Abstract
The purpose of this study was to study the age-dependence of the optomechanical properties of human lenses during simulated disaccommodation in a mechanical lens stretcher, designed to determine accommodative forces as a function of stretch distance, to compare the results with in vivo disaccommodation and to examine whether differences exist between eyes harvested in the USA and India.
Post-mortem human eyes obtained in the USA (n=46, age = 6 to 83 years) and India (n=91, age = 1 day to 85 years) were mounted in an optomechanical lens stretching system and dissected to expose the lens complete with its accommodating framework, including zonules, ciliary body, anterior vitreous and a segmented rim of sclera. Disaccommodation was simulated through radial stretching of the sectioned globe by 2 mm in increments of 0.25 mm. The load, inner ciliary ring diameter, lens equatorial diameter, central thickness and power were measured at each step. Changes in these parameters were examined as a function of age, as were the dimension/load and power/load responses.
Unstretched lens diameter and thickness increased over the whole age range examined and were indistinguishable from those of in vivo lenses as well as those of in vitro lenses freed from zonular attachments. Stretching increased the diameter and decreased the thickness in all lenses examined but the amount of change decreased with age. Unstretched lens power decreased with age and the accommodative amplitude decreased to zero by age 45-50. The load required to produce maximum stretch was independent of age (median 80 mN) whereas the change in lens diameter and power per unit load decreased significantly with age.
The age related changes in the properties of human lenses, as observed in the lens stretching device, are similar to those observed in vivo and are consistent with the classical Helmholtz theory of accommodation. The response of lens diameter and power to disaccommodative (stretching) forces decreases with age, consistent with lens nuclear stiffening.
Keywords: human lens, accommodation, ageing, dimensions, EVAS stretching device, nucleus
1. INTRODUCTION
Accommodation is the dioptric change in the power of the eye required to bring near objects into focus. This change is brought about by an increase in lens thickness, a decrease in lens diameter, and an increase in curvature of the lens anterior and posterior surfaces in response to contraction of the ciliary muscle ([Young, 1793], [Cramer, 1853], [Helmholtz, 1855], [Koretz et al., 1984], [Atchison, 1995] and [Glasser and Kaufman, 2003]). The accommodative amplitude of humans begins to decline early in life and is almost completely lost by the age of 50 to 55 years ([Donders, 1864], [Duane, 1912] and [Kaufman, 1992]. This loss of accommodative ability with age is referred to as presbyopia. It affects virtually everyone and is the most common ocular affliction in the world.
The etiology of presbyopia is still not clearly defined and alterations in every component of the accommodative apparatus have been invoked ([Atchison, 1995], [Fincham, 1937], [Glasser and Kaufman, 2003] and [Strenk, Strenk and Koretz, 2005]), but most of the recent observations and current theories implicate age-related changes in the lenticular component. Lens hardening, in particular, has long been considered to be a major factor. Support has come from numerous investigations, although some suffer from drawbacks which make the data unreliable or difficult to compare (Burd et al., 2006). Recent dynamic mechanical analyses have revealed the age-related development of a stiffness gradient and substantial stiffening of the lens nucleus ([Heys et al., 2005] and [Weeber et al, 2005]). In view of the involvement of nuclear deformation in accommodation (Patnaik, 1967) and the demonstration that stretching stresses are distributed around the corticonuclear boundary (Belaidi and Pierscionek, 2007), it would be expected that the force required for accommodation would have to increase with age. What this force may be and whether the ciliary body is able to deliver it remain to be established. Such information would be of considerable value for understanding the causes of presbyopia and for the development of devices or strategies for restoring accommodation, especially lens refilling protocols in which the hard contents of the old lens are removed and replaced with a softer, more pliable gel ([Kessler, 1964] and [Parel et al., 1986]).
Several in vitro studies have examined the role of the different components of the ocular system in the accommodative process and attempted to assess the forces required. In early studies, disaccommodation was simulated by attaching weights to the ciliary body, by uniaxial loading experiments or by applying centrifugal forces to isolated lenses ([Fisher, 1971], [Pau, 1951], [Schachar, 2004] and [Stadfeldt, 1896]). Centrifugation has again been used more recently to assess the deformability of decapsulated lenses (Burd et al., 2011) These were followed by the development of various mechanical devices for radially stretching the lens while still attached to the accommodative apparatus ([Fisher, 1977], [Sunderland and O'Neill, 1976] and [van Alphen and Graebel, 1991]). An electronically driven instrument was constructed by Pierscionek (1993, 1995) and, since then, several stretching devices, designed to address specific aspects of the accommodative process, have been developed in other laboratories ([Ehrmann et al., 2008], [Gerometta et al., 2007], [Glasser and Campbell, 1998], [Koopmans et al., 2003], [Manns et al., 2007] and [Reilly et. al., 2008]) The Pierscionek instrument measured thickness and curvature (Pierscionek, 1993, 1995), while that designed by Glasser and Campbell (1998) was able to provide information on focal length and spherical aberration, as well as thickness and diameter in a later design (Koopmans et al., 2003). However, other than the device constructed by Reilly et al., (2008), these later instruments did not permit a quantitative assessment of the relationship between the forces applied and the optical or biometric responses. The only device that did allow measurement of force was used only on a small number of porcine lenses (Reilly et al., 2009).
Information on the effect of increasing age on the forces required to stretch lenses and on the response of the lens to those forces would be of particular value for understanding the causes of presbyopia and for the development of devices or strategies for restoring accommodation. In particular, knowledge of the forces involved in lens stretching would greatly facilitate the selection of gels with appropriate visco-elastic properties to be used as replacements for the ageing lens contents. Such information can be obtained with an instrument (EVAS, Ex Vivo Accommodation System) developed in our laboratories (Manns et al., 2007). The EVAS system was designed to measure lens diameter, thickness and power and, simultaneously, the force required to change these. The instrument was also designed for development and investigation of the phaco-ersatz procedure (Parel et al., 1986) in which the contents of the lens are removed through a small capsulorhexis and replaced with a suitable gel, after which the optomechanical responses can again be assessed.
A first generation of the EVAS system has been used to examine small numbers of grouped monkey and human lenses (Manns et al., 2007). This initial study showed that the EVAS lens stretcher produces changes in lens diameter and power that are consistent with in vivo measurements and with the Helmholtz theory of accommodation, but the number of lenses was insufficient to characterize the age-dependence of the responses. The purpose of the present study was to examine the age dependence of the optical and mechanical responses of a large number of post-mortem human lenses, using the EVAS lens stretching system and to evaluate possible differences between lenses obtained in the USA and India.
2. MATERIALS AND METHODS
2.1 Tissue preparation
Post-mortem human eyes were obtained from donors, ranging in age from 1 day to 85 years, and used in compliance with the guidelines of the Declaration of Helsinki for research involving the use of human tissue. Upon arrival at the laboratory, eyes were either directly prepared for the stretching experiment or refrigerated at 4°C. The cause, time of death and time to enucleation had been recorded for each eye. Where detailed medical histories were available, it was possible to eliminate eyes from donors having had uncontrolled diabetes, head or ocular trauma or prior eye surgery. Other contraindications included death through electrocution, liver failure, kidney failure, poisoning and sepsis, as well as treatments and medications which affect collagen and elastin or have the potential to damage tissue, such as chemotherapy, radiation and immunosuppressive therapy.
The eyes were dissected according to the protocol described previously (Manns et al., 2007). In short, after exposing a clean dried scleral surface, eight custom-made annular segments of PMMA termed “scleral shoes” were bonded with cyanoacrylate adhesive onto the anterior scleral surface of the intact globe to form a circumferential band located ~ 1 mm posterior to the limbus to 3 mm above the equator. The shoes were custom-designed to match the anatomy of the eyes and prevent deformation of the globe during dissection as well as providing attachment points to mount the tissue in the lens stretcher (EVAS).
The posterior segment of the eye was then circumferentially dissected and the excess vitreous was removed until the vitreous surface was flush with the scleral shell. Special care was taken to leave the anterior vitreous and the hyaloid membrane near the lens and ciliary body untouched. The tissue chamber was then ¾ filled with Dulbecco's Modified Eagle's Medium (DMEM/F-12, D8437, Sigma, St Louis, MO) in order to minimize the risk of zonular damage and to prevent the lens from drying (Rosen et al., 2005). After removing the fixation pins, the ring was placed with the anterior surface facing upward and the dissected eyeball was transferred to the tissue chamber and connected to the lens stretcher (EVAS) via stainless steel hooks. The cornea was removed at the level of the limbus and the iris was carefully dissected out at its root. Eight segments were then produced by making full-thickness meridional incisions in the sclera between adjacent shoes, avoiding damage to the ciliary body, zonule and lens capsule. At the completion of the dissection, the chamber was completely filled with DMEM. The final preparation was carefully inspected under the operating microscope (BPEI: OMS-300, Topcon, Tokyo, Japan; LVPEI: OPMI 1-FR, Carl Zeiss, Germany) to ensure all components of the accommodative apparatus were intact before stretching commenced.
