Abstract
Purpose
Hypoxia-inducible factor 1 (HIF-1) α is frequently overexpressed in human tumors and is associated with angiogenesis and metastasis. Topotecan, a topoisomerase I inhibitor, has been shown to inhibit HIF-1α expression in preclinical models. We designed a pilot trial to measure HIF-1α inhibition in tumor biopsies from patients with advanced solid tumors over-expressing HIF-1α, after treatment with oral topotecan.
Experimental Design
Topotecan was administered orally at 1.6 mg/m2 once daily for 5 days/week for 2 weeks, in 28-day cycles. Objectives were to determine inhibition of expression of HIF-1α and HIF-1 target genes in tumor; to assess tumor blood flow by dynamic contrast–enhanced magnetic resonance imaging (DCE-MRI); and to measure pharmacokinetics. Tumor biopsies were collected at baseline and during the second cycle of treatment.
Results
Sixteen patients were enrolled. The dose of topotecan was reduced to 1.2 mg/m2/day due to myelosuppression. Seven patients had paired tumor biopsies. In four patients, HIF-1α nuclear staining became undetectable after treatment (7.5%–50% staining at baseline). Decreased levels of VEGF and GLUT-1 mRNA were measured in four patients; the changes were concordant with reduction in HIF-1α in three patients. Decreased tumor blood flow and permeability were observed by DCE-MRI in seven of ten patients after one cycle. One patient had a partial response accompanied by inhibition of HIF-1α in tumor and reduction in tumor blood flow on DCE-MRI.
Conclusions
This multihistology, target assessment trial of a small molecule inhibitor of HIF-1α demonstrated that topotecan could decrease HIF-1α expression in advanced solid tumors.
Keywords: Topoisomerase inhibitor, HIF-1, topotecan, pharmacodynamics, VEGF
INTRODUCTION
Hypoxia-inducible factor 1 (HIF-1) is a basic helix-loop-helix PAS (Per-Arnt-Sim) transcription factor composed of an α subunit, whose levels are tightly regulated by changes in oxygen concentration, and a β subunit, which is constitutively expressed. The α subunit of HIF-1 is rapidly degraded under normal oxygen conditions by ubiquitination and proteasomal degradation in a reaction that requires oxygen, iron, and binding to the product of the tumor suppressor pVHL (1). Several growth factors (such as epidermal growth factor, heregulin, insulin-like growth factor 1 and 2) induce the expression of HIF-1α protein in non-hypoxic conditions, via a PI3K-AKT-mTOR–dependent pathway. Besides physiological stimuli, genetic abnormalities frequently detected in human cancers, including gain of function (e.g., c-src, ras) and loss of function (e.g., VHL, p53, and PTEN) mutations, are associated with the induction of HIF-1α activity and expression of HIF-1–inducible genes. Over-expression of HIF-1α has been found in many human cancers and their metastases (1, 2) and is associated with the induction of genes implicated in angiogenesis (e.g., VEGF), tumor metabolism, invasion (e.g., c-met and CXCR4), and metastasis (3), contributing to poor patient survival (4–9). Thus, inhibition of HIF-1 is an attractive strategy for cancer therapy (10).
Topotecan, a topoisomerase I–targeted inhibitor, is an S-phase specific agent that stabilizes the topoisomerase I enzyme on DNA, causing DNA damage and cell death in the presence of DNA replication. However, we have shown that topotecan also inhibits the accumulation of the α subunit of HIF-1 by a mechanism independent of DNA replication–mediated DNA damage (11–13). Two features of the ability of topotecan to inhibit HIF-1α in vitro are relevant to the design of this clinical trial: the effect is rapidly reversible with removal of the drug (as early as 2 hours), and the daily addition of topotecan to cells cultured under hypoxic conditions significantly decreases the IC50 values for the inhibition of HIF-1α (11–14). Treatment of tumor-bearing animals with low-dose topotecan daily for 10 days resulted in the reduced expression of HIF-1α protein and HIF-1–inducible genes, e.g., VEGF and GLUT-1, in tumor tissues (14). These findings suggest that protracted administration of topotecan may be necessary to achieve a sustained inhibition of HIF-1. Therefore, in this study we decided to administer the injectable formulation of topotecan orally at 1.6 mg/m2/day for 5 days/week for 2 weeks, in 28-day cycles. This is lower than the per-day, US Food and Drug Administration–approved dosage of oral topotecan of 2.3 mg/m2/day once daily for 5 consecutive days, every 21 days, but allowed chronic administration of a potentially safe dose of topotecan.
Based on these preclinical data, we initiated a pilot trial in patients with tumors over-expressing HIF-1α protein to determine whether chronic oral administration of topotecan inhibits HIF-1α expression in tumor biopsy samples; to assess tumor angiogenesis by dynamic contrast–enhanced magnetic resonance imaging (DCE-MRI); and to determine the pharmacokinetics of topotecan administered orally.
