximum uptake observed at the final measurement. D, An example of a fluorescence biodistribution pattern derived from ex vivo scanned organs. Tumors extracted 2 hours post-injection were immunostained using anti-Affibody antibody (Abcam, Cambridge, MA) for HER2-Affitoxin detection (E) and Herceptest™ (Dako, Carpinteria, CA) for HER2 staining (F).
Efficacy of HER2-Affitoxin
Mice bearing subcutaneous BT-474 tumors were divided into 3 experimental groups treated with: (i) saline, (ii) Taq-Affitoxin, and (iii) HER2-Affitoxin. The drugs were injected in 6 fractions every third day to the total dose of 1.5 mg/kg. The average initial volume of the BT-474 tumors was 400 mm3 and continued to grow in both the vehicle-treated control group (saline) and in mice injected with Taq-Affitoxin. By day 24, animals in these groups had to be sacrificed because tumor sizes were approaching the dimension, which, according to Animal Care and Use Committee recommendations and our protocol, mandates euthanasia. Conversely, mice treated with HER2-Affitoxin showed immediate reduction in tumor size. Three days after the first dose of HER2-Affitoxin injection, tumors shrank on average to 60% of their initial size (Figure 3A). By the end of the treatment, the remaining tumor volume in HER2-Affitoxin-treated mice was barely 5% of that measured before the treatment. Moreover, the animals injected with HER2-Affitoxin did not show tumor re-growth within the following 76 days (Figure 3A). All animals tolerated the treatment well and showed only slight (~10%) weight loss.
Figure 3.
Efficacy of HER2-Affitoxin and Taq-Affitoxin in subcutaneous xenografts.
A, Three groups of mice (n = 7–9) bearing BT-474 tumors on the forelimb received 6 intravenous injections of HER2-Affitoxin, Taq-Affitoxin (0.25 mg/kg), or saline every third day, as indicated by arrows. B, Mice bearing relatively large tumors were intravenously injected with 6 doses of HER2-Affitoxin every third day, as indicated by arrows. C, Mice bearing SK-OV-3 tumors (n = 6–7) received 6 doses of HER2-Affitoxin at 0.125 and 0.25 mg/kg, as indicated by arrows. D, Mice with NCI-N87 subcutaneous tumors (n = 4–5) were injected with 3 doses of HER2-Affitoxin at 0.25 and 0.5 mg/kg, as indicated by arrows. Tumor growth and body weight were measured twice a week. The p value was calculated by the one-tailed t-student test (GraphPad Software, Inc., San Diego, CA) *p<0.05, **p<0.01, ***p<0.001. Error bars represents standard deviation (SD) of the average tumor volume.
After the course of treatment (6 × 0.25 mg/kg every third day) was completed, liver enzyme levels were analyzed to assess the potential hepatotoxicity of the drug. The results revealed that the average alanine aminotransferase (ALT) level in the plasma was approximately 3 times higher in drug-treated than in control animals, whereas for aspartate aminotransferase (AST), the ratio was less than 2. No significant differences in the alkaline phosphatase (ALKP) or bilirubin levels were observed (Supplementary Table 4).
It is particularly noteworthy that when mice bearing extremely large tumors (average volume above 1,000 mm3) received six 0.1-mg/kg doses of HER2-Affitoxin, the tumors responded immediately, and the tumor volume, after completion of the treatment, was on average reduced to 100 mm3. These tumors did not re-grow during the following 2 weeks (Figure 3B).
HER2-Affitoxin efficacy was also tested using ovarian cancer xenografts. Three doses of HER2-Affitoxin (0.25 mg/kg, every second day) administered to mice bearing SK-OV-3 tumors (volume < 100 mm3) resulted in a significant delay in tumor growth (p < 0.001). However, 1 month post-injection, the tumors started to re-grow. Repeated treatment with HER2-Affitoxin (3 doses of 0.25 mg/kg each, administered every second day) resulted in further growth delay but, unlike the case of BT-474, it did not completely eradicate the tumors (Figure 3C). Additionally, unlike the case of BT-474 tumors, lower doses of HER2-Affitoxin failed to affect the SK-OV-3 tumor growth. As depicted on Figure 3D treatment of mice bearing NCI-N87 tumors treated with HER2-Affitoxin at 0.5 and 0.25 mg/kg significantly delayed the tumor progression.
