Abstract
Background
The role of natural aeroallergen exposure in modulating allergen-specific immune responses is not well understood.
Objective
To examine relationships between mouse allergen exposure and mouse-specific immune responses.
Methods
New employees (n=179) at a mouse facility underwent repeated assessment of mouse allergen exposure, skin prick testing (SPT), and measurement of mouse-specific IgG. Relationships between the mean level of exposure, variability of exposure (calculated as log standard deviation), and time to development of immunologic outcomes were examined using Cox proportional hazards models.
Results
By 24 months, 32 (23%) participants had developed a +SPT and 10 (8%) had developed mouse-specific IgG4. The incidence of a +SPT increased as levels of exposure increased from low to moderate, peaking at 1.2 ng/m3 and decreased beyond this point (p=.04). The more variable the exposure was across visits, the lower the incidence of a +SPT (HR [95% CI]: 0.17 [0.07–0.41]). Variability of exposure was an independent predictor of +SPT in a model that included both exposure metrics. In contrast, the incidence of mouse-specific IgG4 increased with increasing levels of mouse allergen exposure (2.9 [1.4–6.0]), and there was evidence of a higher risk of mouse-specific IgG4 with greater variability of exposure (6.3 [0.4–95.2]).
Conclusion
Both level and variability of mouse allergen exposure influence the humoral immune response, with specific patterns of exposure associated with specific immunophenotypes. Exposure variability may be a more important predictor of +SPT, while average exposure level may be a more important predictor of mouse-specific IgG4.
Keywords: mouse allergen, IgE, IgG4, laboratory animal allergy
Introduction
In recent years, our understanding of how natural animal allergen exposure modulates the humoral immune response has grown.1–9 However, the relationship between allergen exposure and adaptive immune responses is quite complex and many questions remain. Allergen exposure has generally been conceived of as intensity or concentration of exposure, but other aspects of exposure also may influence the allergen-specific immune response. For example, variability of allergen exposure may influence the type of humoral immune response that develops, yet there is little published regarding this relationship. In addition, although the relationship between intensity or concentration of allergen exposure and immune responses has been examined in many studies, these studies have largely been cross-sectional;7, 10–13 therefore, conclusions from these studies come with many caveats. Addressing these questions about the relationship of exposure and immune response is critical to understanding whether modification of animal allergen exposure is a scientifically supported approach for prevention of allergic sensitization and disease.
Prospective cohort studies with frequent, repeated assessments of exposure and allergic status are essential for gaining insight into the true nature of the exposure-response relationship, but are difficult to conduct in community-based populations. On the other hand, an occupational setting is ideal for the intensive prospective assessments required, and thus is an appealing model of animal allergen exposure-immune response relationships. We therefore conducted a prospective cohort study of new workers at a mouse research and production facility to test the hypothesis that the level and variability of exposure to mouse allergen influences mouse allergen-specific humoral responses.
Methods
Study Population
As a part of routine procedures at The Jackson Laboratory Health Office, health screening of new employees includes skin prick testing and venipuncture for collection of serum. New employees screened between July 2004 and December 2007 were approached by study staff to determine interest and eligibility for the JAXCohort Study. All new, non-temporary, full-time employees at least 18 years of age were eligible to participate. Interested participants were scheduled for a baseline study visit during which written informed consent was obtained. Consent included permission to use the skin testing results and serum that were collected at their initial health screening visit. The JAXCohort Study was approved by Institutional Review Boards at the Johns Hopkins Medical Institutions and The Jackson Laboratory.
Two hundred sixty of 374 eligible new employees consented to a baseline screening visit. One hundred ninety-six met the eligibility criteria for this analysis, which included: (1) absence of any evidence of a mouse-specific humoral response at baseline, and (2) completion of the first exposure assessment at six months. One hundred seventy-nine (90%) of the 196 eligible employees had valid personal mouse allergen exposure data and comprised the population for this analysis.
Clinical Assessments
Skin testing was performed at baseline and every six months. Skin prick testing (SPT) was performed to 14 allergens at the baseline visit and six allergens at the follow-up visits using the MultiTest II device (Lincoln Diagnostics, Decatur, IL), with a positive histamine control and a negative glycerol control. The allergens tested at the baseline visit were: mouse, rat, cat, dog, D. pteronyssinus, D. farinae, pine, birch, oak, orchard grass, Alternaria, Aspergillus, Penicillium, and ragweed. The allergens tested at the follow-up visits were: mouse, rat, cat, dog, dust mite mix, and pine. A positive skin test was defined as an orthogonal wheal size of 3 mm or greater than the negative control.