2.2 Lens stretcher (EVAS: Ex Vivo Accommodation Simulator)
The tissue assembly was connected to the motorized translation stage of the stretcher (EVAS) using an assembly of strings and pulleys. The arrangement of the various elements of the instrument is shown in Figure 1.
Figure 1.
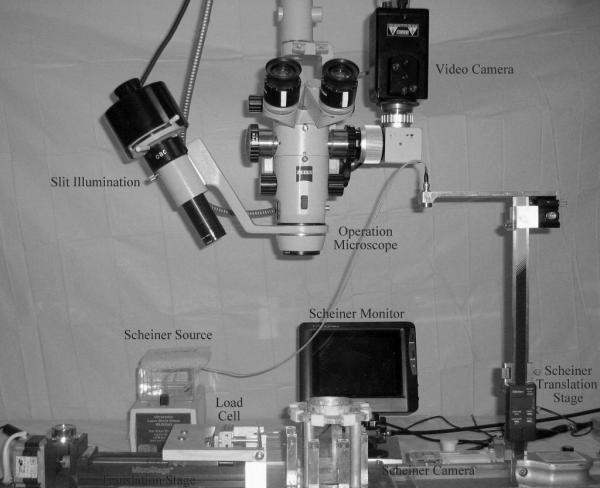
The EVAS lens stretching instrument showing the arrangement of the various elements used for measuring dimensions, power and loads.
The apparatus has been described in detail (Manns et al., 2007). The translational movement of the T-bar produces a radial stretching force equally distributed among the eight segments. A load cell with a range of 100 g and resolution of 0.01 g was mounted on the T-bar to record the total load during the experiment. The recorded load was converted from grams (g) to milliNewtons (mN) using the conversion factor, 1 g = 9.81 mN. Mechanical testing of the lens stretcher, to ensure that reasonable load values were obtained, was done before each series of experiments, using a 70 μm thick annulus made of medical grade silicone elastomer (PDMS, Elastic modulus: 1.7 MPa) with a rectangular cross-section and 17 mm inner diameter and 19 mm outer diameter.
The amount of stretching to be applied was determined in preliminary experiments by monitoring ciliary body condition in response to stretching over a variety of distances. At 4 mm radial stretch, the ciliary body was damaged so that a second stretching experiment yielded different results for the changes in lens parameters. By reducing the amount of stretch until successive runs generated identical results, it was determined that 2 mm was optimal. The translation stage was programmed to move outwards by a total of 2 mm in 0.25 mm increments at a speed of 0.5 mm/s, corresponding to a diameter increase of the outer sclera by 4 mm in 0.5 mm steps. The translation stage is stopped for 10 s at the end of each step to permit measurements of the diameters of the lens and inner ciliary ring and the power of the lens (see below). The load, at equilibrium after each step, was also recorded. At the end of the last step, the translation stage is returned to its initial position at 0.5 mm/s and the dimensions and power were measured again. If these had returned to the original starting values, indicating the stretching had not altered the tissues permanently, the stretching protocol was repeated a further minimum of two times and the data were averaged. Slit-lamp images of the lens cross-section were obtained in separate stretching cycles to ensure that there was no significant axial movement or tilting of the lens. Pre-loading was done to ensure uniform stretching and to ensure that there were no problems with the pulley transmission system, the attachment of the shoes, or with the tissue. One or two pre-conditioning runs were performed to verify symmetrical stretching and to ensure there was a stable response in the load versus stretched distance.
It was generally at the first step that problems arose which necessitated the abandonment of the experiment. Most common were ciliary body detachment at the corneo-scleral spur, with stretching, and lens prolapse during the dissection. By careful exclusion of eyes according to the contraindications listed above, the attrition rate was reduced to less than 25% of all eyes received. Experiments were made on 46 eyes in the Ophthalmic Biophysics Center, Bascom Palmer Institute, University of Miami, USA. The instrument was then shipped to the LV Prasad Eye Institute in Hyderabad, India, where it was used for the assessment of 91 Indian eyes.
2.3 Measurement of dimensions and power
At each step of the stretching cycles, images of the lens with ciliary body were captured at high magnification under retroillumination, using a digital camera mounted on the operation microscope. Diameters were measured along the horizontal and vertical axes in units of pixels using graphics software Canvas 9.0 (ACD Systems, Miami, FL). An image of a metric ruler with 0.5-mm graduations captured before each experiment was used to convert pixel units to length units. The mean of the horizontal and vertical measurements was used as a measure of the diameter.
The apparent lens thickness (Ta) was measured on images recorded using the same camera in a separate stretching cycle, with the lens illuminated with a slit at an angle of 31 ± 1°. A factor to convert apparent thickness to true thickness was derived following a paraxial analysis of lens changes in the stretcher. The first step of the analysis is a forward ray-trace through the immersed lens to determine the points of intersection of the slit beam with the anterior and posterior lens surfaces. The second step is calculation of the position of the virtual aerial reflected image of these two points, which is the image viewed through the microscope. The analysis showed that as a first approximation, the true thickness is directly proportional to the apparent thickness. Changes in the anterior curvature during stretching or with increasing age, and in the fluid layer thickness were found to have only a small effect on the proportionality factor. We therefore derived a fixed proportionality factor, with a different value for the Indian and American eyes, to take into account differences in the slit angle at the two sites, by comparison of the apparent thickness from the slit lamp images with the averaged values from all published in vitro measurements, over the age range of 25-65 years where the change in thickness is approximately linear (Augusteyn, 2010). For nine Indian eyes, not stretched because of ciliary body detachment, it was also possible to compare the slit lamp dimensions with those obtained with calipers. A first order propagation of errors showed that these conversion factors provide thickness values (total accuracy as standard deviation or 95% c.i.) with an uncertainty of ± 7%.
The lens power during stretching cycles was measured with an optical system based on the Scheiner principle. Four parallel laser beams were refracted through the lens and brought to a point focus, the position of which was detected by a miniature board-level camera and the axial position of the focal plane was accurately measured with a height gauge. The refractive power of the immersed lens (in DMEM/F-12; assumed n = 1.332 at 635 nm) was then determined using an optical model.
2.4 Data analysis
Lens diameter (D), apparent thickness (Ta), focal distance and the applied stretching load (L) were measured at the beginning of each cycle and at each step during the stretching. Ta was converted to actual thickness (T) and focal distance to power (P), after which D, T and P were plotted against L to produce Diameter-load, Thickness-load and Power-load response curves. The slopes of the linear portions of these plots, as well as D, T and P, were analyzed as a function of the age since conception (Augusteyn, 2010) since lens growth commences early in gestation.
First, to test for significance of the correlation between the measured parameters and age since conception and postmortem time, all measured and derived parameters were grouped into trivariate sets, involving age since conception, parameter and postmortem time. Then, a trivariate Partial Correlation Analysis was performed to generate Spearman's Ranked Partial Correlation Coefficients and the corresponding p-values for each trivariate set, and the Bonferroni method was employed to allow for multiple comparison while assuring that an overall confidence level (α = 0.05) is maintained. No parameter showed a significant correlation with postmortem time (p > 0.1) and, in all cases, the correlation with age since conception was much more significant than with postmortem time. Therefore, postmortem time was eliminated as an effector and not considered in subsequent analysis.
Then, to determine if any significant differences exist between the two populations (Indian and USA), all measured and derived parameters were paired with the age of the eye to give bivariate data for both the Indian and USA samples and used to generate scatter plots. Parameters that were found to correlate with age were checked for normality by plotting a histogram, a normal plot and by performing the Shapiro-Wilk normality test. Parameters that were found not to correlate with age were compared directly using the Wilcoxon Rank Sum Test. Data that were not normally distributed were transformed by the application of a deterministic mathematical function to each point in the data set to obtain a normal distribution. Then, normalized non-linear data were transformed in the same fashion to obtain a linear trend. Once the data were both normalized and linearized, a least squares linear regression was performed. Using this regression, the residuals and standardized residuals were computed, and any residual outside of the 99% confidence interval (2.575σ) was removed as an outlier. Finally, the slopes and slope errors of each data set were used to construct hypothesis tests on the age-dependent trends using Student-t statistics and, once again, the Bonferroni method was applied. The results of this analysis provided the basis for subsequent grouping of both Indian and USA eyes for further age-dependent analyses. The final numbers of data displayed in the figures and used for analysis are included in the legends to the figures.
