Abstract
Background
Epidemiologic evidence suggests that non-steroidal anti-inflammatory drugs (NSAIDs) delay onset of Alzheimer’s dementia (AD), but randomized trials show no benefit from NSAIDs in symptomatic AD. ADAPT randomized 2,528 elderly persons to naproxen or celecoxib vs. placebo for two years (s.d. 11 months) before treatments were terminated. During the treatment interval, 32 cases of AD revealed increased rates in both NSAID-assigned groups.
Methods
We continued the double-masked ADAPT protocol for two additional years to investigate incidence of AD (primary outcome). We then collected cerebrospinal fluid (CSF) from 117 volunteer participants to assess their ratio of CSF tau to Aβ1–42.
Results
Including 40 new events observed during follow-up of 2,071 randomized individuals (92% of participants at treatment cessation), there were now 72 AD cases. Overall NSAID-related harm was no longer evident, but secondary analyses showed that increased risk remained notable in the first 2.5 years of observations, especially in 54 persons enrolled with Cognitive Impairment – No Dementia (CIND). These same analyses showed later reduction in AD incidence among asymptomatic enrollees given naproxen. CSF biomarker assays suggested that the latter result reflected reduced Alzheimer-type neurodegeneration.
Conclusions
These data suggest a revision of the original ADAPT hypothesis that NSAIDs reduce AD risk, thus: NSAIDs have an adverse effect in later stages of AD pathogenesis, while asymptomatic individuals treated with conventional NSAIDs like naproxen experience reduced AD incidence, but only after 2 – 3 years. Thus, treatment effects differ at various stages of disease. This hypothesis is consistent with data from both trials and epidemiological studies.
1. Background
As populations age, Alzheimer’s dementia (AD) presents an enormous threat to public health [1]. The pathogenesis of AD includes pre-symptomatic and prodromal stages that together last a decade or more before onset of dementia [2]. Functional neuroimaging data suggest that pre-symptomatic AD is characterized by changes in synaptic function [3], possibly induced by oligomers of the Alzheimer amyloid peptide Aβ [4]. The familiar pathological hallmarks of AD – senile plaques, neurofibrillary tangles, and neuronal degeneration – become preponderant later, typically in the prodromal and dementia stages [5, 6]. This evolution in AD pathogenesis suggests that interventions may vary in their effects at different stages of disease. Such a varied response to treatment has been observed, for example, in the transgenic R1.40 mouse model of AD, where the nonsteroidal anti-inflammatory drugs (NSAIDs) ibuprofen and naproxen suppress new neuronal ectopic cell cycle events induced by Aβ oligomers, but fail to reverse existing events [7].
In many observational studies, users of NSAIDs have been found to develop AD with reduced frequency, but there has been no such association with exposure to these drugs shortly before dementia onset [8, 9]. Several randomized trials have assessed the possible neuroprotection suggested by these observational study results. With the single exception described here, however, those trials have all been conducted in symptomatic individuals. Results have been disappointing. Trials of NSAIDs in patients with established dementia showed no mitigation of symptom progression [10, 11]. Even in patients with milder cognitive symptoms, incidence of AD was increased in those who received the cyclooxygenase-2- (COX-2)-selective NSAID rofecoxib [12]. A synthesis of the laboratory, observational, and trial data therefore suggests that NSAIDs may provoke contrasting effects at different stages of AD pathogenesis, with neutral or adverse influence on the risk of dementia onset in people with symptoms, but possible protection against onset in those with healthier brains.
2. Methods
2.1 Approach
We extended the observation period of the previously reported randomized, controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT), designed to test the hypothesis that sustained use of naproxen or celecoxib would reduce incidence of AD in healthy elders [13]. Concerns about safety had led to termination of the ADAPT treatments late in 2004, after an average 24 (s.d. 11) months of treatment assignment [13,14]. At that point an analysis of 32 incident cases of AD (the trial’s primary outcome) had suggested a possible increase in risk of dementia with either NSAID, as reflected by a hazard ratio vs. placebo (HR) of 1·99 (95% CI 0·80 – 4·97; P= 0·14) for celecoxib and 2·35 (CI 0·95 – 5·77; P = 0·06) for naproxen [13]. Following the principle of intention-to-treat (ITT), the primary analysis then included seven individuals who had been randomized before we learned that they had dementia at baseline. Because these seven were not at risk of incident dementia, we also excluded them in a secondary analysis, planned prior to completion of data collection and unmasking of treatment assignment. The latter analysis produced a stronger suggestion of harm, with HRs of 4.11 (CI 1.30 – 13.0; P = 0.02) for celecoxib and 3.57 (CI 1.09 – 11.7; P = 0.04) for naproxen [13].
To evaluate the longer-term effects of the ADAPT treatments, particularly including harm, we followed participants off-treatment for another 18–24 months. Then, in post-treatment months 21 – 41, after completion of clinical follow-up, we collected cerebrospinal fluid (CSF) from 117 non-demented volunteer ADAPT participants for evaluation of treatment effects on biomarkers of AD pathogenesis.
2.2 Standard protocol approvals, registrations and participant consents
All human subject research procedures for ADAPT (clinicaltrials.gov identifier: NCT00007189) were approved by the relevant Institutional Review Boards of the University of Washington (VA Committee, for the ADAPT Chairman’s Office), the Johns Hopkins Bloomberg School of Public Health (Coordinating Center), and each of the six ADAPT field sites. All participants were capable of providing informed consent at enrollment and at each subsequent point of data collection, and did so. We obtained separate consent from the 117 volunteers for lumbar puncture (LP) and measurement of AD biomarkers in CSF.
2.3 Methods of data collection
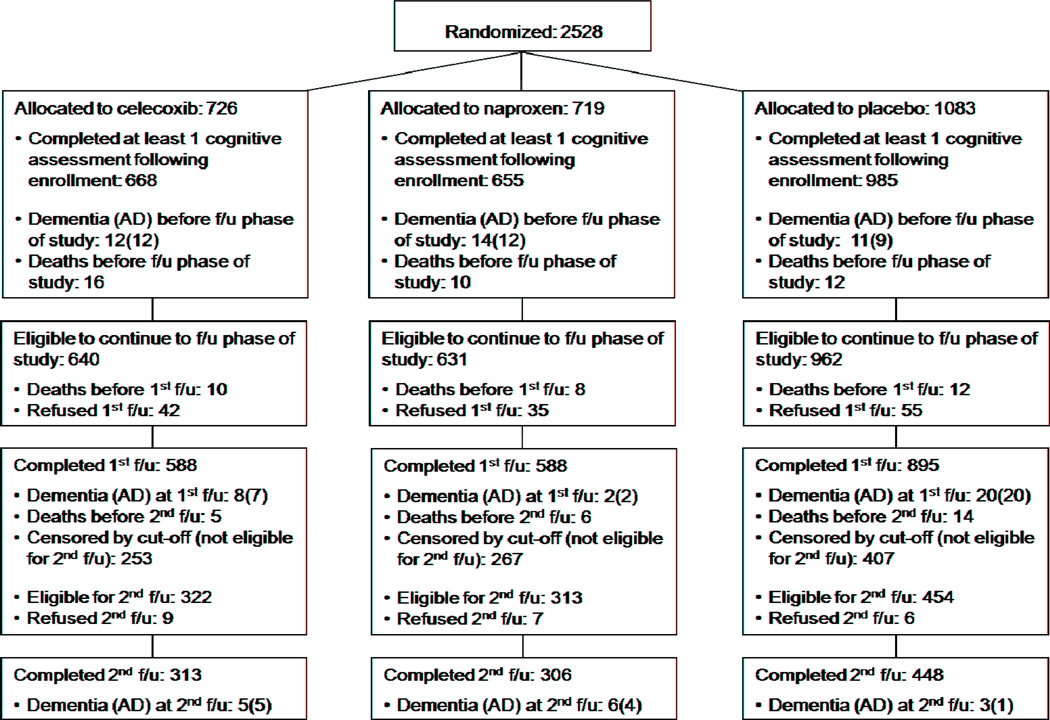
ADAPT data gathering methods have been described previously [13,15,16]. Briefly, 2,528 healthy elderly individuals with a family history suggesting increased risk of AD were enrolled if they met entry criteria that included a minimum score on a brief cognitive screening battery. Enrollees were randomized to naproxen sodium 220 mg twice daily, celecoxib 200 mg twice daily, or placebo [17]. They then underwent more thorough cognitive screening and a full evaluation for dementia when indicated, at baseline and annually thereafter. The primary efficacy outcome throughout was onset of AD, diagnosed using standard research criteria by multidisciplinary expert consensus panels at each of the six field sites. In one change of procedure, however, the present analyses dated outcome events to the screening cognitive assessment or clinical report that triggered a dementia evaluation resulting in the diagnosis. Figure 1 extends our previously published CONSORT-style chart [13], showing participant flow during the period of follow-up data collection. Throughout these observations investigators and participants remained masked to treatment assignment.