Venipuncture was performed at baseline and every six months. Mouse-specific and total IgE were measured by quantitative ImmunoCAP (Phadia, Uppsala, Sweden). A value ≥0.10 kUA/L for mouse-specific IgE was considered positive. Levels of mouse-specific IgG and IgG4 were measured in serum samples using a solid phase antigen-binding assay as previously described.14 The limit of detection for the mouse-specific IgG assay was 20 arbitrary units (AU)/ml, and for the mouse-specific IgG4 assay, 15 AU/ml (1 AU is approximately equivalent to 1.75 ng). Since the mouse allergen-specific IgG assay detects all IgG isotypes, a mouse-specific IgG1-3 response was defined as an IgG response that did not include IgG4.
The baseline questionnaire was administered by study staff and covered demographic information, pulmonary and allergic history, smoking history, and occupational and family history. The follow-up questionnaire was administered every six months and captured interval allergic history and occupational history.
Exposure Assessments
Airborne monitoring to assess breathing zone mouse allergen exposure was conducted starting at the six-month study visit and every six months thereafter. Personal air samples were collected during two full eight-hour shifts within a one week time period, once every six months, using Buck VSS-12® personal sampling pumps with flow rates of two liters per minute. The mean of these two measurements was used to represent average exposure for the worker. Protein was extracted from the filters using standardized procedures.15, 16 Mus m 1, the major mouse allergen, was quantified immunochemically by sandwich ELISA.17, 18 Mouse allergen exposure data were considered invalid if there was evidence of pump malfunction or compromise of the filter (such as a torn filter).
Statistical Analyses
The data were first examined using summary statistics and plots such as histograms and scatter plots to check for non-normal distributions and to explore bivariate relationships. Because the mouse allergen data were highly right skewed, a log transformation was taken and all subsequent analyses were conducted with the log-transformed data.
To assess the relationship between mouse allergen exposure and positive skin prick test (+SPT), Cox proportional hazards models were employed with the outcome being the time until development of +SPT. The same modeling approach was used to estimate the relative risk of developing mouse-specific IgG1-3 and IgG4. A participant was considered to have developed mouse-specific IgG1-3 if he/she had detectable mouse-specific IgG but undetectable mouse-specific IgG4. A participant was considered to have developed mouse-specific IgG4 if he/she had detectable mouse-specific IgG4. Natural splines were used to estimate potential nonlinear exposure-response functions for each of the three outcomes, and partial likelihood ratio tests were used to test for statistically significant nonlinear relationships. For each analysis, the proportional hazards assumption was checked using scaled Schoenfeld residuals.19 Joint models estimating the combined effect of long-term variability and mean level of exposure were fit by including both exposure metrics into the model simultaneously. Goodness of fit of the joint model relative to the single exposure models was assessed with Akaike’s information criterion (AIC), where smaller values of AIC indicate better-fitting models.
Two different metrics of exposure to mouse allergen were used in the statistical analyses: 1) the level of exposure represented by the mean log10 Mus m 1 level across all visits preceding the development of the outcome (e.g. SPT+, IgG1-3, or IgG4); and (2) the long term variation of exposure represented by the log10 standard deviation of log10 Mus m 1 levels across visits preceding development of the outcome. The first metric measures the typical level of Mus m 1 to which each subject was exposed, and the second measures the longer term variability of Mus m 1 exposure across study visits (six month, twelve month, etc.). To calculate the measure of long term variability, at least two visits were required for each subject (n=123). For all analyses, a two-tailed p-value < 0.05 was considered statistically significant. All analyses were performed with R version 2.11.1 (R Development Core Team, 2010).
Results
Study Population
The 179 members of the JAXCohort Study were predominantly white, with a slight preponderance of females and a mean age of 32 years. Prevalences of reported current asthma (10%) and ever smoking (45%) were similar to other US adult populations.20–22 Fifty-one percent had at least one positive skin test on a panel of aeroallergens at baseline, with 35% positive to dust mite, 16% to cat, and 31% to pollens. (Table I) The study population included animal caretakers, administrative/support personnel, scientists, laboratory technicians, and materials/supplies handlers; 61% of the population handled mice. (Table I) Of the 179 subjects analyzed, 43 had worked with mice prior to joining the Jackson Labs.
Table I.
