Abstract
The neuromodulator serotonin regulates auditory processing and can increase within minutes in response to stimuli like broadband noise as well as non-auditory stressors. Little is known about the serotonergic response in the auditory system to more natural stimuli such as social interactions, however. Using carbon-fiber voltammetry, we measured extracellular serotonin in the auditory midbrain of resident male mice during encounters with a male intruder. Serotonin increased in the inferior colliculus (IC) over the course of a 15 minute interaction, but not when mice were separated with a perforated barrier. Several behaviors, including the amount of immobility and anogenital investigation performed by the resident, were correlated with the serotonergic response. Multiple intrinsic factors associated with individual mice also correlated with the serotonergic response. One of these was age: older mice had smaller serotonergic responses to the social interaction. In a second interaction, individual identity predicted serotonergic responses that were highly consistent with those in the first interaction, even when mice were paired with different intruders. Serotonin was also significantly elevated in the second social interaction relative to the first, suggesting a role for social experience. These findings show that during social interaction, serotonin in the IC is influenced by extrinsic factors such as the directness of social interaction and intrinsic factors including age, individual identity, and experience.
Keywords: serotonin, social interaction, inferior colliculus, auditory, neuromodulation
A major function of sensory systems is to distinguish salient from extraneous information. The difficulty and importance of this task is demonstrated by the diversity of presensory, sensory, and neural mechanisms that selectively filter environmentalin formation (for example, Briscoe & Chittka, 2001; Hurley et al., 2004; Suga & Horikawa, 1986; Warrant, 2001). One complication is that the types of information that are most salient may depend upon the behavioral context. Distinct behavioral situations such as interactions with conspecifics are often associated with species-specific sets of signals which must be correctly interpreted to ensure appropriate behavioral responses (Bohn et al. 2008; Miranda & Liu, 2009; Miranda & Wilczynski, 2009; Seyfarth & Cheney, 2009). Neural mechanisms that optimize the processing of context-specific signals on a time scale fitting these contexts are therefore highly advantageous. Some of the mechanisms responsible for this sort of context-dependent processing act rapidly and are dependent on behavioral state or changes in attention (Berridge & Waterhouse, 2003; Castro-Alamancos, 2009; Edeline, 2003; Fritz et al., 2007; Suga & Horikawa, 1986;). Here, we address the influence of a distinct behavioral context, interaction with a conspecific of the same sex, on the release of the neuromodulator serotonin in the auditory midbrain.
Serotonin is well-suited to modulate sensory filtering in the auditory system and other sensory systems. In vertebrates, serotonin-producing neurons send projections throughout the brain and spinal cord from centralized locations in the raphe nuclei (Jacobs & Azmitia, 1992). These serotonergic inputs occur at multiple levels from primary sensory cells through cortex and are thus in a position to have widespread effects on neural processing (Gil-Loyzaga et al., 2000; Gu et al., 1990; Kaiser & Covey, 1997; Kirifides et al., 2001; Klepper & Herbert, 1991; Massey & Redburn, 1987; Matsutani & Yamamoto, 2008; Nagai et al., 1996; Thompson et al., 1994). The release of serotonin can occur relatively rapidly in response to stressful or social situations and also in response to simple sensory stimuli (Hall et al., 2010; Inoue et al., 1994; Pum et al., 2008). Such changes in serotonergic signaling can vary across regions of the brain, even in response to the same stimulus(Inoue et al., 1994; Pum et al., 2008).
Furthermore, the degree of activation of the serotonergic system, from its release at local terminals to receptor binding, incorporates information about social interactions in a range of vertebrate species. For example, dominant and subordinate male anolis are characterized by varying patterns of changes in serotonin across different regions of the nervous system (Edwards & Kravitz 1997; Ling et al., 2009; Summers et al., 2005). The experience of social defeat increases the expression of serotonergic regulatory proteins in mice (Filipenko et al., 2002). Factors associated with the outcome of social interactions, such as age or sex, are also correlated with differences in the expression of serotonin receptors in multiple regions of the brain in rats and mice (Seebart et al., 2007), including the auditory midbrain (Tadros et al., 2007). Whether social experience or intrinsic individual characteristics influence the serotonergic signal within sensory systems during social encounters, however, has not been assessed.
Here we address these issues in the auditory midbrain of mice. Social interactions among mice are mediated by signals across multiple modalities, and there is an increasing appreciation of the prevalence and complexity of vocalizations used by mice in specific social contexts (Holy & Guo, 2005; Liu et al., 2003; Nyby et al., 1976; Shepard & Liu, 2010). The inferior colliculus (IC) is a site in which selective neural responses to species-specific vocalizations are created by the convergence of excitation and inhibition in multiple species including mice and bats (Holmstrom et al., 2007, 2010; Klug et al., 2002). Within the IC of mice, serotonin release increases gradually with the level of behavioral arousal, within minutes in response to stressful situations, and relatively selectively in response to simple sensory stimuli (Hall et al., 2010). Moreover, in at least one species, Mexican free-tailed bats (Tadarida brasiliensis), the local application of exogenous serotonin increases the selectivity of neural responses for species-specific vocalizations(Hurley & Pollak 2005).
To understand the impact of social interaction on serotonergic regulation in the auditory pathway, we measured extracellular serotonin in the IC of resident male mice presented with a novel intruder. Extracellular serotonin was monitored in the resident using carbon fiber voltammetry. Short-term changes in serotonin were compared to the behavioral performance of both members of the social dyad, as well as with the serotonergic response of the same resident mouse over separate experiments in response to different intruders.
METHODS
Animals
Data were obtained from 38 male CBA/J mice (Mus musculus, Jackson Laboratory, Bar Harbor, ME, USA). Mice ranged in age from 7–18 weeks (average 10.6+/−0.6 weeks standard error of the mean (s.e.m.)). Mice were housed individually on a 14/10 light-dark cycle and supplied with food and water ad libitum. All procedures were approved by the Bloomington Institutional Animal Care and Use Committee.
Surgery
Voltammetric headstages were surgically mounted on the skull overlying the IC. After brief exposure to isoflurane fumes, mice were anesthetized with an intraperitoneal injection of ketamine (120 mg/kg) and xylazine (5 mg/kg). Anesthetic state was determined by lack of response to tail pinch. The hair on top of the head was removed with depilatory cream and mice were placed in a stereotaxic device for surgery (Stoelting; Wood Dale, IL, USA). Bregma and lambda were placed in the horizontal plane and holes approximately 1.5 mm in diameter were drilled over the left and right IC (1.1 mm posterior, 1.6 mm lateral to lambda). Custom-made Teflon hubs (Rebec et al., 1993) were placed above each hole at an angle of 15º from vertical and secured to the skull with two stainless steel bone screws (PlasticsOne Inc.; Roanoke, VA, USA) and dental cement. Between experiments, the hubs were plugged with Teflon screws. Mice were allowed to recover from surgery for at least 5 days before recording.
Voltammetry
Carbon fiber electrode construction
Carbon fiber electrodes were prepared from pulled capillary glass. A single carbon fiber (11 μm in diameter, Thornell P25; Cytec Industries Inc., West Paterson, NJ, USA) was threaded through the tube until it protruded from the end of the glass 100–150 μm. The carbon fiber was sealed in place with epoxy resin (Miller-Stephenson Chemical Company, Inc.; Danbury, CT, USA) and soldered with low melting-temperature bismuth alloy (Small Parts; Miramar, FL, USA) to a length of wire that protruded from the back of the electrode.
