Abstract
The relationship between blood pressure (BP) and clinical outcomes among hemodialysis patients is complex and incompletely understood. This study sought to assess the relationship between blood pressure changes with hemodialysis and clinical outcomes during a 6-month period. This study is a secondary analysis of the Crit-Line Intradialytic Monitoring Benefit Study, a randomized trial of 443 hemodialysis subjects, designed to determine whether blood volume monitoring reduced hospitalization. Logistic regression was used to estimate the association between BP changes with hemodialysis (Δsystolic blood pressure = postdialysis–predialysis systoic BP (SBP) and the primary outcome of non-access-related hospitalization and death. Subjects whose systolic blood pressure fell with dialysis were younger, took fewer blood pressure medications, had higher serum creatinine, and higher dry weights. After controlling for baseline characteristics, lab variables, and treatment group, subjects whose SBP remained unchanged with hemodialysis (N = 150, ΔSBP −10 to 10 mm Hg) or whose SBP rose with hemodialysis (N = 58, ΔSBP ≥ 10 mm Hg) had a higher odds of hospitalization or death compared to subjects whose SBP fell with hemodialysis (N = 230, ΔSBP ≤ −10 mm Hg) (odds ratio: 1.85, confidence interval: 1.15–2.98; and odds ratio: 2.17, confidence interval: 1.13–4.15). Subjects whose systolic blood pressure fell with hemodialysis had a significantly decreased risk of hospitalization or death at 6 months, suggesting that hemodynamic responses to dialysis are associated with short-term outcomes among a group of prevalent hemodialysis subjects. Further research should attempt to elucidate the mechanisms behind these findings.
Keywords: SRD, hemodialysis, blood pressure, epidemiology and outcomes, hypertension
Significant controversy surrounds the issue of hypertension and outcomes among hemodialysis (HD) patients. Unlike the general population,1 a direct association between elevated blood pressure (BP) and cardiovascular mortality has not been clearly identified in dialysis patients.2–12 Although long-term studies are required to define the association between hypertension and outcomes,11 pronounced mortality rates and the presence of comorbid conditions that contribute to high mortality among HD patients may limit the ability to detect an independent association between hypertension and outcomes.
A number of studies have been published investigating the associations between BP and outcomes among end-stage renal disease (ESRD) patients.2–6,8–14 The available observational studies suggest that the relationship between BP and outcomes is complex and differs from the general population. Unfortunately, there are a number of difficulties associated with studying the association between BP and outcomes among HD patients. First, it remains unclear which BP parameter to use in these studies: predialysis, postdialysis, and intradialytic changes in BP are all available, yet which parameter is most strongly associated with detrimental outcomes remains uncertain. Second, clinician’s ability to change BP is limited in HD patients due to high frequency15 and severity16 of BP, as well as due to changes in BP associated with interdialytic weight gain, which is directly related to mortality risk.17–19
Clinically, physicians are carefully balancing the relationship between intradialytic weight loss and BP. In some ESRD patients, BP is unaffected by ultrafiltration and hemodialysis, where as other patients experience a more pronounced hemodynamic response with hemodialysis. Differences in clinical characteristics between such patient groups have not been fully described nor has the relationship between BP responses to ultrafiltration with hemodialysis and outcomes been characterized to date.20,21
Owing to the complex relationship between BP, weight gain, and mortality, we postulated that the association between hemodynamic changes and outcomes might be best assessed using other parameters such as hospitalization. Herein, we undertook a secondary analysis of CLIMB (the Crit-line Intradialytic Monitoring Benefit Study) to assess whether BP responses to hemodialysis are associated with differential short-term outcomes while controlling for interdialytic weight gain, case-mix, and other BP parameters.
RESULTS
Baseline characteristics
Baseline characteristics of subjects enrolled in the CLIMB study have been previously reported.22 Two hundred and thirty subjects (52.5%) had a fall in systolic blood pressure (SBP) associated with HD (ΔSBP ≤ −10 mm Hg), 150 subjects (34.2%) did not have a significant change in SBP from pre- to post-HD (ΔSBP −10 to 10 mm Hg), and 58 subjects (13.2%) exhibited a paradoxical rise in SBP with HD (ΔSBP ≥ 10 mm Hg) (Table 1). Subjects whose SBP fell with HD were younger and were on less antihypertensive medications. They also had higher predialysis systolic and diastolic BP, lower postdialysis systolic and diastolic BP, higher serum creatinine, and higher dry weights. There was a trend toward a higher prevalence of male subjects and a higher prevalence of diabetes mellitus among subjects whose SBP decreased during HD. Subjects whose SBP were unchanged with dialysis had the lowest prevalence of diabetes mellitus and the highest rates of catheter use compared to subjects whose SBP fell with HD or whose SBP rose with HD.
Table 1.
