Abstract
The goal of this pilot study was to assess the effects of acute hypoglycemia on retinal function and contrast sensitivity in individuals with and without diabetes. Hyperinsulinemic hypoglycemic and euglycemic clamp procedures were performed in subjects without diabetes (n=7) and with controlled type 1 diabetes (n=5). Mean age was 28 years, and none had retinal disease. During euglycemia (glucose 95–110 mg/dl) and acute hypoglycemia (glucose 50–55 mg/dl), contrast sensitivity was measured and spatial retinal responses were recorded with multifocal electroretinograms (mfERG), a rapid technique for mapping sensitivity from the foveal, macular and peripheral areas of the retina. During hypoglycemia, retinal responses (mfERG P1 wave) were decreased in both type 1 diabetes subjects and subjects without diabetes. The dominant effect was in the amplitude of the responses in the central macular retina, not in their temporal properties. Responses from the central region, central 10 degrees, were on average 1.8-fold lower than those from the periphery for both groups. All diabetes subjects and 3/7 without diabetes reported central scotoma. Decreases in mfERG amplitude were accompanied by decreases in contrast sensitivity. These changes were immediately reversed with the restoration of euglycemia. Overall, this study demonstrates that the acute effects of hypoglycemia in the human eye predominantly involve central vision, and these visual effects originate, at least in part, in the retina. The association between low blood glucose levels and impaired central vision underscores the importance of avoiding when possible and promptly treating hypoglycemia, particularly in individuals with diabetes.
1. INTRODUCTION
Visual disturbances occur in individuals with diabetes during hypoglycemia (Goldgewicht et al., 1983; Hepburn et al., 1991; McCrimmon et al., 1996; Tabandeh et al., 1996). Acute decreases in blood glucose levels have been associated with diplopia, dimness of vision, blurred vision and loss of contrast sensitivity. In the isolated cat eye, decreased glucose concentrations were associated with reductions in retinal sensitivity, with rods being more affected than cones (Macaluso et al., 1992). Mice with chronic hypoglycemia have loss of vision, reduction of retinal responses and increased retinal cell death (Umino et al., 2006). The direct effects of hypoglycemia on retinal function in humans are unclear.
We report, for the first time, the effects of acute hypoglycemia on the human retina under photopic conditions in subjects with and without diabetes. The effects of acute hypoglycemia on the amplitude of retinal responses and their spatial distribution were examined using the multifocal electroretinogram (mfERG). Using a complex, time-dependent visual stimulus in combination with a standard ERG contact lens recording system, the mfERG provides a rapid technique for mapping sensitivity from the foveal, macular and peripheral areas of the retina (Bearse and Sutter, 1996; Curcio et al., 1990; Hood, 2000; Marmor et al., 2003; Sutter and Tran, 1992). We also assessed the effects of acute hypoglycemia on visual contrast sensitivity.
2. MATERIALS AND METHODS
2.1 Subjects
Participants with type 1 diabetes (mean hemoglobin A1c 7.2%) were treated with multiple daily insulin injections or insulin pump therapy. Exclusion criteria included a history of cardiovascular or cerebrovascular disease, uncontrolled hypertension, seizures, head trauma, eye disease, nephropathy, neuropathy, or hypoglycemia unawareness, abnormal electrocardiogram, hemoglobin A1c > 8%, anemia, positive pregnancy test, renal or electrolyte abnormalities. A dilated eye examination was performed by an ophthalmologist prior to study to exclude retinal disease including retinopathy. This study was approved by the Institutional Review Board for the Protection of Human Subjects at SUNY Upstate Medical University. All subjects signed informed consent.
2.2 Hyperinsulinemic Hypoglycemic and Euglycemic Clamp Procedure (Andres et al., 1966)
Participants arrived at the Clinical Research Unit after an overnight fast. Vital signs were recorded and an intravenous catheter inserted in the left hand dorsal vein in a retrograde fashion for blood glucose sampling. The hand was placed in the hand warming box maintained at 55° C to arterialize the venous blood (Liu et al., 1992). Another intravenous catheter was inserted in the right antecubital vein for insulin and 20% dextrose infusion. Glucose levels were measured every five minutes at the bedside using the glucose oxidase method (Beckman Instruments, Fullerton, CA). A primed continuous infusion of 80- mU · m−2 · min−1 of Regular human insulin was used; 20% dextrose infusion was adjusted to achieve euglycemia with a target blood glucose level of 90 – 110 mg/dl (5 – 6.1 mmol/l). After an equilibration phase of 45 minutes, the 20% dextrose infusion rate was decreased slowly over a period of 45–60 minutes to attain hypoglycemia with a target blood glucose level of 50 – 55 mg/dl (2.7 – 3.1 mmol/l). The hypoglycemic phase was maintained for 20 minutes. The rate of dextrose infusion was then increased over 30–45 minutes to re-attain euglycemia. Subjects were offered meals at the conclusion of the study. Subjects remained rested and blinded to their blood glucose concentrations throughout the study.