Figure 1.
The figure shows numbers originally randomized to the three treatment groups as well as numbers eligible to continue to follow-up observation after the end of the treatment interval. The latter include subjects whose first annual follow-up visit occurred during the follow-up period, and therefore differ from the final numbers remaining in the CONSORT-style figure of Reference 13.
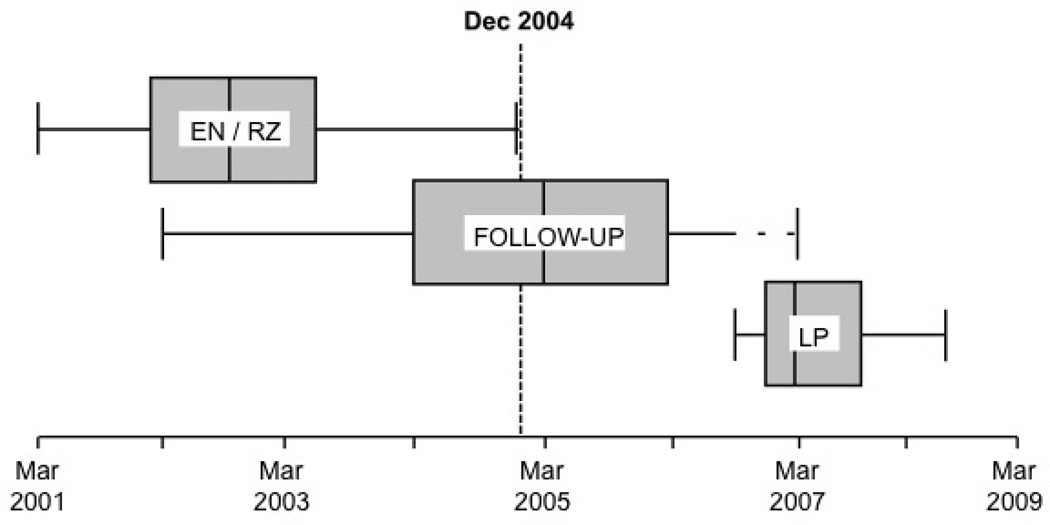
Over a 21-month period after completion of cognitive follow-up, we collected CSF from volunteer dementia-free ADAPT participants (Figure 2A). The CSF donors’ treatment assignments remained unknown to field site staff. LPs were performed following an overnight fast, using techniques identical to those described elsewhere [18]. Sequentially numbered 0.5 ml aliquots of CSF were promptly frozen and shipped on dry ice to storage facilities in Seattle where they were stored at −80°C until analysis.
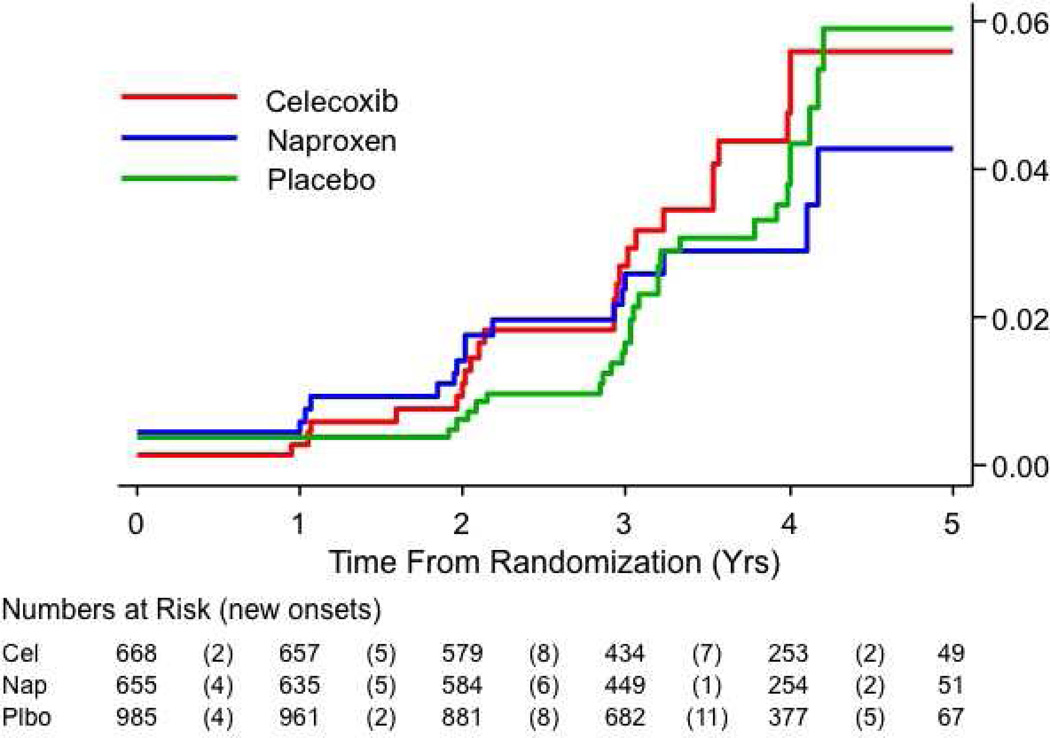
Figure 2.
A, Box-plots showing periods of enrollment and randomization (EN-RZ), follow-up evaluations (FU), and lumbar punctures (LP). Enrollment began in March 2001. LPs commenced in September 2006 in participants for whom clinical follow-up was complete (dashed line).
B, Estimated cumulative incidence of AD (primary analysis). Only 3 new cases appeared among 447 naproxen-assigned individuals remaining after 3 years. The lines’ non-zero origins reflect inclusion in this analysis of 8 prevalent dementia cases.
2.4 Methods for analyses of CSF biomarkers
These methods have been published [19]. Briefly, CSF from the 4th or 5thml collected was assayed for concentration of the Alzheimer amyloid peptide Aβ1–42 and total tau protein (T-tau, there being no evident advantage in our experience for assays of individual phosphorylated tau species such as 181P-tau.) We used Luminex reagents from the Biosource Division of Invitrogen, Camarillo, CA, and X-MAP technology, the LiquiChip workstation from Quiagen, Valencia, CA, following the manufacturer’s instructions exactly. Standard curves were constructed using authentic standards included with each kit, 7 – 5,000 pg/ml for both Aβ1–42 and T-tau. The lower limits of quantitation were 20 pg / ml for Aβ1–42 and 20 pg / ml for T-tau. Each sample was analyzed in duplicate for both endpoints, and the average was taken for statistical analysis using GraphPad Prism, San Diego, CA.