PATIENTS AND METHODS
Eligibility criteria
Patients (age ≥18 years) were eligible if they had pathologically confirmed metastatic malignancy for which there were no acceptable standard therapies; had malignancy that expressed HIF-1α protein as measured by immunohistochemistry (IHC) on archival tissue (defined as ≥ 10% of cells showing positive nuclear staining for HIF-1α); had an Eastern Cooperative Oncology Group performance status ≤ 2; and had adequate organ and marrow function defined as absolute neutrophil count ≥ 1,500/μL, platelets ≥ 100,000/μL, total bilirubin ≤ 1.5 X the upper limit of normal (ULN), aspartate aminotransferase and/or alanine aminotransferase <2.5 X ULN, and creatinine <1.5 X ULN. Patients were required to have tumor lesions amenable to biopsy and be willing to undergo paired tumor biopsies.
Patients must have completed prior anticancer therapy at least 4 weeks before starting the study drug and have recovered to eligibility levels from toxicities of prior treatment. Patients were excluded if they had an uncontrolled intercurrent illness; were pregnant or lactating; had received treatment for brain metastases within the past 3 months; or had prior therapy with topotecan.
This trial was conducted after institutional review board approval. The protocol design and conduct followed all applicable regulations, guidances, and local policies. ClinicalTrials.gov Identifier NCT00182676.
Trial design
This was an open-label, pilot trial of oral topotecan in patients with refractory advanced solid neoplasms expressing HIF-1α as defined above. Topotecan was administered orally, initially at the dose of 1.6 mg/m2 once daily for 5 consecutive days, followed by 2 days without drug, and then another 5 consecutive days of treatment; cycle length was 28 days. Restaging by radiologic imaging was performed every two cycles until disease progression. Each vial of the injectable formulation obtained from commercial sources was reconstituted with 4 mL of bacteriostatic water to yield a drug concentration of 1 mg/mL in oral syringes stored at 4°C for up to 14 days. Immediately prior to administration, the appropriate dose was mixed with 30 mL of either apple, orange, or grape juice (acidic medium) and given orally.
Patients were asked to undergo a new tumor biopsy prior to the start of study treatment (baseline) and then at the end of treatment on cycle 2 (day 12 or 13 of cycle 2). All tumor biopsy samples were obtained from metastatic sites by experienced interventional radiologists. During each biopsy procedure, two cores of tumor tissue were obtained (18-gauge in diameter and at least 1 cm in length). One core was processed for evaluation of HIF-1α levels by IHC, and the other core was processed for RNA extraction and evaluation of mRNA expression of HIF-1 target genes. DCE-MRI scans were performed as described in the Supplementary Data, prior to the start of study treatment, at the end of treatment in cycle 1 (day 9 or 10), and at the end of treatment in cycle 2 (day 12 or 13) to assess changes in tumor blood flow. Patients were considered evaluable if they completed two cycles of therapy and had paired biopsy samples collected.
The dose of topotecan was reduced (dose level -1, 1.2 mg/m2/day, or dose level -2, 0.9 mg/m2/day) for grade 4 thrombocytopenia, grade 4 neutropenia lasting longer than 5 days, and/or grade 3 non-hematologic toxicities (except nausea, vomiting, and diarrhea that responded to supportive measures). All toxicities had to resolve to grade ≤1 or pre-treatment baseline levels prior to administering further therapy on study.
Safety and efficacy evaluations
History, physical examination, and complete blood counts with differential and serum chemistries were performed at baseline, and complete blood counts were repeated once a week on treatment. Radiographic evaluation was performed at baseline and every two cycles to assess tumor response based on the Response Evaluation Criteria in Solid Tumors version 1.0 (15).
Pharmacokinetics
Blood samples (7 mL) for topotecan pharmacokinetic analysis were collected on cycle 1 day 1 in tubes containing lithium heparin as anticoagulant. Samples were collected immediately prior to dosing, and at 0.5, 1, 1.5, 2, 3, 4, and 6 hours after administration of topotecan. Plasma concentrations of both the lactone (pharmacologically active form) and carboxylate forms of topotecan were determined simultaneously by high-performance liquid chromatography with fluorescence detection (see Supplementary Data) (16). Compartmental pharmacokinetic analysis was performed on the first oral dose using WinNonlin, version 4.1 (Pharsight). Pharmacokinetic parameters of interest included maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), area under the concentration-time curve from zero to infinity (AUCo-∞), apparent oral clearance (Cl/F), and terminal half-life (T1/2). Absolute doses were used to calculate Cl/F.
Immunohistochemistry
A paraffin-embedded block, or a minimum of five unstained sections, was required for IHC analysis prior to enrollment. For enrollment purposes the archival material was scored solely as positive (defined as at least 10% of cells with positive nuclear staining for HIF-1α) or negative. Intensity of staining was noted, but not used as an entry parameter. Only patients whose tumors scored as positive were eligible for enrollment.
Tumor biopsies obtained on study were scored according to the nuclear staining for HIF-1α in tumor cells. The percentage of tumor cells showing positive nuclear staining for HIF-1α was calculated; cytoplasmic staining and positive stromal cells were not considered. If a patient had several slides with a range of positivity, the highest value was reported.
