Abstract
Purpose
We have previously reported that many types of tumors are able to induce changes in human T cells that lead to the acquisition of suppressive function as well as phenotypic alterations resembling those found in senescent T cells. In the present study we find a role for IL-7 in protecting T cells from these changes, and further define involved signaling pathways.
Experimental Design
We evaluated the ability of IL-7 treatment to prevent the gain of suppressive function and phenotypic alterations in human T cells after a short co-culture with tumor cells in vitro. We then used inhibitors of components of the PI3K/AKT pathway and siRNA knock-down of Mcl-1 and Bim to evaluate the role of these signaling pathways in IL-7 protection.
Results
We found that IL-7 inhibits CD27/CD28 loss and maintains proliferative capacity, IL-2 production, and reduced suppressive function. IL-7’s protective ability depended on activation of the PI3K/AKT pathway, which inhibited activation of glycogen synthase kinase 3β (GSK3β) which subsequently prevented the phosphorylation and loss of Mcl-1. We further demonstrated a key role for Mcl-1 in that its knock-down or inhibition abrogated the effects of IL-7. Additionally, knock-down of the Mcl-1 binding partner and pro-apoptotic protein Bim protected T cells from these dysfunctional alterations.
Conclusion
These observations confirm the role for Bcl-2 family members in cytokine signaling, and suggest that IL-7 treatment in combination with other immunotherapies could lead to new clinical strategies to maintain normal T cell function and reduce tumor-induced generation of dysfunctional and suppressor T cells.
Keywords: Interleukin-7, tumor immunology, immunosuppression, Mcl-1, immunotherapy
Introduction
The immune response plays an important role in detecting and killing tumor cells and the use of immunotherapies to take advantage of this process has been a major goal of medical research. Despite some successes, obstacles such as multiple processes of T cell regulation and dysfunction remain unresolved. Recent studies have identified significantly increased populations of T cells in human patients that display many characteristics of senescent T cells normally associated with aging (1–3). While the hallmark of these cells is the loss of CD27 and CD28 expression, they also possess other features of senescence, including shortened telomeres (4) and reduced proliferative capacity and cytokine production (5), as well as suppressor activity not associated with traditional senescence. These findings suggest that increased numbers of dysfunctional, senescent-like T cells directly support the local and systemic immunosuppression observed in cancer patients (3, 6). Indeed, increased numbers of these cells correlates with a poor prognosis and contributes to general immune hyporesponsiveness (7–9), making these patients poor candidates for immunotherapy (2, 3, 10).
Working from the hypothesis that the tumors themselves were capable of inducing these dysfunctional changes in T cells, we have previously characterized an in vitro model where normal human T cells were induced to become dysfunctional suppressors after a brief interaction with tumor cell lines of multiple origins (1). This model mimics a process in patients where normal T cells travel to the tumor microenvironment, are altered by tumor-generated factors, and then re-enter the periphery where phenotypic alterations can be used to track them. We found that a brief co-incubation with tumor cells was sufficient to induce a significant loss of CD27 and CD28 expression in both CD4+ and CD8+ T cells as well as other senescence-related markers including telomere shortening, nuclear heterochromatization, and increased expression of p53, p21, and p16. Most significantly, we found that this process involved a soluble factor secreted by tumor cells (but not untransformed fibroblasts), and that both CD4+ and CD8+ populations gained a potent suppressive ability. These data suggest that this process of tumor-induced suppression could be a mechanism by which tumors could not only blunt T cell responses against them directly, but also enhance systemic hyporesponsiveness by redirecting effectors to become regulatory.
Several recent studies suggest that cytoprotective cytokines such as IL-7 and IL-12 may prevent the induction of altered T cell function. For instance, IL-7 and IL-12 can induce telomerase activity, a key enzyme in maintaining telomere length (11–13). IL-12 has been found to restore CD28 expression in CD4+CD28null T cells sorted from human PBMCs (13). Further, in a recent clinical trial of cancer patients, exogenous IL-7 maintained CD27 expression in peripheral blood T cells (14). Therefore, we hypothesized that this cytokine could have the capacity to prevent the dysfunctional alterations we observed in tumor-exposed T cells.
Using our previously described in vitro model of tumor-induced T cell dysfunction, we now show that IL-7 prevents the development of both phenotypic alterations and suppressive function in tumor-exposed T cells. We further show that this protection relies on the PI3K/AKT signaling pathway. Once activated, AKT inhibits the more downstream molecule GSK3β, preventing it from phosphorylating the cell survival molecule Mcl-1. Finally, we demonstrate that Mcl-1 plays a non-apoptotic role as a key downstream effector of IL-7 signaling by preventing tumor-induced dysfunction of T cells through well known pro-survival pathways. These findings suggest that IL-7 is a potent adjuvant for preventing the development of tumor-induced suppressor T cells and may assist in cancer treatment, either alone or with other immunotherapies.
Materials and Methods
Cell culture
Peripheral blood T cells from healthy donors were isolated as described previously (1). CD45RA+ T cells were enriched from purified donor T cells by positive magnetic bead selection (Miltenyi). Human solid tumor cell lines MCF-7, MEL-624, and Tu167 were cultured for 24h up to a 90% confluence. To induce suppressor T cells, purified, naive, human T cells were added to wells containing solid tumor cells at a ratio of 1:1 for 6h. T cells were then collected, washed, and cultured for 7 days in a complete medium with or without each of recombinant human IL-2 (rIL-2, 100U/ml, NCI BRB Preclinical Repository), IL-7 (rIL-7, 20ng/ml, BD Pharmingen), IL-12 (rIL-12, 20ng/ml, R&D Systems), IL-15 (rhIL-15, 10ng/ml, eBioscience).
The following inhibitors at the indicated concentrations were used: 20µM LY294002 (Sigma); 0.5µM AKT inhibitor IV (Cal Biochem); 20µM and 2µM GSK3β inhibitor X; and 5µM rapamycin (Cal Biochem). 20nM TW37 was a gift from Shaomeng Wang (University of Michigan, Ann Arbor, MI), and 10µM ABT737 provided by Abbott Laboratories (Abbott Park, IL).