Carbon fiber electrodes were electrically and chemically treated prior to use in order to increase the sensitivity to and selectivity for serotonin, as previously described (Hall et al., 2010). All electrodes were electrically treated in phosphate-citrate buffered saline using an e-Corder controlled by Chart software (EDAQ; Denistone East, Australia). Electrodes were then coated with Nafion (Sigma-Aldrich, St. Louis, MO, USA) to increase electrode specificity for serotonin (Brazell et al., 1987; Rivot et al., 1995), which was confirmed by testing each electrode prior to use (Sigma-Aldrich, St. Louis, MO, USA) (Hall et al., 2010).
Recording
Immediately prior to recording, mice were anesthetized with ketamine (90 mg/kg) and xylazine (1 mg/kg) for the placement of the electrodes. The carbon fiber recording electrode was mounted in a custom-made Teflon microdrive, and lowered into the IC. The Ag/AgCl reference electrode was lowered until it came into contact with the cerebrospinal fluid above the contralateral IC. Immediately after electrode placement, mice were returned to their home cage. The cage was placed in a Faraday cage and a light-weight, flexible tether connected the recording and reference electrodes to a bipotentiostat (EI-400; Cypress Systems, Chelmsford, MA, USA) through an electric swivel (PlasticsOne Inc.; Roanoke, VA, USA). This allowed the mouse to move freely around the cage during the recording session. Voltammetric recordings began as soon as the electrodes were connected to the tether. A staircase waveform (−300 mV to +600 mV to −300 mV, 10 mV steps, 30 mV/s2, 1 minute duration) was applied with 5 minutes between each waveform, so that one serotonin measurement (trace) was made every 6 minutes. All manipulations were performed more than one hour after the mice walked off of the laboratory tissue.
Voltammetric recording occurred over two sessions, one in each IC, separated by five days. The hemisphere of first recording was chosen randomly for each mouse; there were no hemispheric differences in serotonergic responses (GLM; F(1,87)=1.81, p=0.182). Separate electrodes were implanted for each session. After the second experiment only, a current (~0.3 mA) was passed through the electrode for up to 5 seconds using a lesion maker (Grass Instruments; Quincy, MA, USA) to confirm localization of the electrode in the IC. All lesions were in the IC, as determined by inspection of perfused brains sliced in the transverse plane.
Social interaction
Intruder mice were males that varied in age and mass relative to the resident. Intruders ranged in age from 56 days younger to 28 days older (average 4.1 days younger +/− 5.6 days s.e.m.) and from 8.3 g lighter to 8.1 g heavier (average 0.4 g lighter +/− 1.1 g s.e.m.) than the resident mouse. All intruders were naïve, used for only one interaction, and not reused as residents in future interactions.
Indirect Interaction (n=8)
A transparent perforated Plexiglas barrier was used to divide the home cage into two equal regions 45 minutes after the resident recovered from anesthesia. After 36 minutes (6 voltammetric traces) in this condition, an intruder was placed in the cage on the other side of the Plexiglas barrier. The two mice interacted through the barrier for 36 minutes (6 traces). The intruder and then the barrier were removed and the resident interacted with the entire empty cage for 45 minutes (7 traces). The barrier attenuated tones from 10–30 kHz played through a speaker (Infinity Emit B; Harman International Industries, Woodbury, NY, USA) by an average of 7 to 7.2 dB, depending on the location of the calibrating microphone (ACO Pacific PS9200 kit; Belmont, CA, USA).
Direct Interaction (n=22)
The transparent perforated Plexiglas barrier was used to divide the home cage into two equal regions 45 minutes after waking from anesthesia. After 36 minutes, an intruder was placed on the other side of the Plexiglas barrier. The 2 mice were recorded in this condition for 6 minutes (1 trace). Two minutes before the next trace, the barrier was removed and the resident and intruder interacted for 15 minutes (3 traces). Direct interactions were limited to 15 minutes in order to reduce the possibility of aggressive interactions and harm to the mice or recording equipment. The intruder was then removed and the resident interacted with the empty cage for 45 minutes. Overt aggressive displays such as lunging or biting were cause for immediatetermination of the experiment (1 of 23 direct interactions).
Control (n=8)
In control experiments, resident mice were not presented with an intruder. These experiments were performed with random placement during the first or second recording session of mice that were also exposed to indirect social interaction. In control experiments, the transparent perforated Plexiglas barrier was used to divide the home cage into two equal regions 45 minutes after waking from anesthesia. The resident mouse was recorded for 72 minutes (12 traces) in this condition. The barrier was then removed and the mouse interacted with the empty cage for an additional 45 minutes.
Repeated Direct Interaction (n=15)
Some mice exposed to direct social interaction during the first recording session were exposed to another direct interaction in their second recording session. The 7 mice that were included in the first but not the second experiment were all removed due to technical problems with carbon fiber electrodes during their second experiments. These mice did not differ from the other mice in their age, mass, and serotonergic or behavioral responses to the first direct interaction and experiments were conducted as they were in the first session (Student’s t-tests; all ps>0.05). Again, all intruders were naïve so no resident was exposed to the same intruder twice.
Behavior
Mouse behavior was recorded using a CCD video camera, DVR PCI card and software (Q-See, Digital Peripheral Solutions Inc., Anaheim, CA, USA). The duration of all behaviors was scored by an observer using ODLog software (Macropod Software).
Behaviors scored both during and outside of social interactions were: 1) Rearing – head and forepaws were elevated off the bedding or forepaws were placed on the side wall of the cage 2) Immobility - no observable movement except breathing 3) Digging – disruption of bedding with forepaws 4) Locomotion – horizontal displacement. During direct interactions, the following social behaviors were scored: 1) Anogenital investigation - nose of one mouse contacting the rear of the other. Anogenital investigations by the resident and intruder were scored separately 2) Facial investigation -nose of one mouse contacting face or nose of the other 3) Mounting - at least one paw on top of partner. This was sometimes accompanied by pelvic thrusting. Residents performed this behavior infrequently (1/22 interactions), so only the mounting behavior of intruders was included. During analysis, the cage was divided into four quadrants of equal size to score: 1) Proximal – intruder and resident in the same quadrant of the cage 2) Distal – intruder and resident not in the same quadrant of the cage.
DATA ANALYSIS
Voltammetry
The concentration of extracellular serotonin was measured as the height of the serotonergic oxidation peak in the first derivative of the current trace (Chen et al., 2007; Hall et al., 2010). Analysis of repeated measures was performed on untransformed data, but to account for differences in the sensitivities of individual electrodes when analyzing differences between groups, serotonergic values were normalized to the average of two measurements taken just prior to the introduction of the intruder mouse.
All statistics were performed with MINITAB (Minitab Inc., State College, PA, USA), Stata (StataCorp LP, College Station, TX, USA) or SPSS® (IBM® SPSS® Statistics, Chicago, IL, USA) software. To investigate the relationship between serotonin and behavior, we compared the total duration or count of each behavior displayed by the resident (in seconds) to the serotonergic measurements taken during the 15 minute interaction. Relationships between serotonin, age, mass and behavior were examined using multivariable regression. The sample size was insufficient to support large numbers of independent variables, therefore reduced models using the step down method were used (α for removal=0.05). Changes in behavioral performance during direct interactions were evaluated using one-way analysis of variance (ANOVA), with Bonferroni pair-wise comparisons to determine patterns of change. For voltammetric recordings, differences between groups were evaluated using a general linear model (GLM) with Bonferonni post-hoc tests. Differences within groups were evaluated using a GLM with mouse identification number as a random factor and Bonferonni post-hoc tests. All error bars in figures represent the s.e.m.