Study Population Characteristics of 179 Participants
| Characteristic | n (%) |
|---|---|
| Sex, female | 96 (53.6) |
| Race, white | 162 (90.5) |
| Educational attainment | |
| ≤ High school graduate | 36 (20.1) |
| Some college | 41 (22.9) |
| College graduate | 50 (27.9) |
| Post graduate work | 52 (29.1) |
| Smoking status | |
| Current smoker | 45 (25.1) |
| Former smoker | 35 (19.6) |
| Never a smoker | 99 (55.3) |
| Allergic History | |
| Asthma, ever | 18 (10.1) |
| Hayfever, ever | 26 (14.5) |
| Sensitization/atopic characteristics | |
| Total IgE (kU/L), median (25–75th%) | 13 (5.2–35.8) |
| Atopic (≥1+SPT) | 91 (50.8) |
| Skin test sensitivity | |
| Cat | 29 (16.2) |
| Dog | 2 (1.1) |
| Dust mite | 63 (35.3) |
| Pollen | 56 (31.3) |
| Mold | 16 (8.9) |
| Job category | |
| Animal caretaker | 66 (36.9) |
| Administrative/support staff | 40 (22.3) |
| Scientist | 33 (18.4) |
| Laboratory technician | 21 (11.7) |
| Materials/supplies handler | 16 (9.0) |
| Other | 3 (1.7) |
The study lasted 42 months; the median time each employee was followed was 23 months (17–30 months; 25–75th%). Seventy-two participants left The Jackson Laboratory during the study, and on average, those who left had higher mean exposure (1.16 ng/m3) to mouse allergen while enrolled in the study compared to those who stayed (0.55 ng/m3; p = 0.06). However, of the 64 (89%) who participated in an exit interview, only one reported leaving employment because of mouse allergy.
Mouse Allergen Exposure
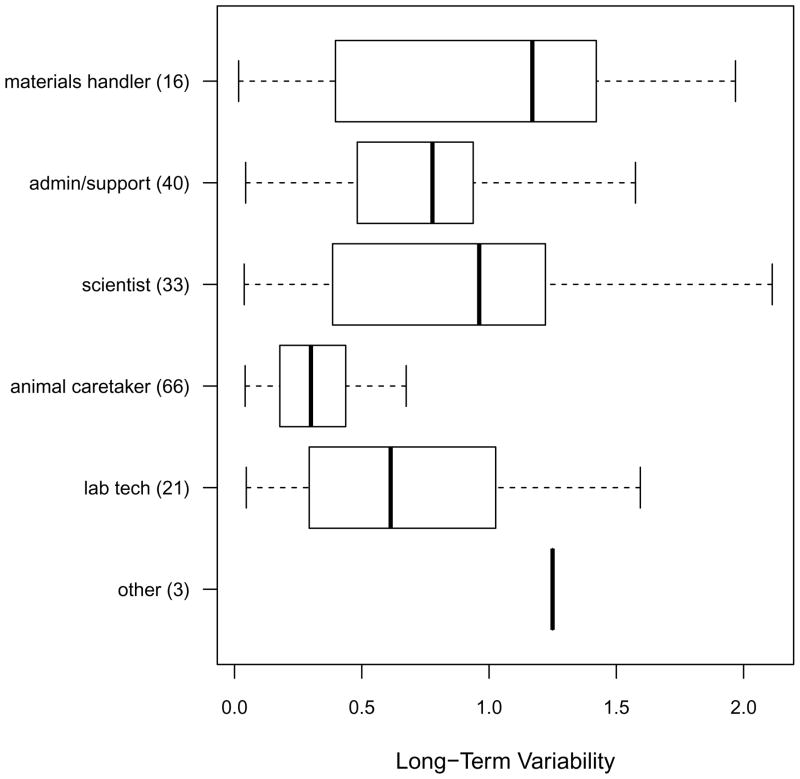
Every six months, participants underwent two days of personal air monitoring to measure mouse allergen exposure. For each six-month assessment, the mean of the first day and second day measurements was calculated for each participant and was taken as an estimate of mouse allergen exposure in the previous six months. This two-day mean mouse allergen concentration was averaged across visits for each participant. For the entire study, the average number of exposure assessments per participant was 2.8, with a range of 1–7 assessments. The median (25th–75th%) of the average mouse allergen concentration across visits was 0.69 ng/m3 (0.09–9.88). The median (25th–75th%) long-term log10 standard deviation was 0.51 (0.29–0.98), so that on average, subjects typically experienced a 3-fold range of variation in their exposure concentrations across their follow-up period. Long-term variability tended to be larger among materials handlers and scientists (≥10-fold range in exposure) and smaller among animal caretakers (2-fold variation in exposure; Figure 1).
Figure 1.
Boxplots of long-term variability of mouse allergen exposure (expressed as the log10 (standard deviation of log10 exposure) by job category. Each box plot indicates the minimum, 25th percentile, median 75th percentile, and the maximum of the data.