Finally, to examine the nature of the age-dependence, all measured and derived parameters were paired with the age of the eye to give bivariate data, with the Indian and USA samples grouped together. Then, the aforementioned protocol concerning normalization, linearization and outlier discrimination was employed on the grouped data sets, where data points before 20 years of age were excluded from the final analysis to eliminate the complexity of rapid early growth from the analyses presented herein.
3. RESULTS
3.1 Unstretched dimensions
The dimensions of the ciliary body and lens were measured, prior to application of stretching forces, with the tension adjusted until the zonules were slightly taut, but not stretched.
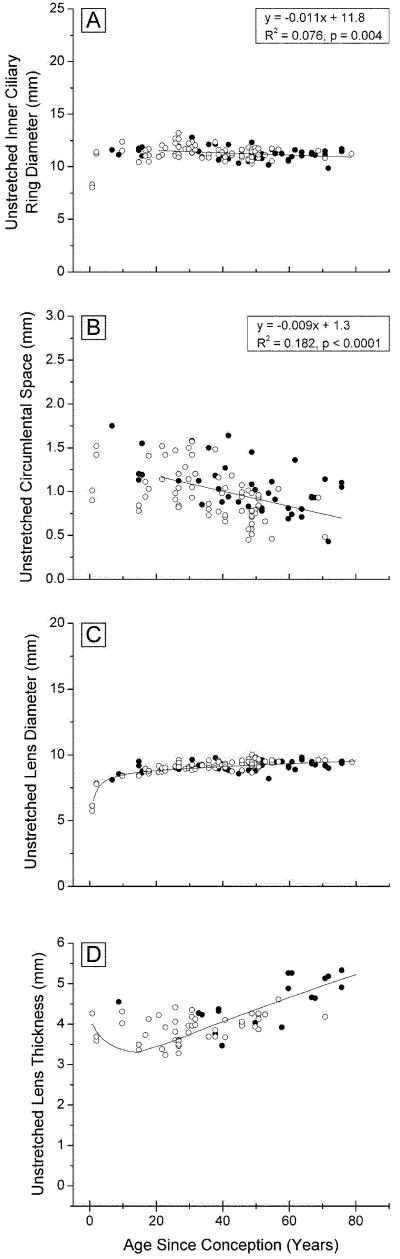
The inner ring diameter of the unstretched ciliary body decreases slightly with age (Fig 2A). In eyes over 20 years, Diameter = 11.8 - 0.011×Age (p=0.004). A decrease has also been observed in vivo but the actual dimensions differ. Strenk et al. (1999) found that, with an 8 D accommodation stimulus, the inner ciliary ring diameter decreased from around 13.3 mm at age 20 years to 12 mm at 83 years.
Figure 2. Ocular dimensions prior to stretching, as a function of age.
inner ciliary ring diameter (A, 127 points) circumlental space (B,116 points), lens diameter (C, 131 points), and lens central thickness (D, 65 points), for Indian (○○○) and USA (●●●) eyes mounted in the EVAS apparatus. Included in C and D are the lines obtained from analysis of all published dimensions for in vitro lenses (Augusteyn 2010).
The distances between the inner margin of the ciliary body (CB) and the lens (the circumlental space) are shown in Figure 2B. In 20 years-old eyes, the distance of around 1.2 mm would correspond to the average length of the zonules. With increasing age, this distance decreases to 0.6-0.7 mm. Calculations from the in vivo measurements of Strenk et al. (1999) suggest a similar age-related decrease but all the in vivo distances appear to be about 0.5 mm greater than those observed in the present study.
The lens diameters (Fig 2C) are indistinguishable from the published data on isolated in vitro lenses (solid line) (Augusteyn, 2010), indicating that the lenses were in their fully accommodated state prior to commencement of the stretching experiments. They are also very similar to the (accommodated) in vivo measurements (Strenk et al., 1999). The data show that lens diameter increases continuously throughout life; between the ages of 50 and 85 years, this amounted to 0.3 mm.
Central lens thicknesses are presented in Figure 2D together with the previously described trend derived from all published in vitro measurements (solid line, Augusteyn, 2010). Although our data are too few to reveal convincing trends, they are consistent with previous observations that central thickness decreases from birth to the teenage years, followed by an increase for the rest of life.
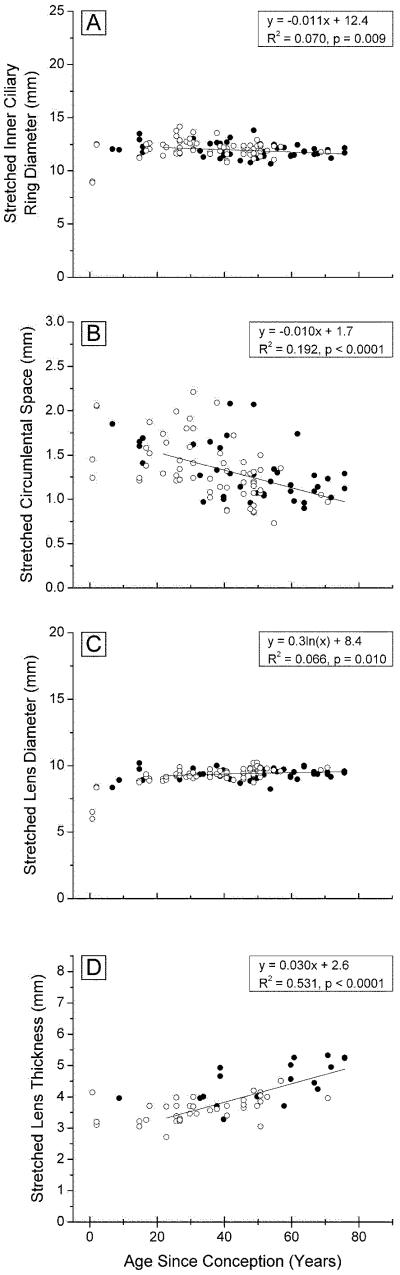
3.2 Stretched dimensions
The dimensions of the ciliary body, lens and circumlental space, after stretching, are presented in Figure 3 and the changes from the unstretched to the stretched state in Figure 4. Comparison of the stretched (Fig 3) with the unstretched (Fig 2) dimensions reveals that the overall trends with age were unaltered but there were age-dependent differences in the responses to stretching.
Figure 3. Ocular dimensions after 2mm radial stretch, as a function of age.
inner ciliary ring diameter (A, 117 points) circumlental space (B, 99 points), lens diameter (C, 118 points), and lens central thickness (D, 56 points), for Indian (○○○) and USA (●●●) eyes mounted in the EVAS apparatus.
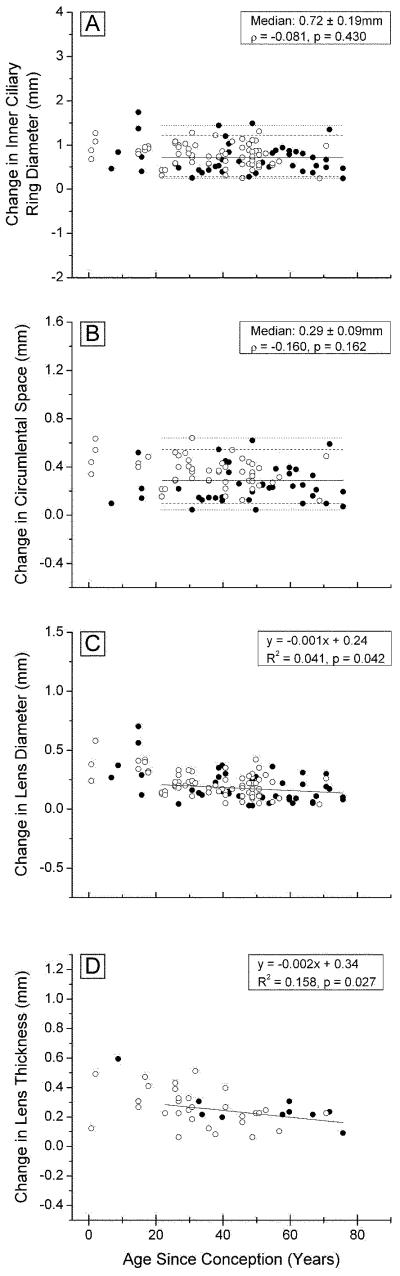
Figure 4. Changes in ocular dimensions with 2 mm radial stretch, as a function of age.
inner ciliary ring diameter (A, 117 points) circumlental space (B, 89 points), lens diameter (C, 117 points), and lens central thickness (D, 49 points), for Indian (○○○) and USA (●●●) eyes mounted in the EVAS apparatus
Although there is considerable variation, it would appear that stretching increased the inner ring diameter of young (<20 years) ciliary bodies by approximately 1 mm and by around 0.7 mm for those over 20 years (Fig 4A). These are similar to the increases observed with in vivo MRI measurements (Strenk et al., 1999), suggesting that the forces being applied to the ciliary body and lens were similar to those applying in vivo.
