Abstract
Twenty years ago, adipocytes were largely considered inert energy storage depots. Today, we know that fat cells are highly insulin sensitive with significant endocrine functions. Alterations in adipocyte development or function can contribute to metabolic disease, in particular Type 2 diabetes. The current obesity epidemic that plagues many nations provides a strong rationale for understanding basic adipocyte biology. The JAK–STAT signaling pathway mediates the action of a variety of hormones that have profound effects on adipocyte development and function. In addition, adipocytes secrete hormones that utilize this signaling pathway. This review summarizes research on the expression and function of JAKs and STATs in adipocytes and highlights the roles of JAK–STAT-activating cytokines in adipose tissue.
The JAK–STAT Pathway
The janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway was originally identified through studies of the transcriptional activation of a number of genes in response to a variety of cytokines, growth factors, and hormones (reviewed by [1,2]). Hormones and cytokines induce a broad range of effects on a number of biological processes and activate receptors that do not contain catalytic cytoplasmic domains. To date, there are four identified members in the JAK kinase family (JAKs 1–3 and Tyk2), which associate with cytokine and growth factor receptors. JAK mediated signaling results in the activation of a signal transduction cascade involving STAT proteins. The STAT family of mammalian transcription factors is comprised of seven proteins (STATs 1, 2, 3, 4, 5A, 5B, and 6) that can be tyrosine phosphorylated in response to ligand induced receptor stimulation. Tyrosine phosphorylation results in dimer formation and translocation to the nucleus where STATs bind DNA and positively or negatively regulate transcription. The specificity of STAT activation and function is not completely understood. However, specificity is determined, at least in part, by the receptor and the specific STAT protein, whose distribution and function are each unique [3]. Other conditions such as serine phosphorylation, dimer composition, and the presence of other proteins associated with the STAT dimers may also confer STAT specificity. Hence, the regulation of tissue-specific genes and the ability to have cell specific tasks appears to be an important physiological role of the JAK–STAT pathway. As discussed herein, the expression of several STATs is modulated during adipogenesis and an adipogenic role for STAT5 is well established. Additional functions of JAK–STAT signaling in adipocytes include the transcriptional regulation of genes involved in insulin action and lipid and glucose metabolism.
Expression and function of JAKs in adipocytes
There have been very few studies focusing on JAK expression, activation, and function in fat cells and adipose tissue. In general, these kinases are largely controlled by tyrosine phosphorylation, rather than by expression levels. The ubiquitously expressed JAKs 1 and 2 are present at similar levels in preadipocytes and adipocytes [4], and they are expressed in adipose tissue in vivo [5]. There is one study that indicates Tyk2 and JAK3 are expressed in adipose tissue [5]. However, adipose tissue is comprised of many cell types, and there is no evidence that these two JAK family members are expressed in mature fat cells. Thus, it is highly likely that JAK–STAT signaling in adipocytes occurs primarily via JAKs 1 and 2.
Both preadipocytes and adipocytes are responsive to hormones and growth factors which activate JAKs 1 and 2, including growth hormone (GH), prolactin (PRL), interferon gamma (IFNγ), leukemia inhibitory factor (LIF), oncostatin M (OSM), cardiotrophin-1 (CT-1), ciliary neurotrophic factor (CNTF) [6–15]. Currently, there is no evidence that JAKs play a STAT independent role in modulating adipocyte differentiation. However, several cytokines that inhibit adipogenesis, including IFNγ [16,17], OSM [18,19], and neuropoietin (NP) [20], are potent activators of JAK kinases. To date, only JAKs 1 and 2 have been detected in adipocytes and their roles are solely attributed to their ability to be activated by cytokines and confer STAT activation. There is one exception, however, in which JAK2 was shown to physically interact with aP2 in adipocytes [21]. The aP2 protein is a lipid binding protein that is highly expressed in fat cells and is likely the most abundantly expressed protein in mature adipocytes. It has been shown that aP2 interacts with the unphosphorylated form of JAK2, and the results of this study suggest that ligand-bound aP2 decreases JAK2 signaling [21]. In summary, there is a paucity of data regarding the role of JAKs in adipocytes. Thus, additional investigations will be required to further elucidate the STAT-dependent and/or independent functions of these kinases in fat cells.
STAT expression and function in adipocyte development
The first studies on STAT expression in adipocytes, performed in murine 3T3-L1 cells, demonstrated that the levels of STATs 1, 3, 5A and 5B are modulated during adipogenesis, whereas STAT6 expression is not regulated during fat cell development and is uniform in preadipocytes and adipocytes [22,23]. To date, there is no evidence that STATs 2 and 4 are expressed in fat cells. In cultured murine and human adipocytes, the protein levels of STATs 3 and 5 are upregulated during differentiation [22,23], implicating a role for these STATs in adipogenesis. STAT1, on the other hand, may not play the same role during murine and human adipocyte differentiation. STAT1 exhibits opposing differentiation-dependent expression patterns in 3T3-L1 fat cells [22] and in cultured subcutaneous human adipocytes [23]. Also, mice with a targeted disruption of the Stat1 gene do not exhibit di fferences in weight gain compared to wild type mice, and with the exception of IFN-dependent responses, biologic responses to other cytokines were not defective [24]. While the relevance of modulation of STAT1 expression during adipocyte development has not yet been revealed, several studies discussed herein examined the contribution of STAT3 and STAT5 on fat cell differentiation.
Adipogenesis is governed by a highly coordinated and temporally defined series of events. The JAK2/STAT3 pathway is activated (i.e. STAT3 is tyrosine phosphorylated and translocated to the nucleus) early during adipogenesis (first 2 – 48 hours following induction of preadipocyte differentiation) [25–27] and is involved in achieving maximal adipocyte differentiation potentially through modulation of C/AAAT enhancer binding protein β (C/EBPβ) transcription [27]. Selective inhibitors of the JAK2/STAT3 signaling pathway and STAT3 activation, as well as STAT3 siRNA and a dominant-negative STAT3, all suppressed adipogenesis in vitro [26]. Additionally, activation of protein inhibitor of activated STAT3 (PIAS3), a constitutively expressed repressor of STAT3, is associated with inhibition of adipogenesis in 3T3-L1 cells [28]. Of note, a peroxisome proliferator-activated receptor gamma (PPARγ) agonist, which did not alter RNAi-induced STAT3 inactivation eliminates the suppression of preadipocyte differentiation mediated by inhibition of STAT3, suggesting that STAT3 regulation of adipogenesis occurs upstream of PPARγ activation [26]. Collectively, these studies indicate a role of STAT3 activation in the modulation of adipogenesis. Additional studies have shown that activation of STAT3 may promote adipogenesis via a critical role in mitotic clonal expansion, a proliferative phase that occurs immediately following induction of adipogenesis and is necessary for differentiation of 3T3-L1 fat cells [25,29,30]. Further investigations probing JAK/STAT3 signaling at various stages of adipogenesis in vitro and in vivo are necessary to better understand the role of STAT3 in fat cell development.