Flow Cytometry
Dysfunctional phenotypic markers were evaluated by CD27/CD28 staining and telomere length as described previously (1). CD3+CD27+CD28+ subsets were collected by FACS sorting. For the intracellular Mcl-1 staining, cells were incubated with Mcl-1 antibody or IgG control (Sigma) followed by fluorescein (FITC) labeled secondary antibody (Jackson ImmunoResearch). For IL-2 staining, tumor-exposed cells +/− IL-7 were washed and stimulated with 1µg/ml anti-CD3 mAb overnight; BD Golgi Stop (0.7µl/ml) was added for 4h followed by intracellular staining with APC-anti-IL-2 and APC-anti-rabbit IgG (BD Pharmingen). For CFSE staining, T cells were labeled with 2µmol/L CFSE (Molecular Probes, Eugene, OR) for 8 minutes at 37°C and then washed 3 times with 10% RPMI 1640 at room temperature. 2×105 of CFSE labeled fresh responder cells were placed in 96-well plates with coated anti-CD3 mAb (1µg/ml, BD Pharmingen) and co-cultured with tumor-exposed cells ±IL-7 for 48 hours (T cell ±IL-7: responder=1:3). Apoptosis in responder cells was assessed by staining FITC-Annexin V and 7-AAD (BD Phamingen) per manufacturer’s instructions. All samples were analyzed on a BD LRSII with FACS Diva Software (BD Bioscience). PBMC’s from head and neck cancer patients were collected and frozen under an institutional review board-approved protocol at the University of Maryland, Baltimore. Informed consent was obtained from all subjects. Expression of T cell surface markers was evaluated by staining with PerCP-anti-CD3 (BD), PEcy7-anti-CD4 (BD), APC-eFluor® 780-anti-CD8 (eBioscience), PE-anti-CD127 (BD), APC-anti-CD28 (BD), and FITC-anti-CD27 (BD).
Western blot analysis and co-immunoprecipitation (co-IP)
Western blot analysis was performed as previously described (1) using the following antibodies: anti-AKT1, anti-phospho-AKT Ser473 and Thr308, anti-Mcl-1, anti-phospho-Mcl-1 (Ser159/Thr163), anti-GSK3β, anti-phospho-GSK3β (Ser9), anti-Bim, anti-STAT5 and anti-phospho-STAT5 (Cell Signaling Biotechnology). For co-IP, cell lysates were precleared with protein G beads and incubated overnight at 4°C with 1µg of anti-Mcl-1 (Santa Cruz Biotechnolgy) or anti-Bim (Alexix Biochemicals, San Diego, CA). Immunoprecipitates were collected by adding 30µl protein G agarose beads (Sigma Aldrich, Saint Louis, MO) and incubated for 1h at 4°C.
Proliferation suppression assay
Autologous T cells (responder cells) were plated at a density of 2×105 cells per well in a flat-bottomed 96-well plate coated with 1µg/ml of anti-CD3 mAb (BD Pharmingen). The next day, tumor-exposed T cells +/− IL-7 were washed and added to the cultures of responder cells for an additional 72h, 1µCi (0.037 MBq)/well [3H]-thymidine was pulsed overnight, and incorporation was measured with a scintillation counter.
RNAi transfection
siRNAs for Mcl-1, Bim and non-targeting were purchased from Dharmacon and Santa Cruz Biotechnology. Purified primary T cells were transfected using the Amaxa Nucleofector Kit (Amaxa Biosystem, Gaithersburg, MD) and analyzed 36h after transfection.
Statistical analysis
The Student’s t-test was used to evaluate the significance of differences between sample means. Statistical significance was defined at P <0.05. All experiments were repeated at least three times with error bars representing the SD.
Results
IL-7 protects human T lymphocytes from tumor-induced T cell supression
We have previously reported that tumor cells can directly induce suppressor T-cells in vitro, which could be a mechanism that contributes to a tumor’s ability to evade immune recognition and killing (1). The cytokines IL-2, IL-7 or IL-12, which are known for their cytoprotective effects on T cells and are currently in clinical trials, were used to determine whether they can prevent these phenotypic and functional alterations. Initially, we treated human peripheral blood T cells with IL-2, IL-7 or IL-12 before and concomitantly with different tumor cell lines, which were chosen based on our previous study (1). T cells were then recovered from co-culture, washed and plated in complete media in the presence or absence of cytokines for seven days. Dysfunctional suppressor T cells were identified by the loss of CD27 and CD28 or telomere shortening. Prior research had found tumor-exposed T cells undergo a 40–60% greater decrease in CD27 and CD28 expression compared to control T cells not exposed to tumor (1). The new findings demonstrate that IL-7 treatment (and to a lesser degree IL-2, but not IL12) consistently protects tumor-exposed T cells from CD27/CD28 loss (Fig. 1A and B). However, only IL-7 treated, tumor-exposed T cells maintained a telomere length comparable to unexposed controls (Fig.1C). We then established that the best time for IL-7 treatment is immediately post co-incubation with tumor (Supplemental Fig 1.), and for this reason all subsequent experiments used this time point. We also determined that the combination of IL-7 with other common receptor gamma-chain cytokines IL-2 and IL-15 did not result in any additive effect over IL-7 alone (Supplemental Fig 2). In order to test whether IL-7 treatment could equally benefit naive versus memory T cells, naive CD45RA+ T cells were enriched from purified T cells by positive magnetic bead selection before both CD45RA-enriched and depleted T cells were incubated with tumor and cultured in IL-7 (Supplemental Fig 3). We found that the degree of CD27/CD28 loss was greater in naive CD45RA+ T cells versus CD45RO+ memory T cells, but that IL-7 treatment was protective in both. These results suggest that naive T cell populations may be more succeptible tumor-induced alterations.
Figure 1. IL-7 prevents tumor-induced T-cell CD27/28 loss and telomere shortening induced by both hematopoietic and solid tumors in vitro.