RESULTS
Extracellular serotonin in the IC changes during social interactions
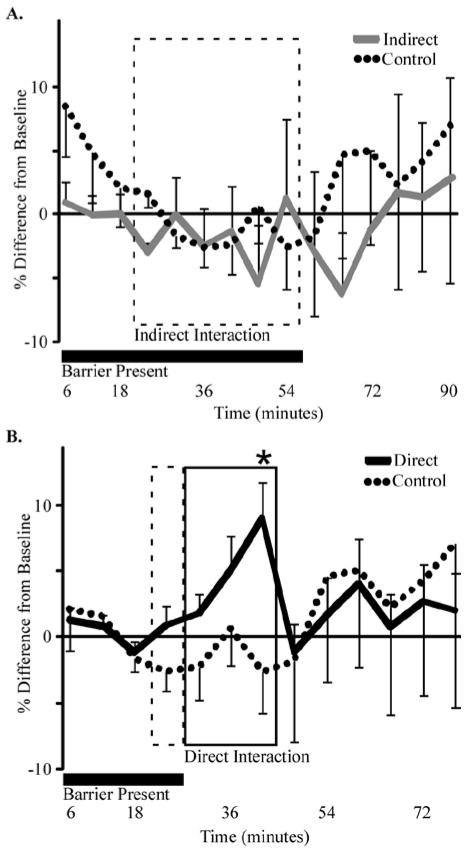
To investigate whether serotonergic neuromodulation in the auditory system is altered during social interactions, we used carbon fiber voltammetry to measure extracellular serotonin in the IC of resident male mice presented with intruders. Three experimental paradigms were tested: 1) controls in which a perforated Plexiglas barrier subdivided the home cage of the resident male, but no other male was placed in the cage; 2) indirect interactions in which intruder males were placed on the other side of the barrier; and 3) direct interactions in which intruder males were placed on the other side of the barrier, and the barrier was then removed. Figure 1 illustrates the serotonergic signal recorded over time. During controls and indirect interaction, placement of the barrier had no effect on the serotonergic signal (paired t-test immediately before versus after barrier placement, p>0.05). During indirect interactions (Fig. 1A, gray line), serotonin did not change relative to the average of the two measurements taken just before the presence of the intruder, or control recordings (Fig. 1A, dashed line; GLM; F(1,88)=0.43, p=0.514). Thus, neither the placement of the barrier, the presence of the intruder mouse (dashed box), nor the removal of the intruder and the barrier influenced extracellular serotonin during indirect interactions.
Figure 1. Extracellular serotonin in the IC increases during direct social interactions.
A) Male mice were exposed to 30 minutes of a transparent, perforated barrier (broken line, n=8), or to an intruder on the other side of the barrier (gray line, n=8). During the presence of the intruder (broken rectangle) extracellular serotonin in the IC was not different relative to pre-interaction levels, post-interaction levels, or the control group. B) Male mice (n=22) were exposed to 5 minutes of indirect social interaction through a barrier (broken rectangle) followed by 15 minutes of direct social interaction with no barrier (solid rectangle). Serotonin did not change when an intruder was presented through the barrier. The serotonin signal during direct interaction (solid line) was significantly elevated above pre-interaction levels by the third measurement (asterisk, GLM, Bonferroni, F(1,86)=8.74, p=0.004, t=2.96, p=0.004) and was significantly greater than the response of control mice (broken line, GLM; F(1,119)=9.53, p=0.003). The serotonin signal in the IC returned to baseline after the interaction. For (A) and (B), values are means, error bars represent s.e.m., and the presence of the barrier is indicated with a horizontal black bar. Control recordings were the same for (A) and (B).
In contrast, serotonin increased in the IC of resident mice during direct interactions with intruders. Similar to the indirect interaction group, resident mice displayed no significant change in serotonin when the intruder was present on the other side of the perforated Plexiglas barrier relative to pre-intruder values (paired t-test, t=−1.56, p=0.133). Once the barrier was removed, however, serotonin increased continuously over the course of the 15 minute interaction (Fig. 1B, black line). This increase was significant relative to both pre-interaction serotonin levels (GLM; F(1,86)=8.74, p=0.004) and resident mice in the control condition (Fig. 1B, dashed line; GLM, F(1,119)=9.53, p=0.003). By the time of the third measurement during direct interaction, 15 minutes after the removal of the barrier separating the two mice, serotonin was 9.34% greater than the pre-interaction baseline (Fig. 1B, asterisk; GLM Bonferroni; t=−2.96, p=0.004). After the intruder was removed, extracellular serotonin returned to pre-interaction levels by the time of the next measurement, within 5 minutes.
Behavioral performance correlates with the serotonergic response during direct interactions
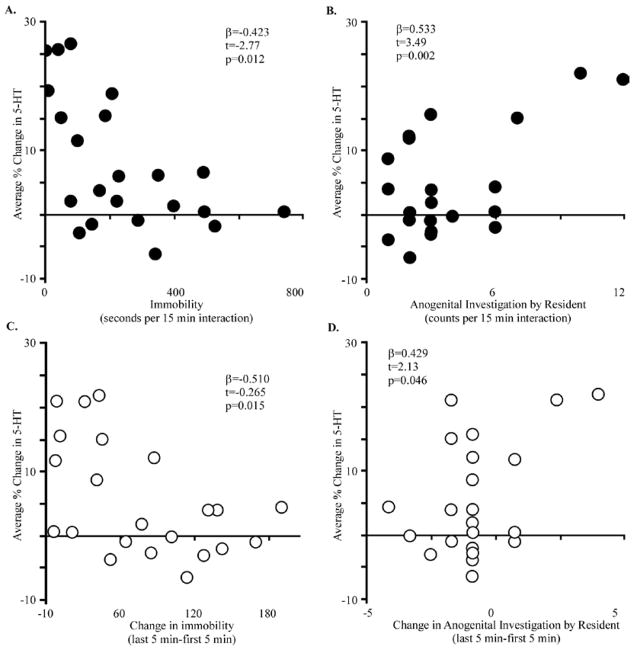
The size of the serotonergic response during direct interaction varied among mice. To determine whether this was correlated with the behavioral response to the social interaction, we measured both social and non-social behaviors performed during the interaction, including anogenital and facial investigation, mounting, time spent proximal or distal to the social partner, rearing, digging, locomotion, and time spent immobile. We further asked whether the observed changes in serotonin were correlated with changes in behavior over the course of the direct interaction, since the serotonergic response emerged over the timecourse of the interaction. To evaluate whether the serotonergic response to an intruder was correlated with overall behavioral performance during direct interaction, the average of the 3 serotonin measurements made in each resident during the direct interaction were compared to the total duration of each of the behaviors monitored during that 15 minute interaction using multivariable regression. Of all the behaviors measured, only two were correlated with the size of the serotonergic response across the resident population: immobility and anogenital investigation by the resident (regression; F(2,19)=13.95, p=0.002, adjusted R2=0.5522). Serotonergic responses tended to be lower in mice that spent more time immobile (Fig. 2A, regression; β=−0.423, t=−2.77, p=0.012) and greater in mice that spent more time investigating the anogenital region of the intruder (Fig. 2B, regression; β=0.533, t=3.49, p=0.002). During these direct social interactions, our measure of immobility is likely to represent resting behavior, because mice spent less time per minute immobile during the social interaction than either before (paired t-test; p<0.001) or after (paired t-test; p=0.023). In contrast, neither the duration of locomotion, digging, or rearing were correlated with the serotonergic response.
Figure 2. Increases in serotonin during social interactions were correlated with behavior.
The average change in the serotonergic signal from each mouse during the 15 minute social interaction (n=22) relative to pre-interaction measurements were negatively correlated with the performance of immobility (A) and correlated with the performance of anogenital investigation by resident mice (B). Further, the serotonin response was correlated with changes in immobility (C) and anogenital investigation by the resident (D) over the course of the interaction (trace 3- trace 1).β, t -and p -values were calculated using multivariable regression.