Immunohistochemistry for HIF-1α was performed using clone H1α67 (Novus Biologicals) at a dilution of 1:2,000. Prior to primary antibody incubation, the slides were subjected to a heat-induced antigen retrieval step consisting of 4 minutes of boiling in a solution of 0.1 mmol/L EDTA, in a microwavable pressure cooker (Nordicware) at full pressure. The primary antibody was incubated overnight, and detection was carried out with the CSA I detection kit (Dako) on an automated robot (Autostainer) according to the manufacturer’s recommendations. When necessary to reduce background staining, additional dilutions of the primary antibody up to 1:8,000 were used.
RNA extraction and real-time PCR analysis
After collection, tumor biopsy samples were submerged in RNAlater RNA Stabilization Reagent (Qiagen), kept at 4°C for 24 hours, and then stored at −20°C. Samples were subsequently disrupted and homogenized in RNA Lysis Buffer (RNeasy Mini Kit, Qiagen) using FastPrep (Lysing Matrix D columns, MP Biomedicals). Total RNA was extracted according to the manufacturer’s procedure. Genomic DNA was digested using RNase-Free DNAse Set (Qiagen) during RNA extraction. RNA integrity was evaluated using RNA 6000 Nano Assay in an Agilent 2100 Bioanalyzer (Agilent Technologies).
RT-PCR was performed using an RT-PCR kit (PE Biosystems) according to the manufacturer’s procedure. VEGF and GLUT-1 expression was assessed by real-time PCR using a 7500 Real-Time PCR System (Applied Biosystems). Typically, 5 ng of reverse-transcribed cDNA per sample was used to perform real-time PCR in triplicate samples. Primers and probes used are listed in Supplementary Table S1.
Detection of 18S rRNA, used as internal control, was performed using premixed reagents from Applied Biosystems. Detection of VEGF and 18S rRNA was performed using TaqMan Universal PCR Master Mix (Applied Biosystems), whereas GLUT-1 detection was performed using Sybr Green PCR Master Mix (Applied Biosystems). Values are expressed as percent change relative to pre-treatment samples for each patient.
Statistical methods
For the purpose of sample size determination, there was one primary endpoint evaluated: expression of HIF-1α protein as determined by IHC. DCE-MRI was evaluated as a secondary endpoint. Results for the primary endpoint were scored on a continuous scale from 0 to 100 (based on the mean percent of cells that stain positive in each biopsy evaluated), and the changes between pre-treatment and the end of treatment on cycle 2 were evaluated. Patients were considered evaluable to assess this primary objective if they completed treatment on cycles 1 and 2 and had paired biopsy specimens available for analysis. With 13 evaluable subjects, there would be 90% power to detect an effect equal to one standard deviation of the differences, using a two-tailed 0.05 alpha-level paired t-test. Since the HIF-1α differences were found to not be normally distributed, a Wilcoxon signed rank test was used instead. In addition, accrual up to 20 patients was permitted to allow for replacement of patients without paired biopsies.
RESULTS
A total of 16 patients were enrolled; the median age was 54 years (Table 1). Patients were heavily pre-treated, with a median of four prior therapies. Eleven patients received at least two cycles of therapy, and of these, seven had paired tumor biopsies and were considered evaluable per protocol.
Table 1.
Patient characteristics
| Number of patients enrolled/evaluable | 16/7 |
| Male/female | 9/7 |
| Median age (range), years | 54 (26–70) |
| ECOG performance status | |
| 0 | 2 |
| 1 | 10 |
| 2 | 4 |
| Median number of prior therapies (range) | 4 (2–8) |
| Diagnosis | |
| Colorectal carcinoma | 5 |
| Ovarian cancer | 2 |
| Adrenocortical cancer | 2 |
| Sarcoma | |
| Alveolar soft part sarcoma | 1 |
| Leiomyosarcoma | 1 |
| Melanoma | 1 |
| Small cell lung cancer | 1 |
| Pancreas adenocarcinoma | 1 |
| Head and neck squamous cell cancer | 1 |
| Bladder transitional cell cancer | 1 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
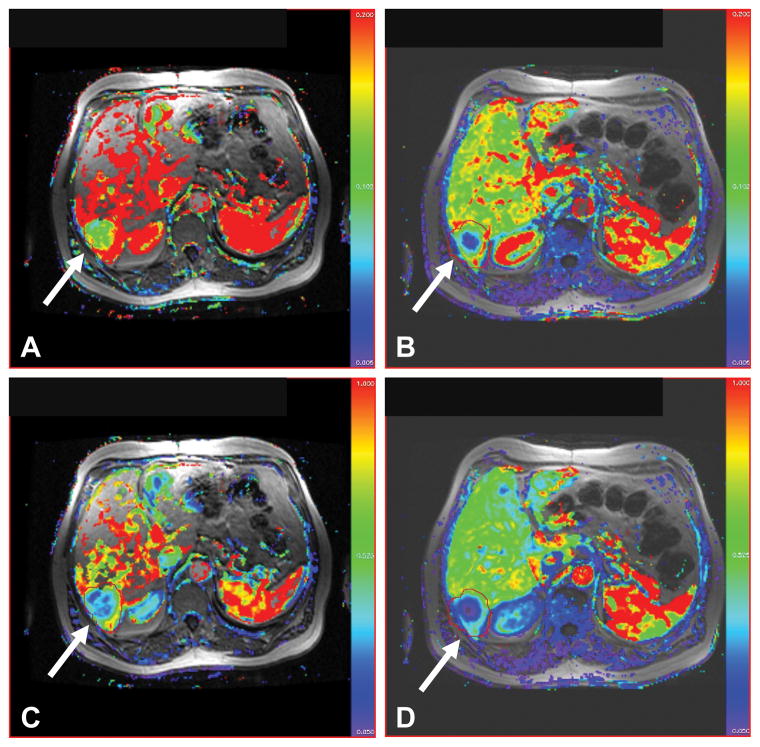
The first two patients received the dose of 1.6 mg/m2/day, and developed grade 4 neutropenia. The dose of topotecan was reduced for subsequent patients to 1.2 mg/m2/day, since the intent was to develop a regimen of oral topotecan that could be safely administered chronically without severe toxicity. There were no unexpected toxicities, with the most common toxicity being myelosuppression, even at the lower dose of 1.2 mg/m2/day (Table 2). A 63-year-old man with metastatic small cell lung cancer, status post disease progression following four cycles of cisplatin and etoposide, received oral topotecan at 1.2 mg/m2/day. He had a partial response to study treatment lasting six cycles, with evidence of inhibition of HIF-1α in tumor biopsy samples (Fig. 1A), and a reduction in tumor blood flow on DCE-MRI (Fig. 2).