The circumlental space increased with each step of stretching. Since the tension at the sclera was adjusted to ensure that some opposing zonules were taut prior to stretching, the increase would represent zonular stretch. Figure 4B indicates there was an increase of 0.29 mm, around 15% of the CB-lens distance, for the 2 mm of stretch applied at the sclera, and that this was independent of age.
The total change in lens diameter achieved with stretching is shown as a function of donor age in Figure 4C. As much as 0.7 mm of stretch was observed with lenses under age 20 years, but this decreased rapidly to an almost constant 0.16 ± 0.1 mm in lenses older than 40. During in vivo disaccommodation, lens diameter increases by 0.6-0.8 mm in young lenses reducing to 0 by age 50 (Strenk et al., 1999).
The amount of lens thinning with stretching decreased from near 0.5 mm, for 2 year-old lenses, to under 0.2 mm, after age 50 (Fig 4D). In vivo MRI measurements, for an accommodation stimulus of 6D, yielded values ranging from a 0.5 mm increase in central thickness at age 25, to 0 mm after age 50 (Strenk et al.,1999). It may also be calculated from the Scheimpflug data of Dubbelman et al. (2005) that, for a 20 year-old, 6 D of accommodation stimulus results in a 0.3 mm increase in lens thickness.
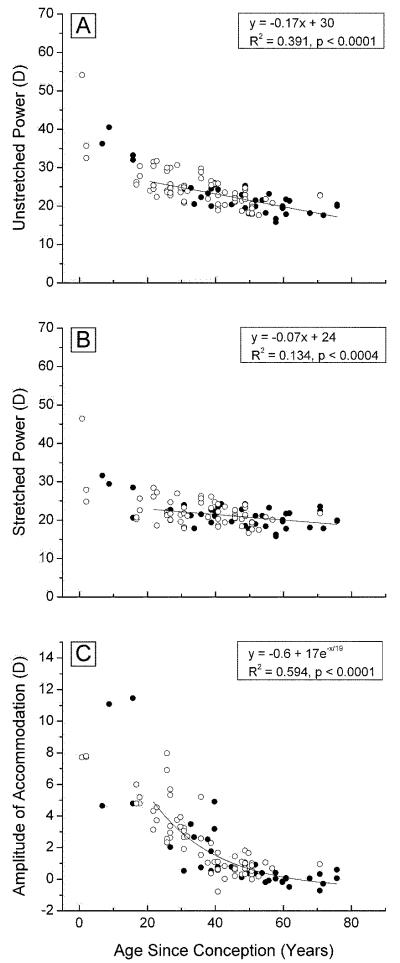
3.3 Lens optical power
The optical power of the unstretched lens was observed to decrease with age from 40-50 D at birth to ~20 D after age 50 (Fig 5A). This is comparable to the observations of Borja et al. (2008) who found that the power of the isolated human lens decreased from around 45 D at age 6 years to 20 D at age 60, although, we found no evidence for the apparent increase in power after this age, reported by these authors. The focal lengths, calculated from our data (F= 1.336/P), range from 27-40 mm near birth to 65-80 mm after age 50. These are similar to the values reported by Glasser and Campbell (1998).
Figure 5. Optical power.
The optical power of accommodated (A, 108 points) and disaccommodated with 2mm stretch (B, 101 points) lenses, and the accommodative amplitude (C, 90 points), as a function of age, for Indian (○○○) and USA (●●●) eyes mounted in the EVAS apparatus.
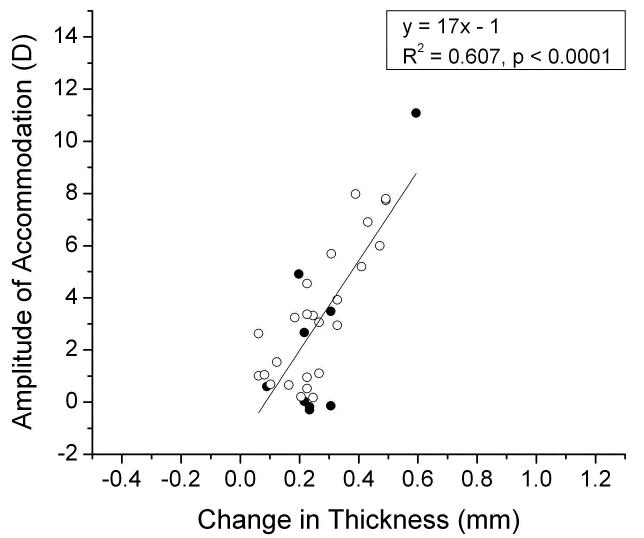
The power of stretched lenses also decreased with age, virtually leveling off at around 20 D after age 50 (Figure 5B), the same as for the unstretched lenses. The differences between the data in Figures 5A and 5B indicate that the accommodative amplitude that could be achieved in the EVAS stretcher, decreased from 12-14 D at birth to around 0 D by age 40-50 (Fig 5C). Using a simple paraxial eye model, it may be estimated that the in vivo accommodative amplitude is ~80% of the change in lens power. Our data yield an estimate of 8-10 D for the accommodative amplitude of the newborn eye, in very close agreement with the objective in vivo measurements of Anderson et al. (2008). The decrease with age is essentially identical to the in vivo accommodative loss ([Anderson et al., 2008], [Hamasaki et al., 1956], [Koretz et al., 1989],]). It is clear from Figure 6 that the accommodative amplitude is directly correlated to the response of lens thickness to stretching, averaging 1 D change for every 0.06 mm of central thickness change. This is comparable to the 0.045 mm/D observed in vivo (Dubbelman et al., 2005). It may be noted that several lenses stretched by up to 0.3 mm with no or very small accommodative change. A similar response was seen with the diameter which averaged around 1 D/0.08 mm, including several lenses which appeared to change by up to 0.3 mm with no discernable accommodative change.
Figure 6.
The accommodative amplitude as a function of central thickness change (D) for Indian (○○○) and USA (●●●) eyes mounted in the EVAS apparatus.
3.4 Stretching loads
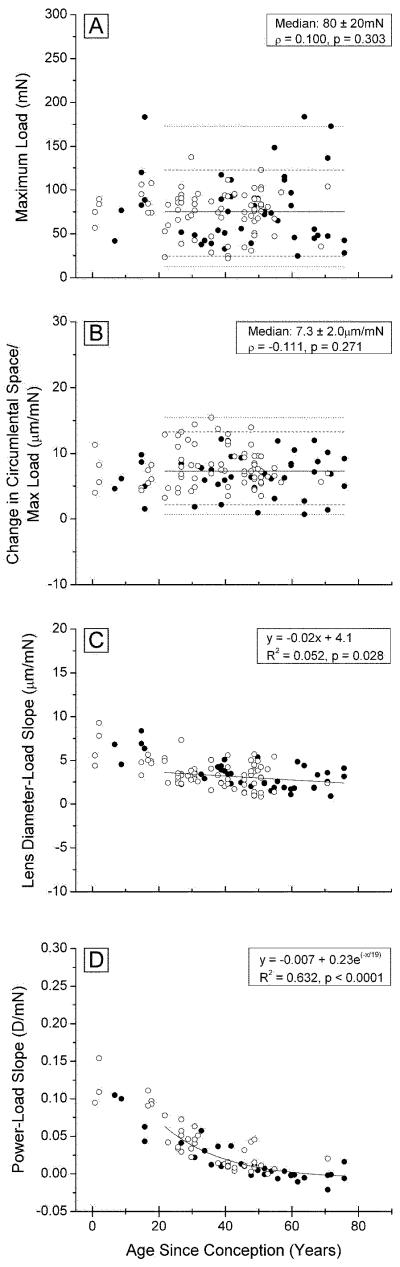
Figure 7A demonstrates that the maximum load achieved with 8 stretching steps was independent of age, with a median of 0.08 ± 0.02 mN (σ = − 0.1; p = 0.3) for the combined Indian and USA eyes. In all cases, the maximum load occurred at the maximum stretch. Although the measured changes in the various parameters provide a very useful view of the effects of stretching, the end points may be influenced by variations in the starting points, due, for example, to differences in the initial zonule tension or eye mounting. Therefore, for each eye, the observed lens diameters, ciliary ring diameters, ciliary body-lens distances and optical powers after every stretching step, were plotted against the force (load) required for the step. It was found in agreement with the observations of Manns et al. (2007) that, once stretching started, the parameter being observed increased or decreased linearly with the applied load (R >0.85; p<0.05) but the magnitude of the response to a given load (Parameter/Load) decreased with increasing age. This can be seen from the slopes of the various Parameter/Load plots, presented in Figures 7B-D.