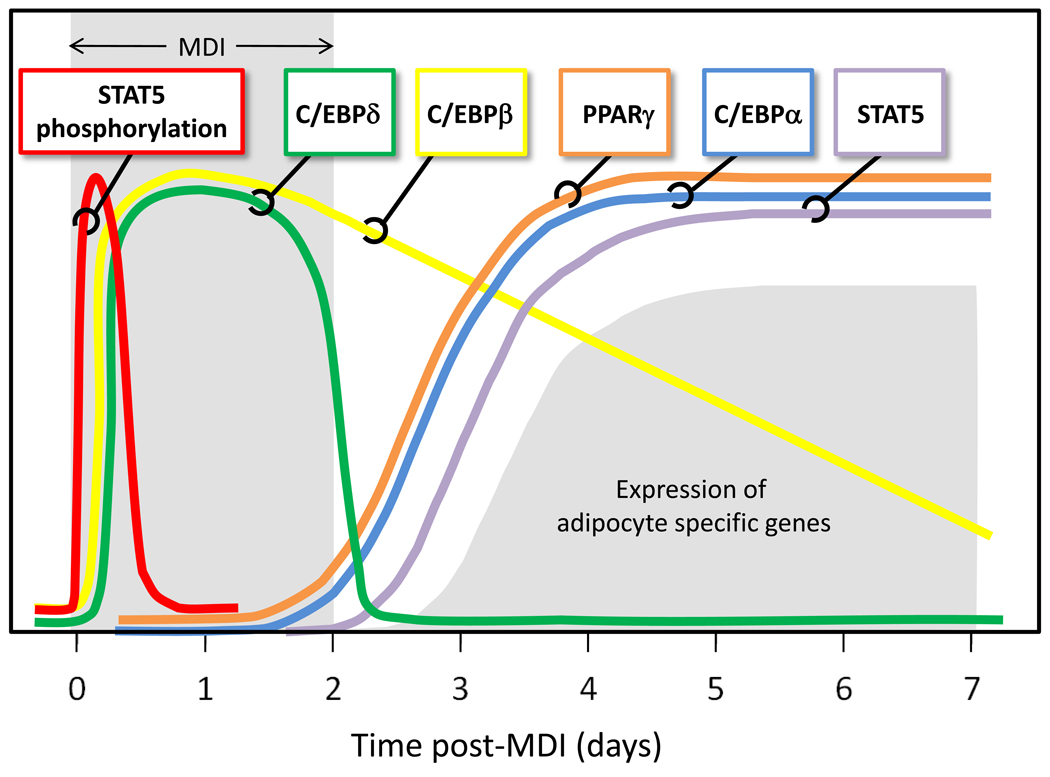
Although STAT3’s involvement in fat cell differentiation is not well defined, a role for STAT5 in adipogenesis is strongly supported (Table 1). Numerous lines of evidence have revealed the importance of STAT5 proteins, in addition to C/EBPs α, β, and δ, PPARγ, and several other adipogenic factors, as transcriptional regulators of fat cell development (reviewed by [20]). As shown in Figure 1, which depicts the expression profiles of several important adipogenic transcription factors, the induction of STAT5 protein expression is coordinately regulated with both PPARγ and C/EBPα. These corresponding regulation patterns have been demonstrated under a variety of conditions in 3T3-L1 cells [4]. Ectopic expression of C/EBPs β and δ in non-precursor cells confers adipogenesis [31], which is accompanied by STATs 5A and 5B accumulation [32]. It should be noted that the increased expression of STAT5 proteins is not typically observed until after the induction of C/EBPα and PPARγ, yet the activation of STAT5 proteins during adipogenesis occurs prior to the upregulation of PPARγ expression in 3T3-L1 cells [33] (Figure 1). In fact, both STAT5 proteins are tyrosine phosphorylated and translocate to the nucleus within 15 minutes after preadipocytes are induced to differentiate [33,34].
Table 1.
Evidence supporting adipogenic nature of STAT5.
| Model System | Evidence to support STAT5 is adipogenic | Reference | |
|---|---|---|---|
| MOUSE | 3T3-L1 | STAT5A and STAT5B expression induced during adipogenesis | [22] |
| Ectopic expression promotes adipogenesis in preadipocytes | [37] | ||
| Activation of STAT5 proteins occurs early in adipogenesis | [33] | ||
| Dominant negative STAT5 attenuates PPARγ activity | [8] | ||
| 3T3-F442A | STAT5 antisense blocks GH dependent adipogenesis | [35] | |
| Constitutively active STAT5 promotes adipogenesis | [36] | ||
| NIH 3T3 | Ectopic expression of STAT5A alone, or with STAT5B, confers adipogenesis in these non-precursor cells | [33] | |
| Balb/c | Ectopic expression of STAT5A alone, or with STAT5B, confers adipogenesis in these non-precursor cells | [33] | |
| Athymic Mice | Ectopic STAT5A expression in Swiss 3T3 cells promotes adipogenesis in vivo | [38] | |
| Transgenic Mice | Knockout of STAT5A and STAT5B results in mice with fat pads 1/5 normal size | [39] | |
| HUMAN | Human Preadipocytes | STAT5A and STAT5B expression induced during adipogenesis | [23] |
Figure 1. STAT5 activation and expression during adipogenesis.
STAT5 represents both STAT 5A and STAT 5B. Both of these STAT5 proteins are activated by tyrosine phosphorylation immediately after the initiation of adipocyte differentiation in 3T3-L1 cells. The activation of STAT5 proteins precedes the induction of C/EBPβ and C/EBPδ, two transcription factors that are induced early during adipogenesis. Increased expression of STAT 5 proteins correlates with induction of both PPARγ and C/EBPα. PPARγ and C/EBPα are important transcription factors in adipocyte development. MDI refers to the hormonal cocktail used for the induction of fat cell differentiation. This cocktail contains methylisobutylxanthine, dexamethasone, and insulin in FBS (fetal bovine serum).
Additionally, several studies investigating the action of growth hormone in promoting adipocyte differentiation have demonstrated a vital role of STAT5 in mediating the action of GH and conferring adipogenesis. In 3T3-F442A preadipocytes, the ability of growth hormone to modulate adipogenesis is attenuated by STAT5 anti-sense oligonucleotides [35], and constitutively active STAT5 is capable of replacing the requirement for growth hormone in adipogenesis of these cells [36]. Ectopic expression studies of STATs 5A and 5B further establish the adipogenic capacity of STAT5 proteins in vitro and in vivo. Ectopically expressed STAT5A induces adipocyte conversion of 3T3-L1 preadipocytes [37] and two different non147 precursor cell lines [33]. Interestingly, STAT5B was not capable of conferring adipogenesis in non-precursor cells [33]. We have also demonstrated that ectopic expression of STAT5A in Swiss 3T3 cells results in the formation of ectopic fat pads in vivo in athymic mice [38].