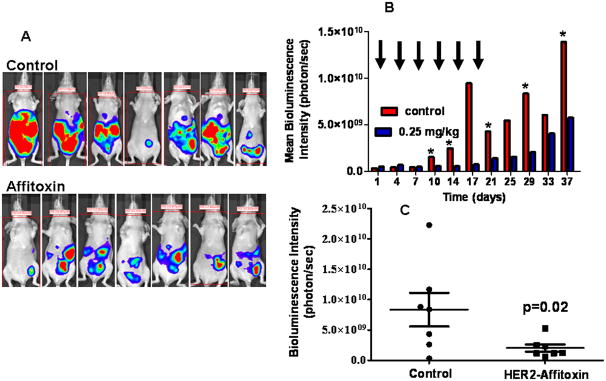
To assess the efficacy of HER2-Affitoxin in treating disseminated cancer, luminescent SK-OV-3 cells were injected into the peritoneal cavity of mice, and the treatment was initiated 1 week later. Two groups of mice received either HER2-Affitoxin or saline (Figure 4A). Tumor progression was measured every 3–4 days with bioluminescence imaging (BLI) and showed that HER2-Affitoxin significantly slowed the tumor progression (Figure 4B and C). However, like in the subcutaneous model, the treatment failed to completely eradicate the tumors.
Figure 4.
Treating disseminated ovarian SK-OV-3 tumors with HER2-Affitoxin.
Mice bearing peritoneal SK-OV-3-luc-D3 tumors were treated with vehicle (control) or received 6 doses of HER2-Affitoxin (0.25 mg/kg) every third day, as indicated by arrows. A, BLI images taken 11 days after the last dose of HER2-Affitoxin was injected. B, Changes in mean bioluminescence intensity during the course of the experiment (arrows indicate HER2-Affitoxin injection time points and asterisks represent the statistical significance between groups; p < 0.05, as determined by t-student test). C, Average and signal distributions in control- and HER2-Affitoxin-treated groups 11 days after the last dose was injected (p value was determined by one-tailed t-student test). Control and treated groups of mice were injected IP with D-Luciferin solution (75 mg/kg). Images were acquired using IVIS Lumina imaging systems 8–10 minutes after D-Luciferin administration.
Immunogenicity of HER2-Affitoxin
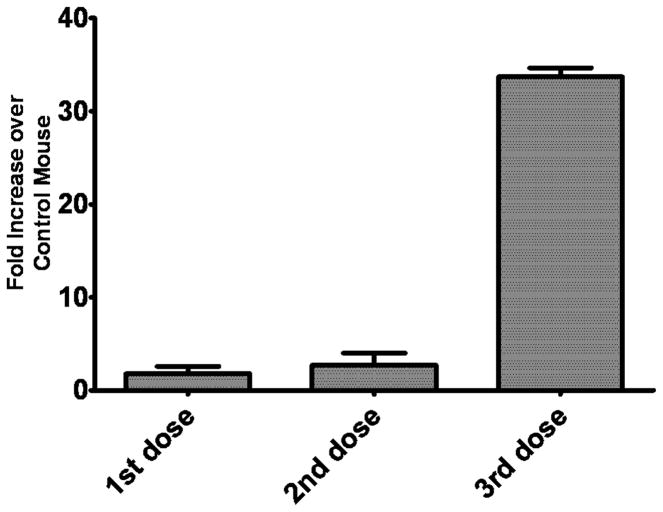
To evaluate the immunogenicity of HER2-Affitoxin, immunocompetent mice received 3 doses of the protein 2 weeks apart (22). The development of HER2-Affitoxin-specific antibodies and the proliferative ability of splenocytes to HER2-Affitoxin were investigated 10 days post-injection of each dose. After the first and second administration, anti-HER2-Affitoxin antibody levels were similar to vehicle-only control animals. A considerable increase in anti-HER2-Affitoxin antibodies was observed only after the third dose of the drug (Figure 5).