Mouse Skin Test Sensitivity
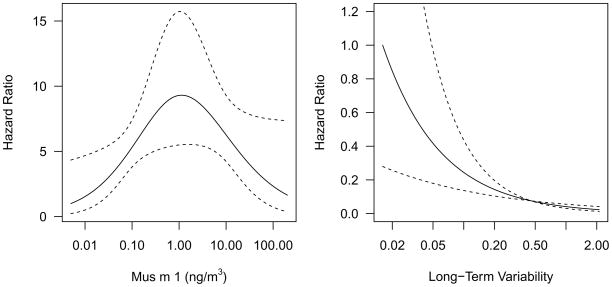
By 24 months, 23% (95% CI: 15–30%) of participants had developed a positive SPT. The risk of a positive mouse SPT was nonlinear, increasing from low to moderate levels of exposure, peaking at approximately 1.2 ng/m3, and then decreasing from moderate to high levels of exposure (p = 0.04; Figure 2a). The analyses of long-term variability of exposure included 123 participants with ≥2 exposure measures. This subset did not differ from the larger study population in terms of population characteristics or mouse allergen exposure. (Table EI) For long-term variability of exposure, the risk of developing a positive mouse SPT decreased with increasing long-term variability in mouse allergen exposure (HR [95% CI]: 0.17 [0.07–0.41]; Figure 2b). We conducted a sensitivity analysis by examining the subset of participants who had not worked with mice prior to joining the Jackson Labs. In this subset the relationships between mean mouse allergen and +SPT and long-term variability and +SPT were similar to the main analysis. Amongst the participants that developed a +SPT, 7 also developed rhinoconjunctival symptoms (sneezing, watery eyes, runny nose) or lower respiratory symptoms (cough, wheezing, chest tightness).
Figure 2.
Plots depicting relationships between (a) level of exposure and (b) and long-term variability of exposure and +SPT. The relationships predicted by the statistical models are indicated by solid lines and 95% confidence intervals are indicated by dotted lines.
Mouse-specific IgG4 and IgG1-3
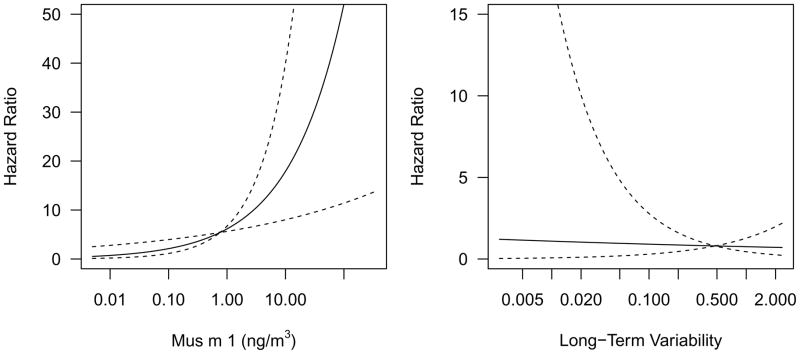
By 24 months, 8% of participants developed mouse-specific IgG4 (95% CI: 3–16%). In models using only one of the exposure measures, high levels of mouse allergen exposure were positively associated with the development of mouse-specific IgG4 (HR [95% CI]: 2.86 [1.39–5.86]), but variability of exposure was not (0.83[0.16–4.32]; Figure 3). By 24 months, 10% of participants developed mouse-specific IgG1-3 (95% CI: 5–15%). Although higher average exposure and lower variability were associated with developing a mouse-specific IgG1-3, these relationships were not statistically significant. (Tables EV-EVI)
Figure 3.
Plots depicting relationships between (a) level of exposure and (b) long-term variability of exposure and mouse-specific IgG4. The relationships predicted by the statistical models are indicated by solid lines and 95% confidence intervals are indicated by dotted lines. Long-term variability is expressed as the log10 (standard deviation of exposure).