2.5 Statistical analyses
Excepting individuals with dementia at baseline, outcome analyses were limited to participants who completed at least one annual cognitive assessment after enrollment. Analyses considered each participant’s time at risk up to his or her last cognitive assessment. Ten participants with dementia not attributed to Alzheimer’s disease were censored at the point their dementia was identified (5 had vascular dementia; others had rarer conditions). Their treatment assignments (10 to placebo, 4 to naproxen, 1 to celecoxib) were proportional to the trial’s randomization ratio (exact P > 0.5). By design, active treatments were compared with placebo and not with each another. Time-to-occurrence of AD was first evaluated using Kaplan-Meier plots and associated log-rank tests. We then employed Cox proportional hazards regression models to seek semi-parametric estimates of differences by treatment assignment. Such models assume a relatively constant ratio of incidence rates over the time of observation (proportional hazards assumption). We tested this assumption using Schoenfeld residuals, with P < 0.05 indicating that the hazard ratio (HR) varied more over the interval of observations than could likely be explained by chance. When the assumption was violated, we examined whether the effects of treatment varied systematically over time following randomization by evaluating the data for presence of a statistical interaction with (continuous) time in Cox models. Provided such interaction was present, we then examined whether a similarly improved fit to the data was evident in more intuitive models that dichotomized the observation period into early vs. later time intervals. The dichotomous models used maximum likelihood methods to estimate the optimum dividing point, concurrently using the statistical interaction term to estimate HR estimates for the early vs. later time intervals. In each instance, we also verified that the early and the later interval data fulfilled the proportional hazards assumption. Following the data analytic plan of the ADAPT protocol, we adjusted all Cox models for stratification variables of five-year age group and field site. Adjusted HRs were estimated with 95% confidence intervals and Wald P values. All tests for statistical significance employed a two-tailed alpha of 0.05.
Again following the principle of intention-to-treat (ITT), the extended primary analysis considered all AD cases discovered following randomization. Also as before, a planned secondary analysis examined rates of incident AD by excluding participants with prevalent dementia at baseline. Other secondary analyses, conducted ad hoc, examined outcomes in subgroups who had Cognitive Impairment – No Dementia (CIND) at baseline (their randomized group proportions were similar to those in ADAPT; exact P = 0.26), and in the cohort after exclusion of participants with either prevalent AD or CIND. CSF levels of T-tau and Aβ1–42 and their ratio were compared across randomized groups by analysis of variance (ANOVA) employing the same covariates as the Cox models. All analyses were conducted using STATA version 10.1 (Stata Corp., 2007, College Station, TX).
3. Results
3.1 Participant characteristics and cognitive outcomes
Demographics of the ADAPT participant cohort have been previously described [13]. Characteristics of the cohort at the beginning of the follow-up period are shown in Table 1. The primary ITT analysis results over the entire observation period are shown in Table 2, lines 1 – 7, and graphically in Figure 2B. The crude rate ratios suggested no net effect of either NSAID. Corresponding log-rank P-values were 0.73 for naproxen and 0.50 for celecoxib. The planned secondary analysis now excluded eight individuals with prevalent dementia rather than seven, because one additional enrollee had received a diagnosis of dementia after the cut-off date for our earlier analyses. Unlike earlier results, the secondary analysis of incidence now showed no increase in risk among the NSAID-assigned groups, but it was otherwise inconclusive (Table 2, lines 9–10; log-rank P-values 0.66 for naproxen, 0.31 for celecoxib). However, the events represented in line 9 of Table 2 violated the proportional hazards assumption (for naproxen, P = 0.01; for celecoxib, P = 0.03). Subsequent analyses showed a significant interaction with time entered as a continuous variable (for naproxen, P = 0.01; for celecoxib, P = 0.04), and similarly with time dichotomized into earlier and later observation intervals (Table 2, line 11). Based on inspection of Figure 2B, the latter analysis divided the observation period into times before or after 2.5 years following randomization (this dichotomous approach was insensitive to the choice of dividing point, yielding identical results for points between 2.2 and 2.8 years following randomization). Within the dichotomized models, all data now satisfied the proportional hazards assumption (data not shown). The separate estimates for early and later HRs are shown in Table 2, lines 12 – 13. As in our earlier analysis, assignment to either treatment led to excess risk early in the trial (less conclusively so for naproxen), but the naproxen-assigned group results now suggested an opposite trend later (Figure 3A, P = 0.07).
Table 1.
Demographics and selected characteristics of the ADAPT cohort at the time all had completed their first follow-up assessment off-treatment
| Naproxen | Celecoxib | Placebo | Total | |
|---|---|---|---|---|
| Participants at treatment termination | 640 | 645 | 957 | 2242 |
| Completed first follow-up (see Figure 1) | 588 | 588 | 895 | 2071 |
| Demographics for subjects who completed first follow-up | ||||
| Age, percentiles (yrs) | ||||
| 50 | 78 | 77 | 78 | 78 |
| 25, 75 | 75, 81 | 76, 81 | 75, 81 | 75, 81 |
| Range | 71 – 92 | 71 – 94 | 71 – 95 | 71 – 95 |
| Gender, % | ||||
| Female | 46% | 47% | 43% | 45% |
| Male | 54% | 53% | 57% | 55% |
| Ethnic group, n | ||||
| White, non-Hispanic | 569 | 565 | 870 | 2004 |
| African American | 12 | 12 | 10 | 34 |
| Hispanic | 1 | 8 | 6 | 15 |
| Other | 5 | 2 | 8 | 15 |
| Refused | 1 | 1 | 1 | 3 |
| Marital Status, n | ||||
| Married | 438 | 417 | 638 | 1493 |
| Widowed | 98 | 116 | 165 | 379 |
| Divorced/separated | 35 | 39 | 70 | 144 |
| Never married | 17 | 16 | 22 | 55 |
| Education, n | ||||
| Less than high school | 27 | 20 | 31 | 78 |
| High school degree | 95 | 112 | 176 | 383 |
| College, no degree | 160 | 164 | 224 | 548 |
| College degree | 99 | 116 | 189 | 404 |
| Post-graduate | 207 | 176 | 275 | 658 |
| History of medical conditions, n | ||||
| Myocardial infarction | 20 | 28 | 53 | 101 |
| Diabetes | 48 | 45 | 67 | 160 |
| Hypertension treatment | 249 | 245 | 374 | 868 |
| Heart failure | 4 | 8 | 5 | 17 |
| Transient ischemic attack | 21 | 18 | 37 | 76 |
| Stroke | 6 | 7 | 9 | 22 |
Table 2.