Purified T cells were incubatd with various tumor cell lines (Tu167, MCF7 and 624mel) at a ratio of 1:1 for 6 hours and then washed and cultured for another 7 days with or without cytokines (100U/ml IL-2, 20ng/ml IL-7, IL12). On day 7, T cell controls and tumor-exposed cells were evaluated for the loss of CD27 and CD28. Representative dot plots depicting CD27−CD28− T cells induced by Tu167 are shown in (A). Tumor-exposed T cells incubated with different tumor cells (from at least three independent experiments) in the presence or absence of different cytokines are shown in (B), error bars represent standard deviation. (C) Analysis of telomere length with freshly isolated T cells, T cells on day 7 and tumor-exposed T cells on day 7 (+/−IL-2, IL- 7, or IL-12). The bar chart shows the standard error of the mean (SEM) relative telomere length (RTL) in cells from 3 independent experiments.*, P=0.027 versus control tumor-exposed cells. NS, no statistical significance between IL-7 treated, tumor-exposed cells and untreated T cells alone.
Our data also show that IL-7 alone promoted T cell survival (Supplemental Fig. 4A) and did not induce proliferation of tumor-exposed T cells (Fig. 2A) or T cells alone (data not shown). These findings suggest that IL-7 protects against T cell dysfunction by inhibiting its development rather than causing the proliferation of non-dysfunctional T cells. To address whether IL-7 treatment is able to reverse the dysfunctional phenotype, we evaluated tumor-exposed T cells (untreated) seven days after co-incubation and added IL-7. CD27/CD28 expression was checked at several time points after the introduction of IL-7, but only minor increases in expression of both markers was observed (data not shown). To determine whether loss of the IL-7 receptor (IL-7R) could account for this finding, we examined the expression of this receptor (CD127) on tumor-exposed T cells. We observed that the loss of CD27 and CD28 correlated with significantly lower CD127 expression (supplemental Fig. 5). To test if the observed decrease of CD127 expression after tumor-exposure has clinical relevance, we obtained peripheral blood from human head and neck cancer patients collected under an IRB-approved protocol before chemotherapy treatment, and examined CD127 expression on CD27−/CD28− T cells among the total CD8+ population as compared to healthy, age-matched donor controls. We chose to focus on the CD8+ T cell population as these cells display greater levels of CD27/CD28 loss in vivo versus CD4+ cells (9). Similar to our in vitro observations, the CD27/CD28 double-negative CD8+ T cell population in these patients also had very low CD127 expression (Supplemental Fig. 6). These results suggest that the benefit of IL-7 therapy would be the protection of CD127+ T cells from tumor-induced dysfunction rather than the reversal of already-dysfunctional cells due to their lack of CD127 expression. Overall, our results demonstrate that IL-7 protects against tumor-induced phenotypic alterations, but that once T cells lose CD27 and CD28 expression, further IL-7 exposure is unlikely to have any effect.
Figure 2. IL-7 enhances the proliferation of CD3-activated, tumor-exposed T cells and restores proliferation of responder cells in co-culture with tumor-exposed cells.
(A) T cells were stained with 2µM CFSE and then co-cultured with Tu167 at a ratio of 1:1 for 6h, washed and cultured in anti-CD3 mAb coated plates with or without IL-7 (20ng/ml). Cell proliferation was examined by flow cytometry on day 1, 4, and 7. Tumor-exposed T cells cultured without anti-CD3 in medium alone (untreated) with IL-7 served as negative controls. (B) Tumor-exposed cells cultured with or without IL-7 for seven days were washed and stimulated with anti-CD3 mAb for 24h, and then IL-2 expression was detected by intracellular cytokine staining and FACS. (C) Tumor-exposed T cells were incubated +/− IL-7 for seven days and then washed and placed into the cultures of normal donor responder cells pre-stimulated for 24h with anti-CD3 antibody at tumor-exposed cell:responder cell ratio of 1:3. Responder T cell proliferation was evaluated by [3H]thymidine incorporation after 72h and represented as the mean of cpm±SD from 3 independent experiments (left panel). The percentage of proliferation inhibition of responder T cells by tumor-exposed T cells and IL-7 treated tumor-exposed T ells was calculated from 3 independent experiments (*, P= 0.027). Each bar represents the mean of triplicate values ±SD. (D) Tumor-exposed T cells cultured seven days ± IL-7 were added to CFSE-labeled responder cells at a ratio of 1:3 and stimulated with anti-CD3 mAb for 48h. Apoptosis of the CFSE-labeled responder cells was evaluated by Annexin V and 7-AAD staining. The bar graph shows the percentage of double positive staining cells from 3 independent experiments. Error bars represent the standard deviation (T: control T cells, R: Responder cells).
IL-7 prevents the functional changes associated with tumor-induced dysfunction
In addittion to the loss of CD27 and CD28 expression, we have also found that tumor-exposed T cells are hypoproliferative when stimulated with anti-CD3. To evaluate whether IL-7 treatment could affect this aspect of tumor-induced dysfunction, we evaluated the proliferative capacity of tumor-exposed T cells after seven days in vitro culture with or without IL-7. Using CFSE-labeling and plate-bound anti-CD3 antibody, we observed an increase in the proliferation of tumor-exposed, IL-7 treated cells, but not in tumor-exposed T cells stimulated with anti-CD3 Ab or IL-7 alone (Fig. 2A).
To further validate this observation, we repeated the experiment using 3H-thymidine incorporation as a read-out for proliferation. Initially, we did not observe proliferation in any of the treatment groups (data not shown). Since IL-7 treated T cells contain small numbers of CD27/CD28 double-negative T cells, we hypothesized that the lack of proliferation could be due to suppression by these cells. In order to evalutate this possibility, CD27+CD28+ T cells were electronically sorted from IL-7 treated or untreated groups seven days after tumor-exposure, and stimulated with plate-bound anti-CD3 antibody for 72h (Supplementary Figure 2B). Interestingly, we observed that CD27+CD28+ T cells not recieving IL-7 treatment were hypoproliferative. This observation could be due to the fact that tumor-induced dysfunction is a process, and that the loss of proliferative capacity preceeds the loss of CD27 and CD28 expression. Tumor-exposed CD27/CD28 double-positive cells treated with IL-7 displayed proliferation similar to control T lymphocytes not exposed to tumor. As expected, the CD27−CD28− population from both untreated and IL-7 treated, tumor-exposed T cells did not proliferate (data not shown). These observations indicate that IL-7 prevents tumor-induced dysfunction in most, but not all T cells, and IL-7 treated CD27+CD28+ T cells have normal proliferative capacity.