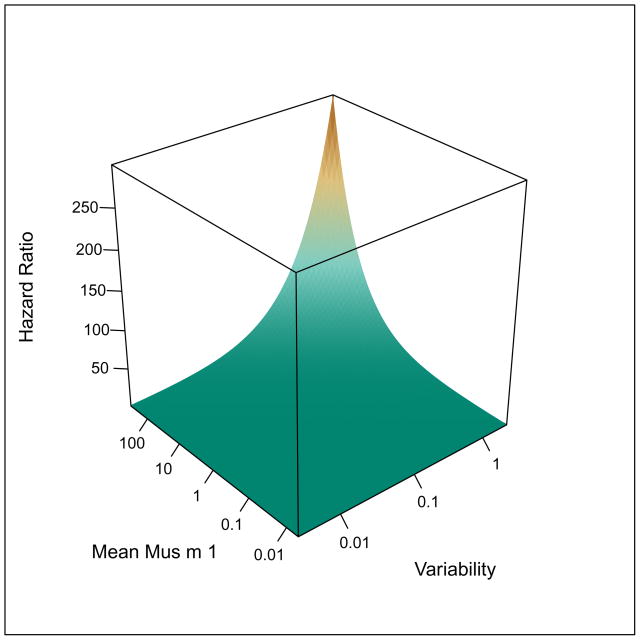
Combined Effects of Variability and Level of Exposure
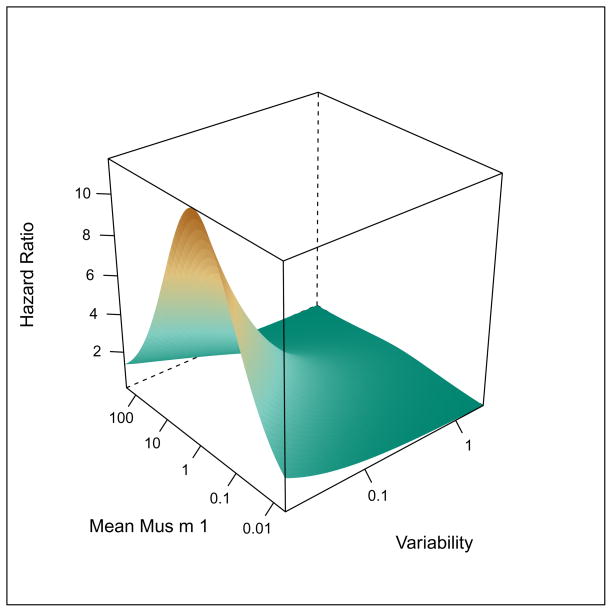
The combined effects of long-term variability and mean level of exposure were modeled using Cox proportional hazard models. (Figure 4) The highest risk of skin test sensitivity was observed with low variability and moderate level of exposure (Figure 4a), but only long-term exposure variability remained an independent predictor of incident skin test sensitivity. Mouse-specific IgE was correlated with mouse skin test sensitivity (cor = 0.44, p < 0.01), but was a rarer event than skin test sensitivity with 5% of participants developing mouse-specific IgE by 24 months. Relationships between average exposure and long term variability of exposure and mouse-specific IgE were consistent with those observed for skin test sensitivity, but were not statistically significant (data not shown).
Figure 4.
Surface plots depicting relationships between level of exposure, variability of exposure and risk of (a) +SPT and (b) mouse-specific IgG4. Long-term variability is expressed as the log10 (standard deviation of exposure).
The highest risk of mouse-specific IgG4 in the models for combined effects was observed with high variability and high level exposure (HR [95% CI]: 6.34 [0.42–95.19]; 3.59 [1.71–7.53, respectively; Figure 4b), but only the level of exposure remained an independent predictor of mouse-specific IgG4. However, this joint model for risk of IgG4 was considered by the Akaike’s information criterion (AIC) to be superior to models including mean exposure or variability alone. Relationships observed between the exposure metrics and +SPT and mouse-specific IgG4 were robust to adjustment for potential confounders, including age, sex, smoking status, total IgE, and respiratory protection use (Online Repository). In addition, atopy, which has been shown to be a risk factor for sensitization and allergen-specific IgG4 in previous studies,8, 23, 24 was also a predictor of these outcomes in this study population (Online Repository).
Skin Test Sensitivity and Mouse-specific IgG Responses
Most study participants developed neither skin test sensitivity nor mouse-specific IgG4. Of the 42 participants who developed either a positive skin test or mouse-specific IgG4, only 2 developed both skin test sensitivity and IgG4 and the remaining 40 developed either skin test sensitivity or mouse-specific IgG4, but not both, suggesting that these two immune responses are inversely related to one another. A similar pattern was observed for skin test sensitivity and mouse-specific IgG1-3.
Discussion
In this occupational JAXCohort Study, both the level and variability of mouse allergen exposure was associated with the pattern of mouse-specific immune responses. A pattern of stable, moderate exposure was most strongly associated with the development of allergic sensitization; a pattern of variable, high level exposure was most strongly associated with an IgG4 response. Further, long-term exposure variability was the more important predictor of skin test sensitivity, while the level of exposure was the more important predictor of mouse-specific IgG4. These findings extend previous work by highlighting the potential role of exposure variability in modulating the risk of allergic sensitization as well as the capacity of multiple characteristics of exposure to simultaneously influence the immune response.