To evaluate whether the serotonergic response in the IC, which developed over 15 minutes of direct interaction, was correlated with changes in behavior over the same time course, we asked: first, whether behavioral performance changed over time and, second, whether changes in behavior were correlated with changes in serotonin. Both locomotor and social behaviors changed over the course of the interaction. Time spent immobile increased (ANOVA; F(2,62)=8.12, p=0.0007) while time spent mobile decreased (ANOVA; F(2,62)=4.16, p=0.0201) and both facial investigation and anogenital investigation by the intruder decreased (ANOVA; F(2,62)=7.67, p=0.0011, F(2,62)=18.58, p<0.0001). These changes in behavior were coincident with an increase in serotonin in the IC. To investigate whether such changes in locomotor or social behavior over time were individually correlated with changes in serotonin, we used regression analysis with change in serotonin as the dependent variable and the difference in behavior between the last and first five minutes of the social interaction as independent variables. Changes in serotonin were correlated with changes in immobility and anogenital investigation. Mice with the largest increases in serotonin showed the smallest increases in immobility (Fig. 2C; F(1,20)=7.04, p=0.0152, adjusted R2=0.224, t=−0.265, p=0.015, β=−0.5104), and also the largest increases in anogenital sniffing (Fig. 2D; regression; F(1,20)=4.52, p=0.0461, adjusted R2=0.1437, t=2.13, p=0.046, β=0.429).
Relative age and mass of resident and intruder
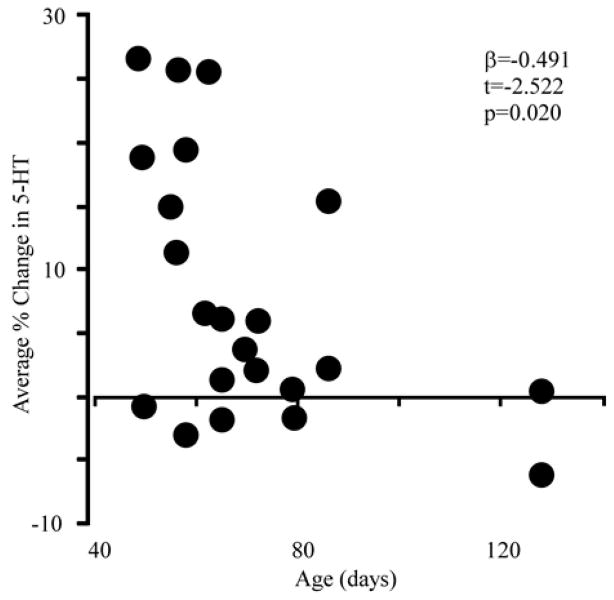
Social dominance is correlated with serotonergic signaling in the central nervous system in a wide variety of species (Blanchard et al., 1993; Edwards & Kravitz 1997; Summers 2001), so we investigated the relationship between the size of the serotonergic response and behavioral indicators of dominance. The relative age and mass of social partners is often related to the behavioral outcome of male-male resident-intruder interactions, with older or larger individuals tending to be dominant (Hilakivi-Clarke & Lister, 1992; Latham & Mason, 2004). We observed age- and mass-related patterns of behavior. Older residents spent more time immobile during direct interactions (regression; F(1,20)=7.78, p=0.0113, adjusted R2=0.244; β=0.529, t=2.79, p=0.011). Similarly, interactions between younger residents and older intruders featured more mounting by the intruder and less immobility by the resident (regression; F(2,19)=6.16, p=0.0087, adjusted R2=0.3934: mounting β=0.382, t=2.13, p=0.047; immobility β=−0.535, t=−2.98, p=0.008). The only significant correlation between serotonin and the physical characteristics of either mouse in the social challenge was for the age of the resident; the amplitude of the serotonergic response decreased with age (Fig. 3; regression; F(1,20)=6.363, p=0.020, adjusted R2=0.203; β=−0.491, t=−2.522, p=0.020).
Figure 3. Resident age is correlated with serotonergic responses to an intruder.
Average serotonergic changes, were negatively correlated with age (days). β, t- and p-values were calculated using multivariable regression.
Individual serotonergic changes and behavior correlate across social interactions
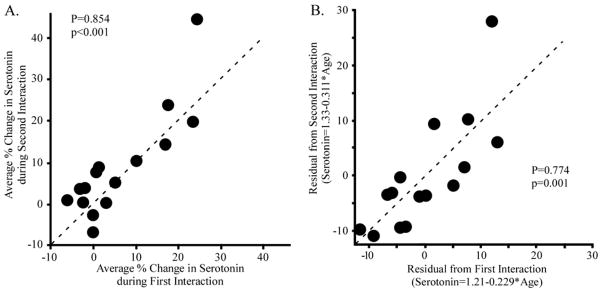
Individual behavioral and physiological responses to environmental challenges can be consistent over time (Koolhaas et al., 2007; Sih et al., 2004). To investigate whether the response characteristics of an individual presented with different social partners were consistent, we exposed some resident mice (n=15) to a direct interaction with a novel intruder, one week after the first interaction. The average serotonergic changes during the first and second social interactions were then compared across the population (Fig. 4A). The magnitude of the two serotonergic responses were highly correlated, with some individuals showing weak responses and some individuals showing strong responses in each interaction (Table 1; Pearson’s P=0.854, p<0.001). Some behavioral responses in each social interaction were also similar. Individual mice performed similar amounts of locomotion and rearing across the two interactions (Table 1; Pearson’s P=0.640, 0.601, p=0.010, 0.018, respectively) and there was a trend towards a correlation in immobility (Table 1; Pearson’s P=0.490, p=0.061). Thus, individual residents showed consistent changes in serotonergic response and behavior across the two behavioral challenges.
Figure 4. Changes in serotonin are similar across multiple direct interactions.
A) The average change in serotonin during the first of two direct interactions separated by one week was correlated with the change in serotonin during the second direct interaction (n=15). The broken line indicates the expected result if the two serotonergic changes were equal. B) Residuals from linear regressions of serotonin versus age in the first and second interactions were also highly correlated.
Table 1.
Serotonergic and behavioral responses of individual mice (n=15) compared across two direct interactions with different intruders.
| Correlation | Difference | ||||
|---|---|---|---|---|---|
| Pearson’s | p-value | Avg. Change (2nd-1st) | t-value (paired) | p-value | |
| 5-HT | 0.854 | <0.001 | 4.04 | −2.300 | 0.037 |
| Immobility Time | 0.495 | 0.061 | −104.13 | 2.010 | 0.064 |
| Locomotion Time | 0.640 | 0.010 | 44.03 | −3.029 | 0.009 |
| Rearing | 0.601 | 0.018 | 2.06 | −2.750 | 0.016 |
| Digging | 0.457 | 0.087 | 2 | −2.010 | 0.068 |
| Nose to Nose | 0.157 | 0.576 | 6.2 | −1.172 | 0.251 |
| Anogenital investigation by Intruder | −0.232 | 0.405 | 1.07 | −0.514 | 0.615 |
| Anogenital investigation by Resident | 0.082 | 0.923 | −0.73 | 0.030 | 0.539 |
| Mounting by Intruder | 0.257 | 0.772 | −0.93 | 0.362 | 0.723 |
| Time Proximal | 0.453 | 0.090 | 25.71 | −0.825 | 0.423 |
| Time Distal | 0.177 | 0.528 | 8.95 | −0.259 | 0.800 |
Left columns describe the Pearson’s correlation between individual performance in two direct interactions. Right columns describe the difference in individual performance with the results of paired t-tests. Time is reported in seconds.
Because the serotonergic response systematically varies with age, the standard period of time between the two experiments could potentially account for the correlation in individual serotonergic responses. To assess this possibility, we stripped the factor of age from the data by calculating the residuals of the age-serotonin regression for each of the two social challenges. These residuals from the first and second interaction were still highly correlated (Fig. 4B, Pearson’s correlation; P=0.774, p=0.001), suggesting that a factor other than age contributes to the individual consistency in serotonergic response.