Figure 7. Stretching loads.
Alterations in the load (force) required for 2 mm radial stretch (A, 114 points) and in the slopes of the circumlental space/load (B, 81 points), lens diameter/load (C, 107 points) and optical power/load (D, 85 points), as a function of age, for the combined Indian and USA eyes mounted in the EVAS stretching apparatus.
There appears to be no change with age in the force required to stretch the zonules (Figure 7B), as judged from the slopes of the Circumlental space/Load plots. An average of 7.3 ± 2 μm/mN was obtained for the whole age range.
By contrast, although there is considerable scatter in the data, it is clear that the slopes of the Lens diameter/load plots decrease with age (Fig 7C), indicating that the response of the lens to a given stretching force decreases with increasing age. The decrease is most rapid in the first 30-40 years of life and appears to level off at an average of 2.4 μm/mN for lenses over 50 years, similar to the 2.9 μm/mmN (0.029 mm/g), previously observed (Manns et al., 2007).
A similar age-related trend is seen in the Optical power/Load data (Fig 7D) in the two populations. There is a gradual decrease in the slope from −0.1 to −0.15 D/mN, in lenses under 10 years of age, to 0 D/mN after age 50.
4. DISCUSSION
4.1 Lens quality and numbers
Data have been obtained on the effects of stretching on ciliary body and lens dimensions, optical power and the forces required to alter these during in vitro stretching of lenses from donors, aged 0-85 years. Although human lens stretching studies, similar to that presented here, have been conducted previously ([Gerometta et al., 2007], [Koopmans et al., 2003], [Manns et al., 2007] and [Pierscionek, 1993]), they were conducted with small numbers of eyes, ranging from 2 to 27, and, in some cases, assessed fewer and different properties. Because of the small sample numbers ([Koopmans et al., 2003] and [Pierscionek, 1993, 1995]) or the grouping of data (Manns et al., 2007), it is difficult to identify age-related trends. In the present study, the 137 eyes examined were distributed over a broad age range, with 100 in the 20-60 year range, which is of most interest in relation to presbyopia.
Particular care was taken in the present study to ensure that the tissues used were in good condition. Consequently, about 25% of available eyes were rejected because of contraindications known to weaken the ciliary body or zonules. Eyes were also monitored during the experimental procedures to ensure they remained undamaged and lenses were carefully examined for evidence of swelling. It is not clear if such precautions were taken in previous studies. Given the variability observed in lenses obtained from eye bank eyes (Augusteyn et al., 2006), it would be expected that a significant number of the lenses examined in previous studies may have been swollen and could have yielded unreliable data. It is suggested that future reports on human eyes include a description of parameters used for the inclusion or exclusion of eyes.
4.2 Indian and USA eyes
The data from experiments conducted in Miami and in Hyderabad were originally analyzed separately in order to determine if there might be differences between eyes obtained from the relatively homogeneous Indian population and the racially more diverse USA donors. In addition, post mortem times for eyes examined in India were significantly shorter than those from the USA. Our results indicate there are no differences in any of the parameters examined. Therefore, all analyses and interpretations were based on the combined data.
4.3 Prestretching
The present estimates of inner ciliary ring diameter are 10% lower than those observed in vivo (Strenk et al., 1999). The difference might be related to the disruption of the scleral wall and/or changes in the geometry of the accommodative structures in the absence of intraocular pressure when mounting the eye in the EVAS device. Nevertheless, in agreement with in vivo observations ([Strenk et al., 1999] and [Tamm et al., 1992]), we found that, after age 20, inner ciliary ring diameter decreases with age.
Our observations on the lens, prior to stretching, are very similar to those made previously. Thus, we find, in agreement with in vitro studies on the isolated lens ([Augusteyn, 2010] and [Borja et al., 2008]), that the fully accommodated diameter increases throughout life, central thickness decreases from birth to the late teens and increases thereafter, and power decreases from birth onwards. The patterns of changes in these parameters are also very similar to those observed for the accommodated lens in vivo ([Jones et al., 2007] and [Strenk et al., 1999]). Such observations do much to reinforce confidence in the ability of the EVAS apparatus to preserve the natural shape of the lens and, therefore, to provide reliable information on (dis)accommodation.
The shape of the human lens is known to change with age. At birth, it has an aspect ratio (T/D) near 0.6, which decreases to 0.4 in the teenage years. Thereafter, the ratio gradually increases to just above 0.5 late in life. This shape change would be expected to significantly affect the curvature of the lens, especially in the early years and, consequently, alter optical power. Coupled with the increase in inner ciliary ring diameter up to adolescence and its possible effect on zonular tension, this makes it difficult to assess the effects of early growth on the optical properties of the eye. Therefore, analysis of the data, with respect to presbyopia development, was restricted to eyes over 20 years of age. However, all data have been shown.
There have been reports, based on in vivo observations ([Jones et al., 2007] and [Strenk et al., 1999]), that the diameter of the human lens does not increase beyond age 50. It has also been reported that there are no age-related diameter increases in old Rhesus monkeys (Wendt et al., 2008). However, these conclusions were based on small numbers of unaccommodated eyes. As pointed out previously (Augusteyn, 2010), in vivo lens dimensions are dependent on interaction of the lens with other ocular components and these interactions may change with age, accommodative and visual stimuli as well as other factors. Our data, obtained with 37 fully accommodated lenses, aged over 50 years, while still attached to the accommodative apparatus, are comparable to the dimensions obtained in several laboratories with in vitro lenses, freed from vitreous and zonules and are consistent with continued growth of the lens diameter. It might be argued that our estimates are high because the lenses were partly stretched when tensioning the zonules, prior to the commencement of the experiments. However, stretching loads were <1 mN when diameters were measured, insufficient to stretch any of the components of the accommodative apparatus, except for the lax ciliary body, emptied of aqueous humor as the tight junctions of the blood-aqueous barrier are breached after death. Furthermore, in a few cases, dimensions were measured again, using calipers at LVPEI and shadowphotogrammetry at BPEI, after removal of the lens from the eye and stretching apparatus. No differences were observed.
Between the ages of 20 and 40 years, there is a reduction of 0.4-0.5 mm in the stretched ciliary body-lens distance, the same as observed in vivo (Strenk et al., 1999). A decrease in inner ciliary ring diameter (0.4-0.5 mm/2) and an increase in lens diameter (0.4-0.5 mm/2) contribute equally to this reduction. This suggests that zonular tension might decrease over this age range, so that the relaxed lens could assume a more accommodated (rounded) shape and ciliary muscle contraction might, then, produce less shape alteration. However, since the zonular insertion band moves onto the lens surfaces, remaining at a fixed distance from the lens poles (Farnsworth and Shyne, 1979) only the ciliary body diameter decrease would affect zonular tension. At age 40-50, the ciliary ring diameter still increases by an average of 0.7 mm, with in vitro stretching, and by 0.6 mm during in vivo disaccommodation (Strenk et al., 1999), ample to overcome any age-related zonular slackness.
As has been observed in many other studies, unstretched (accommodated) lens power decreases relatively smoothly from birth until age 40-50 years, by which time accommodative amplitude has been completely lost. In young lenses, before the adolescent change in growth pattern which leads to an increase in the aspect ratio, unstretched power decreases faster (in D/year) than in the adult lens. This implies that the complex growth changes before adulthood accelerate the loss of power. The accelerated decrease could be due either to a faster rate of surface flattening in the young lens, or to a greater effect of changes in the refractive index gradient profile.
It appears unlikely that alterations in the location of the ciliary body are responsible for this loss of accommodative amplitude with age. During in vivo accommodation in young eyes, the ciliary body moves forward and inwards (Tamm et al., 1992), thereby releasing tension on some of the zonules and facilitating lens rounding. From age 30-80, the ciliary muscle continuously atrophies and adopts an anterior-inward position (Tamm et al., 1992), suggesting that zonular tension and, therefore, the forces which can be exerted on the lens would be significantly reduced. This does not appear to be the case for monkeys where the ciliary body is fixed in a far position (Tamm et al., 1992). In the stretching device, the ciliary body moves radially outwards and there is no vitreous humour to support the lens. Despite this, it is possible to closely reproduce the in vivo age-related changes, in particular, the accommodative loss. Thus, lenticular changes, alone, are probably responsible for the loss of accommodative amplitude as well as the unaccommodated power loss.
4.4 Stretching
The mechanical stretching in the EVAS instrument affects the 3 principal components of the accommodative apparatus, the ciliary body, the zonules and the lens. Each responds differently and some of their responses change with age.