These findings have potentially important implications for primary and secondary prevention of allergic respiratory disease. For primary prevention, tailoring job duties or career paths to avoid moderate, stable exposure may help reduce the incidence of sensitization. For secondary prevention, our findings support the notion that high-level exposure may steer an allergic immune response towards an IgG4 response, thus providing a rationale for evaluating the role of immunotherapy in preventing the development of allergic disease in workers with early evidence of an allergic immune response.
Our findings are consistent with observations made on the humoral response to other allergens in healthy adult populations. For example, beekeepers, who receive intermittent stings separated by periods of no exposure, have a pattern of variable, high-level exposure and primarily mount an IgG4 rather than an IgE response to the honeybee antigens.25 This finding is also consistent with work demonstrating that the development and regulation of IgE follows a unique program that is associated with class switching outside of germinal centers and often involves sequential mu → gamma → epsilon class switching.26 Both poor germinal center development and IgE class switching are favored during chronic exposure of weakly pro-inflammatory antigens,27 as those with stable, moderate dose exposure in this cohort experienced. This observation also suggests that exposure variability may influence the T cell response such that high dose, variable exposure might favor the development of IL-10, which has effects on class switching, plasma cell differentiation and survival favoring IgG4 production over IgE.28
Some previous studies of mouse allergen exposure are consistent with our findings from the JAXCohort Study. In a cross-sectional study, Jeal et al. reported that high rat exposure (more than 50 rats handled per day) was associated with a higher risk of IgG and IgG4 responses, and in contrast, a lower risk of an IgE response and allergic symptoms.10 Results from other occupational studies,6, 7, 29 including a previous cross-sectional study at The Jackson Laboratory,13 differ from results of the JAXCohort Study, generally finding a monotonically increasing relationship between various exposure metrics and risk of sensitization (and IgG or IgG4 for some studies). In one recent study, a higher number of mouse contact hours per months was associated with a greater risk of sensitization, but there was no relationship between this exposure metric and mouse-specific IgG4 levels.30 In this study, participants all handled mice and could have worked at the facility for as long as 18 months at the time of enrollment. Not surprisingly, a significant proportion of participants had mouse-specific IgG4 at enrollment. In contrast, our study included new workers (including non-mouse handlers) with no evidence of a mouse-specific humoral response, so that our findings pertain to exposure-response relationships during an earlier time period in the development of allergen-specific immune responses. Other likely explanations for the apparent discrepancies between some of these other studies and ours include potential bias due to cross-sectional study designs, estimated versus measured allergen exposure, and differences in exposure metrics.6, 7 It is also important to note that many of these other studies reported attenuated risk of some outcomes at the highest levels of exposure, which was sometimes attributed to survival bias.7, 29, 31 In our study, however, there is no evidence to suggest that the findings are a result of survival bias (healthy worker effect, specifically). We interviewed 89% of the participants who left the study, and only one participant reported leaving because of mouse allergy. Although it is possible that workers modify their behavior to reduce exposure when they develop allergic symptoms, only a very small number of sensitized participants had also developed mouse-associated allergic symptoms, so this is unlikely to have biased our results.
Although occupational mouse allergen exposure does not perfectly mimic community exposure, the occupational setting can serve as a model of allergen exposure-immune response relationships. Results of community-based studies of allergen exposure-immune response relationships have been mixed. For example, birth cohort studies have found an increasing prevalence of IgG responses to cat over time32 and dose-dependent relationships between cat allergen exposure and cat-specific IgE and IgG4 responses.33 In contrast, one study of mouse allergen exposure and mouse-specific humoral responses in inner-city preschool children suggested that children with the highest levels of home exposure were less likely to have both sensitization and mouse allergen-specific IgG responses than children with moderate levels of exposure.11 Combined with results of the JAXCohort Study, there is evidence to support further studies of mouse allergen exposure and immune responses in community populations, with the objective of determining whether immune biomonitoring and early immunotherapy are viable research avenues to pursue.
Although our occupational setting can serve as a model for mouse allergen exposure in other settings, we acknowledge two limitations related to the study population and immune response evaluation. Our findings cannot be directly applied to populations that differ from our healthy, predominantly white, worker population. And, while the temporal relationships between our measures of exposure and skin test sensitivity and IgG4 outcomes provide strong evidence for a causal relationship between long-term variability and level of exposure and allergen-specific humoral responses, cellular immune responses were not evaluated, so no conclusions can be drawn regarding the possible cellular immune mechanisms that may be at play.