Coupled with observations in STAT5 null mice, which have fat pads one-fifth normal size [39], the data strongly support that activation of STAT5 proteins is an important driver of adipogenesis both in vitro and in vivo. Moreover, this hypothesis is supported by work demonstrating that STAT5 induces PPARγ expression in coordination with C/EBPβ/δ and also directly stimulates PPARγ transcriptional activity [8], thus suggesting that STAT5 activation drives adipogenesis by inducing PPARγ expression and activity. Furthermore, it has been shown in vitro that STAT5B, but not 5A, binds and transactivates a region of the PPARγ3 promoter [40], an alternative promoter restricted to adipose tissue, macrophages, and colon that contributes to PPARγ1 expression [41]. Of note, a mutation based on a naturally occurring polymorphism that is associated with altered lipid homeostasis is located within the putative STAT binding site of this promoter and eliminates STAT5B binding and activation of a PPARγ3 reporter construct [40].
In summary, the expression of STATs 1, 3, 5A and 5B are modulated during adipocyte development. Whereas the adipogenic capabilities of STATs 1 and 3 are not well defined, STAT5 proteins, particularly STAT5A, are activated and induced during adipogenesis and play an important role in adipose tissue development (Table 1). As discussed in the next section, to date only a few STAT target genes in adipocytes are known. Identification of the STAT-regulated genes in fat cells and examination of how these gene targets might differ during the adipogenic program will be required to fully understand the primary functions of STAT family members in adipocytes.
STAT target genes in mature adipocytes
The tissue distribution of each STAT is unique, and it is widely accepted that STAT proteins have cell-specific functions. Thus, the regulation of tissue-specific genes may be a physiological role for these proteins. Target genes for STATs 1 and 5 in adipocytes have been identified, and their gene products influence adipogenesis, insulin action, and fat and carbohydrate metabolism.
As indicated, studies have revealed the importance of STAT5 proteins during adipogenesis in vitro and in vivo [33,39]. STAT5 proteins are capable of directly binding the PPARγ3 promoter [40] and can transactivate the PPARγ2 and PPARγ3 promoters [8,40]. Although a number of transcription factors have profound effects on adipogenesis, PPARγ is a critical transcriptional regulator facilitating fat cell differentiation. The gene encoding PPARγ is a STAT5 target during adipocyte development and its modulation by STAT5 likely plays a role in the ability of STAT5 to promote adipocyte differentiation.
In adipocytes, studies also identified PPARγ as a STAT1 target gene. Based on the consensus sequence of interferon-γ-activated site (GAS) elements, which are known to mediate IFNγ-sensitive regulation in a STAT-dependent manner, a potential STAT1 binding site was identified in the PPARγ2 promoter. Indeed, STAT1 homodimers bind an IFNγ responsive site within the PPARγ2 promoter in 3T3-L1 adipocytes [42]. These data suggest that IFNγ-induced repression of PPARγ2 [43] is mediated by the direct action of STAT1 on the PPARγ2 promoter. Modulation of both PPARγ activation pathways and IFNγ signaling has been associated with the development of insulin resistance [9,43,44]. Accordingly, STAT1 likely mediates the ability of IFNγ to induce insulin resistance [9,43,45,46] and block adipogenesis [16,17] via transcriptional regulation of PPARγ levels. In another study, an IFNγ-sensitive binding site for STAT1 was discovered in the murine lipoprotein lipase (LPL) promoter [47]. LPL is the rate-limiting enzyme that catalyzes the hydrolysis of serum triglycerides from lipoproteins into free fatty acids for uptake and storage in adipose tissue (reviewed in [48]). In 3T3-F442A adipocytes, IFNγ-activated STAT1 binds to the LPL promoter in a manner that is consistent with IFNγ-induced repression of LPL expression and inhibition of LPL activity [16,49] and lipolysis [50]. While STAT3 also exhibits tyrosine phosphorylation and nuclear translocation in response to IFNγ, STAT1 is a more robust mediator of IFNγ signaling in murine and human adipocytes [6,9,10]. As such, STAT3 was unable to bind to the identified STAT1 binding sites within the PPARγ promoter [42], and LIF, a potent STAT3 activator, does not confer binding of STAT3 to the IFNγ sensitive region of the LPL promoter [47].
Additional studies have focused on the identification of STAT 5 target genes in mature fully differentiated adipocytes. The promoter for acyl CoA oxidase (AOX), the rate limiting enzyme in peroxisomal fatty acid β-oxidation, contains a STAT5 binding site that modulates its gene expression in fat cells [51]. Transfection studies have shown that the promoter activity of aP2, an abundantly expressed lipid binding protein in fat cells, can be activated by STAT5 [52]. Conversely, STAT5 mediates the inhibition of aP2 expression in rat primary preadipocytes [53], which was the first study to suggest that STAT5 proteins could act as transcriptional repressors. Since that time, our own research has revealed that STAT5A can act as a transcriptional repressor in adipocytes. A STAT5A binding site in the murine fatty acid synthase (FAS) promoter mediates the repression of FAS transcription that occurs with prolactin treatment [54]. FAS catalyzes the synthesis of long chain fatty acids and is the key enzyme in de novo lipogenesis. In addition to modulation of genes associated with lipid metabolism, STAT5 can also modulate pyruvate dehydrogenase kinase (PDK)-4, a known regulator of glycolysis, that is highly induced in adipocytes by GH or PRL in a STAT5-dependent manner [55]. Under these conditions, the induction of PDK4 is accompanied by insulin resistance. It is well known that PRL and GH are important modulators of lipid metabolism and are also potent inducers of STAT5 in adipocytes [6,52]. Hence, many of the metabolic actions of these hormones could be mediated by STAT5’s direct modulation of target genes. Unfortunately, relatively few STAT5 target genes have been identified in adipocytes. Nonetheless, we hypothesize that several other STAT5A target genes that play a role in lipid or glucose metabolism will be identified.
STAT3 is abundantly expressed in adipocytes [22,23], mediates the action of numerous cytokines in fat cells (Table 2), and as reviewed in the previous section may play a role in adipogenesis. However, with the exception of C/EBPβ as a potential STAT3 gene target activated early in the adipogenic program [27], to date no adipocyte-specific direct target genes have been identified for STAT3. Although STAT6 is equivalently expressed in preadipocytes and throughout fat cell differentiation [22], only IL-4 has been shown to activate this transcription factor in 3T3-L1 preadipocytes but not in adipocytes [56]. Thus, activators, functions, and gene targets of STAT6 in both preadipocytes and adipocytes remain to be elucidated. Overall, relatively few STAT-regulated genes have been identified in adipocytes. Nonetheless, the STAT 1, 3, and 5 target genes identified thus far encode proteins that are important for fat cell development and for adipocyte-specific functions, such as insulin sensitivity and lipid and carbohydrate metabolism. Although STATs were originally identified as positive regulators of transcription, they act as both transcriptional activators [8,27,40,51,52,55] and repressors [42,47,53,54] in fat cells. Further studies in both cultured adipocytes and in adipose tissue are needed to reveal the complete regulatory potential of the STAT family members in adipocytes.