Diametrical stretching by 4 mm increased the inner ciliary ring diameter by approximately 0.8 mm in the youngest eyes and 0.4 mm in the oldest. These are comparable to the in vivo responses, to an 8D accommodative stimulus, of 1.0-1.2 mm at age 25 years and 0.2-0.3 mm at 83 years (Strenk et al., 1999). The similarity between the changes suggests that the effect of the stretcher on the inner portion of the accommodative apparatus (ciliary ring, zonules and lens) is similar to that in vivo, despite the major differences in the way these changes are produced (radial stretching versus muscle contraction). The ciliary body changes are matched by increases in the circumlental space and lens diameter of 0.25 and 0.55 mm, respectively, in the youngest lens and 0.25 and 0.2 mm, respectively, in the oldest. The fact that the change in the circumlental space remains constant is expected, since we found that the force remains constant with age and there is no change in the force required to stretch the zonules. The reduced lens diameter change, with age, reflects the increased resistance of the lens to stretching.
As noted above, the distance between the ciliary body and lens increased by 0.25 ± 0.1 mm during stretching, independent of age. A similar change has been observed previously with a smaller number of eyes (Manns et al., 2007) and can also be deduced from the in vivo MRI observations (Strenk et al., 1999). The increase appears to be independent of age and takes place early in the stretching protocol, before any changes are detected in the lens diameter. It is probable that this represents stretching and re-alignment of the zonules as they take up tension. Because of the variety of insertion and anchorage points, differing angles between the zonules and lens and involvement of the hyaloid membrane ([Bernal et al., 2006] and [Farnsworth and Shyne, 1979]), it is likely that the loads on some zonules change and different zonules come into play as the ciliary body moves outwards during in vivo disaccommodation. The forces generated would change with age since some zonular insertion points move onto the surfaces of the lens as the diameter increases (Farnsworth and Shyne, 1979). Our data suggest that this actually has little measurable effect on the circumlental space, probably because there are some zonules anchored near the equator that remain there, even in old lenses.
4.4.1 Lens dimensions
A large decrease, from 0.7 to 0.2 mm, was observed with increasing age in the amount of lens diameter stretch which could be generated and a comparable decrease of 0.5 to 0.2 mm in the thickness change. These alterations indicate that the lens becomes more resistant to stretching with increasing age. This resistance is probably responsible for the 0.4 mm decrease in ciliary ring diameter displacement. Interestingly, there appeared to be around 0.2-0.3 mm of residual stretch in the thickness of old lenses, suggesting that the lens retains some flexibility, possibly in the outer, soft cortex, and can still be slightly altered by accommodative forces after age 40-50. Although this has not been observed in vivo for humans, there is a suggestion in the data of Bito et al. (1982) that this could also be the case in rhesus monkey. However, the small change in shape did not appear to translate into any discernable change in power. This may be a function of the limitations of the power measurements (accurate to ± 1.5 D, based on calibration with known power lenses) or of errors in the measurement of dimensions. It is also possible that the changes in lens curvature and, hence, power were in the outer cortex, beyond the measurement zone of our Scheiner optical system which employed four parallel beams effectively representing a pupil size of 3 mm. For future studies, more comprehensive characterisation across a wider extent of the lens, by the incorporation of systems that are able to quantify the topography of the entire lens surface ([Manns et al., 2004] and [Uhlhorn et al., 2008]), would be beneficial.
4.4.2 Power changes
As reported in numerous studies, both in vivo and in vitro, we found that the power of the accommodated lens decreases with age from around 45 D at birth to 20 D at age 40. Thereafter, lens power changes only slightly, in vitro or in vivo, as the lens continues to grow and change shape. The age-related in vivo decline has been variously attributed to changes in lens surface curvature and refractive index distributions as well as, but less likely, alterations in other ocular components ([Atchison, 1995], [Fincham, 1937], [Glasser and Kaufman, 2003] and [Strenk et al., 2005]). Recent studies on isolated lenses ([Borja et al., 2008] and [Jones et al., 2005]) indicate that the surfaces contribute only 20-40% of accommodated power change, most from the anterior surface ([Dubbleman et al., 2005], [Fincham, 1937], [Garner and Yap, 1997] and [Koretz et al., 2002]) and this changes little with age. Thus, alterations in the refractive index distribution, such as the development of the nuclear refractive index plateau (Augusteyn et al., 2008), would be major factors responsible for the loss of power.
Extrapolation from our data indicates that the power of newborn lenses can change by up to 12-14 D. This would correspond to 8-10 D for the accommodative amplitude of the newborn eye, in very close agreement with the objective in vivo measurements of Anderson et al. (2008). We found lens power change declines to near 0 D by age 40-50 years, similar to previous in vitro and in vivo observations ([Borja et al., 2008], [Duane, 1912], [Glasser and Campbell, 1998], [Hamasaki et al., 1956], [Koretz et al., 1989] and [Manns et al., 2007]). The power change appears to be directly related to the responses of lens shape to stretching forces. Our measurements show that a change of around 0.06 mm in the central thickness (and diameter) altered the amplitude by 1 D, close to the 0.045 mm/D estimated by Dubbelman et al. (2003) for the in vivo nucleus and the 0.05mm/D reported by Jones et al. (2007) for the lens.
Accommodation involves changes in the dimensions of the nucleus (Patnaik, 1967). Except for the residual cortical and nuclear stretch, referred to earlier, the alterations we observe in lens dimensions are matched by similar changes previously observed in the nucleus ([Dubbelman et al., 2003], [Kasthurirangan et al., 2008], [Patnaik, 1967] and [Weeber and van der Heijde, 2007]). Therefore, it is likely that the 12-14D lens power loss can be attributed to the inability of the nucleus to change shape because of stiffening ([Weeber and van der Heijde, 2007] [Heys et al., 2005] and [Weeber et al., 2005]). The decrease in the Lens diameter/Load slopes would be consistent with this conclusion although a much larger decrease than the observed 4-fold might have been expected for the reported 'massive increase in nuclear stiffness'. This could suggest that the stiffness of the nucleus may not matter as much as was previously thought.
In addition to the loss of accommodative amplitude, a further 10-15 D of lens power is lost in the unstretched (fully accommodated) lens before age 40-50, apparently at the same rate as the accommodative amplitude loss, suggesting that both may be a consequence of the same events taking place in the nucleus. These events result in formation of a refractive index plateau and stiffening of the nucleus, as well as development of a diffusion barrier between nucleus and cortex. It may be that the additional power loss is due to the equivalent refractive index reduction, associated with growth of the plateau.
4.4.3 Load
EVAS delivered a constant maximum load of approximately 80 mN which was dissipated in different ways according to the age of the eye. In the younger eyes, substantial stretching of the lens resulted. In the older eyes, lens stretch was reduced and, instead, the ciliary body stretched more. This result was unexpected. One would expect the load to increase with age since the radial displacement of the shoes is fixed at 4 mm and the lens becomes stiffer. This apparent discrepancy can be explained using a simple model of the tissue where the lens, zonules and ciliary body are represented by three springs in series. According to our results, the spring constant of the zonules is independent of age, approximately equal to 137 mN/mm (1/7.3 μm/mN). The fact that the force remains constant therefore suggests that the equivalent stiffness of the lens-ciliary body system remains constant with age. This is consistent with our observations that the stiffness of the ciliary body seems to decrease with age while the stiffness of the lens increases with age. In addition, the lens is much stiffer than the ciliary body. The equivalent spring constant of the lens-ciliary body system is therefore dominated by the spring constant of the ciliary body and changes in lens stiffness will have small effects on the total stretching force measured in EVAS.
Since the changes in the EVAS stretching device are the same as in vivo, it is tempting to speculate that the observed load is similar to that generated in vivo. This would facilitate the selection of appropriate replacement gels in the Phaco-ersatz procedure (Parel et al., 1986). However, we cannot be certain that the endpoint in the stretcher corresponds to the relaxed in vivo lens. In particular, we find that the stretched lens power decreases by several diopters between 20 and 40 years of age, whereas the in vivo relaxed lens power is approximately constant. This suggests that lenses were slightly understretched, compared to in vivo disaccommodation. We can therefore deduce only that the in vivo force of accommodation will either increase slightly with age or remain constant. A constant force would be consistent with the FEM model of Hermans et al. (2009).
However, caution should be exercised when interpreting in vitro data since the geometry of the forces applied on the lens in the stretching device will be different from those in vivo. The sclera does not move in vivo and the ciliary body diameter is changed through contraction and relaxation of the longitudinal, circular and reticular muscle components. In the EVAS device, the sclera moves and the intact ciliary body width increases by as much as 1.7 mm in order to produce changes in the ciliary body diameter, similar to those observed in vivo. The in vivo forces applied through zonules inserted on the anterior and posterior surfaces, central to the lens equator, may not be operative in vitro.