Figure 5.
Induction of HER2-Affitoxin antibodies.
Mice were injected with 0.25 mg/kg of HER2-Affitoxin, or vehicle alone (control) every 2 weeks for a total of 6 weeks. Plasma samples were collected 10 days after each injection and assayed with ELISA for the presence of HER2-Affitoxin antibodies. Plasma from control mice was subjected to HER2-Affitoxin (HER2-Affitoxin control). Data are depicted as the average fold increase ± standard error of the mean (SEM) of anti-toxin antibodies measured from mice receiving toxin compared to control mice (n = 3–4 mice per group.)
In contrast, only limited antigen-specific proliferation, as measured in recall assays, was observed in mice after the first injection of HER2-Affitoxin, Second, or third dose of the drug did not lead to increase the stimulation index (Supplementary Figure 6). The limited proliferative response to HER2-Affitoxin was not due to toxicity, since preliminary studies demonstrated that HER2-Affitoxin did not appear to be toxic for splenocytes at tested concentrations. As shown in Supplementary Figure 7, HER2-Afitoxin at 500 ng/ml (~10 nM) reduced splenocytes viability by only 25%, while picomolar concentrations were sufficient to eliminate HER2-positive tumor cells (Figure 1B). In addition, these cells proliferated rigorously in response to mitogens and PMA + ionomycin stimulations, demonstrating the proliferating capabilities of the cells (data not shown).
Discussion
The amplified HER2/neu gene and/or the overexpressed protein have been identified in approximately 20% of invasive breast and non–small lung carcinoma, as well as in ovarian carcinomas and B-cell acute lymphoblastic leukemia (23, 24). Particularly in breast cancer, elevated HER2 status is associated with increased proliferation and survival of cancer cells and contributes to poor therapy outcomes and unfavorable prognosis (25, 26).
Although the clinical use of trastuzumab, a humanized HER2-targeted antibody (27–29), has significantly improved treatment outcome, a large fraction of tumors do not respond to antibody treatment or develop resistance to therapy (30). Resistant tumors have still been shown to express HER2; therefore, HER2 can be used as a target for directed delivery of other therapeutic agents (31). Because HER2-Affitoxin uses a mechanism of cytotoxicity that is distinct from that of trastuzumab, it is a potential alternative or complementary therapy for patients who do not benefit from the traditional therapeutic approach.
In this study, HER2-Affitoxin appeared to be well tolerated by animals at a dosage of 0.5 mg/kg and below. The calculated LD50 value (0.572± 0.051 mg/kg) shows a similar potential to induce acute toxicity, as do monovalent and divalent disulfate-stabilized antibody fragments against HER2 fused to PE38 (dsFv-PE38), according to Bera et al. (32).
Biodistribution data obtained in our study showed that HER2-Affitoxin accumulated in the livers of treated animals. Analyzing liver enzyme levels to assess the potential hepatotoxicity of the drug revealed that only moderate toxicity was induced by HER2-Affitoxin treatment [(for example, mice receiving 1 dose of acetaminophen, 300 mg/kg, by oral gavage had an ALT level ~400 times higher than the control group’s level (33)]. Moreover, the treated mice did not show significant weight loss during the treatment and survived more than 2 months without any signs of organ dysfunctions.
Recently, Weldon et al. reported that deleting a significant portion of the domain II of PE38 resulted in a lysosome-resistant immunotoxin with 10-fold-decreased non-specific toxicity. This new toxin, called HA22-LR, retained excellent biological activity against CD22-positive leukemia cells and showed superior activity in animal models (34). It is likely that similar modifications to the PE38 portion of our HER2-Affitoxin molecule could result in HA22-LR-comparable improvements.