Baseline clinical and demographic characteristics of the study population
| Systolic blood pressure change (ΔSBP) associated with hemodialysis | ||||
|---|---|---|---|---|
| ΔSBP ≤ −10 mm Hg, n=230 |
ΔSBP −10 to 10 mm Hg, n=150 |
ΔSBP ≥ 10 mm Hg, n=58 |
P-value | |
| Age (years) | 57.36 (± 14.97) | 60.34 (± 16.00) | 63.64 (± 16.54) | 0.013 |
| Gender (% male) | 55.2% (127/230) | 48.0% (72/150) | 43.1% (25/58) | 0.16 |
| Race (%) | ||||
| White | 55.2% | 66.0% | 55.2% | 0.14 |
| Black | 38.3% | 28.7% | 38.9% | |
| Others | 6.5% | 5.3% | 6.9% | |
| Tobacco use (vs nonuse) | 27.9% (63/226) | 37.0% (54/146) | 28.1% (16/57) | 0.16 |
| Hispanic ethnicity (vs non-Hispanic) | 3.5% (8/230) | 1.3% (2/150) | 1.7% (1/58) | 0.37 |
| Diabetes mellitus (vs non-DM) | 49.1% (113/230) | 37.6% (56/149) | 46.4% (26/56) | 0.083 |
| Diabetes as cause of ESRD (vs others) | 33.9% (78/230) | 23.3% (35/150) | 36.2% (21/58) | 0.052 |
| Hypertension | 87.4% (201/230) | 92.6% (137/148) | 87.3% (48/55) | 0.24 |
| Antihypertensive medications (vs no antihypertensive use) | 73.9% (170/230) | 74.7% (112/150) | 87.9% (51/58) | 0.074 |
| Arrythmia | 17.2% (39/227) | 18.1% (27/149) | 20.7% (12/58) | 0.83 |
| Cardiac diseasea | 42.8% (98/229) | 35.3% (53/150) | 43.9% (25/57) | 0.30 |
| Chronic obstructive pulmonary disease | 10.0% (23/229) | 14.9% (22/148) | 16.1% (9/56) | 0.26 |
| Cerebrovascular disease | 20.0% (46/230) | 17.5% (26/149) | 14.3% (8/56) | 0.56 |
| Left ventricular hypertrophy | 29.1% (67/230) | 22.7% (34/150) | 24.1% (14/58) | 0.35 |
| Peripheral vascular disease | 20.5% (47/229) | 19.6% (29/148) | 17.9% (10/56) | 0.90 |
| Blood pressure (mm Hg) | ||||
| Predialysis | ||||
| Systolic | 155.46 (± 18.40) | 144.51 (± 22.45) | 139.28 (± 21.62) | < 0.0001 |
| Diastolic | 82.49 (± 11.96) | 77.19 (± 13.52) | 73.76 (± 12.17) | < 0.0001 |
| Postdialysis | ||||
| Systolic | 130.52 (± 17.26) | 142.43 (± 21.88) | 157.92 (± 20.53) | < 0.0001 |
| Diastolic | 71.30 (± 11.00) | 75.48 (± 12.90) | 79.33 (± 12.32) | < 0.0001 |
| ΔSBP | −24.94 (± 12.69) | −2.07 (± 5.61) | 18.64 (± 8.07) | < 0.0001 |
| Pulse pressure | ||||
| Predialysis | 72.97 (± 15.07) | 67.32 (± 15.09) | 65.53 (± 17.29) | 0.0004 |
| Postdialysis | 59.22 (± 13.14) | 66.96 (± 15.41) | 78.59 (± 17.61) | < 0.0001 |
| % of interdialytic weight gain | 3.9% (± 1.28) | 3.8% (± 1.61) | 3.7% (± 1.34) | 0.26 |
| Interdialytic weight gain (kg) | 3.11 kg (± 1.07) | 2.70 (± 1.08) | 2.62 (± 1.11) | 0.0002 |
| Dry weight (kg) | 81.12 (± 23.07) | 73.00 (± 17.55) | 71.45 (± 16.20) | < 0.0001 |
| Baseline laboratoryb | ||||
| Albumin (g/dl) | 3.73 (± 0.49) | 3.77 (± 0.54) | 3.72 (± 0.31) | 0.72 |
| Creatinine (mg/dl) | 9.68 (± 3.20) | 8.85 (± 3.28) | 8.52 (± 2.70) | 0.012 |
| Calcium (mg/dl) | 9.27 (± 0.96) | 9.14 (± 0.89) | 8.96 (± 0.82) | 0.053 |
| Phosphorus (mg/dl) | 5.84 (± 1.81) | 5.62 (± 2.00) | 5.48 (± 1.68) | 0.32 |
| PTH, median (pg/ml) | 187.0 (64.0–435.0) | 193.0 (75.0–382.0) | 147.5 (53.3–214.5) | 0.44 |
| Cholesterol (mg/dl) | 168.80 (± 46.24) | 167.91 (± 37.70) | 171.11 (± 40.02) | 0.95 |
| Hemoglobin (g/dl) | 11.42 (± 1.43) | 11.52 (± 1.33) | 11.11 (± 1.35) | 0.18 |
| URR, median | 0.72 (0.68–0.76) | 0.73 (0.68–0.78) | 0.75 (0.71–0.78) | 0.04 |
| Number of antihypertensive medications (mean ± s.d.) | 1.37 (± 1.14) | 1.47 (± 1.13) | 1.79 (± 1.06) | 0.021 |
| Antihypertensive class (% use) | ||||
| Ace-I | 30.0% (69/230) | 28.7% (43/150) | 32.8% (19/58) | 0.85 |
| Alpha-blocker | 4.4% (10/230) | 6.0% (9/150) | 3.5% (2/58) | 0.67 |
| Beta-blocker | 35.2% (81/230) | 31.3% (47/150) | 39.7% (23/58) | 0.50 |
| CCB | 35.2% (81/230) | 42.7% (64/150) | 44.8% (26/58) | 0.22 |
| Diuretic | 3.5% (8/230) | 3.3% (5/150) | 6.9% (4/58) | 0.50 |
| Nitrate | 11.0% (25/228) | 18.7% (28/150) | 24.2% (14/58) | 0.019 |
| Vasodilator | 17.4% (40/230) | 16.7% (25/150) | 27.6% (16/58) | 0.18 |
| Epoetin use (vs nonuse) | 89.6% (206/230) | 90.0% (135/150) | 87.9% (51/58) | 0.91 |
| Dialysis vintage | ||||
| 0–1 year | 26.6% (60/226) | 31.0% (45/145) | 25.9% (15/58) | 0.60 |
| > 1 year | 73.5% (166/226) | 69.0% (100/145) | 74.1% (43/58) | |
| Years (median ± IQR) | 2.17 (0.97–4.02) | 1.68 (0.85–3.47) | 2.28 (0.96–4.03) | 0.25 |
| Access type | ||||
| AV fistula | 35.8% (82/229) | 32.9% (49/149) | 32.8% (19/58) | 0.023 |
| AV graft | 44.5% (102/229) | 36.9% (55/149) | 55.2% (32/58) | |
| Catheter | 19.7% (45/229) | 30.2% (45/149) | 12.1% (7/58) | |
| Treatment Group (vs usual care) | 52.2% (120/230) | 43.3% (65/150) | 46.6% (27/58) | 0.23 |
Combined history of coronary disease or congestive heart disease.