This prospective JAXCohort Study confirmed findings of previous cross-sectional studies that high level rodent allergen exposure protects against allergic sensitization while promoting allergen-specific IgG4 responses. More importantly, this is the first epidemiologic study, to our knowledge, to find that different characteristics of exposure, namely variability of exposure and level of exposure, can together influence the allergen-specific immune response. Our findings support further examination of the utility of immune biomonitoring and immunotherapy in secondary prevention of rodent allergy.
Supplementary Material
Key Messages.
In this occupational cohort, a pattern of stable, moderate exposure was most strongly associated with the development of allergic sensitization; a pattern of variable, high level exposure was most strongly associated with an IgG4 response.
Since high-level exposure may steer an allergic immune response towards an IgG4 response, allergen immunotherapy should be studied as a possible preventive measure for mouse allergy.
Acknowledgments
Funding: This work was supported by the National Institute of Allergy and Infectious Disease (R01AI070630, R01AI0818451, R03AI0629745, and K23AI060955).
Abbreviations
- AIC
Akaike’s information criterion
- CI
confidence interval
- ELISA
enzyme linked immunosorbent assay
- OR
odds ratio
- SPT
skin prick test
References
- 1.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 2.Custovic A, Hallam CL, Simpson BM, Craven M, Simpson A, Woodcock A. Decreased prevalence of sensitization to cats with high exposure to cat allergen. J Allergy Clin Immunol. 2001;108:537–9. doi: 10.1067/mai.2001.118599. [DOI] [PubMed] [Google Scholar]
- 3.Custovic A, Simpson BM, Simpson A, et al. Current mite, cat, and dog allergen exposure, pet ownership, and sensitization to inhalant allergens in adults. J Allergy Clin Immunol. 2003;111:402–7. doi: 10.1067/mai.2003.55. [DOI] [PubMed] [Google Scholar]
- 4.Almqvist C, Egmar AC, Hedlin G, et al. Direct and indirect exposure to pets - risk of sensitization and asthma at 4 years in a birth cohort. Clin Exp Allergy. 2003;33:1190–7. doi: 10.1046/j.1365-2222.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- 5.Wegienka G, Johnson CC, Havstad S, Ownby DR, Zoratti EM. Indoor pet exposure and the outcomes of total IgE and sensitization at age 18 years. J Allergy Clin Immunol. 126:274–9. 9, e1–5. doi: 10.1016/j.jaci.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portengen L, de Meer G, Doekes G, Heederik D. Immunoglobulin G4 antibodies to rat urinary allergens, sensitization and symptomatic allergy in laboratory animal workers. Clin Exp Allergy. 2004;34:1243–50. doi: 10.1111/j.1365-2222.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 7.Hollander A, Heederik D, Doekes G. Respiratory allergy to rats: exposure-response relationships in laboratory animal workers. Am J Respir Crit Care Med. 1997;155:562–7. doi: 10.1164/ajrccm.155.2.9032195. [DOI] [PubMed] [Google Scholar]
- 8.Heederik D, Venables KM, Malmberg P, et al. Exposure-response relationships for work-related sensitization in workers exposed to rat urinary allergens: results from a pooled study. J Allergy Clin Immunol. 1999;103:678–84. doi: 10.1016/s0091-6749(99)70242-3. [DOI] [PubMed] [Google Scholar]
- 9.Hesselmar B, Aberg B, Eriksson B, Bjorksten B, Aberg N. High-dose exposure to cat is associated with clinical tolerance--a modified Th2 immune response? Clin. Exp Allergy. 2003;33:1681–5. doi: 10.1111/j.1365-2222.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- 10.Jeal H, Draper A, Harris J, Taylor AN, Cullinan P, Jones M. Modified th2 responses at high-dose exposures to allergen: using an occupational model. Am J Respir Crit Care Med. 2006;174:21–5. doi: 10.1164/rccm.200506-964OC. [DOI] [PubMed] [Google Scholar]
- 11.Matsui EC, Eggleston PA, Breysse PN, Rand CS, Diette GB. Mouse allergen-specific antibody responses in inner-city children with asthma. J Allergy Clin Immunol. 2007;119:910–5. doi: 10.1016/j.jaci.2006.12.663. [DOI] [PubMed] [Google Scholar]
- 12.Matsui EC, Wood RA, Rand C, Kanchanaraksa S, Swartz L, Eggleston PA. Mouse allergen exposure and mouse skin test sensitivity in suburban, middle-class children with asthma. J Allergy Clin Immunol. 2004;113:910–5. doi: 10.1016/j.jaci.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Matsui EC, Krop EJ, Diette GB, Aalberse RC, Smith AL, Eggleston PA. Mouse allergen exposure and immunologic responses: IgE-mediated mouse sensitization and mouse specific IgG and IgG4 levels. Ann Allergy Asthma Immunol. 2004;93:171–8. doi: 10.1016/S1081-1206(10)61471-8. [DOI] [PubMed] [Google Scholar]