Proportions, rates, and hazard ratios for ADAPT participants classified by treatment assignment and contrasting intervals of observation.
| Treatment assignment: | Naproxen | Celecoxib | Placebo | |
|---|---|---|---|---|
| Primary intention-to-treat analysis: | ||||
| 1 | No. of participants (person-years) | 655 (2,220) | 668 (2,234) | 985 (3,336) |
| 2 | AD cases (rate) | 18 (0.008) | 24 (0.011) | 30 (0.009) |
| 3 | AD rate ratio vs. placebo (CI) | 0.90 (0.47–1.67) | 1.2 (0.67–2.1) | -- |
| 4 | Deaths (rate) | 24 (0.011) | 31 (0.014) | 38 (0.012) |
| 5 | Death rate ratio vs. placebo (CI) | 0.94 (0.54–1.62) | 1.23 (0.74–2.03) | -- |
| 6 | AD or death (rate) | 50 (0.023) | 60 (0.027) | 77 (0.023) |
| 7 | AD or death ratio vs. placebo (CI) | 0.89 (0.59–1.34) | 1.1 (0.78–1.67) | -- |
| Secondary analyses: | ||||
| 8 | A. Participants (person-years) after exclusion of prevalent AD | 652 (2,220) | 667 (2,234) | 981 (3,336) |
| 9 | AD cases (rate)* | 15 (0.007) | 23 (0.010) | 26 (0.008) |
| 10 | AD rate ratio vs. placebo (CI) | 0.87 (0.43–1.70) | 1.2 (0.72–2.41) | -- |
| 11 | Improvement in proportional hazards models by interaction with time before or after 2.5 yrs. following randomization | P = 0.013 | P = 0.074 | -- |
| 12 | AD cases early (person-years) HR-early (CI) | 9 (1,241) 2.67 (0.88–8.07) | 10 (1,289) 3.01 (1.02–8.94) | 5 (1,876) -- |
| 13 | AD cases late (person-years) HR-late (CI) | 6 (979) 0.43 (0.17–1.08) | 13 (945) 0.93 (0.46–1.87) | 21 (1,460) -- |
| 14 | B. Participants (person-years) with CIND at enrollment | 15 (32) | 16 (47) | 23 (66) |
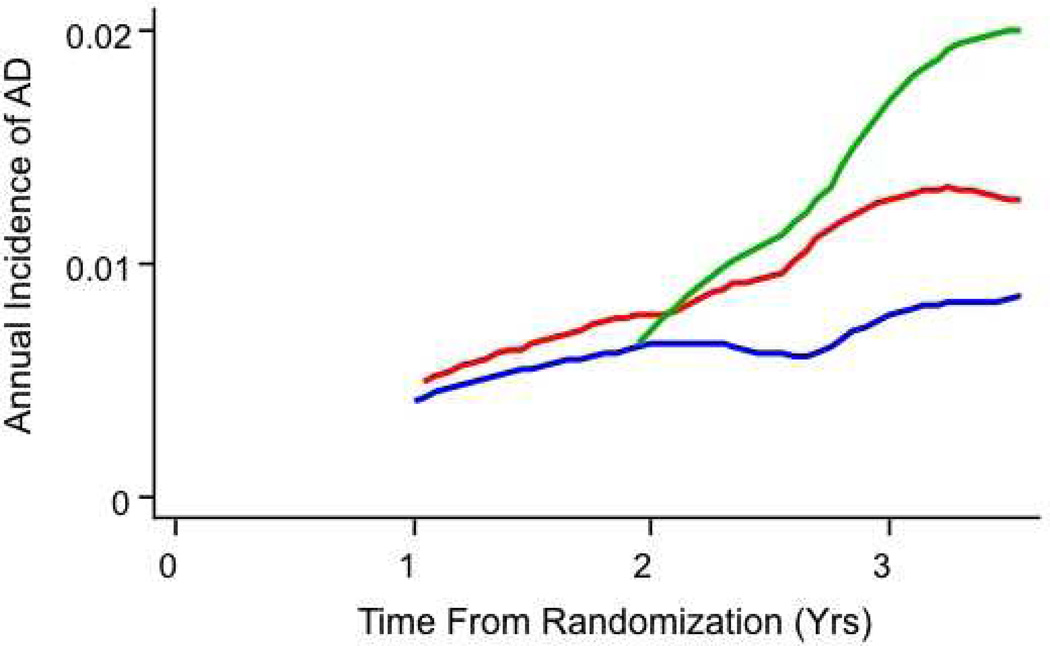
| 15 | AD cases (rate) | 4 (0.12) | 7 (0.15) | 4 (0.06) |
| 16 | HR (CI) | 3.2 (0.72–13.8) | 4.0 (1.00–15.6) | -- |
| 17 | C. Participants (person-years) with no cognitive diagnosis at enrollment | 637 (2,163) | 651 (2,169) | 958 (3,217) |
| 18 | AD cases (rate)* | 11 (0.005) | 16 (0.007) | 22 (0.007) |
| 19 | AD rate ratio vs. placebo (CI) | 0.74 (0.33–1.60) | 1.1 (0.53–2.15) | -- |
| 20 | Improvement in model by term for interaction with time before or after 2.5 yrs. following randomization | P = 0.016 | P = 0.037 | -- |
| 21 | AD cases early (person-years) HR-early (CI) | 7 (1,212) 2.50 (0.72–8.7) | 8 (1,252) 3.11 (0.92–11) | 4 (1,828) -- |
| 22 | AD cases late (person-years) HR-late (CI) | 4 (952) 0.33 (0.11–0.98) | 8 (917) 0.64 (0.28–1.5) | 18 (1,390) -- |
Raw data are shown in conventional font; estimated or derived results in italics. HR = hazard ratio; CI = 95% confidence interval; “early” and “later” identify times before and after 2.5 years following randomization; CIND = cognitive impairment, no dementia.
Proportional hazards assumption violation; we did not calculate HRs for these analyses.
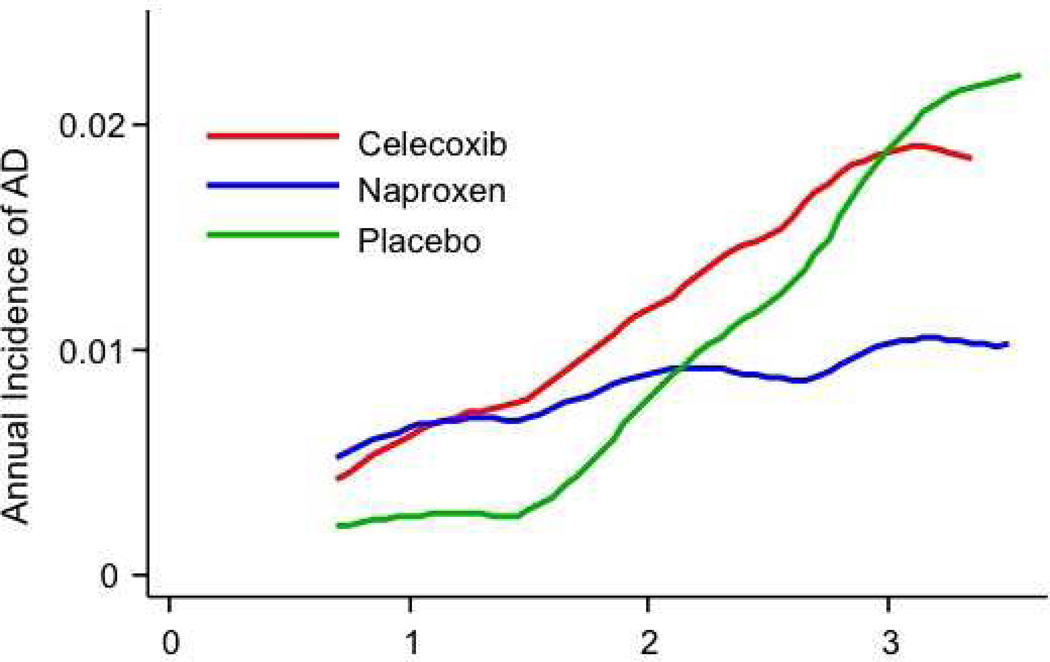
Figure 3.