Seven subjects were missing baseline albumin, 36 missing creatinine, five missing calcium, five missing phosphorus, 217 missing PTH, 208 missing cholesterol, 59 subjects missing hemoglobin, and 44 missing URR.
CCB, calcium channel blocker; AV, arteriovenous; URR, urea reduction ratio; PTH, parathyroid hormone.
Note: to convert albumin in g/dl to g/l, multiply by 10; serum creatinine in mg/dl to mmol/l, multiply by 88.4; calcium in mg/dl to mmol/l, multiply by 0.2495; phosphorus in mg/dl to mmol/l, multiply by 0.3229; PTH in pg/ml to ng/l, multiply by 1; cholesterol in mg/dl to mmol/l, multiply by 0.02586; hemoglobin in g/dl to g/l, multiply by 10.
Unadjusted outcomes
During the 6-month follow-up, 132/438 (30.1%) subjects had a primary event (either non-access-related hospitalization (N = 108) or death (N = 5) or both (N = 19)) (Table 2). Compared to subjects whose SBP fell with HD, subjects whose SBP was unchanged with HD or who had a paradoxical rise in SBP with HD had an increased risk of non-access-related hospitalization or death at 6 months (odds ratio (OR): 1.89, confidence interval (CI): 1.20–2.96, ΔSBP: −10 to 10 mm Hg vs ΔSBP ≤ −10 mm Hg; OR: 2.14, CI: 1.17–3.93, ΔSBP ≥ 10 mm Hg vs ΔSBP ≤ −10 mm Hg, P = 0.0056). Annual non-access-related hospitalization rates were 0.96 (± 2.96 hospitalizations/year) among subjects whose SBP fell with HD compared to 1.55 (± 3.33) among subjects whose SBP was unchanged with HD and 1.90 (± 3.86) among subjects whose SBP rose with HD (P = 0.0083).
Table 2.
Unadjusted comparison of 6-month mortality and non-access-related hospitalization rates among prevalent ESRD subjects grouped by changes in SBP with HDa
| Number of subjects with an event (%) | Odds ratio (95% CI) | P-value | |
|---|---|---|---|
| Combined end point of non-access-related hospitalization or death | |||
| SBP fell with HD (ΔSBP ≤ −10 mm Hg) | 54/230 (23.5%) | 1.00 (reference) | 0.0056 |
| SBP unchanged with HD (ΔSBP −10 to 10 mm Hg) | 55/150 (36.7%) | 1.89 (1.20–2.96) | |
| SBP rose with HD (ΔSBP ≥ 10 mm Hg) | 23/58 (39.7%) | 2.14 (1.17–3.93) | |
| Non-access-related hospitalizationb | |||
| SBP fell with HD (ΔSBP ≤ −10 mm Hg) | 53/230 (23.0%) | 1.00 (reference) | 0.014 |
| SBP unchanged with HD (ΔSBP −10 to 10 mm Hg) | 52/150 (34.7%) | 1.77 (1.12–2.79) | |
| SBP rose with HD (ΔSBP ≥ 10 mm Hg) | 22/58 (37.9%) | 2.04 (1.11–3.77) | |
| Deathb | |||
| SBP fell with HD (ΔSBP ≤ −10 mm Hg) | 7/230 (3.0%) | 1.00 (reference) | 0.046 |
| SBP unchanged with HD (ΔSBP −10 to 10 mm Hg) | 11/150 (7.3%) | 2.52 (0.96–6.66) | |
| SBP rose with HD (ΔSBP ≥ 10 mm Hg) | 6/58 (10.3%) | 3.68 (1.19–11.40) | |
| Combined end point of non-access-related hospitalization or death | |||
| ΔSBP (per 1 mm Hg increase) | 1.020 (1.01–1.03) | 0.0009 |
Reference is ΔSBP ≤ −10 mm Hg.
