Abstract
Introduction
Evidence from previous studies has suggested there may be physical and mental changes in health among testicular cancer survivors. No studies have been conducted in the United States, however.
Methods
Study participants were initially enrolled in the US Servicemen's Testicular Tumor Environmental and Endocrine Determinants (STEED) study between 2002 and 2005. A total of 246 TGCT (testicular germ cell tumor) cases and 236 non-testicular cancer controls participated in the current study, and completed a self-administered questionnaire. Mean time since diagnosis for cases was 14 years, and no less than five for all cases. Component scores determined from responses to questions about physical and mental health on SF36 were tabulated to yield two summary measures, physical component scores (PCS), and mental component scores (MCS). Component and summary scores were normalized to a score of 50 with a standard deviation of 10 by a linear T-score transformation.
Results
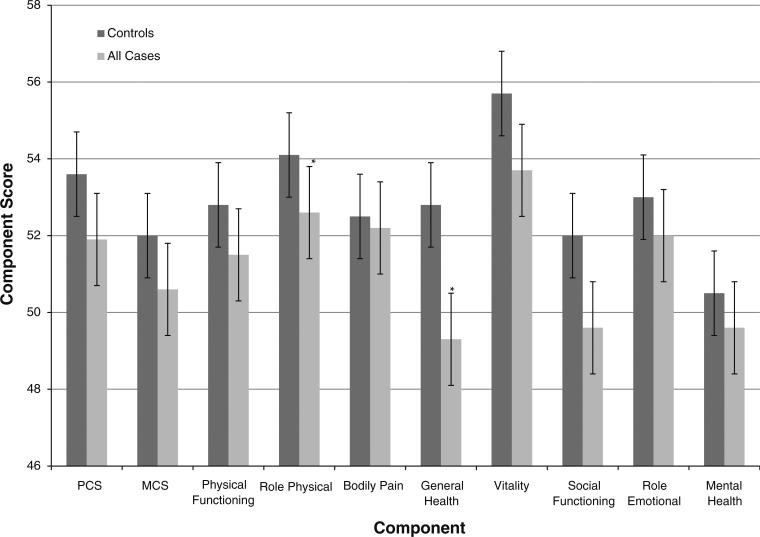
Overall, cases may not suffer greatly in different quality of life than controls. When all cases and controls are compared, TGCT cases had lower PCS (mean: 51.9 95% CI: 50.6–53.2, P value: 0.037) than controls (mean: 53.6 95% CI: 52.7–54.6). MCS were not significantly different (P value: 0.091). In multivariate analyses, several physical health components were worse for TGCT cases such as role-physical (OR 1.19, 95% CI: 1.01–1.39) and general health (OR 1.26, 95% CI: 1.07–1.49) compared to controls. However, TGCT cases treated with chemotherapy had lower PCS (cases: 50.2, 95% CI: 47.6–52.8; controls: 53.6, 95% CI: 52.7–54.6, P value: 0.0032) and MCS (cases: 49.3, 95% CI: 46.5–52.1; controls: 52.0, 95% CI: 50.9–53.2, P value: 0.039). TGCT cases who received treatments other than chemotherapy did not differ from controls in either PCS or MCS.
Discussion
Physical and general health limitations may affect testicular cancer survivors. Men treated with chemotherapy, however, may be most likely to suffer adverse health outcomes due to a combination of body-wide effects on physical and mental factors which affect various aspects of physical health, mental health, and overall quality of life. And in particular, physical functioning, role–physical, and general health are strongly affected.
Keywords: Health status, Quality of life, Testicular cancer
Introduction
Testicular cancer is one of the most treatable cancers. According to the American Cancer Society, the 5-year relative survival rate is over 96%, and more than 140,000 men currently living in the United States are survivors [1]. Because testicular cancer is among the most commonly occurring cancers in young men aged between 15 and 49 years [2], survivors’ quality of life after treatment is an area of particular concern.
Several bodily functions can be affected by testicular cancer treatment [3], which is composed of three primary options: surgery, chemotherapy, and radiotherapy [1]. Surgical procedures, such as retroperitoneal lymph node dissection (RPLND), can lead to an increased risk of sexual and compromised fertility problems [4]; chemotherapy can cause complications with renal, cardiovascular [5–11], neurological, and pulmonary functions in the body; and radiotherapy can cause gastrointestinal problems [3]. Additionally, chemotherapy and radiotherapy have been noted to increase risk of secondary cancers [12].
Quality of life health surveys can assess the impact of cancer diagnosis and treatment on an individual's physical, mental, social, and emotional health [13–15]. Previous studies have suggested limited differences in overall quality of life between cancer survivors and healthy individuals [16–19], although the greatest changes appear to be physical [17, 19] and sexual [16]. However, these studies were conducted in European countries and the results may not be generalizable to a population in the United States due to cultural and therapeutic differences. Additionally, a review of TGCT survivors suggested additional review by treatment type and other well-being aspects should be assessed [20].