Table 2.
Activators of JAK/STAT signaling in adipocytes.
| STATa | Activator | Reference(s) |
|---|---|---|
| STAT1 | IFNγ | [6,9,10] |
| LIF | [6,10,88] | |
| OSM | [6,10] | |
| CT-1 | [14] | |
| GH | [6,89] | |
| IL-11 | [90] | |
| STAT3 | LIF | [6,10,18,88] |
| OSM | [6,10,18,62] | |
| IL-6 | [6,18,91,92] | |
| CNTF | [13,15,63] | |
| CT-1 | [14,63] | |
| IFNγ | [6,9,10] | |
| NP | [62,93,94] | |
| GH | [89] | |
| IL-11 | [90] | |
| STAT5A | GH | [6–8,11,12,89] |
| PRL | [7] | |
| STAT5B | GH | [6–8,11,12,89] |
| PRL | [7] | |
| STAT6 | Unknown | ------ |
Data are mostly from in vitro observations of cultured cells
Distinct functions of STAT5 in preadipocytes and adipocytes
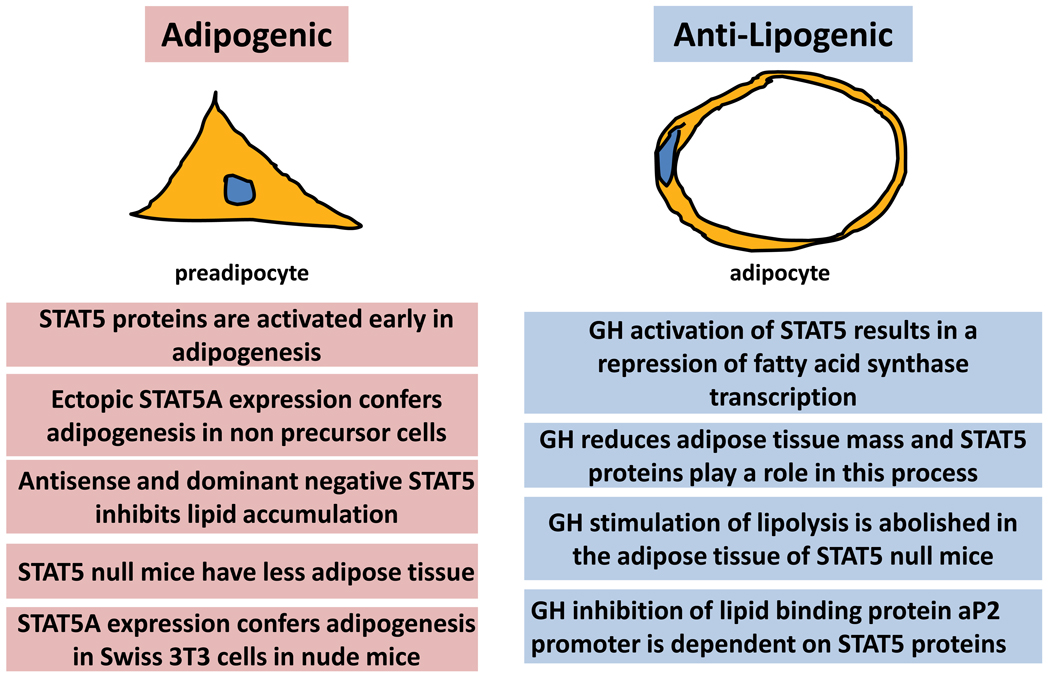
Studies on the identification of STAT5 target genes in adipocytes suggest that these transcription factors modulate gene expression in a manner that favors a reduction in lipid synthesis and/or storage and an increase in lipid release. STAT5A represses the expression of fatty acid synthase [54], an important enzyme in endogenous lipid synthesis. Also, STAT5 inhibits the expression of aP2 [53], a lipid binding protein in adipocytes. The two hormones that have been shown to induce activation of STAT5 proteins in adipocytes are GH and PRL (Table 2). For decades scientists have known that GH reduces adipose tissue mass and there is also evidence of anti-lipogenic effects of PRL [57–60]. A clear role of STAT5 proteins in the ability of GH to induce lipolysis was demonstrated in STAT5 null mice. In these studies, GH was unable to induce lipid release in cells that lack both STAT5 proteins [61]. Collectively, these observations demonstrate anti-lipogenic roles of STAT5 proteins in adipocytes and in adipose tissue.
As already discussed, numerous studies have demonstrated pro-adipogenic effects of STAT5 and have shown that the expression and activation of STAT5 proteins promotes lipid accumulation in a large variety of model systems (Table 1). Therefore, the role of STAT5 is adipogenic in preadipocytes and anti-lipogenic in mature adipocytes (Figure 2).
Figure 2. Opposing functions of STAT5 in preadipocytes and adipocytes.
STAT5 proteins promote adipogenesis and lipid accumulation. Inhibition of STAT5 activation or expression inhibits adipogenesis in vitro and adipose tissue development in vivo. However, in mature adipocytes, there is strong evidence to suggest that STAT5 has anti-lipogenic function.