Another distinguishing characteristic of both senescent and dysfunctional T cell populations in cancer patients and those generated by our tumor-exposure model is reduced production of IL-2 upon stimulation (15). We discovered IL-7 treatment can significantly increase IL-2 production in tumor-exposed T cells after anti-CD3 stimulation (Fig 2B)
Perhaps the most significant functional alteration of tumor-exposed T cells is their ability to suppress the proliferation of normal T lymphocyes in repsonse to anti-CD3 and MLR stimulation in vitro (1). Thus, we examined whether IL-7 treatment could reduce or prevent this suppression. Fresh allogenic T lymphocytes (responder cells) were pretreated with plate-bound anti-CD3 Ab for 24h. Tumor-exposed T cells cultured with or without IL-7 were then added to the responder cells, at a ratio of 1:3. We observed that IL-7 treated cells had less suppressive function than their untreated counterparts (Fig. 2C). We then evaluated whether tumor-exposed T cell-mediated suppression occurs through induction of apoptosis in responder T cells by labeling them with CFSE prior to co-incubation. After 2 days, responder cell apoptosis was measured by 7-AAD and Annexin V staining. The 20% baseline level of apoptosis was expected since anti-CD3 stimulation induces proliferation and activation-induced cell death. In the presence of tumor-exposed T cells, however, apoptosis in responder T lymphocytes nearly doubles, while co-incubation with IL-7 treated, tumor-exposed cells did not significantly increase responder apoptosis (Fig. 2D). Taken together, these results demonstrate that IL-7 inhibits the functional alterations seen in tumor-induced dysfunctional T cells.
PI3K/AKT is required for IL-7 protection against tumor-induced T cell dysfunction
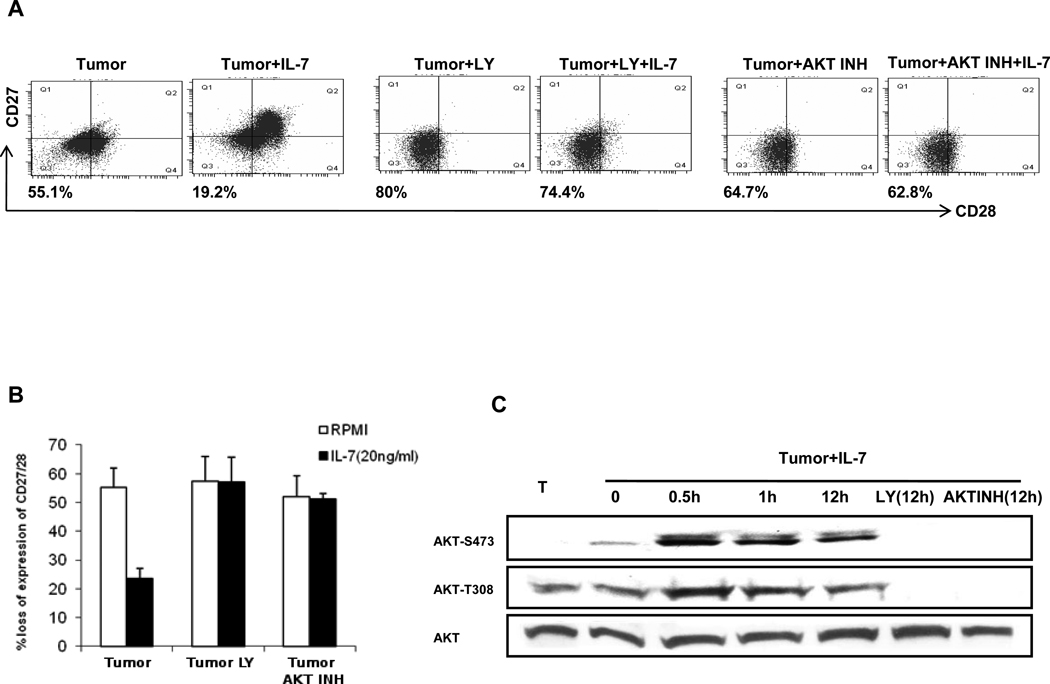
In order to better understand the effects IL-7 treatment has on protecting T cells from tumor-induced dysfunction, we next investigated the role of several pathways known to be involved in IL-7 signaling, namely JNK/STAT5 and PI3K/AKT (16). We found that though inhibition of STAT 5 (17) could block its phosphorylation during IL-7 treatment, the blockade had little effect on the protective ability of IL-7 (Supplemental Fig. 7). We next evaluated the PI3K/AKT pathway, which has been shown to be involved in mediating IL-7-driven T-cell survival (16, 18). AKT is a key downstream target of PI3K, and is activated by dual phosphorylation at Thr-308 and Ser-473. As shown in Fig. 3A and B, IL-7 cannot protect T cells from tumor induced CD27/CD28 loss when cultured with PI3K (LY294002) or AKT inhibitors (AKT inhibitor IV). We then analyzed whether AKT is activated by IL-7 in tumor-exposed cells. As shown in Fig. 3C, IL-7 can induce AKT phosphorylation at Ser473 and Thr308 within 30min, with phosphorylation increasing for at least 12h. The PI3K and AKT inhibitors, as expected, blocked IL-7 induced AKT activation in tumor-exposed cells. Similar results occurred with another inhibitor of PI3K, wortmannin (data not shown).
Figure 3. IL-7-mediated protection from tumor-induced phenotypic alterations depends on PI3K and AKT.