The time courses of the average serotonergic responses across the population were very similar during the two social challenges. During the second interaction, as in the first, the serotonergic signal increased relative to the measurement before the barrier was removed and continued to increase while the mice were allowed to interact (Fig. 5, black line; GLM, F(1,91)=8.71, p=0.004). By the time of the third measurement, extracellular serotonin in the IC increased by an average of 11% (GLM, Bonferroni, t=3.225, p=0.021). As in the first interaction, the extracellular serotonergic signal returned to baseline by the time of the measurement after the intruder was removed. The serotonergic response during the second interaction was greater, however, than the serotonergic response during the first (Fig. 5, compare black line to gray line; GLM, F(1,149)=5.11, p=0.025). Averaging over all time points, the amplitude of the serotonergic response during the second direct interaction was 4% greater than the change measured during the first interaction (Table 1; paired t-test; t=−2.300, p=0.037). This small increase during the second experiment can be seen in figure 4A, in which many of the serotonergic measurements during the second experiment are slightly above the unity line. Further, behavioral differences between the first and second interaction tracked the difference in serotonin (Table 1). Average locomotion and rearing times were consistently longer during the second interaction (paired t-tests; t=−3.029, 2.750; p=0.009, 0.016, respectively) and time spent immobile was consistently, although not significantly, shorter (paired t-test; t=2.010, p=0.064). Thus, both individual behavioral performance and the serotonergic response were increased during the second behavioral challenge relative to the first.
Figure 5. Similar time course of increase in serotonin during repeated interactions.
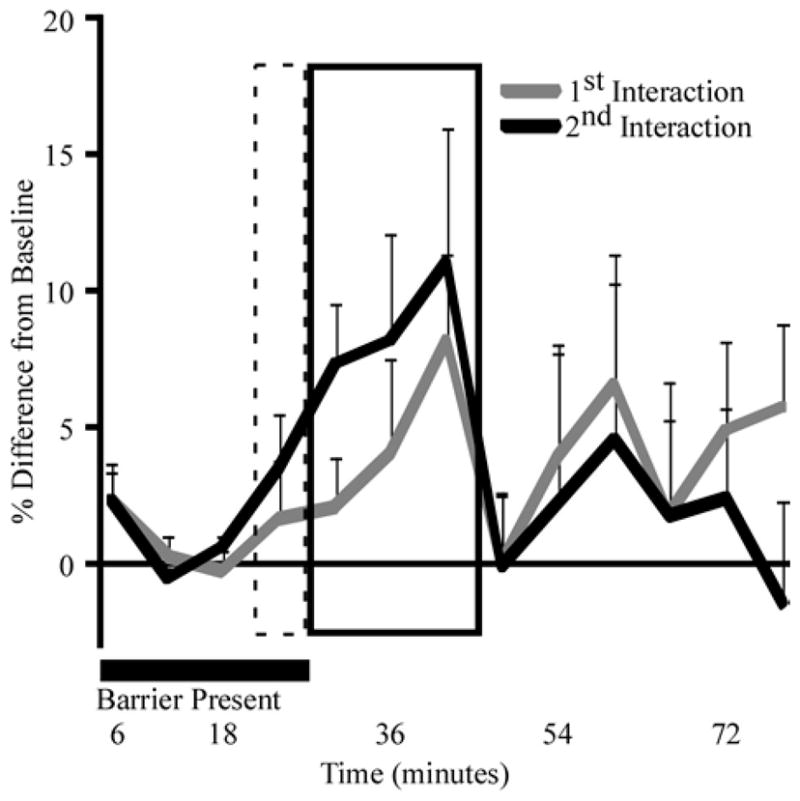
A subset of the mice exposed to direct interaction (grey line, n=15) were exposed to a second direct interaction a week later (black line). As in Fig. 1B, mice were exposed to indirect interaction (broken rectangle) through the barrier (black box) before direct interaction with the intruder (solid rectangle). The serotonergic signal was elevated above control levels during both interactions by the third measurement taken during direct interaction. Values are means; error bars represent s.e.m.
DISCUSSION
We have found that serotonin within the IC of resident male mice increases within minutes during direct social interaction with a novel male intruder. The size of the serotonergic response shows substantial inter-individual variability that correlates with variability in behavior and age, andis consistent in a single individual. The serotonergic response is also facilitated after an initial experience with an intruder. Our results suggest that individual serotonergic responses to the presence of an intruder can shape auditory processing in the midbrain.
Extracellular serotonin in the IC increases only during direct social interaction
Significant increases in serotonin were observed only when male mice interacted directly with each other. When a perforated, transparent barrier was in place, no significant change in serotonin occurred. Thus, elevated serotonin reflected the directness of the social situation. Our experiments did not address which behavioral or sensory cues were important in distinguishing a direct from an indirect interaction. Although the Plexiglas barrier might have selectively removed some tactile cues while allowing the passage of olfactory and acoustic cues, even these latter cues could have been dampened by the barrier (File & Seth, 2003; Latham & Mason, 2004). It is also worth considering that neurons in the dorsal and median raphe nuclei, the major sources of serotonergic fibers in the IC, are not auditory neurons per se. Although some of them respond to auditory stimuli, they are also capable of responding to stimuli of other sensory modalities (Heym et al., 1982; Rasmussen et al., 1986). Thus, the serotonergic neurons could impart general information related to a social situation onto IC neurons rather than information regarding exclusively auditory cues.
The pattern of the serotonergic response during social interaction was different from what we have previously measured in the IC. Extracellular serotonin increased throughout the 15 minute duration of the social interaction, and declined abruptly by the first post-interaction measurement. In contrast, during the presentation of broadband noise or the restriction of movement, extracellular serotonin in the IC peaked within 6 minutes of the manipulation (Hall et al., 2010). Rapid changes in extracellular serotonin have also been reported in mouse hippocampus, lateral septum, and prefrontal cortex during exposure to a predator (Beekman et al. 2005). The contrast between the rapid changes in serotonin observed previously and the gradual increase over the 15 minute interaction with an intruder observed here suggests that different dynamics underlie serotonergic responses in different behavioral situations.
Inter-individualvariation in th e serotonergic response
Although we used the inbred CBA/J line of mice in these studies, inter-individual variation in the serotonergic response was high; some mice showed very little change in serotonin, and some showed increases of up to 40%. These differences among individuals were consistent between two interactions, despite important methodological differences between the experiments. Experiments were separated by 7 days, and performed in contralateral colliculi. Resident mice were also paired with different intruders in the two experiments. Further, separate carbon-fiber electrodes were implanted for each experiment. Since there was a weak relationship between the serotonergic response and age, the ages of the mice could potentially account for the individual consistency. However, when we statistically controlled for age, there was still a high degree of correlation between the individual responses during the first versus second experiments (Fig. 4B). Moreover, inter-individual variation in serotonergic responses was significantly correlated with inter-individual variation in two of the multiple behaviors we measured, immobility and anogenital investigation. These behavioral correlations suggest that the serotonergic increases we measured reflected broad physiological responses to the social situation that varied among individuals. This assessment complements other indications of individual variation in the serotonergic system within the IC, such as an approximately threefold variation in the density of serotonergic fibers across individual mice of the same strain (Papesh and Hurley, unpublished data). For these reasons, we conclude that the variation we observed in the serotonergic response among individualsis behaviorally meaningful.
Individually stable response profiles to behavioral challenges such as encounters with conspecifics or predators are seen in a wide range of animals (Bell et al., 2007; Sih et al., 2004). Several neuromodulators including serotonin have even been associated with particular suites of behaviors. In a study of sticklebacks, the degree of aggression towards conspecifics and boldness in the presence of predators were correlated with activity of the serotonergic and noradrenergic systems in specific brain regions (Bell et al., 2007). This evidence of the involvement of the serotonergic system in individual behavioral differences suggests the possibility that modulation of sensory processing could be part of a suite of physiological changes that correspond to individual behavioral profiles.