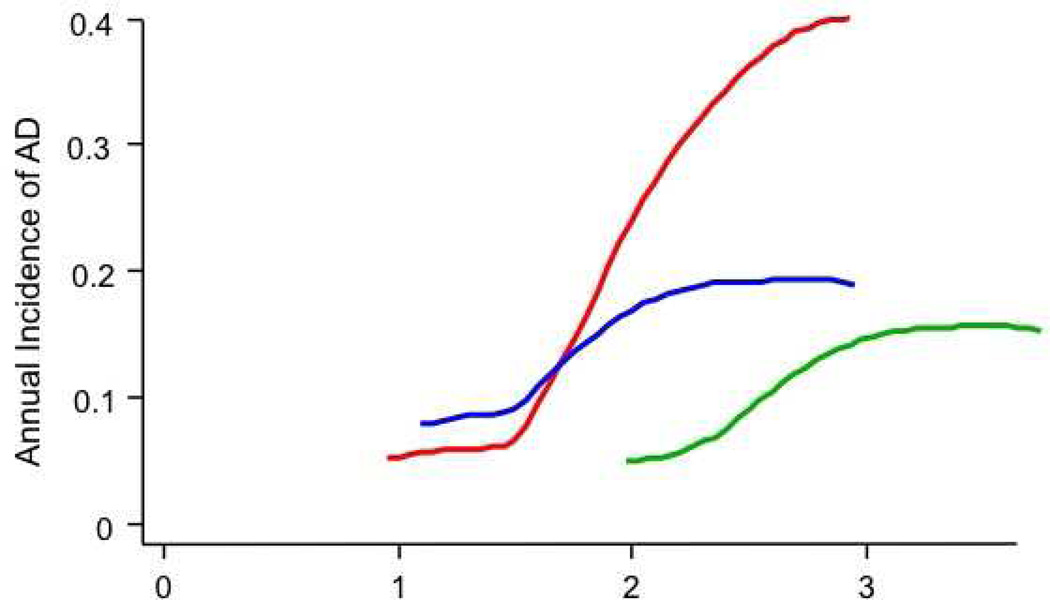
A, Estimated decimal annual incidence for the full cohort (primary analysis). The smoothing program could not accommodate the sparse data from the final year of observations.
B, Annual incidence of AD among 54 participants with CIND at baseline. Note the different scale for the ordinate vs. 3A. The groups assigned to NSAIDs show acceleration of dementia onset by approximately one year, as compared to those who received placebo. The extreme ordinate values for the later observations among celecoxib-assigned participants should probably be ignored.
C, Similar to 3A, but after exclusion of 8 enrollees with AD and 54 with CIND at baseline. An early excess of dementia in both NSAID-assigned groups is still evident, but no participants given placebo developed dementia for 22 months following randomization. New cases of AD increased rapidly thereafter in those assigned to placebo or celecoxib, but incidence among naproxen-assigned participants remained steady at about 0.01/yr.
Like our earlier analyses [13], the present results suggested that the NSAID treatments accelerated the onset of AD early in the trial. AD onset so shortly following randomization suggests that the early-affected participants had substantial Alzheimer neuropathology when enrolled. We tested this idea by examining treatment effects specifically in the 54 participants whose baseline evaluations revealed the presence of CIND. A post hoc analysis of treatment effects in these individuals is shown in Table 2, lines 14 – 16, and in Figure 3B. Complementing these results were hazard ratios after the exclusion of individuals with either prevalent dementia or CIND (a post hoc simulation of a “true” primary prevention trial), as listed on lines 17 – 22 of Table 2, and in Figure 3C. Unexpectedly, throughout the period of observations, many of the participants who developed dementia had not been identified as having CIND at their previous annual assessment [20].
3.2 CSF biomarker analyses
Numbers of CSF donors randomized to celecoxib, naproxen or placebo (38, 25 and 55 respectively) were proportional to the randomization ratio of the parent project (exact P = 0.22). CSF donors did not differ substantially from the full cohort on baseline scores for the modified Mini-Mental State Examination (3MSE) or the original Mini-Mental State Examination (MMSE), both tests of global cognitive function [21, 22]. Mean 3MSEscores were 93.82 vs. 94.23 (P = 0.21); and mean MMSE means were 28.83 and 28.94 (P = 0.42). Although both groups of NSAID-assigned CSF donors trended toward higher baseline scores than placebo-assigned participants on the 3MSE (means of 94.29 and 94.64 for celecoxib and naproxen vs. mean 93.13 for placebo; P = 0.10 and 0.058), the two treatment groups showed no meaningful difference on this measure (P > 0.5). Comparable statistics for the MMSE were P = 0.16, 0.62, 0.36. CSF donors included proportionally more men (71%) than the full sample (55%, P = 0.001), a difference that was driven principally by a larger proportion of men among CSF donors assigned to placebo (78%, exact P= 0.002 vs. sex distribution in full ADAPT cohort). Again, however, there were no meaningful sex differences between the two NSAID-assigned groups of CSF donors (63% and 68% men, P> 0.5). CSF donors’ age at study entry did not differ from the full cohort (P> 0.5), but NSAID-assigned donors tended to be older than those assigned to placebo, with mean ages (s.d.) of 78.8 (3.3) years for placebo vs. 79.4 (4.2, P = 0.42) for celecoxib and 80.6 (3.4, P = 0.03) for naproxen. There was no notable difference in age between the two treated groups (P = 0.25). Finally, the donor group did not differ from the full cohort in percentage with an APOE ε4 allele, nor were there any meaningful differences in this percentage among CSF donors by treatment assignment (all P > 0.5).