Thus, we conducted a case–control study in US military servicemen to address the gap of knowledge in quality of life for testicular cancer survivors in the United States by treatment type and individual components of QoL.
Methods
Study Population
The study population has been previously described [21]. In brief, all study participants were enrolled in the US Servicemen's Testicular Tumor Environmental and Endocrine Determinants (STEED) study between 2002 and 2005. At the time of enrollment, eligible servicemen were aged 46 years or younger and had at least one serum sample stored in the Department of Defense Serum Repository (Walter Reed Army Institute for Research, Silver Spring, MD). Using a person-specific ID, the specimens in the DoDSR computerized database were linked to the defense medical surveillance system (DMSS) and to other military medical databases in order to determine which military personnel had developed TGCT after the date of serum donation while on active duty. TGCT were diagnosed between 1988 and 2002, and limited to classic seminoma or non-seminoma (embryonal carcinoma, yolk sac carcinoma, choriocarcinoma, teratomas, mixed germ cell tumor). Diagnosis and histology were based on the original pathology reports or on review (6.5%) of the pathology slides. A total of 961 eligible cases were identified and 754 were enrolled (78.5%). Men who never had a diagnosis of TGCT and had a blood serum sample in DoDSR were eligible to be controls. Initially, controls were pair-matched to cases based on age (within 1 year), race (white, black, other), and date of serum sample draw (within 30 days), although in this study they are no longer matched. However, these demographic characteristics were not different between cases and controls (Table 1). Of 1,150 potential controls, 928 participated in the study (80.7%).
Table 1.
Selected characteristics of cases and controls
| Characteristic | Controls (n = 236) |
Cases (n = 246) |
||
|---|---|---|---|---|
| Number | Percentage (%) | Number | Percentage (%) | |
| Current age | ||||
| 18–29 | 8 | 39 | 7 | 2.87 |
| 30–39 | 77 | 32.52 | 87 | 35.66 |
| 40–49 | 104 | 44.07 | 100 | 40.98 |
| 50+ | 47 | 19.92 | 50 | 20.49 |
| P value | 0.873 | |||
| Education completed | ||||
| High/vocation school | 75 | 31.78 | 76 | 31.15 |
| College/university | 84 | 35.59 | 101 | 41.39 |
| Graduate/professional | 73 | 30.93 | 64 | 26.23 |
| Missing | 4 | 1.69 | 3 | 1.23 |
| P value | 0.592 | |||
| Income | ||||
| <$15,000–$49,999 | 44 | 17.94 | 49 | 20.01 |
| $50,000–$90,000 | 70 | 29.91 | 89 | 36.33 |
| $90,000+ | 115 | 49.15 | 95 | 38.78 |
| Missing | 7 | 2.99 | 12 | 4.9 |
| P value | 0.105 | |||
| BMI | ||||
| <18.5 | 3 | 1.27 | 4 | 1.63 |
| 18.5–25 | 43 | 18.22 | 47 | 19.11 |
| 25–30 | 101 | 42.8 | 116 | 47.15 |
| >30 | 89 | 37.71 | 79 | 32.11 |
| P value | 0.627 | |||
| Race | ||||
| Black | 3 | 1.27 | 1 | 0.41 |
| White | 222 | 94.07 | 220 | 89.43 |
| Other | 11 | 4.65 | 25 | 10.18 |
| P value | 0.084 | |||
| Years since diagnosis/reference | ||||
| 5–10 | 41 | 17.37 | 35 | 14.23 |
| 10–16 | 134 | 56.78 | 152 | 61.79 |
| >17 | 61 | 25.85 | 59 | 23.98 |
| P value | 0.889 | |||
| Year of treatment/reference | ||||
| Prior to 1990 | 39 | 16.53 | 39 | 15.85 |
| 1990–1995 | 132 | 55.93 | 147 | 59.76 |
| 1996–1999 | 44 | 18.64 | 34 | 13.82 |
| After 2000 | 21 | 8.9 | 26 | 10.57 |
| P value | 0.491 | |||
| Histology | ||||
| Non-seminoma | 0 | – | 134 | 54.47 |
| Seminoma | 0 | – | 112 | 45.53 |
| Treatment* | ||||
| Radiation | 0 | – | 100 | 24.03 |
| Chemotherapy | 0 | – | 75 | 18.02 |
| Surgery | 0 | – | 241 | 57.95 |
Patients may have received more than one treatment (e.g., surgery and radiation)
In May 2008, 1,571 STEED participants with available contact information were mailed a letter of invitation to participate in the current study. The men were also mailed a standardized and validated self-administrated questionnaire on sexual functioning, fertility, and general quality of life. Participants were given the option of completing the questionnaire by phone, although few respondents elected to do so. By the end of April 2009, 559 of these mailings were returned due to undeliverable addresses. A total of 1,012 letters were delivered and 575 responses were received to the questionnaire request (56.8%). From the 575 responses, 24 had died, 69 refused, and 482 completed the questionnaire for a total response rate of 47.6%.