- 14.Witteman AM, Stapel SO, Sjamsoedin DH, Jansen HM, Aalberse RC, Van Der Zee JS. Fel d 1-specific IgG antibodies induced by natural exposure have blocking activity in skin tests. Int Arch Allergy Immunol. 1996;109:369–75. doi: 10.1159/000237265. [DOI] [PubMed] [Google Scholar]
- 15.Wood RA, Eggleston PA, Lind P, et al. Antigenic analysis of household dust samples. Am Rev Respir Dis. 1988;137:358–63. doi: 10.1164/ajrccm/137.2.358. [DOI] [PubMed] [Google Scholar]
- 16.Hollander A, Gordon S, Renstrom A, et al. Comparison of methods to assess airborne rat and mouse allergen levels. I. Analysis of air samples. Allergy. 1999;54:142–9. doi: 10.1034/j.1398-9995.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohman JL, Jr, Hagberg K, MacDonald MR, Jones RR, Jr, Paigen BJ, Kacergis JB. Distribution of airborne mouse allergen in a major mouse breeding facility. J Allergy Clin Immunol. 1994;94:810–7. doi: 10.1016/0091-6749(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 18.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106:1070–4. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 19.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. 1994. pp. 515–26. [Google Scholar]
- 20.McHugh MK, Symanski E, Pompeii LA, Delclos GL. Prevalence of asthma among adult females and males in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2001–2004. J Asthma. 2009;46:759–66. doi: 10.1080/02770900903067895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moorman JE, Rudd RA, Johnson CA, et al. National surveillance for asthma—United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 22.Cory S, Ussery-Hall A, Griffin-Blake S, et al. Prevalence of selected risk behaviors and chronic diseases and conditions-steps communities, United States, 2006–2007. MMWR Surveill Summ. 59:1–37. [PubMed] [Google Scholar]
- 23.Krop EJ, Heederik DJ, Lutter R, et al. Associations between pre-employment immunologic and airway mucosal factors and the development of occupational allergy. J Allergy Clin Immunol. 2009;123:694–700. e1–3. doi: 10.1016/j.jaci.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Gautrin D, Ghezzo H, Infante-Rivard C, Malo JL. Incidence and determinants of IgE-mediated sensitization in apprentices. A prospective study. Am J Respir Crit Care Med. 2000;162:1222–8. doi: 10.1164/ajrccm.162.4.2001023. [DOI] [PubMed] [Google Scholar]
- 25.Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–6. [PubMed] [Google Scholar]
- 26.Erazo A, Kutchukhidze N, Leung M, et al. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aalberse RC, Platts-Mills TA. How do we avoid developing allergy: modifications of the TH2 response from a B-cell perspective. J Allergy Clin Immunol. 2004;113:983–6. doi: 10.1016/j.jaci.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 28.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160:3555–61. [PubMed] [Google Scholar]
- 29.Nieuwenhuijsen MJ, Putcha V, Gordon S, et al. Exposure-response relations among laboratory animal workers exposed to rats. Occup Environ Med. 2003;60:104–8. doi: 10.1136/oem.60.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krop EJ, Doekes G, Heederik DJ, Aalberse RC, Van Der Zee JS. Allergy. IgG4 antibodies against rodents in laboratory animal workers do not protect against allergic sensitization. [DOI] [PubMed] [Google Scholar]
- 31.Cullinan P, Cook A, Gordon S, et al. Allergen exposure, atopy and smoking as determinants of allergy to rats in a cohort of laboratory employees. Eur Respir J. 1999;13:1139–43. doi: 10.1034/j.1399-3003.1999.13e33.x. [DOI] [PubMed] [Google Scholar]
- 32.Jenmalm MC, Bjorksten B. Development of immunoglobulin G subclass antibodies to ovalbumin, birch and cat during the first eight years of life in atopic and non-atopic children. Pediatr Allergy Immunol. 1999;10:112–21. doi: 10.1034/j.1399-3038.1999.00015.x. [DOI] [PubMed] [Google Scholar]
- 33.Lau S, Illi S, Platts-Mills TA, et al. Longitudinal study on the relationship between cat allergen and endotoxin exposure, sensitization, cat-specific IgG and development of asthma in childhood--report of the German Multicentre Allergy Study (MAS 90) Allergy. 2005;60:766–73. doi: 10.1111/j.1398-9995.2005.00781.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.