(A) and (B) Tumor-exposed T cells were cultured with or without IL-7 and with LY294002 (LY) or AKT inhibitor IV. On day 7, cells were stained with CD27 and CD28 antibodies. One representative of the 3 is shown in (A), data from independent 3 experiments are shown in (B), with each bar representing the mean of triplicate values ±SD. (C) T cells alone or tumor-exposed T cells treated with IL-7 or together with LY294002 or AKT inhibitor IV were cultured for the indicated periods. Cell lysates were resolved with 10% SDS-PAGE and immunoblotted with indicated antibodies. Total AKT was used as a loading control.
IL-7 prevents tumor-induced T cell dysfunction through inhibition of GSK3β
We next evaluated downstream cell survival molecules related to the PI3K/AKT pathway by examining the effector molecular complex, mTOR (19, 20). Using the mTOR inhibitor Rapamycin, we did not observe any reduction in IL-7’s protective function (Supplemental Fig. 8), suggesting that mTOR is dispensable for IL-7’s effects. We next studied glycogen synthase kinase 3beta (GSK3β), one of the downstream substrates of PI3K/AKT, which is inactivated by direct phosphorylation on the Ser9 residue, promoting cytokine-driven cell survival (21). These previous reports suggested that GSK3β might effect IL-7 mediated protection through PI3K/AKT pathway activation. We observed that the development of tumor-induced CD27/CD28 loss was prevented by the GSK3β inhibitor X (20µM) even without IL-7 (Fig. 4A and B). We also tested the GSK3β-inhibitor at a lower dose (2µM) and observed less protection. With a lower GSK3β-inhibitor dose, IL-7 could prevent CD27/CD28 loss (data not shown). We also observed that IL-7 causes a time dependent increase in phosphorylation of GSK3β which suggests that this process is part of its protective signaling (Fig 4C).
Figure 4. Inactivation of GSK3β protects against CD27/CD28 loss.
(A) and (B) Tumor-exposed T cells were cultured with medium, IL-7, GSK3β inhibitor X (GSK3β INH), or IL-7 + GSK3β inhibitor X (GSK3β INH+IL-7) for 7 days before staining for CD27/CD28 expression. One representative of the 3 is shown in (A), data from independent 3 experiments are shown in (B), with each bar representing the mean of triplicate values ±SD. (C) T cells alone or tumor-exposed T cells cultured with IL-7 and LY294002 (LY) or AKT inhibitor IV were harvested at the indicated time points. GSK3β phosphorylation was detected by immunolotting with anti-phospho-GSK3β (Ser9) antibody. Blots were re-probed with an anti-GSK3β and anti-β-actin antibodies.
IL-7 up-regulates Mcl-1 expression by dephosphorylation in tumor-exposed T cells
Studies indicate Mcl-1 levels are highly regulated by GSK3β phosphorylation at Ser159 in cytokine-deprived cells, which causes Mcl-1 degradation (22). We observed via Western blot analysis that Mcl-1 phosphorylation occurs in tumor-exposed cells (Tumor 0h) (Fig 5A). IL-7 addition reduced Mcl-1 phosphorylation and up-regulated total Mcl-1 in a time dependent manner. Both PI3K and AKT inhibitors can prevent IL-7-induced Mcl-1 dephosphorylation, while GSK3β inhibitor with IL-7 prevented almost completely Mcl-1 phosphorylation (Fig. 5A). To further evaluate Mcl-1 expression, we used FACS analysis of tumor exposed T cells in a 3 period time course over 1 week. Our data show that fluorescence intensity of Mcl-1 increased and was maintained throughout this course after a single IL-7 administration. As control, we investigated Mcl-1 levels in tumor-exposed cells treated with IL-2 and IL-12 (Fig. 5B), concluding that IL-7 had the greatest impact on Mcl-1 expression. These experiments demonstrate that IL-7 increases Mcl-1 levels by inhibiting its phosphorylation induced by GSK3β, further elucidating IL-7’s role in protecting T cells from tumor-induced dysfunction.
Figure 5. Mcl-1 is up-regulated and dephosphrylated at Ser159/Thr163 by IL-7 in tumor-exposed T cells.
(A) T cells or tumor-exposed T cells harvested immediately (0h) and 12h after co-incubation with Tu167 tumor cells were harvested. Tumor-exposed T cells + IL-7 were harvested 0.5h, 2h or 12h after co-incubation with tumor. Tumor-exposed T cells + IL-7 and LY294002, AKT inhibitor IV, or GSK3β inhibitor X for 12h were harvested. Total Mcl-1, pSer159/Thr163-Mcl-1 and β-actin (loading control) in all samples were analyzed by Western blot. (B) T cells were coincubated with Tu167 tumor cells, washed and cultured in media with or without a single dose of IL-2 (100 U/ml), IL-7 (20ng/ml), or IL-12 (20ng/ml). The expression of Mcl-1 was assessed by flow cytometry on day 2, 4, and 7; the mean fluorescence intensity (MFI) of Mcl-1 is shown. (C) Control and Mcl-1-siRNA transfected T cells were cultured for 36h, then co-incubated with Tu167 for 6h at a ratio of 1:1 and then cultured with or without IL-7. Mcl-1 expression was examined by Western blotting (lower panel). On day 5, we evaluated loss of CD27 and CD28 by flow cytometry. Representative data are shown in the upper panel. (D) TW37 but not ABT737 abrogates IL-7 protection. Tumor-exposed T cells +/− IL-7 with and without TW37 or ABT737 were cultured for 7 days before staining for loss of CD27 and CD28 expression. Data from 3 independent experiments are shown in (D), with each bar representing the mean of triplicate values ±SD.