A general factor that could contribute to the variability in the serotonergic responses that we observed is the previous experience of the animal. Mice showed a significantly larger serotonergic response during the second experiment than the first, although the overall time courses of serotonergic responses in each experiment were similar (Fig. 5). This effect was in the opposite direction as the age-related decrease in the amplitude of the serotonergic response. The larger serotonergic response in the second experiment also corresponded to changes in behavior, making it unlikely that a technical factor such as electrode-induced damage in the contralateral IC could account for the increased response. The specific features of the initial experiment that led to the increased response in the second are not clear. Previous studies have demonstrated an increase in the responsiveness of the serotonergic system over repeated social interactions (Beitia et al., 2005; Devoino et al., 2003; Filipenko et al., 2002). The stress of being handled and anesthetized in the first experiment could also have contributed to the effect of experience (Levine 1959; Kanno et al. 2003). Although the change we observed was relatively small, it raises the possibility that the mice experienced other events prior to our study that could have contributed to inter-individual differences in the responsiveness of their serotonergic systems to social intrusion.
Behavioral correlation with the serotonergic response
Comparison of the serotonergic response with behavioral measurements allows us to evaluate how our findings conform to several existing hypotheses on serotonergic function. In particular, the inter-individual correlation between serotonin and two behaviors, anogenital investigation and immobility, are suggestive of the social status and motor hypotheses of serotonergic function.
In some studies, the dominance status established during a social interaction has correlated with serotonergic transmission (Ling et al., 2009). Males engaging in aggressive interactions show rapid changes in brain serotonin, with dominant and subordinate individuals characterized by different spatiotemporal patterns of changes in serotonin across different regions of the nervous system (Edwards & Kravitz, 1997; Ling et al., 2009; Summers et al., 2005). Whether corresponding changes in serotonin occur within sensory systems during such social encounters has not been assessed. In the current study, we observed a positive correlation between serotonin and anogenital investigation, which has been interpreted in some studies as a pre-aggressive behavior in individually-housed male mice (Cairns & Nakelski, 1971). Analysis of the emergence of this behavior over the social interaction also supports the social status hypothesis in some ways. Although the mean level of anogenital sniffing by residents did not change over the course of the social interaction, individual residents with the largest increases in serotonin also increased their level of anogenital sniffing in parallel (Fig 2AC , ).
Other evidence, however, does not support a social status hypothesis. In particular, mounting is one of the most unambiguous indicators of dominance status (File & Seth, 2003; Hilakivi-Clarke & Lister, 1992). Although our resident mice did not show mounting behavior, likely due to the presence of headstages, they were mounted by intruders. The amount of mounting behavior was predicted by the age of the intruders, with older intruders displaying more mounting; this is evidence that hierarchical social interactions occurred in our experiments. However, being mounted by intruders was not correlated with the serotonergic response of the resident. Furthermore, the change in mounting by individual intruders across the behavioral interaction, which could indicate the development of dominance of the intruder, was not correlated with serotonin. These relationships do not fully support the hypothesis that serotonergic neuromodulation in the IC is correlated with social status, but certainly suggest that social interest or social investigation plays an important role.
The motor hypothesis of serotonergic function states that serotonin facilitates motor output, while at the same time suppressing sensory responses (Jacobs & Fornal 1993). Thus, one expectation of the motor hypothesis is that serotonin should increase during enhanced motor activity. We did not observe a direct relationship between serotonin and any motor activity we measured, or the total time spent in all motor activities, a strong argument against the motor hypothesis. We did, however, observe greater serotonergic responses during second direct interactions, where the resident also performed more locomotion and rearing. A weaker version of the motor hypothesis would predict an inverse relationship between time spent immobile and serotonin. We did note a correlation between total time spent immobile and the serotonergic response, such that higher serotonin corresponded to less immobility, as the motor hypothesis would predict. Examination of the timecourse of the changes in serotonin and immobility over the social interaction did not support the motor hypothesis as well, however. Although there was a significant inverse correlation between individual serotonergic changes and changes in immobility, the individuals with the largest increases in serotonin showed no change in immobility over the course of the direct interaction, while individuals with smaller or no increases in serotonin showed increases in immobility (Fig. 2C,D ). In a strict sense, this evidence does not support a direct relationship between increased movement and serotonin. A further point to consider is that immobility is a behavior that is interpreted differently depending on context. In resident-intruder interactions, a potentially stressful context, immobility is often interpreted as a measure of fear or anxiety, rather than the lack of movement. This evidence, combined with the immediate reduction in serotonin after the removal of the intruder, suggests that increases in serotonin in the IC are more directly influenced by the presence of the intruder than achange inthe residents ’motor output.
Thus, although some aspects of our findings support these specific hypotheses of serotonergic function, this support is disputable. A more general alternative to these two hypotheses is that serotonergic increases are associated with some types of stressful situations that require elevated levels of behavioral arousal (Haller et al., 2005; Reuter & Jacobs, 1996; Chaouloff et al., 1999; Jacobs & Azmitia, 1992). In other behavioral paradigms in the same strain of mice, serotonin increases slowly in the IC of mice that are waking, and rapidly (< 5 min) in mice that are placed in a small arena that limits their movement, or are exposed to loud broadband noise (Hall et al. 2010). As in the current study, these increases are characterized by substantial inter-individual variation. Encountering an intruder could likewise lead to variable levels of stress that trigger the release of serotonin. The significant relationships we have observed between serotonin and investigation of the intruder mouse or the amount of time spent immobile are consistent with this hypothesis in a general way. Future studies examining the correlation between serotonin and activation of the hypothalamopituitary-adrenal axis could address this hypothesis more directly.
Implications of serotonergic response for auditory processing
Several types of evidence suggest that increases in serotonin during social interactions should influence the processing of auditory stimuli by IC neurons. Local application of serotonin and the release of endogenous sources of serotonin both profoundly influence the auditory response properties of IC neurons (Baldan Ramsey et al. 2010; Bohorquez & Hurley, 2009; Hall & Hurley, 2007; Hurley, 2007). Serotonin-evoked changes in auditory responses are graded, in that they are dependent on the amount of serotonin applied (Hurley & Pollak, 2001; Hurley, 2006). Thus, although the increases in serotonin we observed may have been modest, even relatively small perturbations in available serotonin would still be expected to influence the activity of large numbers of neurons. Because the relatively long carbon fiber electrodes we used likely integrated the serotonergic signals through a large volume of the IC, more localized but also more sizable increases in serotonin could also have occurred, but have been averaged out of our measurements.
One class of stimuli with particular importance to a socially-triggered increase in serotonin is species-specific vocalization. Male mice produce multiple classes of ultrasonic vocalizations during resident-intruder interactions (Gourbal et al., 2004; Sales, 1972), and the IC plays an important role in encoding such social signals (Klug et al., 2002). In the mouse IC in particular, both individual neurons and neuron populations can selectively encode vocalizations as a function of spike rate and timing (Holmstrom et al., 2010) . A prediction that arises from these studies is that an increase in serotonin during social interactions should influence the selectivity of IC neurons for species-specific signals. In fact, exogenously applied serotonin in the IC of one species, the Mexican free-tailed bat, does make responses to species-specific vocalizations generally more selective (Hurley & Pollak, 2005). In addition to vocalizations, males also produce a variety of non-vocal cues during interactions associated with factors such as movement of the substrate. It is possible that serotonin also modulates responses to these categories of sounds. Overall, our findings suggest that a direct social challenge triggers the release of serotonin within the auditory system, subsequently increasing neural selectivity for acoustic signals or other types of sounds in an individually variable manner. Such a coordinated response could facilitate the optimal processing of sensory information as well as behavioral output during a social challenge.