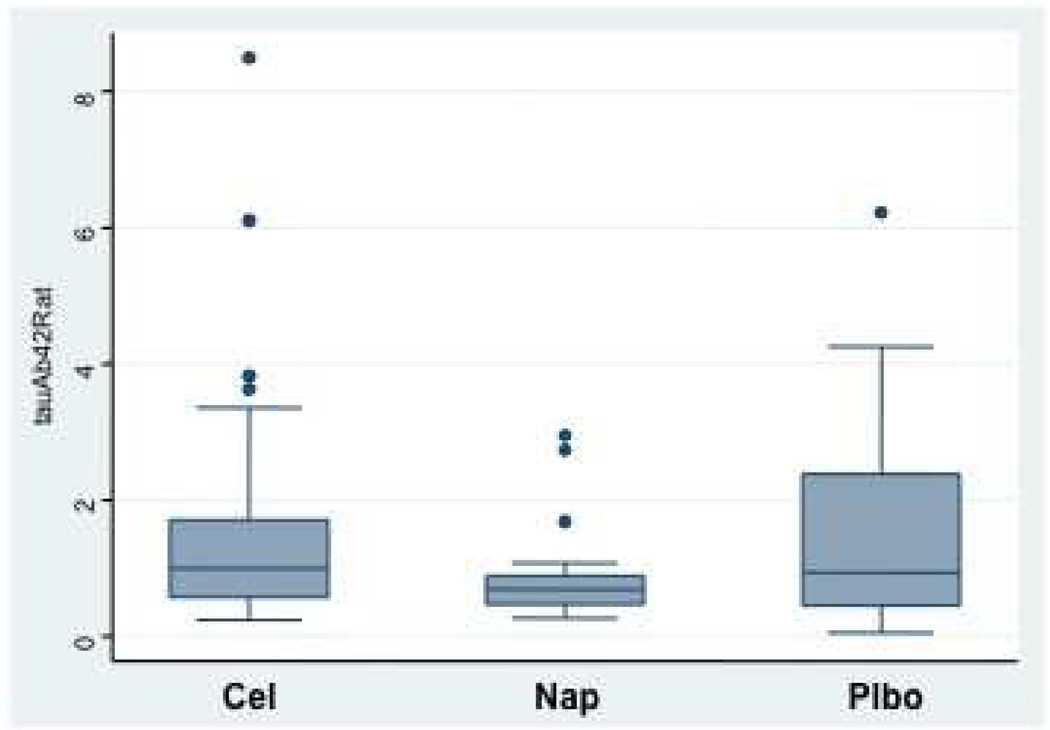
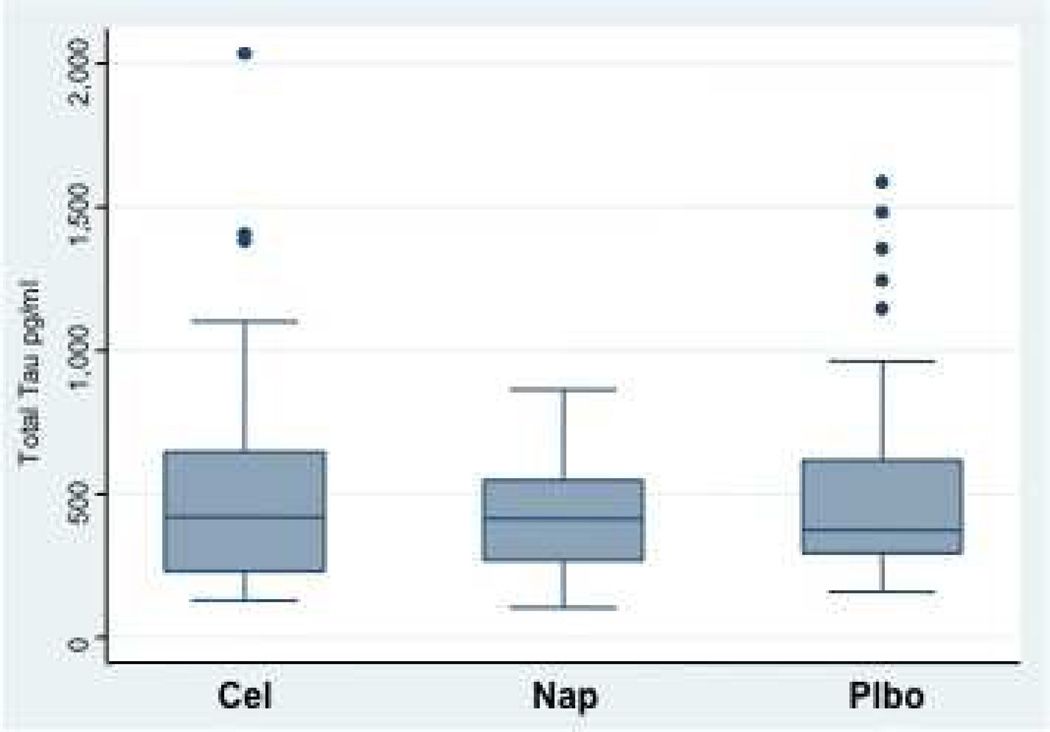
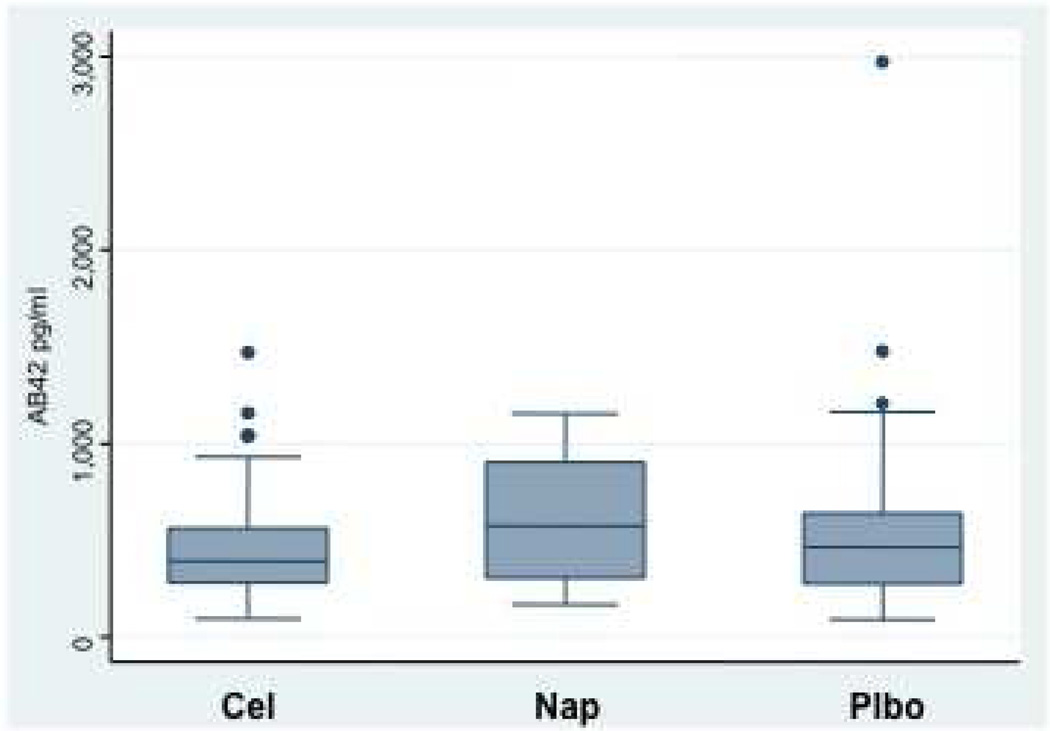
Figure 4 shows box plots for the biomarkers T-tau and Aβ1–42 as well as their ratio, by treatment assignment from the CSF obtained after completion of clinical follow-up. Neither individual biomarker showed a strong treatment effect, although trends were consistently in the direction of improvement (lower T-tau and higher Aβ1–42) among naproxen-assigned participants. Stronger differences were seen in the T-tau / Aβ1–42 ratio, which was reduced by 40% in naproxen-assigned participants compared to placebo (P = 0.02 in models adjusted for age, APOE ε4 status, education, and gender). No attenuation was evident in the naproxen effect on this ratio over the 20-month interval during which CSF was sampled (data not shown). As expected, APOE ε4 status was associated with an increased T-tau / Aβ1–42 ratio (P < 0.001), but the naproxen effect was unchanged in models after adjustment for this and the other covariates (all P > 0.5).
Figure 4.
A, Box plots showing distribution of concentrations for total tau (T-tau) for each of the three treatment groups. The “boxes” indicate the range of values between the 25th and 75th percentiles. “Whiskers” indicate the range of values that did not lie outside the interquartile range (IQR, between 25th and 75th percentile) by more than 1.5 X the IQR. Although statistically anomalous, the apparent outliers were probably individuals whose elevated tau concentration was a realistic signal of neurodegenerative illness. All values were considered in the statistical analyses.
B, Box plots showing distributions of concentrations for CSF Aβ1–42 by treatment assignment. The meaning of the outlying values here is less clear. All values were again considered in the analyses.
C, Box plots showing distribution by treatment assignment for the ratio of T-tau to Aβ1–42 concentrations. The ratios for celecoxib- and placebo-assigned participants were typical of other cognitively normal elderly subjects analyzed in the same laboratory [19]. Ratios for naproxen-assigned participants were reduced, compared to placebo, by 40%.