The study was approved by Institutional Review Boards of the Yale University, New Haven, CT, National Cancer Institute, Rockville, MD and the Walter Reed Army Institute for Research, Forest Glen, MD.
Data Collection
The short form health survey 36-item (SF-36) was utilized to measure participants’ overall physical and mental health, and the influence of their health status on different elements of life, for example work and social activities [13]. The SF-36, provides a generic measure of quality of life that assesses eight health scales: physical functioning (ability to perform physical tasks), role–physical (influence of physical health on work or other regular daily activities), bodily pain (pain or limitations due to pain), general health (self evaluation of physical state), vitality (pep and energy level), social functioning (ability to engage in social activity without pain), role–emotional (influence of emotional on accomplishing tasks), and mental health (self evaluation of mental state) perceptions. Each section consists of several individual questions, and a combination score for each category is determined from the responses within that category. The physical health summary consists of: physical functioning, role–physical, bodily pain, and general health. The mental health summary consists of: vitality, social functioning, role–emotional, and mental health. The resulting score for each section is then tabulated into one of two component summary scores, physical health (PCS) and mental health (MCS). All component and summary scores are normalized to a score of 50 with a standard deviation of 10 by a linear T-score transformation [22]. The means and standard deviations used in scoring were obtained from the 1998 general US population, and the factor score coefficients from the 1990 general US population. Previous studies have established high reliability and quality of data from the SF-36, supporting this survey as a sound tool for assessing health status [14, 15]. The advantage of the standardization and norm-based scoring of the PCS and MCS is that one measure can be compared meaningfully with the other, and their scores have a direct interpretation in relation to the distribution of scores in the general US population [22].
Statistical Analysis
The means of component summary scores (PCS/MCS) were compared using T-test. Statistical significance was assessed based on P values of Wald chi-square tests. In multivariate analyses, two models were built. Scores were assessed as continuous variables. All scores utilized were linearly transformed to a mean of 50 and standard deviation of 10. Each score was analyzed by utilizing an unconditional logistic regression model controlling for age (continuous), race (white, other), BMI (<25, 25–30, >30), income (<$50,000, $50,000–$70,000, >$70,000), years since treatment/reference (continuous), and smoking (never, former, current), to estimate adjusted odds ratios between cases and controls for each unit change in component scores. Multivariate analyses were utilized to assess the impact of covariates on the statistical significance of the summary and component score differences. To obtain P values for trend, the health variable was entered as a continuous variable in the logistic model and examined by the Wald chi-square test, where appropriate. In all analyses, including sub-analyses by treatment and histology type, all controls served as the comparison. Univariate analyses were conducted to compare selected characteristics between cases and controls. All P values were two-sided. All analyses were conducted using SAS statistical software (version 9.1.3; SAS Institute, Cary, NC, USA).
Results
As shown in Table 1, there were no statistically significant differences between cases and controls in the distributions of current age (P = 0.873), income (P = 0.592), education (P = 0.105), BMI (P = 0.627), race (P = 0.084), mean time since diagnosis/reference date (P = 0.889), or year of treatment/reference date (P = 0.491). For cases, the median time between diagnosis and interview was 14 years (mean = 13.7 years). All cases were diagnosed at least 5 years prior to interview.
The distributions of the above mentioned variables in the original STEED population were similar to the distributions in the current study population. For example, the mean reference age at diagnosis/reference for STEED cases and controls were 27.8 and 27.9 years, respectively, and 29.3 years and 29.1 years for the current participants, respectively. The percentages of overweight (BMI = 25–30) individuals in STEED were 43.2% (cases) and 47.5% (controls) while the percentages in the current study were 47.2% (cases) and 42.8% (controls).