JAK–STAT activators in adipose tissue
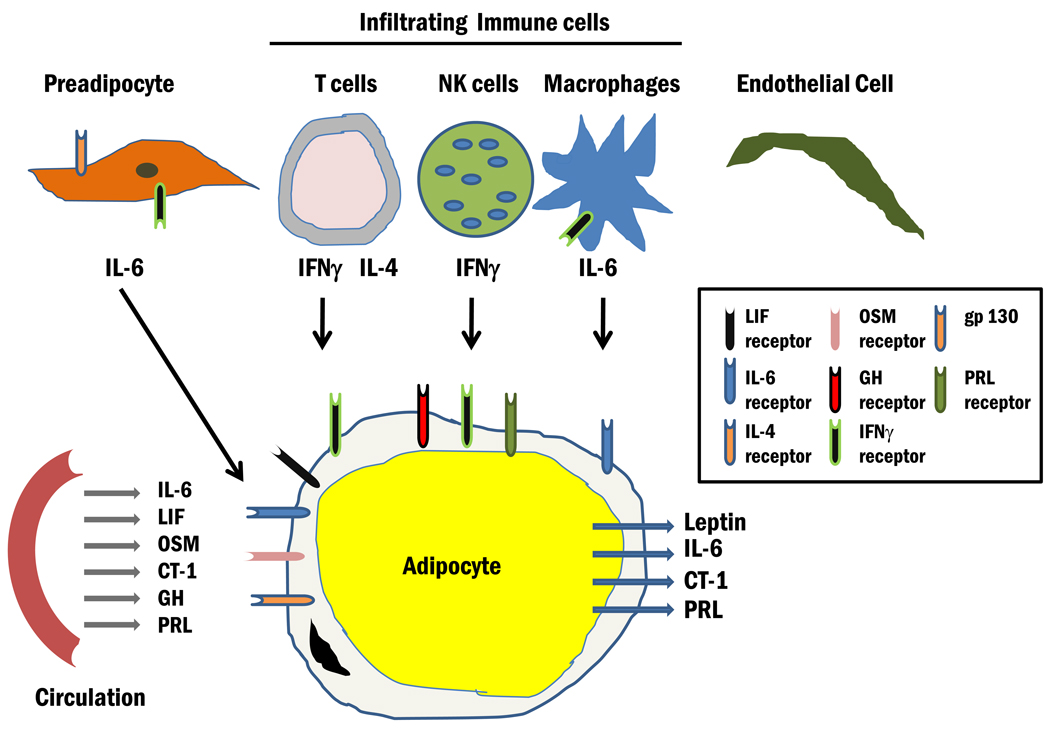
As indicated, adipocytes are responsive to several JAK/STAT activating cytokines and hormones including LIF, OSM, CT-1, interleukin (IL)-6, CNTF, NP, GH, PRL, and IFNγ (Table 2). Most of these observations are based on in vitro studies of cultured adipocytes. However, there is also sufficient and compelling evidence to show that adipose tissue in vivo is responsive to these JAK–STAT activators [12,62,63]. As shown in Figure 3, adipocytes contain receptors for these ligands, most of which are present in circulation. An important function of adipocytes is the production of a variety of endocrine mediators. Of note, there are four JAK–STAT activating hormones which have been shown to be produced from adipocytes (Figure 3), one being leptin. Leptin is an important endocrine hormone that serves as an adiposity signal and can affect food intake and energy expenditure. Of note, the majority of leptin is produced and secreted from adipocytes and the primary target tissue is the arcuate nucleus. Leptin binding to its receptor within this feeding center in the hypothalamus results in JAK2, STAT3, and STAT5 activation. In leptin receptor-deficient mice, analysis of mutant leptin receptor knock-ins has revealed distinct roles of STAT3 and STAT5 in leptin action [64–67]. Besides leptin, other JAK–STAT activating hormones have also been shown to be produced from adipocytes including IL-6 [68,69], CT-1 [70], and PRL [71–74].
Figure 3. The JAK/STAT microenvironment in adipose tissue.
Adipose tissue is largely comprised of adipocytes but also contains preadipocytes, endothelial cells, and infiltrating immune cells, including NK cells, T cells, and macrophages. Adipocytes are highly responsive to many hormones and growth factors that utilize the JAK/STAT pathway. The receptors for these ligands are indicated in the diagram. Infiltrating immune cells also produce cytokines which likely act in a paracrine fashion to induce JAK/STAT signaling in adipocytes. Adipocytes also have important endocrine properties and four JAK/STAT activating hormones have been shown to be produced from adipocytes.
Adipose tissue is largely comprised of adipocytes, but like other tissues contains endothelial cells, connective tissue, and other types of stromal cells. The presence of infiltrating immune cells, such as macrophages and T cells is well documented and studies in the last decade suggest that these cells are modulated in conditions of obesity and Type 2 diabetes. IFNγ is produced from both natural killer (NK) cells [75] and T cells [76–79] present in adipose tissue. IFNγ can inhibit the differentiation of preadipocytes [16,17], induce insulin resistance in mature adipocytes [9,43], and decrease PPARγ expression by targeting this nuclear receptor to the ubiquitin proteasome system for degradation in adipocytes [80]. It is highly likely that the production of IFNγ from infiltrated immune cells acts in a paracrine fashion on adjacent adipocytes to result in insulin resistance. Clearly, JAK–STAT signaling pathways play an important role in the majority of cell types that are found in adipose tissue. Although endothelial cells have endocrine functions, there is no evidence of JAK–STAT producing hormones from these cells. However, it is probable that the production of JAK–STAT ligands from adipocytes and infiltrating immune cells likely impacts the functions of endothelial cells that reside in adipose tissue.
Concluding Remarks
A summary of the current literature on the JAK–STAT signaling pathway in fat cells and adipose tissue reveals several conclusions. First, adipocytes and adipose tissue are highly responsive to a wide variety of hormones and growth factors that utilize the JAK–STAT pathway. Second, adipocytes produce hormones, such as leptin, that can act in an endocrine manner and utilize the JAK–STAT signaling pathway in its target tissues. In addition to leptin, other JAK–STAT ligands including CT-1, PRL, and IL-6 are produced from adipocytes and likely act in autocrine, paracrine, and/or endocrine manners. In general, the cell-specific functions of JAKs and STATs in adipocytes are poorly understood. However, STAT5 clearly plays a role in adipocyte development. Whereas in preadipocytes, STAT5 proteins act in a manner that favors lipid accumulation, in mature adipocytes these transcription factors promote lipid loss. Studies in liver indicate that JAK2 and STAT5 proteins act to prevent hepatic lipid accumulation in mice as the absence of either JAK2 [81] or both STATs 5A and 5B [82,83] results in fatty livers and steatosis. Collectively, these observations suggest that STAT5 proteins in liver and fat are acting to limit lipid accumulation in these two metabolically active, insulin responsive tissues. Studies in mice lacking STAT3 in liver have revealed a role of this STAT in regulating glucose homeostasis by suppressing the expression of gluconeogenic genes in the liver [84]. Ectopic expression of STAT3 in liver shows an increase in the levels of atherogenic lipids, suggesting that STAT3 plays a role in expression of hepatic genes involved in lipid metabolism [85]. Tissue-specific knockouts of STAT3 and STAT5 have revealed roles of these STATs in lipid and glucose metabolism. However, to date, there is only one tissue-specific STAT knockout in adipocytes [86].