2.3 Multifocal Electroretinogram (mfERG) Recording
Local responses from multiple retinal areas were recorded by using the mfERG technique (VERIS; Electro-Diagnostic Imaging, San Mateo, CA, USA). A stimulus array of 103 or 241 hexagons covering 40 degrees of retinal area was displayed at a frame rate of 75 Hz on a Veris stimulator/camera. The hexagonal area was scaled with eccentricity. A binary m-sequence was used. The mfERG stimulator was presented in an otherwise dark-adapted room. Each subject’s right pupil was dilated to a diameter of ≥ 7 mm with 1% tropicamide. After topical anesthesia with 0.5% tetracaine, a Burian Allen bipolar contact lens electrode (Hansen Ophthalmic Laboratories, Iowa City, IA) was placed on the test eye. A few drops of contact lens rewetting solution were applied to the inner surface of contact lens for better corneal contact. A ground electrode was placed on the forehead. The left eye was covered with an eye patch. The focus dial of the Veris refractor camera was adjusted so that the central fixation point marked by an “x” was in the best focus of the subject, as depicted by a sharp image of subject’s eye on the examiner’s monitor. The total duration of one recording (consistent of 16 segments and including breaks) was approximately 15 minutes, including 7 minutes and 17 seconds of recording usable data. Subjects were required to maintain fixation during the recording of a segment. However, they could rest after a segment had been recorded. Subject fixation was monitored by real-time infrared viewing of the eye image displayed on the examiner’s monitoring screen. If loss of fixation or an artifact was observed, the affected segment was discarded and re-recorded. Responses were amplified (Grass instruments), filtered (10–300Hz), sampled and processed on a G4 PowerMac. The Burian Allen contact lens electrode was removed from the eye after obtaining the mfERG during hypoglycemia, and reinserted when euglycemia was achieved.
2.4 Analysis of the mfERG
The typical waveform of the mfERG response is a biphasic wave with an initial negative deflection (N1) followed by a positive peak (P1) wave. The P1 wave corresponds to the b wave of the full field electroretinogram (Hood et al., 1997). The corneal negative a-wave (corresponding to the N1 wave of mfERG) provides information about phototransduction processes. The corneal positive b-wave (corresponding to the P1 wave of mfERG) represents the postphototransduction responses.
Peak-to-peak amplitude from trough of the first negative wave (N1) to the peak of the following positive wave (P1) was measured during hypoglycemia and euglycemia. The P1 wave amplitude for the rings 1, 2 and 3 for 241 setting and rings 1 and 2 for 103 setting was averaged to obtain mean P1wave amplitude for central retinal function. The P1 wave amplitude for rings 7, 8 and 9 for 241 setting and rings 5 and 6 for 103 setting was averaged to obtain mean P1wave amplitude for the peripheral retinal response. The effect of hypoglycemia on P1 wave amplitude of central and peripheral retinal response was determined. The 3-dimensional density response was plotted during hypoglycemia and euglycemia.
Data are expressed as mean ± SEM. Data were analyzed by t tests for comparisons between percent changes in P1 wave amplitudes in central and peripheral retina during hypoglycemia and euglycemia.
2.5 Contrast sensitivity
Subjects read letters on a Pelli-Robson contrast sensitivity chart (Haag-Streit, Essex, UK) from a distance of 1 meter. The pupil of the eye tested was not dilated and the other eye was occluded with an eye patch. At least 20 seconds were allowed for subjects to identify the letters.
3. RESULTS
3.1 Glucose levels of participants
Seven subjects (5 females, 2 males) without diabetes (mean age 28 year; range 22–40 years) and 5 subjects (all males) with type 1 diabetes (mean age 28 years; range 23–35 years) participated. For individuals with and without diabetes, the mean glucose levels during euglyemia were 98 mg/dl and 99 mg/dl, and during hypoglycemia 53 mg/dl and 50 mg/dl respectively.