Table 2.
Adverse events, by patient, grade 2 or greater and at least possibly related to topotecan
| Adverse event | Grade of adverse event by patient | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Anemia | 2 | 2 | 2 | 2 | 2 | 3 | 4 | 3 | 2 | |||||||
| Diarrhea | 3 | 2 | ||||||||||||||
| Fatigue | 2 | |||||||||||||||
| Leucopenia | 2 | 3 | 2 | 3 | 2 | 2 | 3 | 3 | ||||||||
| Lymphopenia | 3 | 3 | 3 | 2 | 3 | |||||||||||
| Nausea | 2 | 2 | ||||||||||||||
| Neutropenia | 4 | 4 | 3 | 2 | 2 | 3 | 3 | |||||||||
| Thrombocytopenia | 2 | 3 | 2 | 3 | 4 | |||||||||||
NOTE: Patients 1 and 2 received 1.6 mg/m2/day topotecan; all other patients received 1.2 mg/m2/day topotecan.
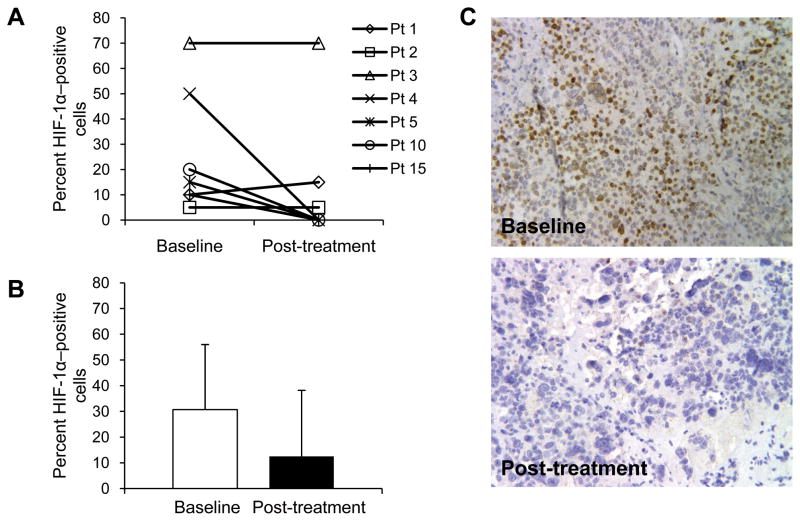
Fig. 1.
Percentage of HIF-1α–positive cells in samples from baseline and post-treametment tumor bisopises from seven evaluable patients, reported per patient (A) and as mean ± SD (B). Immunohistochemistry staining for HIF-1α shown for patient 4 (breast cancer) at baseline and after two cycles of topotecan (C).
Fig. 2.
Ktrans (upper row) and kep (lower row) color maps obtained from DCE-MRI of a 63-year-old man (patient 10) with liver metastases before (A, C) and after treatment during cycle 2 (B, D) show significant reduction in values consistent with response to therapy (arrows); patient had partial response lasting six cycles.
HIF-1α expression
Of the 16 patients who were eligible for the study (HIF-1α nuclear staining in ≥ 10% of cancer cells from archival tissue), 15 underwent a baseline (pre-treatment) biopsy. In these patients, the average percentage ± SD of HIF-1α–positive cells in the archival tissue was 26.4% ± 22.7% (range, 10%–90%) and in the baseline biopsy was 22.1% ± 22.4% (range, 0–70%), suggesting a good correlation between archival tissue and biopsy sample (r= 0.896). Two out of 12 patients whose archival tissue was HIF-1α positive (50% and 10%, respectively) had baseline biopsy samples that did not show any nuclear HIF-1α staining. Seven patients received baseline and post-treatment biopsies and were evaluable for the primary endpoint of the study (Fig. 1). Although there was a decrease in the average percentage of HIF-1α–positive cells in the post-treatment biopsy samples compared to baseline, the difference was not statistically significant (P=0.16) (Fig. 1B). However, in four patients, no HIF-1α nuclear staining was detectable in the post-treatment biopsies, compared with a range of HIF-1α–positive cells of 7.5% to 50% in the baseline biopsies for these patients (Figs. 1A and 1C). In two patients, there was no change in the number of HIF-1α–positive cells between the baseline and post-treatment biopsies.