In order to avoid complications due to any lag in the response of the different parameters to the applied load, as well as differences in the responses, slopes were determined from the parameter versus load plots. It is clear from a consideration of the parameter/load slopes that the responses of the lens to a given load decrease with age, whereas other components of the accommodative apparatus appear to be unaffected. Thus, the Lens power/Load slope decreases from 0.25 D/mN to almost zero by around age 40 while the Lens diameter/Load slope decreases from 8 to 3 μm/mN. By contrast, the slopes for the CB-lens distance (7.3 μm/mN) and CB inner diameter (11 μm/mN) appear to be constant with age. These observations are consistent with a lenticular origin for presbyopia.
An increased resistance of the lens to stretching, rather than alterations in the ciliary body or other parts of the accommodative apparatus is consistent with conclusions from FEM modeling ([Belaidi and Pierscionek, 2007], [Hermans et al., 2009] and [Weeber and van der Heijde, 2007]). Hermans et al. (2009) deduced that the force exerted by the ciliary body is constant with age, while Belaidi and Pierscionek (2007) predicted that the same amount of force would produce less deformation in old lenses, as has been observed in the present study. Interestingly, the latter authors noted that, with radial equatorial stretching, the maximum stress is located around the nuclear-cortical boundary, which lies at about 2/3 of the lens diameter and thickness. This is what one might expect for a mechanism in which the nucleus changes shape during accommodation. The boundary separates the pre- and post-natal tissues, which are characterized by different crystallin distributions (Augusteyn, 2010). The barrier to diffusion of low molecular weight substances is also located in this region (Sweeney and Truscott, 1998), as is the outer edge of the refractive index plateau, both of which develop with increasing age ([Augusteyn et al., 2008] and [Jones et al., 2005]). It may be that the resistance to stretching and the consequential failure of the nucleus to change shape originate in this region and that they are related to the gradually increasing nuclear stiffness ([Heys et al., 2005] and [Weeber et al., 2005]). Weeber and van der Heijde (2007) modeled the effects of lens stiffness and concluded that a changing gradient of stiffness would affect accommodation.
Although the current study has provided a wealth of information on the accommodative apparatus and its age-related changes, variations and shortcomings in some aspects of the measuring protocols have introduced uncertainty into the calculations. In particular, the measurement of lens central thickness using the slit lamp is unsatisfactory because of the need to correct for optical distortion. Optical coherence tomography (OCT) (Uhlhorn et al., 2008) is currently being incorporated into a newer version of the EVAS stretching device (Ehrmann et al., 2008) This will provide far more accurate measures of lens thickness, cross sectional areas, as well as refractive index distributions.
In conclusion, through the use of the EVAS stretching device we have been able to examine aspects of the accommodative system, in vitro, using a large number of eyes, harvested in the USA and India. Our observations on the effect of age on the human accommodative system and the changes which occur during accommodation are very similar to those obtained in vivo and are consistent with the classical Helmholtz theory of accommodation. Our data indicate that the responses of lens diameter and power to disaccommodative (stretching) forces decrease with age and can be primarily attributed to stiffening of the nucleus. Overall, we find that for all measured parameters which change with age, the changes occur progressively after the initial complex growth phase ending at the end of the teenage years. Our results, therefore, also show that presbyopia is a consequence of the normal continued growth of the lens during adulthood.
The information obtained and the protocols developed in the current study will be invaluable for the development of procedures for replacement of the ageing lens contents.
Acknowledgements
This work was supported, in part, by NIH Research Grant RO1EYO14225 and the Australian Federal Government CRC Scheme through the Vision Cooperative Research Centre. We are indebted to the Florida Lions Eye Bank, Miami, USA and the Ramayamma International Eye Bank, Hyderabad, India for the supply of eyes. The authors are grateful for the assistance of David Borja, Mohan Kumar, and Noel Ziebarth with some of the early data collection and Virender Sangwan and Pravin Vaddavalli for performing some of the eye dissections. Mariela Aguilar and Qian Garrett provided invaluable administrative support, while Willi Aumayr, Klaus Ehrmann, Billy Lee and Izuru Nose ensured that instrument functions were optimal. The constant encouragement from Brien Holden was a major stimulus to the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary reports on some of the data were presented at the annual meetings of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, May 2007, 2008, 2009 and 2010.
References
- van Alphen GWHM, Graebel WP. Elasticity of tissues involved in accommodation. Vis. Res. 1991;31:1417–1438. doi: 10.1016/0042-6989(91)90061-9. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Hentz G, Glasser A, Stuebing KK, Manny RE. Minus-lens-stimulated accommodative amplitude decreases sigmoidally with age: A study of objectively measured accommodative amplitudes from age 3. Invest. Ophthalmol. Vis. Sci. 2008;49:2919–2926. doi: 10.1167/iovs.07-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison DA. Accommodation and Presbyopia. Ophthalmic Physiol. Opt. 1995;15:255–277. [PubMed] [Google Scholar]
- Augusteyn RC. On the growth and internal structure of the human lens. Exp. Eye Res. 2010;90:643–654. doi: 10.1016/j.exer.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusteyn RC, Jones C, Pope J. Age-related development of a RI plateau in the human lens: evidence for a distinct nucleus. Clin. Exp. Optom. 2008;91:296–301. doi: 10.1111/j.1444-0938.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- Augusteyn RC, Rosen AM, Borja D, Ziebarth NM, Parel J-M. Biometry of primate lenses during immersion in preservation media. Mol. Vis. 2006;12:740–747. [PubMed] [Google Scholar]
- Belaidi A, Pierscionek BK. Modelling internal stress distributions in the human lens: can opponent theories coexist? J. Vision. 2007;7:1–12. doi: 10.1167/7.11.1. [DOI] [PubMed] [Google Scholar]
- Bernal A, Parel J-M, Manns F. Evidence for posterior zonular attachment on the anterior hyaloid membrane. Invest. Ophthalmol. Vis. Sci. 2006;47:4708–473. doi: 10.1167/iovs.06-0441. [DOI] [PubMed] [Google Scholar]
- Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest. Ophthalmol. Vis. Sci. 1982;23:23–31. [PubMed] [Google Scholar]
- Borja D, Manns F, Ho A, Ziebarth N, Rosen AM, Jain R, Amelinckx A, Arrieta E, Augusteyn RC, Parel J-M. Optical power of the isolated human crystalline lens. Invest. Ophthalmol. Vis. Sci. 2008;49:2541–2548. doi: 10.1167/iovs.07-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd HJ, Wilde GS, Judge SJ. Can reliable values of Young's Modulus be deduced from Fisher's (1971) spinning lens measurements? Vis. Res. 2006;46:1346–1360. doi: 10.1016/j.visres.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Burd HJ, Wilde GS, Judge SJ. An improved spinning lens test to determine the stiffness of the human lens. Exp. Eye Res. 2011;92:28–39. doi: 10.1016/j.exer.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer A. Het accommodatievermogen der oogen physiologisch toegelicht. De Erven Loosjes; Haarlem: 1853. pp. 35–37. [Google Scholar]
- Donders FC. On the anomalies of accommodation and the refraction of the eye. New Sydenham Society; London: 1864. [Google Scholar]
- Duane A. Normal values of the accommodation at all ages. J. Amer. Med. Assn. 1912;59:1010–1013. [Google Scholar]
- Dubbleman M, van der Heijde R, Weeber HA. Change in shape of the ageing human crystalline lens with age. Vis. Res. 2005;45:117–121. doi: 10.1016/j.visres.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Dubbelman M, van der Heijde R, Weeber HA, Vrensen GFJM. Changes in the internal structure of the human crystalline lens with age and accommodation. Vis. Res. 2003;43:2363–2375. doi: 10.1016/s0042-6989(03)00428-0. [DOI] [PubMed] [Google Scholar]