3.2 P1 wave amplitude
The P1 wave amplitude was higher during euglycemia than hypoglycemia and decreased with reduction in blood glucose levels in both the normal volunteers and subjects with diabetes. The magnitude of amplitude changes was about equal for both groups, but the effects of hypoglycemia were not uniformly distributed across the retina. The reduction in P1 wave amplitude in the central retina was significantly greater than that observed in the peripheral retina during acute hypoglycemia in both groups (Table 1). Hypoglycemia modulated the amplitude of the mfERG but not its temporal properties. No detectable changes were recorded in the onset (or implicit times) of the mfERG components. The changes in amplitude reversed immediately with restoration of euglycemia. There were no timing differences between subjects with and without diabetes.
Table 1.
Percent decrease in P1 wave amplitude in central and peripheral retina from euglycemia to hypoglycemia
| Subject | Mean glucose during hypoglycemia (mg/dl) |
Central retina (% reduction) |
Peripheral retina (% reduction) |
|---|---|---|---|
| Normal volunteers | |||
| 1 | 48 | 37 | 17 |
| 2 | 50 | 19 | 12 |
| 3 | 50 | 6 | 3 |
| 4 | 50 | 17 | 6 |
| 5 | 50 | 9 | 4 |
| 6 | 51 | 5 | 5 |
| 7 | 50 | 9 | 9 |
| Mean +/− SEM (p = 0.050) |
50 +/− 0.34 | 14.57 +/−4.24 | 8.00 +/− 1.90 |
| Type 1 diabetes | |||
| 1 | 49 | 24 | 19 |
| 2 | 56 | 19 | 7 |
| 3 | 51 | 11 | 6 |
| 4 | 55 | 11 | 5 |
| 5 | 52 | 10 | 1 |
| Mean +/− SEM (p = 0.050) |
53 +/− 1.3 | 14.75 +/− 2.72 | 7.87 +/− 2.98 |
Normal volunteers: euglycemia: mean glucose 99 mg/dl
Type 1 diabetes: euglycemia: mean glucose 98 mg/dl
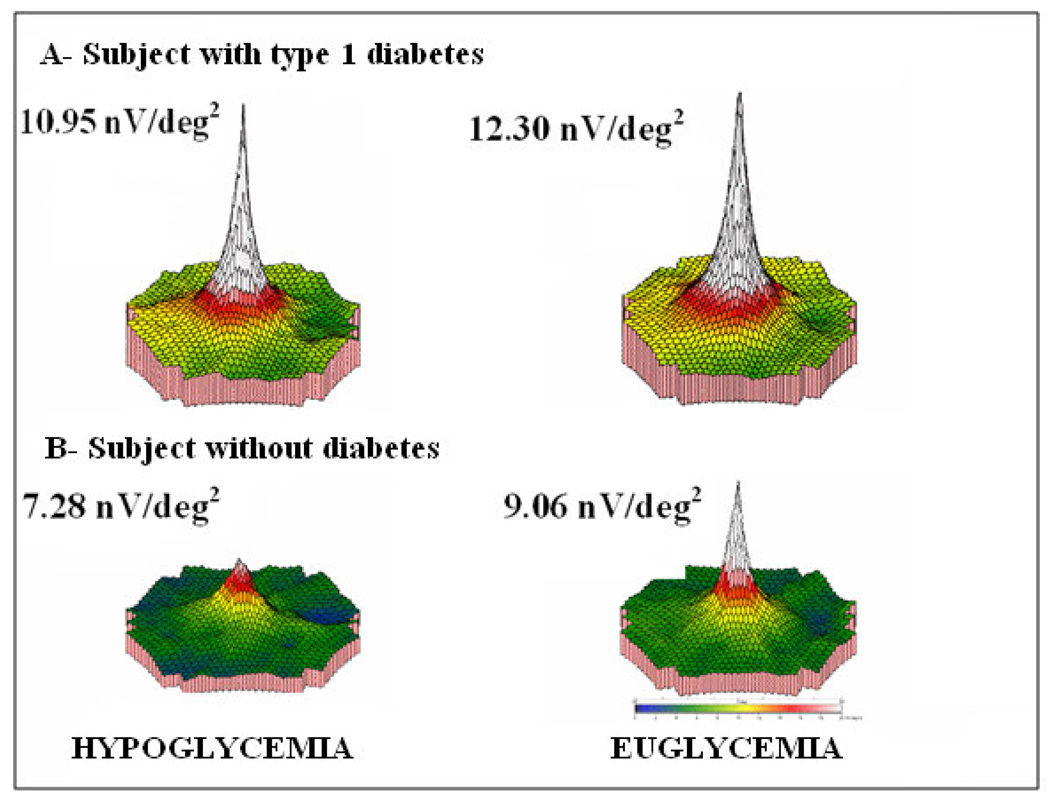
3.3 3-dimensional (3-D) response
The 3-D distribution reflects the electrical response density at different retinal locations. These plots show the overall strength per unit area of retina (combining N and P components) and were obtained by dividing the response amplitude by the area of the hexagon. In a normal subject the highest response densities are achieved in the foveolar region, reflected by a peak in the central area of this electrical map.