HER2-Affitoxin showed a fast rate of clearance from the bloodstream after intravenous administration. The obtained values were comparable to those reported by Bera et al. for the anti-HER2, monovalent e23 dsFv-PE38 fusion (32). This observation, along with biodistribution data (Figure 2), strongly suggests that the filtration through kidney glomerulus is the main clearance mechanism, and seems to be consistent with the HER2-Affitoxin size (46 kDa). On the other hand, relatively fast clearance of the drug from the blood raised the question of whether the drug would be able to reach the tumor tissue and exert its cytotoxic effect. Our in vitro studies showed that HER2-Affitoxin has a high affinity to the receptor (17), which facilitates immediate binding to the cell surface, followed by slower internalization, and, finally, induced cell death. Indeed, HER2-Affitoxin killed nearly 90% of the receptor-positive cell population after 1 minute of exposure, while Taq-Affitoxin required much more time to exert the same effect. Similarly, cells with no receptor expression remained unaffected by both specific and non-specific toxins at up to 1 hour of exposure (Figure 1). These findings suggest that although HER2-Affitoxin is cleared relatively quickly from circulation, it should still have efficacy in vivo. Moreover, normal cells with low HER2 levels should remain unaffected by the toxin.
Biodistribution analysis performed by NIR optical imaging confirmed that an HER2-Affitoxin load given intravenously could be successfully delivered to BT-474 tumors. Tumor immunostaining confirmed retention of HER2-Affitoxin on the cell membrane; however, distribution was not even throughout the tumor tissue (Figure 2E). For comparison, BT-474 tumors extracted from mice injected with HER2-Affibody showed an even distribution of the signal throughout the tissue section (Supplementary Figure 8). This observation is not surprising when the sizes of HER2-Affitoxin and HER2-Affibody are taken into consideration. Approximately 5 times smaller than HER2-Affitoxin, HER2-Affibody molecules show better diffusion, resulting in enhanced tumor penetration.
A high level of drug accumulation was found in the kidneys and liver. Retention in the latter organ may be due to the high dose injected into animals to visualize tumor accumulation; however, a slightly elevated level of the liver enzyme suggests that the liver is involved in HER2-Affitoxin metabolism. It has to be mentioned that HER2-Affibody molecules used for HER2-Affitoxin construction do not cross-react with murine receptors (Supplementary Figure 9). Therefore, in murine models, neither the observed liver and kidney accumulations nor the toxicity can be mediated through HER2. Receptor-dependent toxicity and biodistribution studies of HER2-Affitoxin in normal tissues require the use of other animal models.
In vivo studies demonstrated the therapeutic efficacy of HER2-Affitoxin. HER2-Affitoxin treatment not only resulted in delayed growth of SK-OV-3 and NCI-N87 tumors (Figure 3C and D), but also led to complete remission of relatively large BT-474 subcutaneous tumors. Moreover, the latter tumors did not re-grow 2 months following treatment (Figure 4A). The observed tumoricidal activity of HER2-Affitoxin is mediated by HER2, since mice treated with a non-HER2-specific toxin (Taq-Affitoxin) exhibited the same tumor growth rates as vehicle-injected animals. Interestingly, both SK-OV-3 and NCI-N87 xenografts responded to HER2-Affitoxin treatment when tumor size was relatively small, while larger tumors remained unaffected by HER2-Affitoxin (data not shown). This might result from hampered delivery of HER2-Affitoxin, most likely due to poor drug penetration to the tumor tissue combined with the relatively short half-life of HER2-Affitoxin in circulation. Results from our optical imaging studies support this hypothesis. Even a well-responding BT-474 model showed a much lower accumulation of the toxin than HER2-Affibody molecules, which are approximately 5 times smaller and have a longer, 20-minute circulation half-life (Supplementary Figure 10). On the other hand, BT-474 tumor tissue shows a much higher HER2-Affibody accumulation than what was observed for NCI-N87 or SK-OV-3 tumors, even though, according to our ELISA data, NCI-N87 and SK-OV-3 tumors have higher HER2 expression levels (Supplementary Figure 10). In our previous work, we showed that, while HER2-Affitoxin binding is strictly correlated with HER2 expression levels, the drug response of the cells did not necessarily follow this pattern (17). This indicates that, even in vitro, other cell line–specific factors affect the toxicity of HER2-Affitoxin. The situation is even more complicated in the tumor model level where other factors, including the development of blood vessels and hydrostatic pressure of interstitial fluid, in the tumor tissue may limit the Affitoxin’s delivery and efficacy (35). These microenvironmental characteristics might explain the difference in HER2-Affitoxin’s effectiveness against large NCI-N87 and SK-OV-3 tumors.