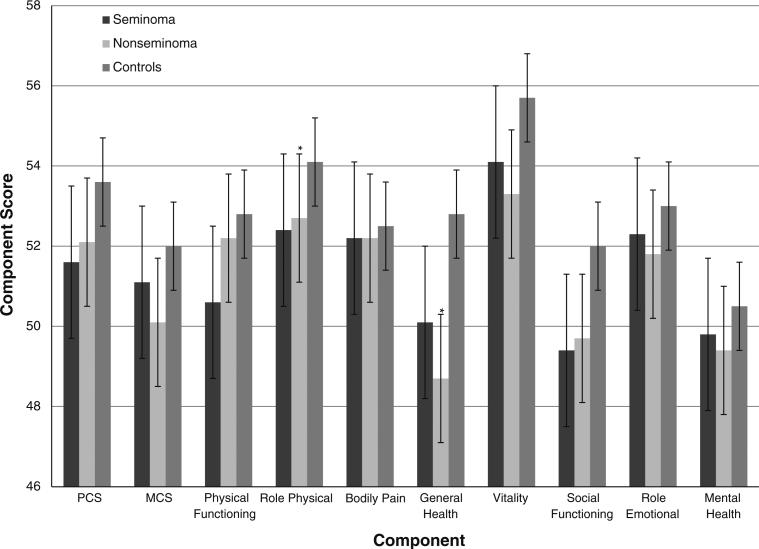
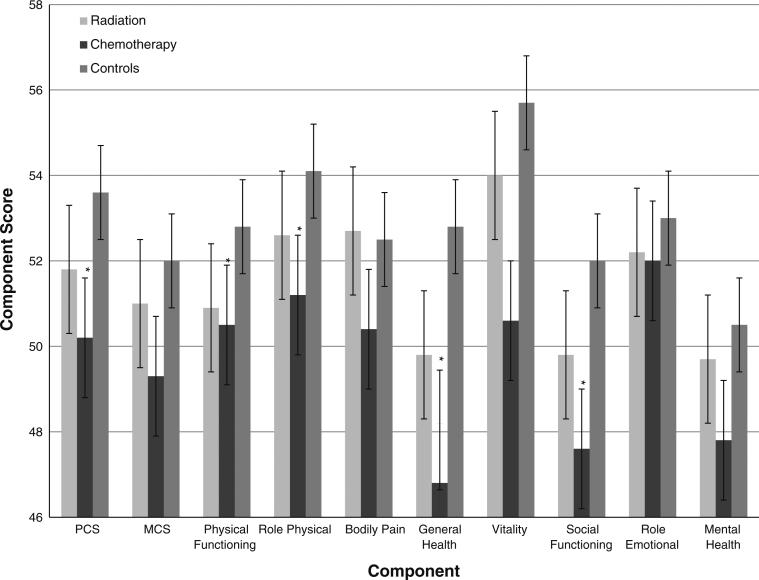
The mean and 95% CI of summary scores, and individual component scores are presented in Figs.1, 2 and 3. Cases had a significantly lower mean PCS compared to controls (P value: 0.037), with the results more pronounced among cases who received chemotherapy (P value: 0.0032). Although the MCS was not significantly different between cases and controls (P value: 0.091), cases who received chemotherapy had a significantly lower MCS compared to controls (P value: 0.039). Cases reported significantly lower mean scores in the role–physical (P value: 0.025), general health (P value: 0.0001), vitality (P value: 0.0058), and social functioning (P value: 0.005) categories. Several of these scores belong in the physical domain, contributing to the lower PCS score among cases. Comparing individual categories, survivors treated with chemotherapy had a lower level of physical functioning (P value: 0.027), role–physical (P value: 0.0044), general health (P value: 0.0001), vitality (P value: 0.011), social functioning (P value: 0.016), and mental health (P value: 0.028) scores. Additionally, general health and social functioning scores were lower for both histologies and radiation treatment (P values: < 0.05).
Fig. 1.
Summary and individual component scores of all cases and controls. * Statistically significant in multivariate analysis
Fig. 2.
Summary and individual component scores by histologic subtype. * Statistically significant in multivariate analysis
Fig. 3.
Summary and individual component scores by treatment group. * Statistically significant in multivariate analysis
In the multivariate analysis of the overall study population, the PCS (P value: 0.84) and the MCS (P value: 0.36) were not statistically different between cases and controls (Table 2). In the continuous variable analysis, role–physical (OR 1.19; 95% CI 1.01–1.39) and general health (OR 1.26; 95% CI 1.07–1.49) were significant, suggesting trends (P values: 0.034 and 0.007, respectively). No other statistically significant relationships were noted in the overall study population.
Table 2.
Multivariate analysis of summary scores and individual component scores
| Overall population | By histological subtype |
By treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Seminoma |
Non-seminoma |
Radiation |
Chemotherapy |
|||||||
| Cases | *OR (95%CI) | Cases | *OR(95%CI) | Cases | *OR(95%CI) | Cases | *OR (95%CI) | Cases | *OR (95%CI) | ||
| PCS | 232 | 241 | 1.02 (0.86, 1.21) | 110 | 0.88 (0.70, 1.11) | 131 | 1.10 (0.90, 1.35) | 99 | 0.85 (0.66, 1.10) | 71 | 1.42 (1.08, 1.86) |
| MCS | 232 | 241 | 1.08 (0.92, 1.27) | 110 | 1.14 (0.93, 1.42) | 131 | 1.06 (0.87, 1.29) | 99 | 1.14 (0.91, 1.44) | 71 | 1.21 (0.93, 1.58) |
| Physical functioning | 237 | 243 | 1.03 (0.88, 1.20) | 112 | 0.98 (0.80, 1.20) | 131 | 1.06 (0.89, 1.28) | 100 | 0.98 (0.79, 1.23) | 72 | 1.41 (1.10, 1.81) |
| Role-physical | 239 | 244 | 1.19 (1.01, 1.39) | 112 | 1.14 (0.94, 1.39) | 132 | 1.21 (1.00, 1.46) | 101 | 1.12 (0.90, 1.39) | 72 | 1.36 (1.08, 1.71) |
| Bodily pain | 239 | 245 | 0.89 (0.70, 1.13) | 113 | 0.82 (0.59, 1.13) | 132 | 0.92 (0.69, 1.22) | 101 | 0.78 (0.54, 1.11) | 73 | 1.20 (0.82, 1.76) |
| General health | 237 | 246 | 1.26 (1.07, 1.49) | 113 | 1.09 (0.88, 1.36) | 133 | 1.38 (1.12, 1.69) | 101 | 1.11 (0.88, 1.41) | 74 | 1.59 (1.20, 2.09) |
| Vitality | 235 | 246 | 1.07 (0.89, 1.29) | 113 | 1.10 (0.86, 1.41) | 133 | 1.08 (0.86, 1.36) | 101 | 1.04 (0.79, 1.36) | 74 | 1.32 (0.97, 1.80) |
| Social functioning | 237 | 245 | 1.48 (0.97, 2.25) | 113 | 1.57 (0.91, 2.70) | 132 | 1.56 (0.95, 2.58) | 100 | 1.52 (0.84, 2.75) | 73 | 2.25 (1.20, 4.22) |
| Role-emotional | 238 | 243 | 1.38 (0.85–2.22) | 111 | 1.50 (0.83–3.00) | 132 | 1.36 (0.76–2.44) | 100 | 1.41 (0.73–2.70) | 72 | 1.41 (0.68–2.92) |