Expression of HIF-1 target genes
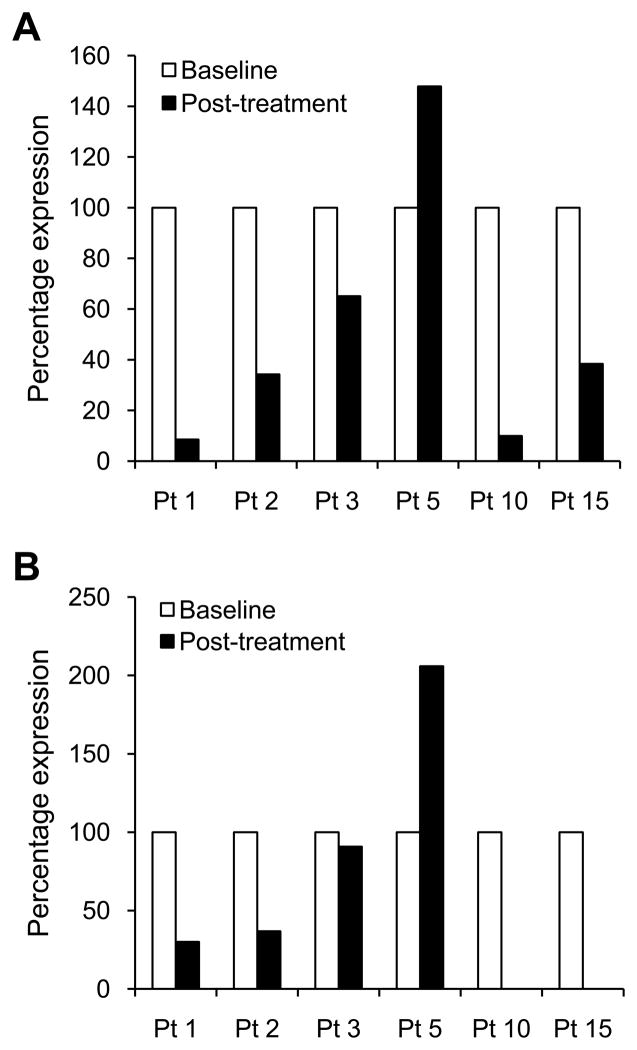
Evaluation of mRNA expression of HIF-1 target genes was performed in six patients for whom RNA was available from paired biopsies. VEGF mRNA expression was decreased in five out of the six evaluable patients (Fig. 3A). Expression of VEGF mRNA, relative to 100% used as an arbitrary value for baseline levels, ranged from 8.5% to 65% in post-treatment biopsies. Patients 1 and 3, for whom no decrease in HIF-1α protein was observed by IHC, had decreased levels of VEGF mRNA expression by 91.5% and 35%, respectively, compared to baseline biopsies. Patient 5 had no decrease in VEGF mRNA expression, despite a decrease in HIF-1α protein to levels undetectable by IHC in the post-treatment biopsy.
Fig. 3.
VEGF (A) and GLUT-1 (B) mRNA epxression in pre- and post-treatment biopsies of six evalubale patients. Results are expressed as percentage mRNA expression realtive to baseline levels, arbitrarily considered equal to 100%. In patients 10 and 15, levels of GLUT-1 mRNA were undetectable in the post-treatment biopsies.
Changes in GLUT-1 mRNA expression (Fig. 3B) were fairly concordant with VEGF mRNA levels. In particular, four out of six patients had decreased levels of both VEGF and GLUT-1 mRNA in the post-treatment biopsies. Patient 5 did not have a decrease in either VEGF or GLUT-1 mRNA expression, and patient 3 had a modest decrease in both VEGF and GLUT-1 by 34.9% and 9.2%, respectively.