Depletion of Mcl-1 abrogates IL-7 protection against tumor-induced dysfunction
To determine whether Mcl-1 plays a role in mediating IL-7’s protective activity, we reduced Mcl-1 levels through siRNA-mediated knock-down and examined the resulting influence on cell survival. T cells were transfected with siRNA-Mcl-1 or a control siRNA. Transfection with Mcl-1 specific siRNA produced a profoundly decreased Mcl-1 expression which IL-7 did not up regulate. Importantly, IL-7’s protective activity was significantly abrogated in T cells with reduced Mcl-1 levels. IL-7 still had a small effect in Mcl-1 knock-down T cells; however this may be due to the fact that the siRNA transfection is not absolute. To further verify the lack of off-target effects, we used a distinct Mcl-1 siRNA from another company and obtained similar expression and functional results (Supplemental Fig. 9). Notably, Mcl-l’s down-regulation in T cells can induce moderate losses of CD27/CD28 compared to controls (Fig.5C). To confirm Mcl-1’s specific role in IL-7 mediated protection, we introduced two pharmacological drugs, TW37 (a nonpeptide pan small-molecule inhibitor of Bcl-2, Mcl-1 and Bcl-xL) and ABT737 (a small-molecule BH3 mimetic that binds to and antagonizes Bcl-2/Bcl-xL). Fig. 5D illustrates that IL-7 can reduce CD27/CD28 loss with ABT737 but not with TW37 treatment. Collectively, these results indicate Mcl-1’s involvement in IL-7-mediated protection from tumor-induced T cell dysfunction.
The importance of Mcl-1’s binding partner Bim in IL-7 protection
After examining Mcl-1’s role in IL-7 mediated protection from tumor-induced T cell dysfunction, we explored its well-known binding partner Bim, whose inhibition is essential in IL-7 mediated lymphocyte survival (23–25). To assess Bim’s role in IL-7 mediated signaling, we acutely knocked-down its expression using siRNA (from two different sources) in tumor-exposed cells with and without IL-7 (Fig. 6A and Supplemental Fig. 9B). Bim loss abrogated tumor-induced CD27/CD28 loss and IL-7 addition could not provide further protection (Fig. 6A). Finally, to assess whether IL-7’s function was dependent on binding of Mcl-1 with Bim, we performed immunoprecipitation (IP) experiments for these proteins. Interestingly, when we performed IP for Mcl-1, almost all Bim was pulled-down in tumor-exposed T cells whether or not IL-7 was administered. In contrast after the IP for Bim, the level of free Mcl-1 was significantly increased by IL-7 in tumor-exposed cells (Fig. 6B and C). These data suggest that IL-7 driven Mcl-1 expression prevents tumor-induced T cell dysfunction, and that the level of free (unbound to Bim) Mcl-1 is key to this process.
Figure 6. Free Mcl-1 is important for IL-7 protection and up-regulated by IL-7 treatment.
(A) T cells were transfected with either control siRNA or Bim siRNA for 36 hours, co-incubated with Tu167 for 6h at a ratio of 1:1 and cultured in fresh medium +/− IL-7 for 5 days. The loss of CD27/CD28 was determined by FACS (upper panel). Bim expression was examined by Western blotting (lower panel). One representative of the 3 is shown. (B) and (C) Tumor-exposed T cells with and without IL-7 were lysed in IP buffer and immunoprecipitated with anti-Mcl-1 and Bim antibody. The immunoprecipitated proteins, total extracts, and depleted supernatant were analyzed by SDS-PAGE and immunoblotting with indicated antibodies.
Discussion
The link between senescence and tumor-induced T cell suppression has yet to be completely understood. Many studies have found elevated levels of T cells bearing the characteristics of replicative senescence normally associated with aged individuals in peripheral blood and tumor-infiltrating lymphocytes of cancer patients (3, 9). Other studies have focused on phenotypically similar populations that exhibit suppressor function.(3) Our previous data suggest that the answer may be more complex. Specifically, we observe in appropriate co-incubation conditions that tumor cells can induce senescent-like changes in T cells with strongly associated gain of suppressor function, in the absence of activation and proliferation.(1) Interaction with tumors has been shown to induce a large array of changes in T cells in addition to the senescent-like dysfunctional changes reported here, including the induction of pre-apoptotic features such as the loss of ζ chain and CCR7 expression.(26) Our previous work has found that the T cell alterations we observe are a process distinct from tumor-induced apoptosis, though we report here that the protection conferred by IL-7 treatment involves several signaling pathways known to be anti-apoptotic. Thus the signaling relationship between tumor-induced apoptosis and dysfunction remains to be completely resolved.
The presence of tumor-induced dysfunctional T cell populations in cancer patients is associated with systemic immunosuppression and poor prognosis. Therefore, regulating their formation could improve clinical outcomes (8, 27). While cytokines are an attractive option for immunomodulation, little is understood about their effects on natural and induced suppressor cell populations. For instance, some cytoprotective cytokines, such as IL-15, induce immunosuppressive cellular responses including increases in senescence-associated markers on T cells (28, 29). Our in vitro model, where tumor cells can induce similar changes in normal donor T cells, provides a platform to identify cytokines which regulate formation of dysfunctional T cells and dissect the molecular pathways responsible for these effects. Using this model we observed that IL-7 is a potent inhibitor of tumor-induced dysfunctional T-cell phenotypic and functional changes. Furthermore, we show that IL-7 exerted its protective function through common molecular pathways involved in preventing apoptosis.
We initially began with three cytokines currently utilized in clinical trials, namely IL-2, IL-7 and IL-12. IL-2 and IL-7 share the same gamma chain receptor but seem to have disparate effects on the immune system (30). For instance, whereas both IL-2 and IL-7 promote T cell survival, IL-2 also causes expansion of natural T regulatory cells. IL-12, a non-gamma chain cytokine, not only promotes anti-tumor immunity but has been shown to increase CD28 expression in CD28null T cells (13, 31). We found a striking difference between these cytokines in that only IL-7 consistently protects against tumor-induced T-cell phenotypic and functional changes, and that combination with other IL-2 family cytokines (IL-2 and IL-15) does not produce an additive effect over IL-7 alone. This observation is notable in that both IL-2 and IL-15 have been shown to enhance the survival of effector and memory T cell populations similar to IL-7. (30) Several phase I clinical trials have been conduced with IL-7 in the context of cancer (14, 32, 33) and HIV (34, 35). In total, these trials demonstrate that IL-7 can be well tolerated by patients with minimal toxicities and induces the expansion of naïve and memory CD4+ and CD8+ T cell populations. However, overall anti-tumor efficacy was nominal whether used alone or with vaccine. Our data showing that induction of a suppressive, dysfunctional phenotype is accompanied by the loss of IL-7R suggests that the timing of administration would be important not in the depletion of existing dysfunctional suppressors, but in protecting subsequent waves of effector T cells induced either endogenously by vaccine or injected via adoptive transfer. Indeed, pre-clinical mouse models have indicated that IL-7 can successfully be combined with a vaccine to significantly boost anti-tumor immunity (36).