| Mental health | 237 | 246 | 1.02 (0.86, 1.20) | 102 | 1.04 (0.83, 1.30) | 133 | 0.99 (0.81, 1.22) | 101 | 1.06 (0.84, 1.35) | 74 | 1.16 (0.88, 1.53) |
Adjusted for age (continuous), race (white, other) BMI (<25, 25–30, >30), income (<$50,000, $50,000–$70,000, [$70,000), years since treatment/reference (continuous), and smoking (never, former, current)
Compared to controls, survivors who had been diagnosed with a non-seminoma TGCT mirrored the associations seen in the overall population. In multivariate analyses, role–physical (OR 1.21; 95% CI 1.00–1)1.46) and general health (OR 1.38; 95% CI 1.12–1.69) were impaired. In comparison, there were no statistically significant differences in risk of reporting poor or very poor results between men who had been diagnosed with seminoma and controls .
The group treated with chemotherapy faced a greater risk of PCS quality of life being impaired (OR 1.42; 95% CI 1.08–1.86). In component analyses, risk of lower physical functioning (OR 1.41; 95% CI 1.10–1.81), role-physical (OR: 1.36; 95% CI 1.08–1.71), general health (OR: 1.59; 95% CI 1.20–2.09), and social functioning (OR: 2.35; 95% CI 1.20–4.22) scores were greater in cases compared to controls. Survivors treated with radiotherapy did not differ significantly from the controls in multivariate analyses.
Discussion
We conducted a study in US-based military men to access quality of life after treatment for testicular cancer. The data suggest that testicular cancer survivors experience some health effects and physical limitations, but overall, their quality of life is not drastically different than that of controls. The PCS was slightly lower for cases compared to controls (+1.7, P: 0.037), but the MCS was comparable (+1.4, P: 0.091). However, individuals who received chemotherapy suffered greater physical morbidity compared to controls. Notably, non-seminoma is the histology most likely to be treated with chemotherapy.
Our study results are generally consistent with previous quality of life studies. Several studies that considered both physical and mental components observed no major difference when comparing cases to controls [16–19]. When assessing the physical component scores, cases reported slightly lower scores in this study and in a study by Mykletun et al. [17], although the study by Mykletun et al. did not observe a difference specifically for men treated with chemotherapy. An interesting finding in a study by Thorsen et al. [19] suggested that survivors incorporated more physical activity into their daily lives, something that was not directly reported in this study. However, the poorer physical health of chemotherapy-treated survivors based on several component scores, as well as the PCS score, in our study suggests that the current study population would not show the same finding. We assessed individual components of the SF36 to determine if individual aspects of quality of life are affected even if overall quality of life is not and found that several areas assessed in the SF36 were impacted.
Significant changes noted in quality of life studies can have perceivable clinical effects. A unit change in the standard error of measurement of a quality of life survey can be tied to patient-driven minimally clinical differences [23]. Assessments in several diseases such as arthritis [24], orthopedics [25], systemic sclerosis [26], and cardiovascular [27] have measureable and often diagnosable clinical outcomes. In this study, physical health components including general health and physical functioning, particularly for chemotherapy patients, were likely to reflect clinically detectable physical ailments. Health risk factors, such as high blood pressure, high cholesterol, and obesity, have had negative impacts on SF-36 scores [27], factors which could be affecting this population, but were not directly assessed in the current study.