For this subgroup analysis alone, 19 subjects had both a hospitalization and death and were included in both analyses.
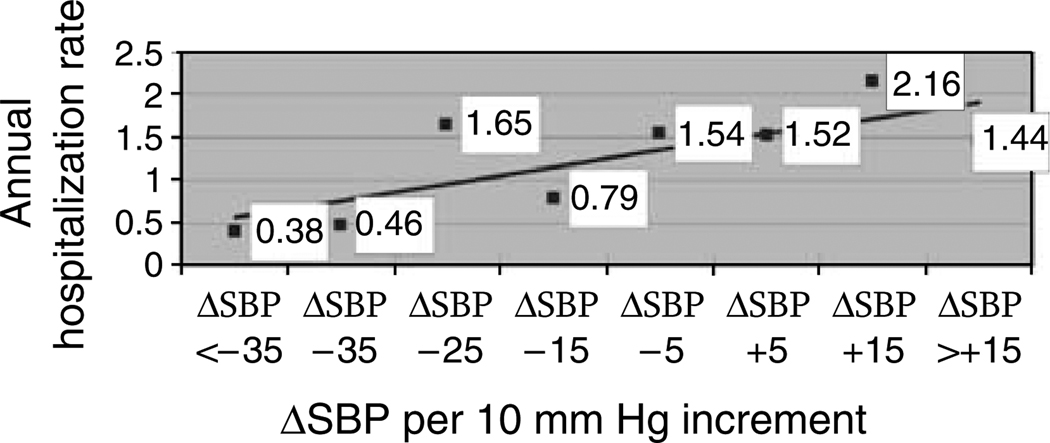
When ΔSBP was modeled as a continuous variable, every 1 mm Hg increase in ΔSBP following HD was associated with an increased odds of a non-access-related hospitalization or death at 6 months (OR: 1.02, CI: 1.01–1.03, P = 0.0009). Thus, a 10 mm Hg increase in SBP with HD was associated with a 20% increased odds of hospitalization or death at 6 months among subjects. The relationship between 10 mm Hg increments of ΔSBP and annual non-access-related hospitalization is plotted in Figure 1.
Figure 1.
Unadjusted annual non-access-related hospitalization rates among prevalent ESRD subjects plotted per 10 mm Hg increment increase in ΔSBP (ΔSBP = postdialysis–predialysis SBP).
Multivariable analysis
After adjusting for relevant confounders, subjects whose SBP was unchanged with HD or whose SBP rose with HD had an increased risk of non-access-related hospitalization or death compared to patients whose SBP fell with HD (P = 0.012) (Table 3).
Table 3.
Adjusted analysis of 6-month mortality and non-access-related hospitalization among prevalent ESRD subjectsa
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| SBP fell with HD (ΔSBP ≤ −10 mm Hg) | 1.00 (reference) | 0.012 |
| SBP unchanged with HD (ΔSBP −10 to 10 mm Hg) | 1.85 (1.15–2.98) | |
| SBP rose with HD (ΔSBP ≥ 10 mm Hg) | 2.17 (1.13–4.15) | |
| Dry weight (per 1 kg increase) | 0.99 (0.97–1.00) | 0.018 |
| Cardiac disease (yes vs no) | 1.70 (1.10–2.64) | 0.018 |
| Treatment group (treatment vs usual care) | 1.62 (1.04–2.51) | 0.033 |
| Phosphorus (per 1 mg/dl increase) | 1.12 (1.001–1.26) | 0.049 |
| Black race (vs non-black race) | 0.65 (0.40–1.06) | 0.084 |
Variables tested for significance included the following: age; race; dry weight; % of interdialytic weight gain; ΔSBP; dialysis vintage; access type; history of arrhythmia, cardiac disease (coronary artery disease or congestive heart disease), diabetes mellitus, hypertension, left ventricular hypertrophy, and peripheral vascular disease; baseline albumin, creatinine, calcium, phosphorus, and urea reduction ratio; number of BP medications; and treatment group.
In adjusted models with ΔSBP as a continuous variable, every 1 mm Hg increase in ΔSBP following HD was associated with a 2% increased odds of non-access-related hospitalization or death (OR: 1.02, CI: 1.01–1.03, P = 0.0022).
In multivariate analyses, other variables associated with an increased risk of hospitalization or death included lower dry weight (P = 0.018), history of coronary artery disease or congestive heart disease (P = 0.018), CLIMB treatment group (P = 0.033), and increasing phosphorus (P = 0.049). There was a trend toward improved outcomes among black subjects (P = 0.084). Variables not associated with an increased risk of the primary outcome included increasing age; % of interdialytic weight gain; dialysis vintage; number of BP medications; access type; history of arrhythmia, diabetes mellitus, hypertension, left ventricular hypertrophy, or peripheral vascular disease; and baseline creatinine, albumin, calcium, or urea reduction ratio.
ΔSBP category did not interact with age (P = 0.76), race (P = 0.92), % of interdialytic weight gain (P = 0.55), history of coronary artery disease or congestive heart failure (P = 0.25), diabetes mellitus (P = 0.42), left ventricular hypertrophy (P = 0.25), peripheral vascular disease (P = 0.33), predialysis SBP (P = 0.14), or predialysis diastolic BP (P = 0.999).