IL-7 is known to activate cell survival signaling pathways, including the JAK/STAT and the PI3K/AKT pathways.(37) However, these pathways may work in concert or be mutually exclusive (38). In addition, consequences of IL-7 signaling can differ depending on the T cell lineage. We hypothesized that the protective effects of IL-7 may be mediated through these known cytoprotective pathways, and identified only the PI3K/AKT pathway as significant. Our data show that PI3K and AKT are required for IL-7 mediated protection from dysfunctional changes, and in doing so causes phosphorylation of AKT at both possible sites, indicating full activation of this molecule. Finally, our findings show that telomere length is maintained with IL-7 administration, which is consistent with AKT’s reported ability to activate telomerase (39).
A number of recent studies have demonstrated that in cancer, constitutive AKT expression promotes cellular senescence (40). Others have shown, however, that AKT can promote proliferation through inhibition of RAF (41). This discrepancy may be a cell-type dependent phenomenon and, at least in mouse and human T cells, AKT appears to maintain normal function. Thus, our data implicating the PI3K/AKT pathway may represent changes in lymphocytes only, or may be related to its function in combination with other IL-7 induced pathways. Ultimately, these findings describe a novel IL-7 mediated T-cell protective pathway dependent on PI3K and AKT.
The AKT molecule has many downstream targets. Two of the most studied are the mTOR complex and GSK3β, which are both involved in promoting T cell proliferation (42, 43). We observe that inhibition of mTOR using rapamycin did not affect IL-7’s protective activities. This observation is interesting since it has been recently reported that AKT induces cellular senescence through mTOR and may also explain why AKT in IL-7 signaling is anti-senescent (44). As a result, we then tested the importance of GSK3β. Our data show that chemical inhibition of GSK3β was sufficient to almost completely inhibit tumor-induced phenotypic alterations in T cells even without IL-7. We further show that IL-7 causes the phosphorylation and inhibition of GSK3β. Overall, our data illustrate that during IL-7 signaling, AKT is activated which then prevents GSK3β signaling and leads to reduced CD27/CD28 loss.
One of the main targets of phosphorylation by GSK3β is the anti-apoptotic Bcl-2 family member Mcl-1 (22). Although there is significant data regarding Mcl-1’s role in apoptosis, there is no data regarding its ability to modulate function and phenotypic changes in T cells. Other anti-apoptotic Bcl-2 molecules, however, were shown to inhibit (Bcl-xL) or promote (Bcl-2) similar processes associated with senescence (45, 46). We observe that IL-7 consistently causes the phosphorylation of AKT (activation) and GSK3β (inhibition) and prevents the increase in phosphorylation of Mcl-1. These findings seem to occur as early as 30 to 60 minutes after administration of IL-7 indicating their temporal proximity. Although the increase in Mcl-1 expression could be due to lack of phosphorylation and degradation, others showed that AKT can induce increases in Mcl-1 expression via a translational mechanism (47). Regardless, the importance of these findings was confirmed through knock-down and chemical inhibition of Mcl-1 in tumor-exposed T cells, demonstrating its importance in preventing dysfunctional phenotypic changes, and indicating a novel non-apoptotic role.
Knowing that Mcl-1 has important function in resisting the tumor-induced T-cell phenotypic changes in our model, we decided to investigate whether a related pro-apoptotic molecule, Bim, also played a role. We and others have already shown Bim to bind to and be inhibited by Mcl-1 in apoptosis (24). Our data show that not only does IL-7 reduce the phosphorylation and increase the overall expression of Mcl-1, but also causes an increase in free Mcl-1 protein (not bound to Bim), resulting in a protective effect.
In summary, we present the novel conclusion that the cytokine IL-7 can prevent formation of dysfunctional T cells that display suppressor function and senescent phenotype. We further show novel data indicating that a major IL-7 induced anti-apoptotic pathway is also protective (see Supplemental Fig. 10 for a diagrammatic overview). Despite recent advances in immunotherapy, successful clinical outcomes remain limited (27, 48), and tumor-induced immune suppression could be a major contributing factor (49). As a result, there is new enthusiasm for cytoprotective strategies that are designed to overcome these processes (50). Cytokines like IL-7 reduce many aspects of cancer immunosuppression, including induction of dysfunctional phenotypic and functional immune changes, like those elucidated in this study. Therefore, future approaches to treat cancer using immune therapies will require the incorporation of cytoprotective strategies, such as IL-7, to bring them to clinical reality.
Statement of Translational Relevance.
In the current study, we provide compelling data that IL-7, a cytoprotective cytokine, is also a potent inhibitor of tumor induced suppressive activity and phenotypic alterations observed in human T cells. We further show that IL-7’s protective activities require involvement of the PI3K/AKT pathway and demonstrate the importance of Mcl-1 as a key regulator of these dysfunctional changes. Together these data provide new insights into the molecular action of IL-7. Perhaps of greater importance is the observation that T-cell alterations similar those reported here are found in cancer patients, and have been shown to be associated with poor prognosis and candidacy for immunotherapy. IL-7 may now have a broader appeal as an adjuvant to immune based cancer treatment.
Supplementary Material
Acknowledgments
This work was sponsored by an award from the Charlotte Geyer Foundation and the National Cancer Institute, National Institutes of Health (grant #5R01CA132796).
Footnotes
Conflict of Interest Statement: Dr. Strome is a co-founder and major stockholder in Gliknik Inc., a biotechnology company. He also receives royalties through the Mayo Clinic College of Medicine for licensure of intellectual property related to B7-H1 (PD-L1) and 4-1BB (CD137) to third parties.