Health effects from the treatment of testicular cancer can be numerous [3]. Surgery can often lead to infertility and sexual dysfunction in RPLND, although since the mid-1990s a nerve-sparing technique has reduced trauma to the lymph nodes in the testicle area [4]. An analysis of the data was restricted to men who were diagnosed and treated after 1990, but the results did not differ significantly. Chemotherapy can be toxic to the urinary, cardiovascular, nervous, and pulmonary systems. Results from this analysis suggest that the general health of chemotherapy-treated individuals was significantly negatively impacted. Radiotherapy can affect the gastrointestinal system. Additionally, 20–30% of patients will exhibit long-term side effects from these treatments, including an increased risk of myeloid leukemia and myelodysplastic syndrome due to chemotherapy, and solid tumors due to radiotherapy [12]. Although specific health outcomes were not directly assessed in this study, worse self-perceived physical and general health was noted compared to the control population.
The cardiovascular system is the most commonly system affected by chemotherapy for testicular cancer. The most frequently occurring complication is Raynaud's phenomenon [5, 6, 11]. A study by Huddart et al. [7] suggested that overall cardiovascular-related morbidities after testicular cancer treatment were increased by 100%. Bokemeyer et al. [5] reported that in addition to Raynaud's phenomenon hypertension, higher serum cholesterol without obesity, ototoxicity, and peripheral neuropathy were present in TGCT survivors. Other studies have reported the presence of excess coronary artery disease, hypercholesterolemia, hypertension, and microalbuminuria [8]. Chemotherapy has also been reported to increase the risk of cardiovascular disease as much as smoking does [9] and increase the risk of myocardial infarction in younger people [10]. These complications might have contributed to the poorer health of TGCT survivors who were treated with chemotherapy, and this may be reflected in the general health and physical functioning aspects of the SF36.
Mental health can have an important impact upon overall health status, and there appeared to be a trend for worsening mental health among men treated with chemotherapy in our study. Past studies suggested anxiety to be present in a greater number of survivors [28–30], and in particular, anxiety about future health [30]. Anxiety is a strong predictor of future chronic fatigue [28] and chronic fatigue can lead to decreased overall health, physical ability, and mental ability [31]. Exhaustion and fatigue were more common among survivors in prior studies [28, 30]. Survivors also rated their stress to be a more pressing issue than controls in a general quality of life study [17], and stress has been considered a factor which negatively affects health [32] and immune function [33]. However, in the current study, these effects were only seen in men treated with chemotherapy, and the difference between cases and controls did not persist after adjustment for potential confounders. Additionally, social functioning was impaired, suggesting that the impacts from treatment could have contributed to inability to integrate and interact with others. This interference could be due to physical ailments, emotional ailments, or a combination of the two.
Whether the poorer quality of life among men treated with chemotherapy is due to chemotherapy treatment or non-seminoma histology is difficult to isolate. TGCT survivors treated with chemotherapy are almost always non-seminoma patients, and most, but not all, non-seminoma patients are treated with chemotherapy. No studies have investigated differential quality of life independent of treatment, and conducting such a study would be very difficult and potentially unethical. In this study, categories that had an increased risk of poorer health, except for physical functioning, occurred in both chemotherapy and non-semonima TGCT survivors.
For military service men, chemotherapy may greatly affect their daily lives. Military men need, particularly those on active duty, to retain their physical and mental abilities more than men in the general population. However, studies of Gulf War military personnel have suggested servicemen may be at greater risk of morbidity than men in the general population [34].
The current study had some limitations. The study did not determine comorbidities or diseases that might be present in the population, and it is impossible to specifically identify what led to poorer quality of life. As previously mentioned, adverse cardiovascular health is not an uncommon health effect of chemotherapy, but the proportion of the reduced quality of life that is attributable to cardiovascular problems is unknown. As with all retrospective questionnaire-based psychometric epidemiologic studies, this study is subject to misinterpretation of aspects of the questionnaire, but the normalization of the scores helps to attenuate this effect. The SF36 allows for assessment of individual aspects of life which contribute to the overall quality of life assessment, but only a few questions per area comprise each aspect and lack the ability to identify psychological adjustment. This study also only looked at long-term follow-up of TGCT survivors, and may not reflect quality of life for patients in early recovery. And while the general health surveys such as the SF-36 are useful tools for measuring self-reflection of health, disease-specific tools to assess related health outcomes measured are often necessary [35]. The response rate was not optimal, but the sampled group was representative of the STEED population as seen by the similar distribution of demographic variables. And the sample size was somewhat small, making stratification of some results leading to small cell sizes which limit the statistical stability of some effect estimates. Multiple comparisons also may have been an issue as a few artifacts in the results are likely due to chance. However, any potentially erroneous findings were de-emphasized.
In conclusion, our study suggests that quality of physical health, but not mental health, among TGCT survivors may be lower than that of controls. Additionally, TGCT survivors treated with chemotherapy may have reduced physical health compared to controls, whereas other treatments did not significantly differ. And in particular, physical functioning, role–physical, and general health are strongly affected.