Despite the absence of these genetically manipulated mouse models, there is sufficient data to predict key functions of STATs in adipocytes. STAT1 is highly activated by IFNγ, whose production is increased from infiltrating immune cells present in adipose tissue. Known effects of IFNγ include an inhibition of adipogenesis and the induction of insulin resistance. Mice lacking STAT1 in adipocytes may be more insulin sensitive, perhaps in a manner similar to adipocytes that lack tumor necrosis factor (TNF) receptors [87]. However, the inhibition in adipogenesis may also result in ectopic lipid accumulation. The loss of STAT3 in adipocytes is less clear since many cytokines and hormones activate this transcription factor in fat cells. Although there is evidence that STAT3 plays a role in adipogenesis, these studies are based solely on in vitro observations and likely due to alterations in clonal expansion, a phenomenon that may only occur in vitro. The primary activators of STAT3 in adipocytes are the gp130 cytokines. Some gp130 cytokines, like NP and OSM, inhibit adipogenesis. Some gp130 cytokines induce insulin resistance yet others act as insulin sensitizers. Since cells lacking STAT3 would lack the ability of the cytokines to signal through their primary signaling pathway, it is challenging to predict the phenotype of mice lacking STAT3 in fat cells. However, there is a transgenic mouse model where STAT3 expression was knocked out with use of aP2 Cre. The primary phenotype of this mouse was increased weight and increased adipose tissue mass, associated with adipocyte hypertrophy [86]. These studies suggest that STAT3 contributes to body weight homeostasis. However, since aP2 can also be expressed in other cells, it is unclear if these observations are solely mediated by the lack of STAT3 in adipocytes. The loss of both STAT5 proteins in adipocytes would likely result in mice that have reduced fat pad size. We hypothesize that these mice might have ectopic lipid accumulation, particularly if placed on a high fat diet, which would result in insulin resistance. The loss of either STAT5A or STAT5B may result in a similar, but less drastic phenotype than the double null mice. Although the generation of fat-specific STAT knockout mice will shed the light on the function of these transcription factors in fat cells, we predict that a variety of in vitro and in vivo approaches will be required to elucidate additional functions of the JAK–STAT pathway in adipocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 2.Schindler CW. Series introduction. JAK-STAT signaling in human disease. J Clin Invest. 2002;109:1133–1137. doi: 10.1172/JCI15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 4.Stewart WC, et al. Regulation of signal transducers and activators of transcription (STATs) by effectors of adipogenesis: coordinate regulation of STATs 1, 5A, and 5B with peroxisome proliferator-activated receptor-gamma and C/AAAT enhancer binding protein-alpha. Biochim Biophys Acta. 1999;1452:188–196. doi: 10.1016/s0167-4889(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 5.Hellgren G, et al. Growth hormone receptor interaction with Jak proteins differs between tissues. J Interferon Cytokine Res. 2001;21:75–83. doi: 10.1089/107999001750069935. [DOI] [PubMed] [Google Scholar]
- 6.Balhoff JP, Stephens JM. Highly specific and quantitative activation of STATs in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1998;247:894–900. doi: 10.1006/bbrc.1998.8890. [DOI] [PubMed] [Google Scholar]
- 7.Fleenor D, et al. Growth hormone and prolactin receptors in adipogenesis: STAT-5 activation, suppressors of cytokine signaling, and regulation of insulin-like growth factor I. Horm. Res. 2006;66:101–110. doi: 10.1159/000093667. [DOI] [PubMed] [Google Scholar]
- 8.Kawai M, et al. Growth hormone stimulates adipogenesis of 3T3-L1 cells through activation of the Stat5A/5B-PPARgamma pathway. J Mol Endocrinol. 2007;38:19–34. doi: 10.1677/jme.1.02154. [DOI] [PubMed] [Google Scholar]
- 9.McGillicuddy FC, et al. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem. 2009;284:31936–31944. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephens JM, et al. Activation of signal transducers and activators of transcription 1 and 3 by leukemia inhibitory factor, oncostatin-M, and interferon-gamma in adipocytes. J Biol Chem. 1998;273:31408–31416. doi: 10.1074/jbc.273.47.31408. [DOI] [PubMed] [Google Scholar]
- 11.Story DJ, Stephens JM. Modulation and lack of cross-talk between signal transducer and activator of transcription 5 and Suppressor of cytokine signaling-3 in insulin and growth hormone signaling in 3T3-L1 adipocytes. Obesity. (Silver. Spring) 2006;14:1303–1311. doi: 10.1038/oby.2006.148. [DOI] [PubMed] [Google Scholar]
- 12.Zvonic S, et al. Growth hormone, but not insulin, activates STAT5 proteins in adipocytes in vitro and in vivo. Biochem Biophys Res Commun. 2003;302:359–362. doi: 10.1016/s0006-291x(03)00179-7. [DOI] [PubMed] [Google Scholar]
- 13.Zvonic S, et al. The regulation and activation of ciliary neurotrophic factor signaling proteins in adipocytes. J Biol Chem. 2003;278:2228–2235. doi: 10.1074/jbc.M205871200. [DOI] [PubMed] [Google Scholar]
- 14.Zvonic S, et al. Effects of cardiotrophin on adipocytes. J Biol Chem. 2004;279:47572–47579. doi: 10.1074/jbc.M403998200. [DOI] [PubMed] [Google Scholar]
- 15.Ott V, et al. Direct effects of ciliary neurotrophic factor on brown adipocytes: evidence for a role in peripheral regulation of energy homeostasis. J Endocrinol. 2002;173:R1–R8. doi: 10.1677/joe.0.173r001. [DOI] [PubMed] [Google Scholar]
- 16.Gregoire F, et al. Interferon-gamma and interleukin-1 beta inhibit adipoconversion in cultured rodent preadipocytes. J Cell Physiol. 1992;151:300–309. doi: 10.1002/jcp.1041510211. [DOI] [PubMed] [Google Scholar]
- 17.Keay S, Grossberg SE. Interferon inhibits the conversion of 3T3-L1 mouse fibroblasts into adipocytes. Proc. Natl Acad. Sci U. S. A. 1980;77:4099–4103. doi: 10.1073/pnas.77.7.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyaoka Y, et al. Oncostatin M inhibits adipogenesis through the RAS/ERK and STAT5 signaling pathways. J Biol Chem. 2006;281:37913–37920. doi: 10.1074/jbc.M606089200. [DOI] [PubMed] [Google Scholar]
- 19.Song HY, et al. Oncostatin M promotes osteogenesis and suppresses adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem. 2007;101:1238–1251. doi: 10.1002/jcb.21245. [DOI] [PubMed] [Google Scholar]
- 20.White UA, Stephens JM. Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol. 2010;318:10–14. doi: 10.1016/j.mce.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson BR, et al. Interaction of adipocyte fatty acid-binding protein (AFABP) and JAK2: AFABP/aP2 as a regulator of JAK2 signaling. J Biol Chem. 2009;284:13473–13480. doi: 10.1074/jbc.M900075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens JM, et al. The expression and regulation of STATs during 3T3-L1 adipocyte differentiation. J Biol Chem. 1996;271:10441–10444. doi: 10.1074/jbc.271.18.10441. [DOI] [PubMed] [Google Scholar]