Sensitivity analysis
Four separate models were tested, which included the addition of predialysis systolic and diastolic BP, postdialysis systolic and diastolic BP, predialysis pulse pressure, and postdialysis pulse pressure. In each model performed, none of the BP parameters were associated with an increased risk of the primary outcome, nor did they significantly modify the effect of ΔSBP on outcomes (data not shown).
Further sensitivity analyses were performed, which excluded subjects without KDOQI-(Kidney Disease Outcomes Quality Initiative) defined hypertension (predialysis SBP < 140 and postdialysis SBP < 130, and predialysis diastolic < 90 and postdialysis diastolic < 80). According to these standards, 343/431 (79%) subjects had hypertension in our cohort. After adjustment for relevant covariates, ΔSBP remained a strong predictor of outcomes among subjects with KDOQI-defined hypertension (Table 4).
Table 4.
Combined outcome of 6-month mortality and non-access-related hospitalization among hypertensive (defined by KDOQI standards) ESRD subjects (N=343/431)
| Adjusted odds ratio (95% CI)a | P-value | |
|---|---|---|
| SBP fell with HD (ΔSBP ≤ − 10 mm Hg) | 1.00 (reference) | 0.006 |
| SBP unchanged with HD (ΔSBP − 10 to 10 mm Hg) | 2.08 (1.19–3.61) | |
| SBP rose with HD (ΔSBP ≥ 10 mm Hg) | 2.61 (1.29–5.27) | |
| ΔSBP (per 1 mm Hg increase) | 1.02 (1.01–1.04) | 0.001 |
Controlled for black race, cardiac disease, baseline phosphorus, dry weight, and treatment group.
Separate analyses included only subjects on antihypertensive medications to assess the impact of medication class on outcomes and to determine if specific antihypertensives modified the effect of ΔSBP on outcomes. In our cohort, 76% of subjects were on antihypertensive medications. None of the classes of antihypertensive agents were associated with the primary outcome (alpha blocker (P = 0.88), angiotensin-converting enzyme-1 (P = 0.30), β blocker (P = 0.85), calcium channel blocker (P = 0.99), diuretic (P = 0.76), nitrate (P = 0.31), or vasodilator (P = 0.81)), nor did they modify the impact of ΔSBP on outcomes (data not shown).
DISCUSSION
Our study demonstrates that hemodynamic responses with hemodialysis are associated with short-term clinical outcomes among a cohort of prevalent dialysis subjects. Although the effect of hypertension on long-term clinical outcomes remains uncertain,2–5,7,8,11 in our investigation, SBP that was unchanged with HD or paradoxically rose with HD was associated with an increased risk of non-access-related hospitalization or death at 6 months compared to SBP that fell with HD. Furthermore, in our investigation, BP changes associated with hemodialysis were more strongly associated with clinical outcomes than pre- or postdialysis systolic BP, diastolic BP, or pulse pressure.
Our study suggests that hemodynamic responses to dialysis may be used to identify subjects at increased risk of important short-term events. Although no study to date has demonstrated this relationship, a recent study by Stidley et al.9 suggests that the association between BP and outcomes varies over time. In their investigation of 16 959 incident hemodialysis patients, elevated predialysis SBP (> 160 vs 140–149 mm Hg) was associated with lower mortality, and low postdialysis SBP (< 110 mm Hg) was associated with increased mortality. However, no models included both pre- and postdialysis SBP to determine whether hemodynamic responses to dialysis were associated with clinical outcomes.
Interestingly, Foley et al.2 analyzed pre- and postdialysis BP parameters in 11 142 prevalent United States Renal Data System (USRDS) patients and found that neither pre- nor postdialysis SBP was significantly associated with all-cause mortality after controlling for demographics, comorbid conditions, and % of interdialytic weight gain. Their investigation suggested that wide pulse pressure, potentially as a marker of vascular compliance, was associated with increased long-term mortality. Unfortunately, direct comparisons between studies are not appropriate given the different duration of follow-up.
The lack of an association between a variety of other BP parameters (such as pre- or postdialysis SBP) and clinical outcomes in our investigation can likely be explained by two factors: (1) the deleterious effects related to hypertension likely require longer follow-up than available in the CLIMB study and (2) recent literature suggests that routine dialysis BP parameters may not be reflective of the hemodynamic burden a patient experiences between dialysis treatments. 11,23,24 Thus, the lack of a significant association between other BP parameters and outcomes does not minimize this relationship, but highlights the importance of hemodynamic responses associated with HD on outcomes over short follow-up periods.