Acknowledgments
These experiments were supported in part by National Institute of Deafnessand Other Communication Disorders grant DC-008963. The authors wish to thank Dr. G. Troy Smith for helpful advice on the manuscript.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Baldan Ramsey LC, Sinha SR, Hurl ey LM. 5-HT1A and 5-HT1B receptors differentially modulate rate and timing of auditory responses in the mouse inferior colliculus. European Journal of Neuroscience. 2010;32(3):368–379. doi: 10.1111/j.1460-9568.2010.07299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman M, Flachskamm C, Linthorst AC. Effects of exposure to a predator on behaviour and serotonergic neurotransmission in different brain regions of C57bl 6N mice. European Journal of Neuroscience. 2005;21:2825–2836. doi: 10.1111/j.1460-9568.2005.04107.x. [DOI] [PubMed] [Google Scholar]
- Beitia G, Garmendia L, Azpiroz A, Vegas O, Brain PF, Arregi A. Time-dependent behavioral, neurochemical, and immune consequences of repeated experiences of social defeat stress in male mice and the ameliorative efects of fluoxetine. Brain, Behavior and Immunity. 2005;19:530–539. doi: 10.1016/j.bbi.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Bell AM, Backström T, Huntingford FA, Pottinger TG, Winberg S. Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiology & Behavior. 2007;91:15–25. doi: 10.1016/j.physbeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research Brain ResearchRev iews. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behavioral Brain Research. 1993;58(1–2):113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Bohn KM, Schmidt-French B, Ma ST, Pollak GD. Syllable acoustics, temporal patterns, and call composition vary with behavioral context in Mexican free-tailed bats. Journal of the Acoustical Society of America. 2008;124(3):1838–1848. doi: 10.1121/1.2953314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohorquez A, Hurley LM. Activation of serotonin 3 receptors changes in vivo auditory responses in the mouse inferior colliculus. Hearing Research. 2009;251(1–2):29–38. doi: 10.1016/j.heares.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazell MP, Kasser RJ, Renner KJ, Feng J, Moghaddam B, Adams RN. Electrocoating carbon fiber microelectrodes with Nafion improves selectivity for electroactive neurotransmitters. Journal of Neuroscience Methods. 1987;22:167–172. doi: 10.1016/0165-0270(87)90011-2. [DOI] [PubMed] [Google Scholar]
- Briscoe AD, Chittka L. The Evolution of Color Vision in Insects. AnnualRev iewof Entomology. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Cortical Up and Activated States: Implications for Sensory Information Processing. Neuroscientist. 2009;15(6):625–634. doi: 10.1177/1073858409333074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormède P. Serotonin and Stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Cairns RB, Nakelski JS. On fighting in mice: ontogenetic and experiential determinants. Journal of Comparative and Physiological Psychology. 1971;74(3):354–364. doi: 10.1037/h0030584. [DOI] [PubMed] [Google Scholar]
- Chen YP, Liu SY, Yu HQ. A simple and rapid method for measuring dissolved oxygen in waters with gold microelectrode. Analytica Chimica Acta. 2007;598(2):249–253. doi: 10.1016/j.aca.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Devoino LV, Al'perina EL, Podgornaya EK, Polyakov OV, Idova GV, Yu R, Il'yuchenok Nature of the distribution of serotonin and a serotonin metabolite in brain structures and the development of immunosuppression in submissive mice. Neuroscience and Behavioral Physiology. 2003;33(5):473–477. doi: 10.1023/a:1023463201122. [DOI] [PubMed] [Google Scholar]
- Edeline JM. The thalamo-cortical auditory receptive fields: regulation by the states of vigilance, learning and the neuromodulatory systems. Experimental Brain Research. 2003;153:554–572. doi: 10.1007/s00221-003-1608-0. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status and aggression. Current Opinion in Neurobiology. 1997;7(6):812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. European Journal of Pharmacology. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Filipenko ML, Beilina AG, Alekseyenko OV, Dolgov VV, Kudryavtseva NN. Increase in Expression of Brain Serotonin Transporter and Monoamine Oxidase A Genes Induced by Repeated Experience of Social Defeats in Male Mice. Biochemistry (Moscow) 2002;67(4):451–455. doi: 10.1023/a:1015238124000. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention — focusing the searchlight on sound. Current Opinion in Neurobiology. 2007;17:437–455. doi: 10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Gil-Loyzaga P, Bartolome V, Vicente-Torres A, Carricondo F. Serotonergic innervation of the organ of corti. Acta Otolaryngologia. 2000;120:128–132. doi: 10.1080/000164800750000757. [DOI] [PubMed] [Google Scholar]
- Gourbal BEF, Barthelemy M, Petit G, Gabrion C. Spectrographic analysis of the ultrasonic vocalisations of adult male and female BALB/c mice. Naturwissenschaften. 2004;91:381–385. doi: 10.1007/s00114-004-0543-7. [DOI] [PubMed] [Google Scholar]
- Gu Q, Patel B, Singer W. The laminar distribution and postnatal development of serotonin-immunoreactive axons in the cat primary visual cortex. Experimental Brain Research. 1990;81:257–266. doi: 10.1007/BF00228114. [DOI] [PubMed] [Google Scholar]
- Hall IC, Hurley LM. The serotonin releaser fenfluramine alters the auditory responses of inferior colliculus neurons. Hearing Research. 2007;288(1–2):82–94. doi: 10.1016/j.heares.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Rebec GV, Hurley LM. Serotonin in the inferior colliculus fluctuates with behavioral state and environmental stimuli. The Journal of Experimental Biology. 2010;213:1009–1017. doi: 10.1242/jeb.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Toth M, Halasz J. The activation of raphe serotonergic neurons in normal and hypoarousal-driven aggression: A double labeling study in rats. Behavioral Brain Research. 2005;161:88–94. doi: 10.1016/j.bbr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Heym J, Steinfels GF, Jacobs BL. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Research. 1982;215:259–276. doi: 10.1016/0006-8993(82)90743-0. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke LA, Lister RG. The role of body weight in resident-intruder aggression. Aggressive Behavior. 1992;18:281–287. [Google Scholar]
- Holmstrom L, Roberts PD, Portfors CV. Responses to social vocalizations in the inferior colliculus of the mustached bat are influenced by secondary tuning curves. Journal ofNeurophysiol ogy. 2007;98(6):3461–3472. doi: 10.1152/jn.00638.2007. [DOI] [PubMed] [Google Scholar]
- Holmstrom LA, Eeuwes LB, Roberts PD, Portfors CV. Efficient encoding of vocalizations in the auditory midbrain. The Journal of Neuroscience. 2010;30(3):802–819. doi: 10.1523/JNEUROSCI.1964-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biology. 2005;3(12):e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Current Opinion in Neurobiology. 2004;14:488–459. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Hurley LM. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Research. 2007;1181:21–29. doi: 10.1016/j.brainres.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. Journal of Comparative Physiology A. 2005;191:535–546. doi: 10.1007/s00359-005-0623-y. [DOI] [PubMed] [Google Scholar]
- Inoue T, Tsuchiya K, Koyama T. Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. Pharmacology, Biochemistry and Behavior. 1994;49(4):911–920. doi: 10.1016/0091-3057(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiological Reviews. 1992;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends in Neurosciences. 1993;16(9):346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith RM, Edwards KS, Givens B, Beversdorf DQ. Combined effect of maternal serotonin transporter genotype and prenatal stress in modulating offspring social interaction. International Journal of Developmental Neuroscience. 2010;28:529–536. doi: 10.1016/j.ijdevneu.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A, Covey E. 5-HT innervation of the auditory pathway in birds and bats. In: Syka JL, editor. Acoustical Signal Processing in the Central Auditory System. Plenum Press; New York: 1997. pp. 71–78. [Google Scholar]