Acknowledgments
This study is supported by grants CA130110 and CA105666 from the National Cancer Institute (NCI) and by Fogarty training grants 1D43TW008323-01 and 1D43TW007864-01 from the National Institute of Health (NIH). This publication was made possible by CTSA Grant number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the NIH and NHL roadmap for medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR. The authors are greatly indebted to the Study participants, without whom, there would have been no study. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Contributor Information
Christopher Kim, Yale School of Public Health, Yale University, 60 College Street, LEPH 440, New Haven, CT 06520, USA.
Katherine A. McGlynn, Department of Health and Human Services, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD, USA
Ruth McCorkle, Yale School of Nursing, Yale University, New Haven, CT, USA.
Ralph L. Erickson, Walter Reed Army Institute of Research, Silver Spring, MD, USA
David W. Niebuhr, Walter Reed Army Institute of Research, Silver Spring, MD, USA
Shuangge Ma, Yale School of Public Health, Yale University, 60 College Street, LEPH 440, New Haven, CT 06520, USA.
Barry Graubard, Department of Health and Human Services, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Briseis Aschebrook-Kilfoy, Department of Health and Human Services, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Kathryn Hughes Barry, Yale School of Public Health, Yale University, 60 College Street, LEPH 440, New Haven, CT 06520, USA; Department of Health and Human Services, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Yawei Zhang, Yale School of Public Health, Yale University, 60 College Street, LEPH 440, New Haven, CT 06520, USA.
References
- 1.Cancer Reference Information. American Cancer Society; 2009. [Google Scholar]
- 2.McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;91:63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- 3.Grosfeld G, Small E. Long-term side effects of treatment for testis cancer. Urologic Clinics of North America. 1998;25(3):503–515. doi: 10.1016/s0094-0143(05)70040-9. [DOI] [PubMed] [Google Scholar]
- 4.Arai Y, Ishitoya S, Okubo K, Aoki Y, Okada T, Maeda H, et al. Nerve-sparing retroperitoneal lymph node dissection for metastatic testicular cancer. Int J Urology. 1997;4(5):487–492. doi: 10.1111/j.1442-2042.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 5.Bokemeyer C, Berger CC, Kuczyk MA, Schmoll HJ. Evaluation of long-term toxicity after chemotherapy for testicular cancer. J Clin Onc. 1996;14:2923–2932. doi: 10.1200/JCO.1996.14.11.2923. [DOI] [PubMed] [Google Scholar]
- 6.Fossa SD, de Wit R, Roberts JT, Wilkinson PM, de Mulder PHM, Mead GM, et al. Quality of life in good prognosis patients with metastatic germ cell cancer: a prospective study of the european organization for research and treatment of cancer genitourinary group/medical research council testicular cancer study group (30941/TE20). J Clin Onc. 2003;21(6):1107–1118. doi: 10.1200/JCO.2003.02.075. [DOI] [PubMed] [Google Scholar]
- 7.Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Onc. 2003;21(8):1513–1523. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- 8.Meinardi MT, Gietema JA, van der Graaf WTA, van Veldhuisen DJ, Runne MA, Sluiter WJ, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Onc. 2000;18(8):1725–1732. doi: 10.1200/JCO.2000.18.8.1725. [DOI] [PubMed] [Google Scholar]
- 9.van den Belt-Dusebout AW, de Wit R, Gietema JA, Horenblas S, Louwman MWJ, Ribot JG, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Onc. 2007;25(28):4370–4378. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- 10.van den Belt-Dusebout AW, Nuver J, de Wit R, Gietema JA, ten Bokkel Huinink WW, Rodrigus PTR, et al. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Onc. 2006;24(3):467–475. doi: 10.1200/JCO.2005.02.7193. [DOI] [PubMed] [Google Scholar]
- 11.Vogelzang NJ, Bosl GJ, Johnson K, Kennedy BJ. Raynaud's phenomenon: a common toxicity after combination chemotherapy for testicular cancer. Annals of Internal Medicine. 1981;95(3):288–292. doi: 10.7326/0003-4819-95-3-288. [DOI] [PubMed] [Google Scholar]
- 12.Kollmannsberger C, Kuzcyk M, Mayer F, Hartmann JT, Kanz L, Bokemeyer C. Late toxicity following curative treatment of testicular cancer. Seminars. 1999;17(4):275–281. doi: 10.1002/(sici)1098-2388(199912)17:4<275::aid-ssu9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 14.McHrney CA, Ware JE, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kosinski M, Keller SD, Hatoum HT, Kong SX, Ware JE. The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: tests of data quality, scaling assumptions and score reliability. Medical Care. 1999;37(5):MS10–MS22. doi: 10.1097/00005650-199905001-00002. [DOI] [PubMed] [Google Scholar]