A potential solution to the problem of limited delivery to the tumor is to combine HER2-Affitoxin treatment with tumor-penetrating peptides. In a recent report published by Sugahara et al., small iRGD peptides binding to αv integrins significantly increased vascular and tissue permeability in a tumor-specific and neuropilin-1-dependent manner (36). Similar findings have been shown using treatments in combination with small molecules (doxorubicin), nanoparticles (nab-paclitaxel and doxorubicin liposomes), or monoclonal antibodies (trastuzumab). In this case, direct conjugation of the peptides to drugs is not necessary because enhanced drug penetration was observed with co-administration (36).
Immunogenicity studies demonstrated that administering HER2-Affitoxin induces humoral immune response but not cellular-mediated immune response. Anti-HER2-Affitoxin antibodies were observed in treated mice. If these antibodies are neutralizing, their generation may limit the effectiveness of repeated treatment cycles. As our effort to improve HER2-Affitoxin therapeutic potency continues, we will consider introducing several mutations into the PE38 part of the protein, as described by Onda et al. Replacing hydrophobic amino acids within B cell epitopes on PE38 resulted in a less immunogenic version of the drug, leaving cytotoxic and antitumor activities uncompromised (22, 37).
Overall, although further studies are needed to investigate the toxicity of HER2-Affitoxin in animals expressing “human-like” (Affibody-binding) HER2 in normal tissues, our data indicate that this molecule is a potent anticancer drug that might prove to be effective against solid tumors in humans. The fact that a dose as low as 0.1 mg/kg was enough to eradicate relatively large tumors, with only mild toxicity observed at higher doses, suggests a therapeutic window broad enough to provide effective and safe treatment of HER2-positive tumors. These promising data will have to be confirmed by clinical trials addressing the obvious concerns regarding Affitoxin immunogenicity and possible liver and kidney toxicity.
Supplementary Material
Translational Relevance.
A large fraction of HER2-positive tumors do not respond or develop resistance to the current HER2-targeted therapies, including trastuzumab and lapatinib. Since at least some of the resistant tumors continue to express HER2, this receptor can be used as a target for directed delivery of other therapeutic agents. This work describes preclinical studies of HER2-Affitoxin, a novel recombinant protein combining an HER2-specific Affibody and modified Pseudomonas aeruginosa exotoxin A (PE 38), which, after binding to HER2, is internalized and delivered to the cytosol of the tumor cell, where it blocks protein synthesis by ADP ribosylation of eEF-2. Our results indicate that this molecule is a potent anticancer agent that is effective against human HER2-positive tumor xenografts in mice. Since HER2-Affitoxin utilizes a mechanism of cytotoxicity that is distinct from trastuzumab, it might become a potential alternative or complementary therapy for patients who do not benefit from the traditional therapeutic approach.
Acknowledgments
Financial support: This research was supported in part by the Center for Cancer Research, an Intramural Research Program of the National Cancer Institute, and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Additional support came from the Breast Cancer Research Stamp Fund awarded through competitive peer review, and was funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contracts N01-CO-12400 and N01-CO-12401.