- Ehrmann K, Ho A, Parel J-M. Biomechanical analysis of the accommodative apparatus in primates. Clin. Exp. Optom. 2008;91:302–312. doi: 10.1111/j.1444-0938.2008.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth PN, Shyne SE. Anterior zonular shifts with age. Exp. Eye Res. 1979;28:291–297. doi: 10.1016/0014-4835(79)90091-5. [DOI] [PubMed] [Google Scholar]
- Fincham EF. The mechanism of accommodation. British J. Ophthalmol. Suppl. 1937;8:5–80. [Google Scholar]
- Fisher RF. The elastic constants of the human lens. J. Physiol. 1971;212:147–180. doi: 10.1113/jphysiol.1971.sp009315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RF. The force of contraction of the human ciliary muscle during accommodation. J. Physiol. 1977;270:51–74. doi: 10.1113/jphysiol.1977.sp011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner LF, Yap MKH. Changes in ocular dimensions and refraction with accommodation. Ophthal. Physiol. Optics. 1997;17:12–17. [PubMed] [Google Scholar]
- Gerometta R, Zamudio AC, Escobar DP, Candia OA. Volume change of the ocular lens during accommodation. Am. J. Physiol. Cell. Physiol. 2007;293:C797–C804. doi: 10.1152/ajpcell.00094.2007. [DOI] [PubMed] [Google Scholar]
- Glasser A, Campbell MCW. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 1998;38:209–229. doi: 10.1016/s0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- Glasser A, Kaufman PL. Accommodation and Presbyopia. In: Kaufman PL, Alm A, editors. Adler's Physiology of the eye, Clinical Application. 10th ed. St Louis, Mo: 2003. pp. 197–203. [Google Scholar]
- Hamasaki D, Ong J, Marg E. The amplitude of accommodation in presbyopia. Am. J. Optom. Arch. Am. Optom. 1956;33:3–14. doi: 10.1097/00006324-195601000-00002. [DOI] [PubMed] [Google Scholar]
- Helmholtz H. The accommodation of the eyes. Alb. Graefes Arch. Klin. Exp. Ophthalmol. 1855;1:1–70. [Google Scholar]
- Hermans EA, Pouwels PJW, Dubbelman M, Kuijer JPA, van der Heijde RGL, Heethaar RM. Constant Volume of the Human Lens and Decrease in Surface Area of the Capsular Bag during Accommodation: An MRI and Scheimpflug Study. Invest. Ophthalmol. Vis. Sci. 2009;50:281–289. doi: 10.1167/iovs.08-2124. [DOI] [PubMed] [Google Scholar]
- Heys KR, Cram SL, Truscott RJW. Massive increase in the stiffness of the lens nucleus with age: the basis for presbyopia? Mol. Vis. 2005;10:256–263. [PubMed] [Google Scholar]
- Jones CE, Atchison DA, Meder R, Pope JM. Refractive index distribution and refractive index properties of the isolated human lens using magnetic resonance imaging (MRI) Vis. Res. 2005;45:2253–2366. doi: 10.1016/j.visres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Jones CE, Atchison DA, Pope JM. Changes in lens dimensions and refractive index with age and accommodation. Optom. Vis. Sci. 2007;84:990–995. doi: 10.1097/OPX.0b013e318157c6b5. [DOI] [PubMed] [Google Scholar]
- Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest Ophthalmol. Vis. Sci. 2008;49:2531–40. doi: 10.1167/iovs.07-1443. [DOI] [PubMed] [Google Scholar]
- Kaufman PL. In: Accommodation and Presbyopia: Neuromusclar and Biophysical Aspects, Adler's Physiology of the Eye. Ninth Edition Hart William M., editor. Mosby; St. Louis, Mo.: 1992. pp. 391–411. [Google Scholar]
- Kessler J. Experiments in refilling the lens. Arch. Ophthalmol. 1964;71:412–417. doi: 10.1001/archopht.1964.00970010428021. [DOI] [PubMed] [Google Scholar]
- Koopmans SA, Terwee T, Barkhof J, Haitjema HJ, Kooijman AC. Polymer refilling of presbyopic human lenses in vitro restores the ability to undergo accommodative changes. Invest. Ophthalmol. Vis. Sci. 2003;44:250–257. doi: 10.1167/iovs.02-0256. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye – aging of the anterior segment. Vis. Res. 1989;29:1685–1692. doi: 10.1016/0042-6989(89)90150-8. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Cook CA, Kaufman PL. Aging of the human lens: changes in lens shape upon accommodation and accommodative loss. J. Opt. Soc. Amer. A. 2002;19:144–151. doi: 10.1364/josaa.19.000144. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Handelman GH, Brown NP. Analysis of human crystalline lens curvature as a function of accommodative state and age. Vis. Res. 1984;24:1141–1151. doi: 10.1016/0042-6989(84)90168-8. [DOI] [PubMed] [Google Scholar]
- Manns F, Fernandez V, Zipper S, Sandadi S, Hamaoui M, Ho A, Parel JM. Radius of curvature and asphericity of the anterior and posterior surface of human cadaver crystalline lenses. Exp. Eye Res. 2004;78:39–51. doi: 10.1016/j.exer.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Manns F, Parel J-M, Denham D, Billotte C, Ziebarth N, Borja D. Fernandez, V., Aly M, Arrieta E, Ho A, Holden B. Optomechanical Response of human and monkey lenses in a lens stretcher. Invest Ophthalmol. Vis. Sci. 2007;48:3260–3268. doi: 10.1167/iovs.06-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parel J-M, Gelender H, Trefers WF, Norton EW. Phaco-ersatz: cataract surgery designed to preserve accommodation. Alb. Graefes Arch. Clin. Exp. Ophthalmol. 1986;224:165–173. doi: 10.1007/BF02141492. [DOI] [PubMed] [Google Scholar]
- Patnaik B. A photographic study of accommodative mechanisms: changes in the lens nucleus during accommodation. Invest. Ophthalmol. 1967;6:601–611. [PubMed] [Google Scholar]
- Pau H. Dependence of the shape of the lens on physical factors. Ophthalmologica. 1951;122:308–314. doi: 10.1159/000301076. [DOI] [PubMed] [Google Scholar]
- Pierscionek BK. In vitro alteration of lens curvatures by radial stretching. Exp. Eye Res. 1993;57:629–635. doi: 10.1006/exer.1993.1168. [DOI] [PubMed] [Google Scholar]
- Pierscionek BK. Age-related response of human lenses to stretching forces. Exp. Eye Res. 1995;60:325–332. doi: 10.1016/s0014-4835(05)80114-9. [DOI] [PubMed] [Google Scholar]
- Reilly MA, Hamilton PD, Ravi N. Dynamic multi-arm radial lens stretcher: A robotic analog of the ciliary body. Exp. Eye Res. 2008;86:157–164. doi: 10.1016/j.exer.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Reilly MA, Hamilton PD, Peery G, Ravi N. Comparison of the behavior of natural and refilled porcine lenses in a robotic lens stretcher. Exp. Eye Res. 2009;88:483–494. doi: 10.1016/j.exer.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Rosen AM, Denham DB, Fernandez V, Borja D, Ho A, Manns F, Parel JM, Augusteyn RC. In vitro dimensions and curvatures of human lenses. Vis. Res. 2006;46:1002–1009. doi: 10.1016/j.visres.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Schachar R. Qualitative effect of zonular tension on freshly extracted intact human crystalline lenses: implications for the mechanism of accommodation. Invest. Ophthalmol. Vis. Sci. 2004;45:2691–2695. doi: 10.1167/iovs.03-1267. [DOI] [PubMed] [Google Scholar]
- Stadfeldt AE. Die veranderung der lines bei traction der zonula. Klin. Monatsbl. Augenheilk. 1896;34:429–431. [Google Scholar]
- Strenk SA, Strenk LM, Koretz JF. The mechanism of presbyopia. Prog. Retin. Eye Res. 2005;24:379–393. doi: 10.1016/j.preteyeres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest. Ophthalmol. Vis. Sci. 1999;40:1162–1169. [PubMed] [Google Scholar]
- Sunderland HR, O'Neill WD. Functional dependence of optical parameters on circumferential forces in the cat lens. Vis. Res. 1976;16:1151–1158. doi: 10.1016/0042-6989(76)90256-x. [DOI] [PubMed] [Google Scholar]
- Sweeney MHJ, Truscott RJW. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp. Eye Res. 1998;67:587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- Tamm S, Tamm E, Rohen JW. Age-related changes of the human ciliary muscle: a quantitative morphometric study. Mech. Ageing Dev. 1992;62:209–221. doi: 10.1016/0047-6374(92)90057-k. [DOI] [PubMed] [Google Scholar]
- Uhlhorn SR, Borja D, Manns F, Parel J-M. Refractive index measurement of the isolated crystalline lens using optical coherence tomography. Vis. Res. 2008;48:2732–2738. doi: 10.1016/j.visres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber HA, Eckert G, Soergel F, Meyer CH, Pechhold W, van der Heijde RGL. Dynamic mechanical properties of human lenses. Exp. Eye Res. 2005;80:425–434. doi: 10.1016/j.exer.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Weeber HA, van der Heijde RGL. On the relationship between lens stiffness and accommodative amplitude. Exp. Eye Res. 2007;85:602–697. doi: 10.1016/j.exer.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Wendt M, Croft MA, McDonald J, Kaufman PL, Glasser A. Lens diameter and thickness as a function of age and pharmacologically stimulated accommodation in rhesus monkeys. Exp. Eye Res. 2008;86:746–752. doi: 10.1016/j.exer.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. Observations on vision. Phil. Trans. Royal Soc. Lond. 1793;83:169–191. [Google Scholar]