The explanation for the association between adverse outcomes associated with SBP that fails to fall with dialysis is unclear. Furthermore, the pathophysiology underlying differential BP responses to dialysis is also unclear. However, it has been postulated that failure to lower BP with dialysis is mediated by enhanced renin–angiotensin system and/or increased sympathetic nervous system activity in response to decreases in blood volume.25 In addition, underlying cardiovascular disease26 or inability to achieve dry weight has also been suggested as causing this effect.27,28 Furthermore, failure to lower BP with HD is also likely independent of vascular calcification, which would stiffen vessels, reduce compliance, and increase differences in SBP after volume reduction.29
A number of studies suggest that patients who do not reach target dry weights may be less likely to respond to HD with an appropriate lowering of BP.27,28 Fishbane et al.27 compared 21 HD patients and found that atrial natiuretic peptide levels were significantly higher postdialysis among patients whose mean arterial pressures (MAP) were unchanged with HD (ΔMAP ~ 1 mm Hg) compared to those whose MAP fell with HD (ΔMAP ~ 20 mm Hg). Furthermore, successful sequential ultrafiltration with lowering of dry weight among three patients converted them to having MAPs that fell with HD and to having lower ANP levels following HD. In another study of seven patients with an increase in BP with dialysis and significant cardiac dilation, intense ultrafiltration over time normalized BP responses and cardiac parameters in most patients.28
Mourad et al.26 analyzed pulse-wave velocity among ESRD patients whose MAP increased 6% with dialysis compared to those whose MAP fell 17% with dialysis, and found that mean pulse-wave velocity was significantly higher among patients whose MAP increased with HD. Prior investigations have found a strong relationship between increasing pulse-wave velocity and higher mortality among ESRD patients.30,31 These studies suggest that possibly under-diagnosed larger artery vascular disease may play a role in the poorer outcomes noted among subjects whose BP failed to fall with dialysis.
Although the findings of this study are novel, this analysis is not without limitations. For example, owing to sample size and missing data, the associations between cholesterol, hemoglobin, and parathyroid harmone and clinical outcomes could not be assessed. Although these parameters may have an effect on arterial compliance, there were no significant differences in the baseline values between groups based on ΔSBP, suggesting that these parameters may not have altered our analyses. Second, given the observational nature of the present study, no conclusions regarding cause and effect can be made. Third, the BP parameters used for this analysis were not standardized and were obtained from an average of four dialysis sessions; however, prior studies have used 1 week averages of routine dialysis BP recordings to assess outcomes, 2,4 and routine dialysis BP parameters are more useful to apply to clinical practice. Fourth, the subjects included in these analyses were prevalent to hemodialysis. Given the known high mortality and morbidity among incident ESRD patients, our cohort likely represents a healthier patient population who survived the initial dialysis period. In addition, the cohort utilized for this analysis was part of a randomized controlled trial, which excluded ‘sicker patients’ (such as those with low serum albumin), and the known volunteer bias likely resulted in the lower than expected mortality in this cohort. These factors likely affect the generalizability of our results to the wider range of prevalent ESRD patients and caution should be used in applying these results to the broader USRDS population.
Hemodynamic responses to hemodialysis are associated with short-term outcomes among a cohort of prevalent hemodialysis subjects. Failure to lower SBP with HD was associated with a significantly increased risk of non-accessrelated hospitalization and death at 6 months in our analysis, which was independent of weight gain and similar among patients taking or not taking antihypertensives. Further research should seek to identify the underlying pathophysiology behind differential hemodynamic responses with dialysis among ESRD patients in order to try to identify modifiable risk factors to target for interventions in this high-risk group.
MATERIALS AND METHODS
Patient population
Subjects for this analysis included 443 patients who were enrolled in the CLIMB study.22 Methods, baseline characteristics, and the results of the CLIMB study have been previously reported.22 Entry criteria included age between 18 and 85 years, ESRD duration for ≥ 2 months, and treatment with in-center hemodialysis three times a week. Exclusion criteria included BP not measurable by standard techniques, active gastrointestinal bleed, severe malnutrition (albumin < 2.6 g/dl), active hematologic disease, patient expected to be unavailable due to moving or living donor renal transplant, malignancy requiring chemotherapy, and inability to provide informed consent. The Institutional Review Board at each of the six participating centers approved the original study protocol and the Duke University Institutional Review Board approved this analysis.
Outcomes
The primary end point of the original CLIMB study was non-access-related hospitalization. For the purpose of this analysis, the primary end point is a combined outcome of non-access-related hospitalization and death at 6 months. Annual non-access-related hospitalization rates and 6-month mortality were also analyzed separately in secondary analyses.
Study measurements
Subjects enrolled in CLIMB were observed for 2 weeks and then randomized to 6 months of intradialytic blood volume monitoring using Crit-Line* (Hema Metrics Inc. (*formerly In-Line Diagnostics), Kaysville, UT, USA) or conventional clinical strategies. Subjects were subsequently followed for 6 months.
At enrollment, the following baseline parameters were obtained and were available for this analysis: demographics (race, age, sex); dialysis vintage; tobacco use (defined as current or quit within last 10 years); dialysis access type; treatment center; past medical history including history of diabetes mellitus, cause of ESRD (diabetes mellitus, hypertension or other), hypertension, bilateral nephrectomy, peripheral vascular disease, chronic obstructive pulmonary disease, coronary artery disease (defined as a history of myocardial infarction, coronary artery bypass graft, or percutaneous coronary intervention), congestive heart failure (defined as a history of congestive heart disease or left ventricular dysfunction), left ventricular hypertrophy (defined by the presence of left ventricular hypertrophy on echocardiography or electrocardiogram), cerebrovascular disease (history of transient ischemic attach or stroke), arrythmia (cardiac arrest, atrial fibrillation/flutter, atrial/ventricular tachycardia, or ventricular fibrillation), malignancy, antihypertensive medication class, and routine laboratory data.
Subjects were seen thrice weekly during routine hemodialysis and the following values were recorded and available for the analysis: pre- and postdialysis BP sitting and standing, lowest BP during treatment, pre- and postdialysis weight, target dry weight (determined by the treating nephrologists), intradialytic interventions, and complications.