The authors would like to thank Affibody AB and Dr. Ira Pastan (NIH) for providing the constructs used to clone the Affitoxin. We also thank Melisa Gregory and Anatoli Malyguine (NCI-Frederick) as well as Dona Butcher, Babak Sabouri, and Alejandra Garcia-Glaessner (NIH) for their technical support and helpful discussions.
Footnotes
Disclaimer: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
References
- 1.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12(3):541–52. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 2.Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11(2):495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 3.Faltus T, Yuan J, Zimmer B, Kramer A, Loibl S, Kaufmann M, et al. Silencing of the HER2/neu gene by siRNA inhibits proliferation and induces apoptosis in HER2/neu-overexpressing breast cancer cells. Neoplasia. 2004;6(6):786–95. doi: 10.1593/neo.04313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nord K, Gunneriusson E, Ringdahl J, Stahl S, Uhlen M, Nygren PA. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat Biotechnol. 1997;15(8):772–7. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- 5.Nygren PA. Alternative binding proteins: affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J. 2008;275(11):2668–76. doi: 10.1111/j.1742-4658.2008.06438.x. [DOI] [PubMed] [Google Scholar]
- 6.Urica N. Radiology and Clinical Immunology, Biomedical. Uppsala University, Medicinska vetenskapsområdet, Faculty of Medicine, Department of Oncology; 2008. EGFR and HER2 Targeting for Radionuclide-Based Imaging and Therapy: Preclinical Studies; p. 50. PhD. [Google Scholar]
- 7.Orlova A, Tolmachev V, Pehrson R, Lindborg M, Tran T, Sandstrom M, et al. Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res. 2007;67(5):2178–86. doi: 10.1158/0008-5472.CAN-06-2887. [DOI] [PubMed] [Google Scholar]
- 8.Ekblad T, Tran T, Orlova A, Widstrom C, Feldwisch J, Abrahmsen L, et al. Development and preclinical characterisation of 99mTc-labelled Affibody molecules with reduced renal uptake. Eur J Nucl Med Mol Imaging. 2008;35(12):2245–55. doi: 10.1007/s00259-008-0845-7. [DOI] [PubMed] [Google Scholar]
- 9.Tran T, Engfeldt T, Orlova A, Sandstrom M, Feldwisch J, Abrahmsen L, et al. (99m)Tc-maEEE-Z(HER2:342), an Affibody molecule-based tracer for the detection of HER2 expression in malignant tumors. Bioconjug Chem. 2007;18(6):1956–64. doi: 10.1021/bc7002617. [DOI] [PubMed] [Google Scholar]
- 10.Tolmachev V, Nilsson FY, Widstrom C, Andersson K, Rosik D, Gedda L, et al. 111In-benzyl-DTPA-ZHER2:342, an affibody-based conjugate for in vivo imaging of HER2 expression in malignant tumors. J Nucl Med. 2006;47(5):846–53. [PubMed] [Google Scholar]
- 11.Tolmachev V, Friedman M, Sandstrom M, Eriksson TL, Rosik D, Hodik M, et al. Affibody molecules for epidermal growth factor receptor targeting in vivo: aspects of dimerization and labeling chemistry. J Nucl Med. 2009;50(2):274–83. doi: 10.2967/jnumed.108.055525. [DOI] [PubMed] [Google Scholar]
- 12.Nordberg E, Orlova A, Friedman M, Tolmachev V, Stahl S, Nilsson FY, et al. In vivo and in vitro uptake of 111In, delivered with the affibody molecule (ZEGFR:955)2, in EGFR expressing tumour cells. Oncol Rep. 2008;19(4):853–7. doi: 10.3892/or.19.4.853. [DOI] [PubMed] [Google Scholar]