References
- 1.Montes CL, Chapoval AI, Nelson J, Orhue V, Zhang X, Schulze DH, et al. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res. 2008;68:870–879. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, et al. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 4.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 5.Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25:599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Karagoz B, Bilgi O, Gumus M, Erikci AA, Sayan O, Turken O, et al. CD8+CD28− cells and CD4+CD25+ regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Med Oncol. 2009 doi: 10.1007/s12032-008-9165-9. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Escribano JA, Hernandez-Caselles T, Campillo JA, Campos M, Frias JF, Garcia-Alonso A, et al. Changes in the number of CD80(+), CD86(+), and CD28(+) peripheral blood lymphocytes have prognostic value in melanoma patients. Hum Immunol. 2003;64:796–801. doi: 10.1016/s0198-8859(03)00122-8. [DOI] [PubMed] [Google Scholar]
- 8.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 10.Powell DJ, Jr., Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 12.Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol. 1998;161:5909–5917. [PubMed] [Google Scholar]
- 13.Warrington KJ, Vallejo AN, Weyand CM, Goronzy JJ. CD28 loss in senescent CD4+ T cells: reversal by interleukin-12 stimulation. Blood. 2003:3543–3549. doi: 10.1182/blood-2002-08-2574. [DOI] [PubMed] [Google Scholar]
- 14.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008 doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, et al. KLRG1 signaling induces defective Akt (Ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009 doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 16.Pallard C, Stegmann AP, van Kleffens T, Smart F, Venkitaraman A, Spits H. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10:525–535. doi: 10.1016/s1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 17.Muller J, Sperl B, Reindl W, Kiessling A, Berg T. Discovery of chromone-based inhibitors of the transcription factor STAT5. Chembiochem. 2008;9:723–727. doi: 10.1002/cbic.200700701. [DOI] [PubMed] [Google Scholar]
- 18.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–29166. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 19.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 20.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Han J, Goldstein LA, Gastman BR, Rabinovitz A, Rabinowich H. Disruption of Mcl-1.Bim complex in granzyme B-mediated mitochondrial apoptosis. J Biol Chem. 2005;280:16383–16392. doi: 10.1074/jbc.M411377200. [DOI] [PubMed] [Google Scholar]
- 24.Han J, Goldstein LA, Gastman BR, Rabinowich H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem. 2006;281:10153–10163. doi: 10.1074/jbc.M510349200. [DOI] [PubMed] [Google Scholar]
- 25.Huntington ND, Labi V, Cumano A, Vieira P, Strasser A, Villunger A, et al. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim sustains B lymphopoiesis in the absence of IL-7. Int Immunol. 2009;21:715–725. doi: 10.1093/intimm/dxp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiteside TL. Immune responses to malignancies. Journal of Allergy and Clinical Immunology. 2010;125:S272. doi: 10.1016/j.jaci.2009.09.045. S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer Regression in Patients After Transfer of Genetically Engineered Lymphocytes. Science. 2006 doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamichi H, Sereti I, Lane HC. IL-15 acts as a potent inducer of CD4(+)CD25(hi) cells expressing FOXP3. Eur J Immunol. 2008;38:1621–1630. doi: 10.1002/eji.200737607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada H, Kaibara N, Okano S, Maeda T, Shuto T, Nakashima Y, et al. Interleukin-15 selectively expands CD57+ CD28− CD4+ T cells, which are increased in active rheumatoid arthritis. Clin Immunol. 2007;124:328–335. doi: 10.1016/j.clim.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Herpen CM, van der Laak JA, de Vries IJ, van Krieken JH, de Wilde PC, Balvers MG, et al. Intratumoral recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies locoregional lymph node architecture and induces natural killer cell infiltration in the primary tumor. Clin Cancer Res. 2005;11:1899–1909. doi: 10.1158/1078-0432.CCR-04-1524. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moller P, Sun Y, Dorbic T, Alijagic S, Makki A, Jurgovsky K, et al. Vaccination with IL-7 gene-modified autologous melanoma cells can enhance the anti-melanoma lytic activity in peripheral blood of patients with a good clinical performance status: a clinical phase I study. Br J Cancer. 1998;77:1907–1916. doi: 10.1038/bjc.1998.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009;15:528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Kittipatarin C, Khaled AR. Interlinking interleukin-7. Cytokine. 2007;39:75–83. doi: 10.1016/j.cyto.2007.07.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, et al. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 40.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 42.Mitsiades CS, Mitsiades N, Koutsilieris M. The Akt pathway: molecular targets for anti-cancer drug development. Curr Cancer Drug Targets. 2004;4:235–256. doi: 10.2174/1568009043333032. [DOI] [PubMed] [Google Scholar]
- 43.Breslin EM, White PC, Shore AM, Clement M, Brennan P. LY294002 and rapamycin co-operate to inhibit T-cell proliferation. Br J Pharmacol. 2005;144:791–800. doi: 10.1038/sj.bjp.0706061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. 2009;2:ra11. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung MS, Jin DH, Chae HD, Kang S, Kim SC, Bang YJ, et al. Bcl-xL and E1B-19K proteins inhibit p53-induced irreversible growth arrest and senescence by preventing reactive oxygen species-dependent p38 activation. J Biol Chem. 2004;279:17765–17771. doi: 10.1074/jbc.M305015200. [DOI] [PubMed] [Google Scholar]
- 46.Nelyudova A, Aksenov N, Pospelov V, Pospelova T. By blocking apoptosis, Bcl-2 in p38-dependent manner promotes cell cycle arrest and accelerated senescence after DNA damage and serum withdrawal. Cell Cycle. 2007;6:2171–2177. doi: 10.4161/cc.6.17.4610. [DOI] [PubMed] [Google Scholar]
- 47.Woodland RT, Fox CJ, Schmidt MR, Hammerman PS, Opferman JT, Korsmeyer SJ, et al. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–760. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 50.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.