Discussion
In a randomized, controlled trial of naproxen and celecoxib for primary prevention of AD, treatments were stopped after an average of two years, but treatment effects were evaluated over an extended interval including an additional 18–24 months. Extended findings on the safety of the treatments, and on the second declared cognitive outcome measure of cognitive trajectory, will be reported separately. Where earlier ITT and, especially, secondary analyses limited to incident cases had suggested harm from either NSAID, both approaches now suggested no net effect. Treatment effects were heterogeneous over time, however, and secondary analyses suggested that harm was limited to early events (similar to the prior analyses of the treatment interval). Inclusion of later events appeared to mitigate this treatment-related risk, especially among participants assigned to naproxen. Subsequent CSF biomarker analyses suggested reduced rates of ongoing AD pathogenesis in those treated with naproxen.
These data suggest a revision of the original ADAPT hypothesis of neuroprotection from conventional NSAIDs (much less was known about “coxibs” such as celecoxib). The revised hypothesis adds the notion that NSAIDs have an adverse effect on AD pathogenesis in its later stages. Thus, early in the ADAPT observation period there was an excess of dementia in both of the NSAID-assigned groups, possibly reflecting adverse effects of NSAID exposure on enrollees who, although not yet demented, had substantial Alzheimer pathology. Limited additional evidence supports this idea. For example, ten of the early-affected cases in the trial had a baseline diagnosis of CIND, a condition often associated with neuropathological characteristics of AD [6]. In ADAPT, assignment of participants with CIND to NSAIDs (especially to celecoxib) accelerated their onset of AD dementia (Figure 2B). Although cognitively “normal,” the 26 other ADAPT enrollees without CIND who nonetheless developed dementia within three years had baseline cognitive function that was lower than that of the remaining cohort: e.g., their mean adjusted score on the 3MSE was 90.1 (s.d. 3.6) vs. 94.3 (s.d. 3.3) for others (P < 0.0001), and comparable results were observed with the MMSE (data not shown). If NSAID treatments do accelerate the expression of AD dementia in people with early symptomatic or late-stage pre-symptomatic AD, this effect would add to concerns about the safety of long-term administration of NSAIDs to the elderly [23, 24].
The remainder of the re-stated hypothesis predicts, as before, that asymptomatic individuals treated with conventional NSAIDs like naproxen will show reduced incidence of AD, but only after an interval of 2 – 3 years. The ADAPT data that suggest this hypothesis are found in Table 2, line 22. These later results are also consistent with observational study results showing that the occurrence of AD in users of NSAIDs was reduced only if such use occurred two or more years before the observation period [25].
Other interpretations of the extended ADAPT results are possible. Notwithstanding the interaction P-values, the contrasts between early and later rates may have occurred by chance. This is a particular concern given the limited number of events and the post hoc nature of the interaction analysis. Even if “real,” the differences in treatment effects may have resulted from the effects of treatment cessation, and not time following randomization. Thus, irrespective of the stage of disease progression, NSAIDs might be harmful when taken but leave a residual neuroprotective effect thereafter, perhaps resulting from selective removal of vulnerable individuals in the NSAID-assigned groups (although the latter part of this interpretation is challenged by the celecoxib results). Other limitations of the ADAPT results include their reliance on findings from an experiment in which treatments were stopped prematurely. Furthermore, the supporting analyses relied on relatively small numbers of events, and on post hoc stratification of observations based upon a finding of statistical interaction with time. In particular, the later results with naproxen need verification by further observations.
Notably, however, the still-later CSF biomarker results do support the second portion of the re-stated hypothesis that naproxen can protect cognitively healthy individuals against AD neurodegeneration. The biomarker data came from an independent sample of 117 dementia-free participants studied 21 – 41 months after the treatments had been terminated. CSF tau rises in patients with AD, and in those with prodromal AD symptoms [26], while Aβ1–42 declines during the development of AD [27]. The ratio of T-tau to Aβ1–42 robustly distinguishes normal elderly from those with AD or milder cognitive symptoms [2]. This ratio is also increased in normal elderly with an ε4 allele at the APOE locus (a genetic risk factor for AD). More importantly, an elevated T-tau / Aβ1–42 ratio identifies those individuals with mild cognitive symptoms most likely to evolve to AD dementia, and even those cognitively “normal” individuals who will shortly develop a syndrome suggestive of prodromal AD [2, 19]. Thus, the ratio of T-tau to Aβ1–42 in CSF is a likely indicator of ongoing AD pathogenesis. Accordingly, the ADAPT biomarker results appear to constitute a separate endpoint that not only suggests a biological treatment effect but also may predict subsequent cognitive benefit from assignment to naproxen.
Mechanisms for a hypothesized disease stage-related contrast in the effects of the ADAPT treatments are unknown. The “classic” activity of NSAIDs is inhibition of cyclooxygenase (COX) isoenzymes that oxidize arachidonic acid to prostaglandin H2. The latter in turn activates G protein-coupled receptors, either directly or by acting as precursor for other eicosanoids that influence a variety of metabolic pathways [28]. One such pathway is COX-1- and COX-2- dependent activation of microglia resulting in release of pro-inflammatory and pro-oxidant factors that are injurious to nearby neurons or dendrites in experimental models [29]. The relative contributions of COX-1 and COX-2 to such microglial-mediated paracrine damage are not entirely clear. However, COX-1 suppression (e.g., by conventional NSAIDs such as naproxen) could protect neurons against such immune-mediated damage in the early stages of AD pathogenesis. By contrast, brain COX-2 is abundant in dendrites [30], where it is essential for transduction of post-synaptic signals from NMDA-type glutamate receptors. Inhibition of COX-2 decreases the efficiency of such signaling [31], and could therefore provoke increased presynaptic stimulation via autoregulatory mechanisms. The latter could conceivably stress neurons that are already dysfunctional (e.g., in brains of patients with early AD, CIND, or late-stage pre-symptomatic AD). COX-2 inhibition, whether by conventional dual-inhibitor NSAIDs or, especially, by potent selective agents, could thereby be deleterious to patients with these conditions.
While other activities have been proposed for some NSAIDs (e.g., some may be PPAR-γ agonists), the importance of these actions in vivo relative to COX inhibition is not established [32]. High doses of some, but not all, NSAIDs can modulate γ-cleavage of the amyloid precursor protein away from production of toxic Aβ1–42 [33]. Neither of the ADAPT treatments modulate γ-secretase activity in this way, however [34], and at present there is no human evidence for a preferential effect of the so-called Aβ-lowering NSAIDs in the treatment of AD [34], or in its prevention [35, 36]. Although neuroprotective effects of NSAIDs have been demonstrated repeatedly in transgenic mouse models of AD [37], including one recent report that tested a strongly selective COX-2 inhibitor [38], the implications of these findings for human AD are unclear.
The possibility of sustained effects of the treatments on cognitive outcomes in the ADAPT cohort is under continuing observation. For now, the trial’s results suggest a hypothesis that may offer improved understanding of an apparently conflicted literature of observational and clinical trial findings on NSAID use and AD. They appear also to indicate a need for further laboratory investigation of NSAID effects on AD pathogenesis.