The mean decrease in the 3-D response from euglycemia to hypoglycemia was 7.73% in normal volunteers and 9.43 % in subjects with diabetes. The 3-D plots showed clearly the demarcated blind spot, confirming subject fixation. The 3-D response analysis demonstrated some loss of central peak in all subjects during hypoglycemia. The 3-D responses in a subject with diabetes and in a subject without diabetes are shown (Figure 1).
Figure 1.
3-Dimenstional response in: A) subject with type 1 diabetes mellitus and B) normal volunteer. During hypoglycemia and euglycemia the blood glucose levels were 50 mg/dl and 97 mg/dl respectively. The 3 dimensional response density plots showed a significant decrease in amplitude during hypoglycemia in the subject with type 1 diabetes and in the normal volunteer.
nV/deg2 = nanovolts per degrees squared.
3.4 Contrast sensitivity
In subjects with and without diabetes, mean actual contrast sensitivity decreased by 0.42 (p=0.0004) and 0.36 (p<0.001) from euglycemia to hypoglycemia respectively. A temporary central scotoma was reported during hypoglycemia in 5/5 subjects with diabetes and 3/7 subjects without diabetes. Hypoglycemia resulted in up to 6-fold decrease in contrast sensitivity.
4. DISCUSSION
This pilot study is the first to demonstrate significant diminution of central retinal function in individuals with and without diabetes during acute hypoglycemia. Central retinal function decreased consistently in all subjects with diabetes and this reduction was significantly greater than the reduction observed in peripheral retinal function. There were also significant reductions in contrast sensitivity during hypoglycemia.
Retinal elements responsible for vision are distributed in an inhomogeneous fashion across the visual field. The fovea has highest cone density; the central part of the fovea is rod free. The increased concentration of cones in the central part of the retina is responsible for the superior acuity, and is associated with high metabolic demands. The foveal area is avascular. Delivery of metabolic substrates to this area is maintained through the extra-retinal, choroidal circulation (Provis, 2001; Wybar, 1954). Glycolytic and oxidative metabolism plays a major role in maintaining retinal function (Winkler, 1981). It is possible that during hypoglycemia, the cells of central retina are not able to meet their metabolic demands due to decreased supply of glucose as well as decreased production of endogenous glucose by glycolytic pathways.
There were two participants without diabetes in whom the decrease in the amplitude of the P1 wave from euglycemia to hypoglycemia was modest and of similar magnitude in both the central and peripheral parts of the retina. In these subjects, the 3-D response decreased in the central rings during hypoglycemia by only <1% and 8%. The reason for this is unclear. In contrast, in all 5 participants with diabetes, there was greater change in central versus peripheral retinal function during hypoglycemia. None of the participants had retinopathy. It is possible that individuals with retinal disease have greater sensitivity to hypoglycemia. This is an area for future study.
The acute adverse effects of low blood glucose levels on vision in healthy individuals with type 1 diabetes have important short-term safety implications (Cryer, 2008; McFarland et al., 1946; Perlmuter et al., 2008). The glycemic threshold for these visual effects is not known. It is important for individuals with diabetes to recognize vision problems to avoid impairment with driving and other activities. There may also be long-term consequences of low glucose levels on vision. Sustained hypoglycemia has been associated with loss of vision and retinal degeneration in glucagon receptor knock out mice (Umino et al., 2006). It is not known if individuals with diabetes who experience frequent and prolonged hypoglycemia are at higher risk for visual loss.
In summary, this pilot study demonstrates for the first time, that the central retina is more sensitive to the effects of hypoglycemia than the peripheral retina, resulting in impaired central vision. Possible long-term reduction in retinal function from chronic hypoglycemia warrants further investigation.
HIGHLIGHTS.
Acute hypoglycemia lowers retinal responses (multifocal electroretinogram PI waves)
Central retina is more sensitive to hypoglycemia than peripheral retina
Acute hypoglycemia impairs contrast sensitivity
Effects observed in humans with and without type 1 diabetes
Acknowledgements
We thank the nurses in the Clinical Research Unit at SUNY Upstate Medical University for their help during the hyperinsulinemic hypoglycemic and euglycemic clamp procedures, and Dr. Barry E. Knox and Dr. Eduardo C. Solessio for their review and editing of this manuscript. This work was supported by funds from the Division of Endocrinology, Diabetes and Metabolism at SUNY Upstate Medical University, Syracuse NY and NIH EY00667.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no potential conflicts of interest.