Definitions
Baseline BP and weight gain parameters used for this analysis were averaged from preintervention and the first week of the study and included four dialysis sessions (1 mid-week dialysis session at preintervention and three dialysis sessions during the first week in the study). BP parameters were obtained by automated devices by dialysis nurses trained at each individual dialysis unit. The following definitions were used:
% of interdialytic weight gain = ((predialysis weight (kg)–previous postdialysis weight (kg))/target dry weight (kg)) × 100.18,19,32,33
Interdialytic weight gain = predialysis weight (kg)–previous postdialysis weight (kg).
ΔSBP = sitting postdialysis SBP–sitting predialysis SBP at the same dialysis treatment session.
Annualized hospitalization rate = (no. of hospitalizations/number of person days) × 365 days/year.
For the purpose of this study, subjects were divided a priori into three clinical groups based on BP changes with HD: (1) SBP fell with HD (ΔSBP ≤ −10 mm Hg) (2) SBP unchanged with HD (ΔSBP −10 to 10 mm Hg), and (3) SBP rose with HD (ΔSBP ≥ 10 mm Hg).
Statistical analysis
Of the 443 subjects initially enrolled in the CLIMB study, one subject was excluded owing to lack of follow-up after enrollment and four subjects were excluded owing to missing pre- or postdialysis BP recordings during enrollment and the first week of the study. The remaining 438 subjects were included in unadjusted analysis. Owing to missing data in seven of these subjects, 431 subjects were included in the final multivariable model.
Descriptive statistics are presented as counts and percentages for discrete variables. Continuous variables are reported as means with standard deviations unless noted otherwise. Categorical variables were compared with χ2 tests. One-way analysis of variance was used to compare normally distributed continuous variables; otherwise, the non-parametric Kruskal–Wallis test (for three-way comparisons) was used.
In unadjusted analysis, logistic regression was utilized to compare differences in clinical outcomes between patients grouped by ΔSBP. All event analyses used logistic regression rather than survival methodology because of the short duration of follow-up and concerns associated with violating proportional hazards assumptions in Cox proportional hazards models. For the primary outcome, only one event per subject was included as an end point. The relationship between ΔSBP and clinical outcomes was also modeled separately as a continuous variable.
In adjusted analysis, logistic regression was used to determine the relationship between ΔSBP and outcomes while controlling for demographics and case-mix. Backward selection was used to identify the final multivariable model. Owing to our small sample size and limited number of clinical events, only parameters deemed clinically relevant or significantly different between groups were initially entered into the full model. Variables with a large number of missing data were not tested for inclusion unless they trended toward a difference among groups (P < 0.15). Variables tested in the final model included age; race; dry weight; % of interdialytic weight gain; ΔSBP; dialysis vintage; access type; history of arrhythmia, cardiac disease (coronary artery disease or congestive heart disease), diabetes mellitus, hypertension, left ventricular hypertrophy, and peripheral vascular disease; baseline albumin, creatinine, calcium, phosphorus, and urea reduction ratio; number of BP medications; and treatment group. Variables that were not significant with a P-value > 0.10 in the model were removed until only variables with a P-value < 0.10 remained. Treatment group was forced into all models. To assess for potential confounding, separate models were tested to examine whether other hemodynamic parameters (pre- or postdialysis SBP, diastolic BP, or pulse pressure) modified the effect of ΔSBP on outcomes or were significantly associated with outcomes. Interaction terms between prespecified parameters and ΔSBP were also tested.
Sensitivity analyses that assessed the relationship between ΔSBP and outcomes only among patients with KDOQI-defined hypertension (predialysis sitting BP ≥ 140/90 or postdialysis sitting BP ≥ 130/80)34 and separately only among patients on antihypertensive medications were performed. Subsequently, the ability of specific antihypertensive agents to modify the effect of ΔSBP on outcomes among patients on antihypertensive agents or were independently associated with the primary outcome was tested.
All statistical analyses were performed using SAS Eguide (version 9.1, SAS Institute, Cary, NC, USA).
ACKNOWLEDGMENTS
This research was presented in abstract form at the Young Investigators Forum at the Southern Society for Clinical Investigators Meeting on March 1, 2006, in Atlanta, GA, USA and at the National Kidney Foundation Young Investigators Competition in Chicago, IL, USA on April 19, 2006. Dr Inrig was supported by NIH Grant K12 RR-017630.
Footnotes
All authors report that they have no financial conflicts of interest to disclose.