Acknowledgements
This work was supported by the U.S. Department of Veterans Affairs Puget Sound Health Care System, Seattle, Washington, by NIH grants U01-AG-15477, R01-AG-24010, and P50-AG-05136, and by the N. Bud Grossman Center for Memory Research and Care, Minneapolis, MN, and McGill University. Celecoxib and matching placebo were provided by Pfizer, Inc. Naproxen sodium and its placebo were provided by Bayer Consumer Healthcare. No funding or supporting agency had any role in the design or conduct of the study; collection, management, or interpretation of the data; or preparation of the manuscript. Courtesy copies of the manuscript were provided to the funding agency and to Pfizer, Inc., and Bayer Consumer Heathcare at the time of submission of the manuscript. Data management, descriptive statistics, and primary analyses for ADAPT were provided by the ADAPT Coordinating Center under direction of Drs. Meinert and Martin. The planned and ad hoc secondary analyses, as well as derivation of additional descriptive statistics, were performed by Dr. Baker. Each of these parties verified the other’s analytic results. We are deeply indebted to Ms. Jane Anau for her superb technical assistance, to the outstanding staff of the ADAPT field sites and Coordinating Center and, especially, to the ADAPT participant cohort for their persistent commitment and generosity of time and effort.
Abbreviations
- 3MSE
modified Mini-Mental State Examination
- Aβ
amyloid beta pepide
- AD
Alzheimer’s dementia (i.e., the dementia of Alzheimer’s disease)
- ADAPT
Alzheimer’s disease anti-inflammatory prevention trial
- C
Celsius
- CI
95% confidence interval
- CIND
cognitive impairment – no dementia
- CONSORT
Consolidated Standards of Reporting Trials
- COX
cyclooxygenase
- CSF
cerebrospinal fluid
- HR
hazard ratio
- ITT
intention-to-treat
- LP
lumbar puncture
- mg
milligrams
- ml
milliliter
- MMSE
Mini-Mental State Examination
- NSAIDs
nonsteroidal anti-inflammatory drugs
- pg
picogram
- PPAR-γ
peroxisome proliferator-activated receptor, gamma-type
- 181P-tau
phospho-tau substituted at the 181st amino acid threonine residue
- s.d.
standard deviation
- T-tau
total concentration of all tau species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Membership of the ADAPT Research Group is provided in Reference 13.
References
- 1.A National Alzheimer's Strategic Plan: The Report of the Alzheimer's Study Group. [Accessed: 04 April 2009];Congressional Task Force on Alzheimer's Disease. http://www.alzstudygroup.org/Portals/0/National_Alzheimers_Strategic_Plan.pdf. [Google Scholar]
- 2.Sonnen JA, Montine KS, Quinn JF, Kaye JA, Breitner JC, Montine TJ. Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurol. 2008;7(8):704–714. doi: 10.1016/S1474-4422(08)70162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192(1):106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278(5337):412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 6.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varvel NH, Bhaskar K, Patil AR, Pimplikar SW, Herrup K, Lamb BT. Abeta oligomers induce neuronal cell cycle events in Alzheimer's disease. J Neurosci. 2008;28(43):10786–10793. doi: 10.1523/JNEUROSCI.2441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szekely C, Thorne J, Zandi P, Ek M, Messias E, Breitner J, Goodman S. Nonsteroidal Anti-Inflammatory Drugs for the Prevention of Alzheimer's Disease: A Systematic Review. Neuroepidemiology. 2004:159–169. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 9.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28(5):639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289(21):2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 11.Soininen H, West C, Robbins J, Niculescu L. Long-term efficacy and safety of celecoxib in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;23(1):8–21. doi: 10.1159/000096588. [DOI] [PubMed] [Google Scholar]
- 12.Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, Assaid C, Nessly ML, Norman BA, Baranak CC, Reines SA. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30(6):1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 13.ADAPT Research Group. Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, Piantadosi S, Sabbagh M. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68(21):1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 14.ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT) PLoS Clin Trials. 2006;1(7):e33. doi: 10.1371/journal.pctr.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65(7):896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ADAPT Research Group. Meinert CL, McCaffrey LD, Breitner JC. Alzheimer's Disease Anti-inflammatory Prevention Trial: design, methods, and baseline results. Alzheimers Dement. 2009;5(2):93–104. doi: 10.1016/j.jalz.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin B, Meinert CL, Breitner J. Double placebo design in a prevention trial for Alzheimer's disease. Control Clin Trials. 2002 Feb;23(1):93–99. doi: 10.1016/s0197-2456(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 18.Peskind ER, Riekse R, Quinn JF, Kaye J, Clark CM, Farlow MR, Decarli C, Chabal C, Vavrek D, Raskind MA, Galasko D. Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord. 2005;19(4):220–225. doi: 10.1097/01.wad.0000194014.43575.fd. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69(7):631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 20.ADAPT Research Group. Breitner JCS. Onset of Alzheimer's dementia occurs commonly without prior cognitive impairment. Alzheimer's & Dementia. 2008;4(4) Supplement 1:T13–T131. [Google Scholar]
- 21.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. Mini-Mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. The Lancet. 1994;343:769–772. doi: 10.1016/s0140-6736(94)91843-0. [DOI] [PubMed] [Google Scholar]
- 24.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296(13):1633–1644. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 25.In 't Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler M, Stricker BHC. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345(21):1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 26.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 27.Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 2008;4(1):38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009 Apr;30(4):174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnen JA, Breitner JC, Lovell MA, Markesbery WR, Quinn JF, Montine TJ. Free radical-mediated damage to brain in Alzheimer's disease and its transgenic mouse models. Free Radic Biol Med. 2008 Aug 1;45(3):219–230. doi: 10.1016/j.freeradbiomed.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufmann WE, Andreasson KI, Isakson PC, Worley PF. Cyclooxygenases and the central nervous system. Prostaglandins. 1997 Sep;54(3):601–624. doi: 10.1016/s0090-6980(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 31.Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, Ross ME, Iadecola C. Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol. 2004 May;55(5):668–675. doi: 10.1002/ana.20078. [DOI] [PubMed] [Google Scholar]
- 32.Kojo H, Fukagawa M, Tajima K, Suzuki A, Fujimura T, Aramori I, Hayashi K, Nishimura S. Evaluation of human peroxisome proliferator-activated receptor (PPAR) subtype selectivity of a variety of anti-inflammatory drugs based on a novel assay for PPAR delta(beta) J Pharmacol Sci. 2003 Nov;93(3):347–355. doi: 10.1254/jphs.93.347. [DOI] [PubMed] [Google Scholar]
- 33.Weggen S, Eriksen J, Das P, Sagi S, Wang R, Pietrzik C, Findlay K, Smith T, Murphy M, Bulter T, Kang D, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001 Nov 8;414(6860):212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 34.Weggen S, Eriksen J, Sagi S, Pietrzik C, Ozols V, Fauq A, Golde TE, Koo EH. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity. J Biol Chem. 2003 Aug 22;278(34):31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- 35.Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009 Dec 16;302(23):2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szekely CA, Green RC, Breitner JC, Østbye T, Beiser AS, Corrada MM, Dodge HH, Ganguli M, Kawas CH, Kuller LH, Psaty BM, Resnick SM, Wolf PA, Zonderman AB, Welsh-Bohmer KA, Zandi PP. No advantage of A beta 42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology. 2008 Jun 10;70(24):2291–2298. doi: 10.1212/01.wnl.0000313933.17796.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zahs KR, Ashe KH. 'Too much good news' -- are Alzheimer mouse models trying to tell us how to prevent, not cure, Alzheimer's disease. Trends in Neurosci. 2010;33(8):381–389. doi: 10.1016/j.tins.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH, Younkin SG, Rowan M, Cleary J, Wallis RA, Sun GY, Cole G, Frautschy S, Anwyl R, Ashe KH. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131(Pt 3):651–664. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]