- 23.Harp JB, et al. Differential expression of signal transducers and activators of transcription during human adipogenesis. Biochem Biophys Res Commun. 2001;281:907–912. doi: 10.1006/bbrc.2001.4460. [DOI] [PubMed] [Google Scholar]
- 24.Meraz MA, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 25.Deng J, et al. Activation of signal transducer and activator of transcription-3 during proliferative phases of 3T3-L1 adipogenesis. Endocrinology. 2000;141:2370–2376. doi: 10.1210/endo.141.7.7551. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, et al. Signal transducer and activator of transcription 3 (STAT3) regulates adipocyte differentiation via peroxisome-proliferator-activated receptor gamma (PPARgamma) Biol Cell. 2010;102:1–12. doi: 10.1042/BC20090070. [DOI] [PubMed] [Google Scholar]
- 27.Zhang K, et al. JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulating C/EBPbeta transcription. J Cell Biochem. 2011;112:488–497. doi: 10.1002/jcb.22936. [DOI] [PubMed] [Google Scholar]
- 28.Deng J, et al. Protein inhibitor of activated STAT3 inhibits adipogenic gene expression. Biochem Biophys Res Commun. 2006;339:923–931. doi: 10.1016/j.bbrc.2005.10.217. [DOI] [PubMed] [Google Scholar]
- 29.Cernkovich ER, et al. Midkine is an autocrine activator of signal transducer and activator of transcription 3 in 3T3-L1 cells. Endocrinology. 2007;148:1598–1604. doi: 10.1210/en.2006-1106. [DOI] [PubMed] [Google Scholar]
- 30.Machinal-Quelin F, et al. Proadipogenic effect of leptin on rat preadipocytes in vitro: activation of MAPK and STAT3 signaling pathways. Am J Physiol Cell Physiol. 2002;282:C853–C863. doi: 10.1152/ajpcell.00331.2001. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, et al. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephens JM, et al. PPARgamma ligand-dependent induction of STAT1, STAT5A, and STAT5B during adipogenesis. Biochem. Biophys. Res. Commun. 1999;262:216–222. doi: 10.1006/bbrc.1999.0889. [DOI] [PubMed] [Google Scholar]
- 33.Floyd ZE, Stephens JM. STAT5A promotes adipogenesis in nonprecursor cells and associates with the glucocorticoid receptor during adipocyte differentiation. Diabetes. 2003;52:308–314. doi: 10.2337/diabetes.52.2.308. [DOI] [PubMed] [Google Scholar]
- 34.Baugh JE, Jr, et al. The modulation of STAT5A/GR complexes during fat cell differentiation and in mature adipocytes. Obesity. (Silver. Spring) 2007;15:583–590. doi: 10.1038/oby.2007.500. [DOI] [PubMed] [Google Scholar]
- 35.Yarwood SJ, et al. Growth hormone-dependent differentiation of 3T3-F442A preadipocytes requires Janus kinase/signal transducer and activator of transcription but not mitogen-activated protein kinase or p70 S6 kinase signaling. J Biol Chem. 1999;274:8662–8668. doi: 10.1074/jbc.274.13.8662. [DOI] [PubMed] [Google Scholar]
- 36.Shang CA, Waters MJ. Constitutively active signal transducer and activator of transcription 5 can replace the requirement for growth hormone in adipogenesis of 3T3-F442A preadipocytes. Mol Endocrinol. 2003;17:2494–2508. doi: 10.1210/me.2003-0139. [DOI] [PubMed] [Google Scholar]
- 37.Nanbu-Wakao R, et al. Stimulation of 3T3-L1 adipogenesis by signal transducer and activator of transcription 5. Mol Endocrinol. 2002;16:1565–1576. doi: 10.1210/mend.16.7.0862. [DOI] [PubMed] [Google Scholar]
- 38.Stewart WC, et al. STAT5A expression in Swiss 3T3 cells promotes adipogenesis in vivo in an athymic mice model system. Obesity. (Silver. Spring) 2011 doi: 10.1038/oby.2011.66. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teglund S, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 40.Meirhaeghe A, et al. A functional polymorphism in a STAT5B site of the human PPAR gamma 3 gene promoter affects height and lipid metabolism in a French population. Arterioscler. Thromb. Vasc. Biol. 2003;23:289–294. doi: 10.1161/01.atv.0000051382.28752.fe. [DOI] [PubMed] [Google Scholar]
- 41.Fajas L, et al. PPARgamma3 mRNA: a distinct PPARgamma mRNA subtype transcribed from an independent promoter. FEBS Lett. 1998;438:55–60. doi: 10.1016/s0014-5793(98)01273-3. [DOI] [PubMed] [Google Scholar]
- 42.Hogan JC, Stephens JM. The identification and characterization of a STAT 1 binding site in the PPARgamma2 promoter. Biochem Biophys Res Commun. 2001;287:484–492. doi: 10.1006/bbrc.2001.5606. [DOI] [PubMed] [Google Scholar]
- 43.Waite KJ, et al. Interferon-gamma-induced regulation of peroxisome proliferator-activated receptor gamma and STATs in adipocytes. J Biol Chem. 2001;276:7062–7068. doi: 10.1074/jbc.M007894200. [DOI] [PubMed] [Google Scholar]
- 44.Barroso I, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 45.Koivisto VA, et al. Effect of interferon on glucose tolerance and insulin sensitivity. Diabetes. 1989;38:641–647. doi: 10.2337/diab.38.5.641. [DOI] [PubMed] [Google Scholar]
- 46.Shiba T, et al. Hyperglycaemia due to insulin resistance caused by interferon-gamma. Diabet. Med. 1998;15:435–436. doi: 10.1002/(SICI)1096-9136(199805)15:5<435::AID-DIA566>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 47.Hogan JC, Stephens JM. STAT 1 binds to the LPL promoter in vitro. Biochem Biophys Res Commun. 2003;307:350–354. doi: 10.1016/s0006-291x(03)01198-7. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 49.Doerrler W, et al. Cytokines induce catabolic effects in cultured adipocytes by multiple mechanisms. Cytokine. 1994;6:478–484. doi: 10.1016/1043-4666(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 50.Feingold KR, et al. Stimulation of lipolysis in cultured fat cells by tumor necrosis factor, interleukin-1, and the interferons is blocked by inhibition of prostaglandin synthesis. Endocrinology. 1992;130:10–16. doi: 10.1210/endo.130.1.1370149. [DOI] [PubMed] [Google Scholar]
- 51.Coulter AA, Stephens JM. STAT5 activators modulate acyl CoA oxidase (AOX) expression in adipocytes and STAT5A binds to the AOX promoter in vitro. Biochem Biophys Res Commun. 2006;344:1342–1345. doi: 10.1016/j.bbrc.2006.04.071. [DOI] [PubMed] [Google Scholar]
- 52.Nanbu-Wakao R, et al. Prolactin enhances CCAAT enhancer-binding protein-beta (C/EBP beta) and peroxisome proliferator-activated receptor gamma (PPAR gamma) messenger RNA expression and stimulates adipogenic conversion of NIH-3T3 cells. Mol Endocrinol. 2000;14:307–316. doi: 10.1210/mend.14.2.0420. [DOI] [PubMed] [Google Scholar]
- 53.Richter HE, et al. The role of signal transducer and activator of transcription 5 in the inhibitory effects of GH on adipocyte differentiation. J Mol Endocrinol. 2003;30:139–150. doi: 10.1677/jme.0.0300139. [DOI] [PubMed] [Google Scholar]