DCE-MRI
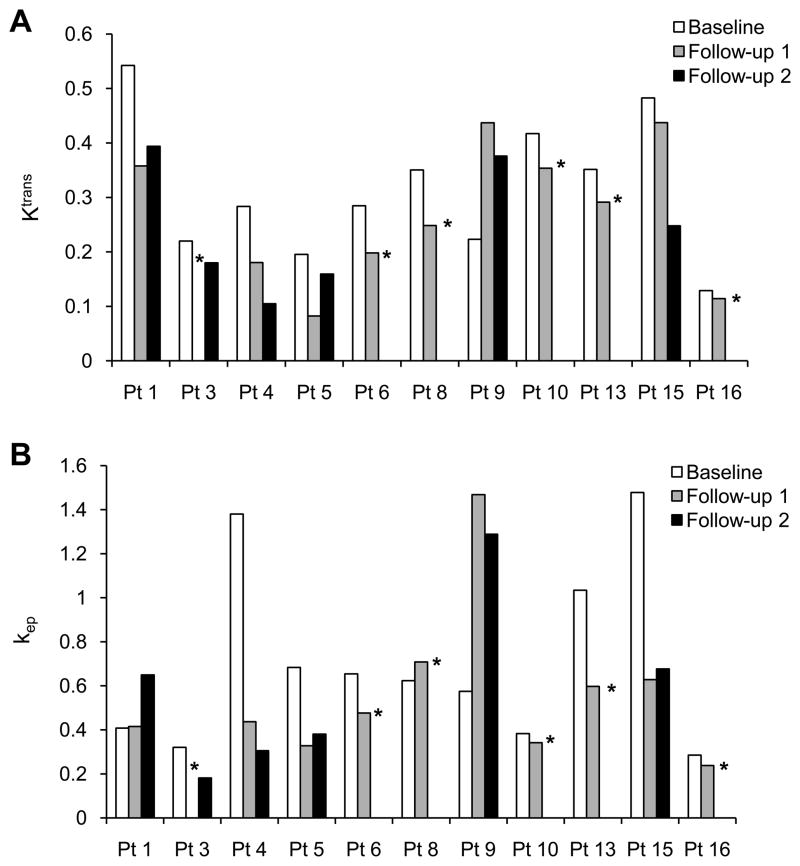
Twelve patients had DCE-MRI scans at baseline. Lesions were found in the liver (5 patients), lung (2 patients), retroperitoneum (2 patients), chest wall (1 patient), axilla (1 patient), and mesentery (1 patient). Eleven patients had at least one follow-up DCE-MRI scan after treatment, and six had two follow-up scans. In one patient, the first follow-up scan was not adequate due to an unsuccessful contrast media injection. Decreases in both Ktrans and kep compared to baseline scan were seen in seven of ten patients who had follow-up DCE-MRI at the first time point and in four of six patients who had follow-up DCE-MRI at the second time point (Figs. 4A and 4B).
Fig. 4.
DCE-MRI results. Ktrans (A) and kep (B) values by patient at baseline and follow-up DCE-MRIs. *No follow-up DCE-MRI at that time point.
Pharmacokinetics
Eleven of the 16 patients enrolled had sufficient pharmacokinetic data available for compartmental analysis. Nine of these patients received topotecan at the dose of 1.2 mg/m2 and two at 1.6 mg/m2, orally in an acidic medium (apple, orange, or grape juice) to prevent hydrolysis into the open/carboxylate form. The mean plasma concentration versus time curves for the closed (lactone) and open (carboxylate) forms of topotecan are shown in Supplementary Fig. S1. The parameters were successfully estimated using a one-compartment model with first-order input. Three patients required a lag-time model to fit the carboxylate plasma-concentration data. All parameters were estimated assuming first-order absorption. Administration of topotecan at 1.2 mg/m2 resulted in higher plasma concentrations for the lactone than the carboxylate form, resulting in 1.5-fold higher Cmax (P=0.019, Mann-Whitney test) and 1.5-fold higher AUC0-∞ (P=0.050, Mann-Whitney test), whereas the Tmax for the appearance of the carboxylate form was 1.27 times longer than the lactone form (P=0.040, Mann-Whitney test) (Supplementary Table S2). There were no significant differences in terminal T1/2 between the two topotecan forms (Supplementary Table S2). Administration of topotecan at 1.6 mg/m2 showed a slightly delayed absorption for both the lactone and carboxylate forms, resulting in a lower Cmax at later Tmax, and lower overall exposure (AUC0-∞/nominal dose) compared to the 1.6 mg/m2 dose group, although given the sample size (n=2), there was limited power to detect significant differences in topotecan absorption or disposition at the higher dose.
The plasma exposure of the active lactone form was correlated with DCE-MRI parameters, Ktrans and kep, the change in percent expression of VEGF and GLUT-1 mRNA, and the change in percentage of HIF-1α–positive cells. No correlation was found between pharmacokinetic metrics of plasma exposure and both the MRI parameters or the change in HIF-1α–positive cells. However, relatively strong correlations were found between the AUC0-∞ and change in percent expression of VEGF (R2=0.70) and GLUT-1 (R2=0.87) (Supplementary Fig. S2), but not with the nominal dose (R2=0.08 and 0.10 respectively), for those patients with a decrease in mRNA expression and evaluable pharmacokinetics (n=4). The plasma exposure revealed no correlation with dose, over the nominal dose range of 1.3 to 3.3 mg (R2=0.016), suggestive of reduced bioavailability (i.e., slight trend observed of an increase in Cl/F with increasing doses; Supplementary Fig. S3).