- Kanno M, Matsumoto M, Togashi H, Yoshioka M, Mano Y. Effects of Acute Repetitive Transcranial Magnetic Stimulation on Extracellular Serotonin Concentration in the Rat Prefrontal Cortex. Journal of Pharmacological Sciences. 2003;93:451–457. doi: 10.1254/jphs.93.451. [DOI] [PubMed] [Google Scholar]
- Kirifides ML, Simpson KL, Lin RCS, Waterhouse BD. Topographic organization and neurochemical identity of dorsal raphe neurons that project to the trigeminal somatosensory pathway in the rat. The Journal of Chemical Neuroanatomy. 2001;435:325–340. doi: 10.1002/cne.1033. [DOI] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of norandrenergic and serotonergic fibers in the cochlear nucleus and the inferior colliculus of the rat. Brain Research. 1991;557:190. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Hurley LM, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. Journal of Neurophysiology. 2002;88:1941–1954. doi: 10.1152/jn.2002.88.4.1941. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Buwalda B, van Reenen K. Individual Variation in Coping with Stress: A multidimensional approach of ultimate and proximate mechanisms. Brain, Behavior and Evolution. 2007;70:218–226. doi: 10.1159/000105485. [DOI] [PubMed] [Google Scholar]
- Latham N, Mason G. From house mouse to mouse house: the behavioural biology of free-living Mus musculus and its implications in the laboratory. Applied Animal Behavior Science. 2004;86:261–289. [Google Scholar]
- Levine S. Emotionality and aggressive behavior in the mouse as a function of infantile experience. The Journal of Genetic Psychology: Research and Theory on Human Development. 1959;94:77–83. doi: 10.1080/00221325.1959.10532436. [DOI] [PubMed] [Google Scholar]
- Ling TJ, Forster GL, Watt MJ, Korzan WJ, Renner KJ, Summers CH. Social status differentiates rapid neuroendocrine responses to restraint stress. Physiology & Behavior. 2009;96:218–232. doi: 10.1016/j.physbeh.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. Journal of the Acoustical Society ofAm erica. 2003;114(6 Pt 1):3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- Massey SC, Redburn DA. Transmitter circuits in the vertebrate retina. Progress in Neurobiology. 1987;28:55–96. doi: 10.1016/0301-0082(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Matsutani S, Yamamoto N. Centrifugal innervation of the mammalian olfactory bulb. Anatomical Science International. 2008;83:218–227. doi: 10.1111/j.1447-073X.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- Miranda JA, Liu RC. Dissecting natural sensory plasticity: Hormones and experience in a maternal context. Hearing Research. 2009;252:21–28. doi: 10.1016/j.heares.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JA, Wilczynski W. Sex differences and androgen influences on midbrain auditory thresholds in the green treefrog, Hyla cinerea. Hearing Research. 2009;252:79–88. doi: 10.1016/j.heares.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Kim DJ, Delay RJ, Roper SD. Neuromodulation of transduction and signal processing in the end organs of taste. Chemical Senses. 1996;21:353–365. doi: 10.1093/chemse/21.3.353. [DOI] [PubMed] [Google Scholar]
- Nyby J, Dizinno GA, Whitney G. Social status and ultrasonic vocalizations of male mice. Behavioral Biology. 1976;18:285–289. doi: 10.1016/s0091-6773(76)92198-2. [DOI] [PubMed] [Google Scholar]
- Paigen B, Ishida BY, Verstuyft J, Winters RB, Albee D. Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis. 1990;10:316–323. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- Popova N. From genes to aggressive behavior: the role of serotonergic system. BioEssays. 2006;28:495–503. doi: 10.1002/bies.20412. [DOI] [PubMed] [Google Scholar]
- Pum M, Huston JP, De Souza Silva MA, Muller CP. Visual sensory-motor gating by serotonin activation in the medial prefrontal and occipital, but not in the rhinal, cortices in rats. Neuroscience. 2008;153:361–372. doi: 10.1016/j.neuroscience.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Strecker RE, Jacobs BL. Single unit response of noradrenergic, serotonergic and dopaminergic neurons in freely moving cats to simple sensory stimuli. Brain Research. 1986;369:336–340. doi: 10.1016/0006-8993(86)90546-9. [DOI] [PubMed] [Google Scholar]
- Rebec G, Langley PE, Pierce RC, Wang Z, Heidenreich BA. Journal of Neurosience Methods. 1–2. Vol. 47. 1993. A simple micromanipulator for multiple uses in freely moving rats: electrophysiology, voltammetry, and simultaneous intracerebral infusions; pp. 53–59. [DOI] [PubMed] [Google Scholar]
- Rivot JP, Cespuglio R, Puig S, Jouvet M, Besson JM. In vivo electrochemical monitoring of serotonin in spinal dorsal horn with nafion-coated multi-carbon fiber electrodes. Journal of Neurochemistry. 1995;65:1257–1263. doi: 10.1046/j.1471-4159.1995.65031257.x. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Research. 1996;739(1–2):57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Animal Behaviour. 1972;20(1):88–100. doi: 10.1016/s0003-3472(72)80177-5. [DOI] [PubMed] [Google Scholar]
- Seebart BR, Stoffel RT, Behan M. Age-related changes in the serotonin 2A receptor in the hypoglossal nucleus of male and female rats. Respiratory Physiology & Neurobiology. 2007;158:14–21. doi: 10.1016/j.resp.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Production, usage, and comprehension in animal vocalizations. Brain and Language. 2009;115:92–100. doi: 10.1016/j.bandl.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Shepard KN, Liu RC. Experience restores innate female preference for male ultrasonic vocalizations. Genes, Brain and Behavior, Article. 2010 doi: 10.1111/j.1601-183X.2010.00580.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. TRENDS in Ecology and Evolution. 2004;19(7):372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Suga N, Horikawa J. Multiple time axes for representation of echo delays in the auditory cortex of the mustached bat. Journal of Neurophysiology. 1986;55(4):776–805. doi: 10.1152/jn.1986.55.4.776. [DOI] [PubMed] [Google Scholar]
- Summers CH. Mechanisms for quick and variable responses. Brain, Behavior and Evolution. 2001;57:283–292. doi: 10.1159/000047246. [DOI] [PubMed] [Google Scholar]
- Summers CH, Korzan WJ, Lukkes JL, Watt MJ, Forster GL, Øverli O, Höglund E, Larson ET, Ronan PJ, Matter JM, Summers TR, Renner KJ, Greenberg N. Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiology and Biochemical Zoology. 2005;78(5):679–694. doi: 10.1086/432139. [DOI] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zettel ML, Zhu XX, Lynch-Erhardt M, Frisina RD. Serotonin 2B receptor: Upregulated with age and hearing loss in mouse auditory system. Neurobiology of Aging. 2007;28:1112–1123. doi: 10.1016/j.neurobiolaging.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Thompson GC, Thompson AM, Garrett KM, Britton BH. Serotonin and serotonin receptors in the central auditory system. Otolaryngology Head & Neck Surgery. 1994;110(1):93–102. doi: 10.1177/019459989411000111. [DOI] [PubMed] [Google Scholar]
- Warrant E. The design of compound eyes and the illumination of natural habitats. In: Barth G, Schmid A, editors. Ecology of Sensing. Springer-Verlag; Berlin, Heidelberg: 2001. pp. 187–214. [Google Scholar]
- Winslow JT. Mouse social recognition and preference. Current Protocols in Neuroscience. 2003;Chapter 8(Unit 8.16):1–16. doi: 10.1002/0471142301.ns0816s22. [DOI] [PubMed] [Google Scholar]