- 54.Hogan JC, Stephens JM. The regulation of fatty acid synthase by STAT5A. Diabetes. 2005;54:1968–1975. doi: 10.2337/diabetes.54.7.1968. [DOI] [PubMed] [Google Scholar]
- 55.White UA, et al. The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes. 2007;56:1623–1629. doi: 10.2337/db06-1286. [DOI] [PubMed] [Google Scholar]
- 56.Deng J, et al. Interleukin-4 mediates STAT6 activation in 3T3-L1 preadipocytes but not adipocytes. Biochem Biophys Res Commun. 2000;267:516–520. doi: 10.1006/bbrc.1999.1993. [DOI] [PubMed] [Google Scholar]
- 57.Ling C, et al. Identification of functional prolactin (PRL) receptor gene expression: PRL inhibits lipoprotein lipase activity in human white adipose tissue. J Clin Endocrinol Metab. 2003;88:1804–1808. doi: 10.1210/jc.2002-021137. [DOI] [PubMed] [Google Scholar]
- 58.Fortun-Lamothe L, et al. Influence of prolactin on in vivo and in vitro lipolysis in rabbits. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:141–147. doi: 10.1016/s0742-8413(96)00069-2. [DOI] [PubMed] [Google Scholar]
- 59.Fielder PJ, Talamantes F. The lipolytic effects of mouse placental lactogen II, mouse prolactin, and mouse growth hormone on adipose tissue from virgin and pregnant mice. Endocrinology. 1987;121:493–497. doi: 10.1210/endo-121-2-493. [DOI] [PubMed] [Google Scholar]
- 60.Flint DJ, et al. Prolactin and the regulation of adipose-tissue metabolism during lactation in rats. Mol Cell Endocrinol. 1981;22:265–275. doi: 10.1016/0303-7207(81)90096-4. [DOI] [PubMed] [Google Scholar]
- 61.Fain JN, et al. Stimulation of lipolysis but not of leptin release by growth hormone is abolished in adipose tissue from Stat5a and b knockout mice. Biochem Biophys Res Commun. 1999;263:201–205. doi: 10.1006/bbrc.1999.1302. [DOI] [PubMed] [Google Scholar]
- 62.White UA, et al. Gp130 Cytokines Exert Differential Patterns of Crosstalk in Adipocytes Both In Vitro and In Vivo. Obesity. (Silver. Spring) 2011 doi: 10.1038/oby.2010.293. ( http://www.nature.com/oby/index.html) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zvonic S, et al. Cross-talk among gp130 cytokines in adipocytes. J Biol Chem. 2005;280:33856–33863. doi: 10.1074/jbc.M508020200. [DOI] [PubMed] [Google Scholar]
- 64.Bates SH, et al. Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Buettner C, et al. Critical role of STAT3 in leptin's metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gong Y, et al. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 67.Piper ML, et al. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol. 2008;22:751–759. doi: 10.1210/me.2007-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flower L, et al. Stimulation of interleukin-6 release by interleukin-1beta from isolated human adipocytes. Cytokine. 2003;21:32–37. doi: 10.1016/s1043-4666(02)00495-7. [DOI] [PubMed] [Google Scholar]
- 69.Fried SK, et al. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 70.Natal C, et al. Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2008;294:E52–E60. doi: 10.1152/ajpendo.00506.2007. [DOI] [PubMed] [Google Scholar]
- 71.Hugo ER, et al. LS14: a novel human adipocyte cell line that produces prolactin. Endocrinology. 2006;147:306–313. doi: 10.1210/en.2005-0989. [DOI] [PubMed] [Google Scholar]
- 72.Brandebourg T, et al. Adipocyte prolactin: regulation of release and putative functions. Diabetes Obes Metab. 2007;9:464–476. doi: 10.1111/j.1463-1326.2006.00671.x. [DOI] [PubMed] [Google Scholar]
- 73.Hugo ER, et al. Prolactin release by adipose explants, primary adipocytes, and LS14 adipocytes. J Clin Endocrinol Metab. 2008;93:4006–4012. doi: 10.1210/jc.2008-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McFarland-Mancini M, et al. Induction of prolactin expression and release in human preadipocytes by cAMP activating ligands. Biochem Biophys Res Commun. 2006;344:9–16. doi: 10.1016/j.bbrc.2006.03.168. [DOI] [PubMed] [Google Scholar]
- 75.O'Rourke RW, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duffaut C, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler. Thromb. Vasc. Biol. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 77.Rocha VZ, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ. Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strissel KJ, et al. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity. (Silver. Spring) 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang H, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 81.Sos BC, et al. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J Clin Invest. 2011 doi: 10.1172/JCI42894. ( http://www.jci.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cui Y, et al. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- 83.Barclay JL, et al. GH-Dependent STAT5 Signaling Plays an Important Role in Hepatic Lipid Metabolism. Endocrinology. 2011;152:181–192. doi: 10.1210/en.2010-0537. [DOI] [PubMed] [Google Scholar]
- 84.Inoue H, et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med. 2004;10:168–174. doi: 10.1038/nm980. [DOI] [PubMed] [Google Scholar]
- 85.Kinoshita S, et al. Role of hepatic STAT3 in the regulation of lipid metabolism. Kobe J Med Sci. 2008;54:E200–E208. [PubMed] [Google Scholar]
- 86.Cernkovich ER, et al. Adipose-specific disruption of signal transducer and activator of transcription 3 increases body weight and adiposity. Endocrinology. 2008;149:1581–1590. doi: 10.1210/en.2007-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sethi JK, et al. Characterisation of receptor-specific TNFalpha functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett. 2000;469:77–82. doi: 10.1016/s0014-5793(00)01250-3. [DOI] [PubMed] [Google Scholar]
- 88.Hogan JC, Stephens JM. Effects o f leukemia inhibitory factor on 3T3-L1 adipocytes. J Endocrinol. 2005;185:485–496. doi: 10.1677/joe.1.05980. [DOI] [PubMed] [Google Scholar]
- 89.Smit LS, et al. The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol Endocrinol. 1996;10:519–533. doi: 10.1210/mend.10.5.8732683. [DOI] [PubMed] [Google Scholar]
- 90.Tenney R, et al. Interleukin 11 signaling in 3T3-L1 adipocytes. J Cell Physiol. 2005;202:160–166. doi: 10.1002/jcp.20100. [DOI] [PubMed] [Google Scholar]
- 91.Rotter V, et al. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 92.Andersson CX, et al. Insulin antagonizes interleukin-6 signaling and is anti-inflammatory in 3T3-L1 adipocytes. J Biol Chem. 2007;282:9430–9435. doi: 10.1074/jbc.M609980200. [DOI] [PubMed] [Google Scholar]
- 93.White UA, et al. Neuropoietin attenuates adipogenesis and induces insulin resistance in adipocytes. J Biol Chem. 2008;283:22505–22512. doi: 10.1074/jbc.M710462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.White UA, Stephens JM. Neuropoietin activates STAT3 independent of LIFR activation in adipocytes. Biochem Biophys Res Commun. 2010;395:48–50. doi: 10.1016/j.bbrc.2010.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]