REFERENCES
- 1.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Herzog CA, Collins AJ. Blood pressure and long-term mortality in US hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002;62:1784–1790. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 3.Zager PG, Nikolic J, Brown RH, et al. U curve association of blood pressure and mortality in hemodialysis patients. Kidney Int. 1998;54:561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 4.Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33:507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 5.Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO study. Kidney Int. 2004;65:2380. doi: 10.1111/j.1523-1755.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 6.Salem MM, Bower J. Hypertension in the hemodialysis population: any relation to one-year survival? Am J Kidney Dis. 1996;28:737–740. doi: 10.1016/s0272-6386(96)90257-7. [DOI] [PubMed] [Google Scholar]
- 7.Salem MM. Hypertension in the haemodialysis population: any relationship to 2-years survival? Nephrol Dial Transplant. 1999;14:125–128. doi: 10.1093/ndt/14.1.125. [DOI] [PubMed] [Google Scholar]
- 8.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 9.Stidley CA, Hunt WC, Tentori F, et al. Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol. 2006;17:513–520. doi: 10.1681/ASN.2004110921. [DOI] [PubMed] [Google Scholar]
- 10.Klassen PS, Lowrie EG, Reddan DN, et al. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA. 2002;287:1548–1555. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R. Hypertension and survival in chronic hemodialysis patients – past lessons and future opportunities. Kidney Int. 2005;67:1–13. doi: 10.1111/j.1523-1755.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 12.Foley RN, Parfrey PS, Harnett JD, et al. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. 1996;49:1379–1385. doi: 10.1038/ki.1996.194. [DOI] [PubMed] [Google Scholar]
- 13.Fleischmann EH, Bower JD, Salahudeen AK. Risk factor paradox in hemodialysis: better nutrition as a partial explanation. Asaio J. 2001;47:74–81. doi: 10.1097/00002480-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45(Part 2):811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 15.US Renal Data Systems. USRDS 2004 annual data report: atlas of end-stage renal disease in the United States. Bdthesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2004. [Google Scholar]
- 16.Griffith TF, Chua BS, Allen AS, et al. Characteristics of treated hypertension in incident hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2003;42:1260–1269. doi: 10.1053/j.ajkd.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Szczech LA, Reddan DN, Klassen PS, et al. Interactions between dialysis-related volume exposures, nutritional surrogates and mortality among ESRD patients. Nephrol Dial Transplant. 2003;18:1585–1591. doi: 10.1093/ndt/gfg225. [DOI] [PubMed] [Google Scholar]
- 18.Kimmel PL, Varela MP, Peterson RA, et al. Interdialytic weight gain and survival in hemodialysis patients: effects of duration of ESRD and diabetes mellitus. Kidney Int. 2000;57:1141–1151. doi: 10.1046/j.1523-1755.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Gomez JM, Villaverde M, Jofre R, et al. Interdialytic weight gain as a marker of blood pressure, nutrition, and survival in hemodialysis patients. Kidney Int. 2005 Suppl 93:S63–S68. doi: 10.1111/j.1523-1755.2005.09314.x. [DOI] [PubMed] [Google Scholar]
- 20.Sherman RA, Daniel A, Cody RP. The effect of interdialytic weight gain on predialysis blood pressure. Artif Organs. 1993;17:770–774. doi: 10.1111/j.1525-1594.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 21.Boon D, van Montfrans GA, Koopman MG, et al. Blood pressure response to uncomplicated hemodialysis: the importance of changes in stroke volume. Nephron Clin Pract. 2004;96:c82–c87. doi: 10.1159/000076745. [DOI] [PubMed] [Google Scholar]
- 22.Reddan DN, Szczech LA, Hasselblad V, et al. Intradialytic blood volume monitoring in ambulatory hemodialysis patients: a randomized trial. J Am Soc Nephrol. 2005;16:2162–2169. doi: 10.1681/ASN.2004121053. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal R, Peixoto AJ, Santos SFF, et al. Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1:389–398. doi: 10.2215/CJN.01891105. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Andersen MJ, Bishu K, et al. Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int. 2006;69:900–906. doi: 10.1038/sj.ki.5000145. [DOI] [PubMed] [Google Scholar]
- 25.National Kidney Foundation. [accessed March 1, 2006];K/DOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. 2000 available: http://www.kidney.org.
- 26.Mourad A, Khoshdel A, Carney S, et al. Haemodialysis-unresponsive blood pressure: cardiovascular mortality predictor? Nephrology (Carlton) 2005;10:438–441. doi: 10.1111/j.1440-1797.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 27.Fishbane S, Natke E, Maesaka JK. Role of volume overload in dialysis-refractory hypertension. Am J Kidney Dis. 1996;28:257–261. doi: 10.1016/s0272-6386(96)90309-1. [DOI] [PubMed] [Google Scholar]
- 28.Cirit M, Akcicek F, Terzioglu E, et al. Paradoxical rise in blood pressure during ultrafiltration in dialysis patients. Nephrol Dial Transplant. 1995;10:1417–1420. [PubMed] [Google Scholar]
- 29.Chen CH, Lin YP, Yu WC, et al. Volume status and blood pressure during long-term hemodialysis: role of ventricular stiffness. Hypertension. 2003;42:257–262. doi: 10.1161/01.HYP.0000085857.95253.79. [DOI] [PubMed] [Google Scholar]
- 30.Blacher J, Guerin AP, Pannier B, et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 31.Blacher J, Safar ME, Guerin AP, et al. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852–1860. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 32.Testa A, Beaud JM. The other side of the coin: interdialytic weight gain as an index of good nutrition. Am J Kidney Dis. 1998;31:830–834. doi: 10.1016/s0272-6386(98)70052-6. [DOI] [PubMed] [Google Scholar]
- 33.Sezer S, Ozdemir FN, Arat Z, et al. The association of interdialytic weight gain with nutritional parameters and mortality risk in hemodialysis patients. Renal Failure. 2002;24:37–48. doi: 10.1081/jdi-120002659. [DOI] [PubMed] [Google Scholar]
- 34.National Kidney Foundation. [accessed January 15, 2006];K/DOQI: Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. 2000 available: http://www.kidney.org.