References
- Andres R, Swerdloff R, Pozefsky T, Coleman D. Manual feedback technique for the control of blood glucose concentration. In: Skeggs L, editor. Automation in Analytical Chemistry. White Plains: Mediad Inc.; 1966. pp. 486–491. [Google Scholar]
- Bearse MA, Jr., Sutter EE. Imaging localized retinal dysfunction with the multifocal electroretinogram. J. Opt. Soc. Am. A. Opt. Image. Sci. Vis. 1996;13:634–640. doi: 10.1364/josaa.13.000634. [DOI] [PubMed] [Google Scholar]
- Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J. Comp. Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Goldgewicht C, Slama G, Papoz L, Tchobroutsky G. Hypoglycaemic reactions in 172 type 1 (insulin-dependent) diabetic patients. Diabetologia. 1983;24:95–99. doi: 10.1007/BF00297389. [DOI] [PubMed] [Google Scholar]
- Hepburn DA, Deary IJ, Frier BM, Patrick AW, Quinn JD, Fisher BM. Symptoms of acute insulin-induced hypoglycemia in humans with and without IDDM. Factor-analysis approach. Diabetes Care. 1991;14:949–957. doi: 10.2337/diacare.14.11.949. [DOI] [PubMed] [Google Scholar]
- Hood DC. Assessing retinal function with the multifocal technique. Progress in Retinal & Eye Research. 2000;19:607–646. doi: 10.1016/s1350-9462(00)00013-6. [DOI] [PubMed] [Google Scholar]
- Hood DC, Seiple W, Holopigian K, Greenstein V. A comparison of the components of the multifocal and full-field ERGs. Vis Neurosci. 1997;14:533–544. doi: 10.1017/s0952523800012190. [DOI] [PubMed] [Google Scholar]
- Liu D, Moberg E, Kollind M, Lins PE, Adamson U, Macdonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia. 1992;35:287–290. doi: 10.1007/BF00400932. [DOI] [PubMed] [Google Scholar]
- Macaluso C, Onoe S, Niemeyer G. Changes in glucose level affect rod function more than cone function in the isolated, perfused cat eye. Invest. Ophthalmol. Vis. Sci. 1992;33:2798–2808. [PubMed] [Google Scholar]
- Marmor MF, Hood DC, Keating D, Kondo M, Seeliger MW, Miyake Y. Guidelines for basic multifocal electroretinography (mfERG) Doc. Ophthalmol. 2003;106:105–115. doi: 10.1023/a:1022591317907. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Deary IJ, Huntly BJ, MacLeod KJ, Frier BM. Visual information processing during controlled hypoglycaemia in humans. Brain. 1996;119:1277–1287. doi: 10.1093/brain/119.4.1277. [DOI] [PubMed] [Google Scholar]
- McFarland RA, Halperin MH, Niven JJ. Visual thresholds as an index of physiological imbalance during insulin hypoglycemia. Am. J. Physiol. 1946;145:299–313. doi: 10.1152/ajplegacy.1946.145.3.299. [DOI] [PubMed] [Google Scholar]
- Perlmuter LC, Flanagan BP, Shah PH, Singh SP. Glycemic control and hypoglycemia. Diabetes Care. 2008;31:2072–2076. doi: 10.2337/dc08-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provis JM. Development of the primate retinal vasculature. Progress in Retinal & Eye Research. 2001;20:799–821. doi: 10.1016/s1350-9462(01)00012-x. [DOI] [PubMed] [Google Scholar]
- Sutter EE, Tran D. The field topography of ERG components in man--I. The photopic luminance response. Vision Res. 1992;32:433–446. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- Tabandeh H, Ranganath L, Marks V. Visual function during acute hypoglycaemia. Eur. J. Ophthalmol. 1996;6:81–86. doi: 10.1177/112067219600600116. [DOI] [PubMed] [Google Scholar]
- Umino Y, Everhart D, Solessio E, Cusato K, Pan JC, Nguyen TH, Brown ET, Hafler R, Frio BA, Knox BE, Engbretson GA, Haeri M, Cui L, Glenn AS, Charron MJ, Barlow RB. Hypoglycemia leads to age-related loss of vision. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19541–19545. doi: 10.1073/pnas.0604478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J. Gen. Physiol. 1981;77:667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybar KC. A study of the choroidal circulation of the eye in man. J. Anat. 1954;88:94–98. [PMC free article] [PubMed] [Google Scholar]