DISCUSSION
This study is, to the best of our knowledge, the first clinical trial to evaluate an inhibitor of HIF-1α in tumor biopsy samples in a prospective, target-driven trial in patients with advanced solid tumors. Eligibility was based on the presence of target, HIF-1α, independent of histology, in archival tissue samples. We report modulation of HIF-1α expression in tumor tissue, in patients with metastatic cancers treated with topotecan, a topoisomerase I inhibitor. Topotecan has been shown by our group to inhibit HIF-1α expression in cell culture and xenograft models (12, 14). Even though we were able to successfully perform paired tumor biopsies and associated evaluation in only 7 of the 16 patients, there are several conclusions that can be drawn from this clinical trial. Complete inhibition of HIF-1α was observed in biopsy specimens from four out of seven evaluable patients after treatment with topotecan. Variability inherent to biopsy sampling may limit the impact of these findings, due to heterogeneous expression of HIF-1α in solid tumors, in addition to technical challenges related to tissue processing and interpretation of IHC. Interestingly, the levels of HIF-1α staining in this study were comparable between archival tissue and the baseline biopsy for 10 out of 12 patients. However, recognizing the heterogeneity in HIF-1 expression in human tumors, we assessed changes in mRNA expression of HIF-1-target genes, as well as changes in DCE-MRI, to provide an overall evaluation of changes potentially associated with HIF-1 inhibition. Results of the DCE-MRI analysis were consistent with modulation of tumor blood flow and permeability, an expected outcome of HIF-1 inhibition in tumor tissue, dependent, at least in part, on inhibition of VEGF, a known HIF-1 target gene.
Concordant results between levels of HIF-1α protein by IHC and VEGF mRNA expression were only observed for three out of six evaluable patients. In two patients, a decrease in VEGF mRNA was detected in the absence of changes in HIF-1α protein levels by IHC, and in one patient, decreased levels of HIF-1α by IHC did not correlate with a decrease in VEGF mRNA expression. In contrast, there was a good correlation between VEGF and GLUT-1 mRNA levels between pre- and post-treatment biopsies. These discrepancies, which remain difficult to interpret given the small number of patients, may be due to HIF-1α–independent regulation of gene expression, (e.g., HIF-2–dependent or HIF-independent) and/or sampling variability, due to heterogeneous expression of HIF-1 in solid tumors. Alternatively, inhibitory effects on mRNA expression of HIF-1 target genes may last longer than those detectable at the protein level, given the short half-life and rapid turnover of HIF-1α with changes in oxygen concentration. Thus, identification and validation of the most reliable and predictive pharmacodynamic endpoint reflective of HIF-1α modulation remains to be defined in future studies.
Of 16 patients enrolled on study, only 7 received paired biopsies, due to patients experiencing progressive disease before a second biopsy could be performed. Patients were selected based on an arbitrary threshold of ≥ 10% HIF-1α–positive cells in archival tumor tissue, without scoring cytoplasmic staining and positive stromal cells. In an analysis by Zhong et al. of HIF-1α expression in 179 tumor specimens from primary or metastatic cancers, approximately 40% of metastatic lesions expressed HIF-1α in ≥ 10% of cells (1). HIF-1α over-expression has also been associated with treatment failure and patient mortality in a variety of cancers (4–9). Given the heterogeneous expression of HIF-1α in human tumors and the known correlation between HIF-1 over-expression and poor prognosis and response to treatment, this study may have selected patients with unfavorable prognostic features.
Pharmacokinetic analysis revealed that oral administration of topotecan at 1.2 mg/m2 resulted in higher plasma concentrations for the lactone (pharmacologically active form) than the carboxylate form. The reversible hydrolysis from lactone to carboxylate is largely pH dependent. Below pH 4, topotecan exists almost exclusively as lactone; however, at pH 7.2 the carboxylate is three times as likely to be present (17). The mean T1/2 of topotecan lactone at a 1.2 mg/m2 dose (3.86 h) was comparable to 4.22 h previously reported for topotecan lactone at the same dose from a phase I clinical trial by Gerrits et al. (18), in which a similar oral formulation of injectable topotecan was administered.
Efforts to identify small molecule inhibitors of HIF-1 have primarily focused on cell-based high-throughput screening assays. The majority of HIF-1 inhibitors described so far lack specificity, raising the question as to whether HIF-1 inhibition does indeed contribute to the clinical activity of these agents (19). In addition, although genetic approaches have demonstrated that HIF-1 deletion is associated with impaired tumor growth in experimental models, the potential therapeutic effects of HIF-1 inhibition in human tumors remains to be demonstrated. Therefore, it is imperative that early clinical development of putative small molecule inhibitors of HIF-1 be guided by pharmacodynamic evaluation of target modulation in tumor tissue.
Topotecan was used in this study as a prototype HIF-1 inhibitor. However, its short half-life and clinical toxicities argue against its use on a chronic schedule to inhibit signal transduction pathways. In addition, topotecan has been tested in renal cell cancer, a disease in which HIF plays an important role, and was found to be inactive, although it was used on a classic cytotoxic schedule (1.5 mg/m2 IV daily × 5 every 28 days) (20). Topoisomerase I inhibitors with longer half-lives and increased tumor retention (21) may be more suitable for HIF-1 inhibition in the clinic. Finally, single-agent inhibition of HIF-1 may not be sufficient to achieve meaningful therapeutic responses, at least in part due to the limited and heterogeneous expression of HIF-1 in solid tumors. HIF-1 expression has been implicated in mediating resistance to chemotherapy and radiation therapy, suggesting that combination studies should be pursued to fully explore the potential therapeutic effects of HIF-1 inhibition in human cancers.