- 16.Joly F, Heron JF, Kalusinski L, Bottet P, Brunce D, Allouache N, et al. Quality of life in long-term survivors of testicular cancer: a population-based case-control study. J Cln Onc. 2002;20(1):73–80. doi: 10.1200/JCO.2002.20.1.73. [DOI] [PubMed] [Google Scholar]
- 17.Mykletun A, Dahl AA, Haaland CF, Bremnes R, Dahl O, Klepp O, et al. Side effects and cancer-related stress determine quality of life in long-term survivors of testicular cancer. J Clin Onc. 2005;23(13):3061–3068. doi: 10.1200/JCO.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 18.Rudberg L, Nilsson S, Wikblad K. Health-related quality of life in survivors of testicular cancer 3 to 13 years after treatment. J Psychosocial Oncology. 2000;18(3):19–31. [Google Scholar]
- 19.Thorsen L, Nystad W, Dahl O, Klepp O, Bremnes RM, Wist E, et al. The level of physical activity in long-term survivors of testicular cancer. European Journal of Cancer. 2003;39(9):1216–1221. doi: 10.1016/s0959-8049(03)00151-5. [DOI] [PubMed] [Google Scholar]
- 20.Fleer J, Hoekstra HJ, Sleijfer DT, Hoekstra-Weebers JEHM. Quality of life of survivors of testicular germ cell cancer: a review of the literature. Supportive Care in Cancer. 2004;12(7):476–486. doi: 10.1007/s00520-004-0646-x. [DOI] [PubMed] [Google Scholar]
- 21.McGlynn KA, Sakoda LC, Rubertone MV, Sesterhenn IA, Lyu C, Graubard BI, et al. Body size, dairy consumption, puberty, and risk of testicular cancer germ cell tumors. American Journal of Epidemiology. 2007;165(4):355–363. doi: 10.1093/aje/kwk019. [DOI] [PubMed] [Google Scholar]
- 22.Ware J, Kosinski M, Dewey J. How to score version 2 of the SF-36 health survey (standard & acute forms) QualityMetric Incorporated; Lincoln, RI: 2000. [Google Scholar]
- 23.Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Medical Care. 1999;37(5):469–478. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Kosinski M, Keller SD, Ware JE, Hatoum HT, Kong SXD. The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis—Relative validity of scales in relation to clinical measures of arthritis severity. Medical Care. 1999;37(5):MS23–MS39. doi: 10.1097/00005650-199905001-00003. [DOI] [PubMed] [Google Scholar]
- 25.Theis JC. Clinical priority criteria in orthopaedics: a validation study using the SF36 quality of life questionnaire. Health Serv Manage Res. 2004;17(1):59–61. doi: 10.1258/095148404322772732. [DOI] [PubMed] [Google Scholar]
- 26.Khanna D, Furst DE, Clements PJ, Park GS, Hays RD, Yoon J, et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. Journal of Rheumatology. 2005;32(5):832–840. [PubMed] [Google Scholar]
- 27.Chambers BA, Guo SS, Siervogel R, Hall G, Chumlea WC. Cumulative effects of cardiovascular disease risk factors on quality of life. J Nutr Health Aging. 2002;6(3):179–184. [PubMed] [Google Scholar]
- 28.Fossa SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J Clin Onc. 2003;21(7):1249–1254. doi: 10.1200/JCO.2003.08.163. [DOI] [PubMed] [Google Scholar]
- 29.Dahl AA, Haaland CF, Mykletun A, Bremnes R, Dahl O, Klepp O, et al. Study of anxiety disorder and depression in long-term survivors of testicular cancer. J Clin Onc. 2005;23(10):2389–2395. doi: 10.1200/JCO.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 30.Arai Y, Mawakita M, Hida S, Terachi T, Okada Y, Yoshida O. Psychosocial aspects in long-term survivors of testicular cancer. Clinical Urology. 1996;155(2):574–578. [PubMed] [Google Scholar]
- 31.Komaroff AL, Fagiolia LR, Doolittle TH, Gandek B, Gleita MA, Guerrieroa RT, I. I, et al. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. The American Journal of Medicine. 1996;101(3):281–290. doi: 10.1016/S0002-9343(96)00174-X. [DOI] [PubMed] [Google Scholar]
- 32.Kasl S. Stress and health. Annual Review of Public Health. 1984;5:319–341. doi: 10.1146/annurev.pu.05.050184.001535. [DOI] [PubMed] [Google Scholar]
- 33.Kubo C. Stress and immune function. J Japan Med Assoc. 2003;46(2):50–54. [Google Scholar]
- 34.Barrett DH, Doebbeling CC, Schwartz DA, Voelker MD, Falter KH, Woolson RF, et al. Posttraumatic stress disorder and self-reported physical health status among U.S. military personnel serving during the Gulf War period. Psychosomatics. 2002;43:195–205. doi: 10.1176/appi.psy.43.3.195. [DOI] [PubMed] [Google Scholar]
- 35.Jenkinson C, Peto V, Fitzpatrick R, Greenhall R, Hyman N. Self-reported functioning and well-being in patients with Parkinson's disease: comparison of the short-form health survey (SF-36) and the Parkinson's Disease Questionnaire (PDQ-39). Age and Ageing. 1995;24(6):505–509. doi: 10.1093/ageing/24.6.505. [DOI] [PubMed] [Google Scholar]