- 13.Kramer-Marek G, Kiesewetter DO, Martiniova L, Jagoda E, Lee SB, Capala J. [18F]FBEM-Z(HER2:342)-Affibody molecule-a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur J Nucl Med Mol Imaging. 2008;35(5):1008–18. doi: 10.1007/s00259-007-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SB, Hassan M, Fisher R, Chertov O, Chernomordik V, Kramer-Marek G, et al. Affibody molecules for in vivo characterization of HER2-positive tumors by near-infrared imaging. Clin Cancer Res. 2008;14(12):3840–9. doi: 10.1158/1078-0432.CCR-07-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundberg E, Hoiden-Guthenberg I, Larsson B, Uhlen M, Graslund T. Site-specifically conjugated anti-HER2 Affibody molecules as one-step reagents for target expression analyses on cells and xenograft samples. J Immunol Methods. 2007;319(1–2):53–63. doi: 10.1016/j.jim.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Friedman M, Lindstrom S, Ekerljung L, Andersson-Svahn H, Carlsson J, Brismar H, et al. Engineering and characterization of a bispecific HER2 × EGFR-binding affibody molecule. Biotechnol Appl Biochem. 2009;54(2):121–31. doi: 10.1042/BA20090096. [DOI] [PubMed] [Google Scholar]
- 17.Zielinski R, Lyakhov I, Jacobs A, Chertov O, Kramer-Marek G, Francella N, et al. Affitoxin-A Novel Recombinant, HER2-specific, Anticancer Agent for Targeted Therapy of HER2-positive Tumors. J Immunother. 2009 doi: 10.1097/CJI.0b013e3181ad4d5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf P, Elsasser-Beile U. Pseudomonas exotoxin A: from virulence factor to anti-cancer agent. Int J Med Microbiol. 2009;299(3):161–76. doi: 10.1016/j.ijmm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6(7):559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 20.Hassan M, Riley J, Chernomordik V, Smith P, Pursley R, Lee SB, et al. Fluorescence lifetime imaging system for in vivo studies. Mol Imaging. 2007;6(4):229–36. [PMC free article] [PubMed] [Google Scholar]
- 21.Gunneriusson E, Nord K, Uhlen M, Nygren P. Affinity maturation of a Taq DNA polymerase specific affibody by helix shuffling. Protein Eng. 1999;12(10):873–8. doi: 10.1093/protein/12.10.873. [DOI] [PubMed] [Google Scholar]
- 22.Stish BJ, Oh S, Chen H, Dudek AZ, Kratzke RA, Vallera DA. Design and modification of EGF4KDEL 7Mut, a novel bispecific ligand-directed toxin, with decreased immunogenicity and potent anti-mesothelioma activity. Br J Cancer. 2009;101(7):1114–23. doi: 10.1038/sj.bjc.6605297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22(42):6570–8. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 24.Meric-Bernstam F, Hung MC. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin Cancer Res. 2006;12(21):6326–30. doi: 10.1158/1078-0432.CCR-06-1732. [DOI] [PubMed] [Google Scholar]
- 25.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200(3):290–7. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 26.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 27.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 28.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 29.Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16(8):2659–71. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 30.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 31.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 32.Bera TK, Viner J, Brinkmann E, Pastan I. Pharmacokinetics and antitumor activity of a bivalent disulfide-stabilized Fv immunotoxin with improved antigen binding to erbB2. Cancer Res. 1999;59(16):4018–22. [PubMed] [Google Scholar]
- 33.Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol. 1998;11(6):604–7. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- 34.Weldon JE, Xiang L, Chertov O, Margulies I, Kreitman RJ, FitzGerald DJ, et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood. 2009;113(16):3792–800. doi: 10.1182/blood-2008-08-173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann M, Guschel M, Bernd A, Bereiter-Hahn J, Kaufmann R, Tandi C, et al. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8(2):89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 328(5981):1031–5. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onda M, Beers R, Xiang L, Nagata S, Wang QC, Pastan I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci U S A. 2008;105(32):11311–6. doi: 10.1073/pnas.0804851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.