This study emphasizes issues related to target-driven clinical trials that not only relate specifically to evaluation of HIF-1 but also, in general, to biomarker evaluation during exploratory clinical studies. Extensive evaluation of target modulation in preclinical models and adequate assay development are essential for a successful clinical study. As discussed above, evaluation of HIF-1α expression poses unique problems due to its heterogeneous expression in tumor tissue. However, a global evaluation of tissue endpoints associated with target modulation, supported by validated assay development, is imperative to provide proof-of-principle in mechanism-based drug development.
Supplementary Material
Translational Relevance.
Although hypoxia-inducible factor 1 (HIF-1) α is an exciting target for cancer therapy, the potential therapeutic effect of HIF-1α inhibition in human tumors remains to be demonstrated. This pilot study is the first target-driven, multihistology clinical trial of a small molecule inhibitor of HIF-1α designed to evaluate inhibition of the target in paired tumor biopsies. The trial serves as a model for incorporating pharmacodynamic evaluation of target modulation in tumor tissue into the clinical development of putative small molecule inhibitors of HIF-1α. In a limited number of tumor samples from patients, we were able to demonstrate changes in expression of HIF-1α and HIF-1 target genes in response to chronic oral administration of the topoisomerase I inhibitor topotecan, which has been shown to inhibit HIF-1α in preclinical models.
Acknowledgments
We thank Gina Uhlenbrauck (SAIC-Frederick, Inc.) for editorial support. We thank Sukyung Woo, Ph.D. (Center for Cancer Research, National Cancer Institute) for assistance in pharmacokinetic analysis.
Grant support: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported by the Center for Cancer Research and the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
References
- 1.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- 2.Bos R, Zhong H, Hanrahan CF, et al. Levels of hypoxia-inducible factor-1α during breast carcinogenesis. J Natl Cancer Inst. 2001;93:309–14. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Hypoxia-inducible factor 1 and cancer pathogenesis. IUBMB Life. 2008;60:591–7. doi: 10.1002/iub.93. [DOI] [PubMed] [Google Scholar]
- 4.Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1α is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–6. [PubMed] [Google Scholar]
- 5.Aebersold DM, Burri P, Beer KT, et al. Expression of hypoxia-inducible factor-1α: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–6. [PubMed] [Google Scholar]
- 6.Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G. Expression of hypoxia-inducible factor 1α in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res. 2001;7:1661–8. [PubMed] [Google Scholar]
- 7.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1α and 2α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–90. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schindl M, Schoppmann SF, Samonigg H, et al. Overexpression of hypoxia-inducible factor 1α is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002;8:1831–7. [PubMed] [Google Scholar]
- 9.Bos R, van der Groep P, Greijer AE, et al. Levels of hypoxia-inducible factor-1α independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–81. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- 10.Melillo G. Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev. 2007;26:341–52. doi: 10.1007/s10555-007-9059-x. [DOI] [PubMed] [Google Scholar]
- 11.Rapisarda A, Uranchimeg B, Scudiero DA, et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62:4316–24. [PubMed] [Google Scholar]
- 12.Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004;64:1475–82. doi: 10.1158/0008-5472.can-03-3139. [DOI] [PubMed] [Google Scholar]
- 13.Rapisarda A, Shoemaker RH, Melillo G. Targeting topoisomerase I to inhibit hypoxia inducible factor 1. Cell Cycle. 2004;3:172–5. [PubMed] [Google Scholar]
- 14.Rapisarda A, Zalek J, Hollingshead M, et al. Schedule-dependent inhibition of hypoxia-inducible factor-1α protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Res. 2004;64:6845–8. doi: 10.1158/0008-5472.CAN-04-2116. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Loos WJ, Stoter G, Verweij J, Schellens JH. Sensitive high-performance liquid chromatographic fluorescence assay for the quantitation of topotecan (SKF 104864-A) and its lactone ring-opened product (hydroxy acid) in human plasma and urine. J Chromatogr B Biomed Appl. 1996;678:309–15. doi: 10.1016/0378-4347(95)00529-3. [DOI] [PubMed] [Google Scholar]
- 17.Underberg WJ, Goossen RM, Smith BR, Beijnen JH. Equilibrium kinetics of the new experimental anti-tumour compound SK&F 104864-A in aqueous solution. J Pharm Biomed Anal. 1990;8:681–3. doi: 10.1016/0731-7085(90)80102-u. [DOI] [PubMed] [Google Scholar]
- 18.Gerrits CJ, Burris H, Schellens JH, et al. Five days of oral topotecan (Hycamtin), a phase I and pharmacological study in adult patients with solid tumours. Eur J Cancer. 1998;34:1030–5. doi: 10.1016/s0959-8049(97)10173-3. [DOI] [PubMed] [Google Scholar]
- 19.Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13:2780–6. doi: 10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law TM, Ilson DH, Motzer RJ. Phase II trial of topotecan in patients with advanced renal cell carcinoma. Invest New Drugs. 1994;12:143–5. doi: 10.1007/BF00874445. [DOI] [PubMed] [Google Scholar]
- 21.Sapra P, Zhao H, Mehlig M, et al. Novel delivery of SN38 markedly inhibits tumor growth in xenografts, including a camptothecin-11-refractory model. Clin Cancer Res. 2008;14:1888–96. doi: 10.1158/1078-0432.CCR-07-4456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.