Abstract
Many vaccines existing today provide strong protection against a wide variety of infectious organisms, and these consist of either live attenuated or inactivated microorganisms. Most of these vaccines were developed empirically and there has not been a clear understanding of the immunological principles that contribute to this success. Recent advances in systems biology are being applied to the study of vaccines in order to determine which immunological parameters are the best predictors of success. New approaches to vaccine development include the identification of peptide epitopes and the manipulation of the immune response to generate the most appropriate response. Vaccines are being developed to prevent and/or treat such conditions as cancer and autoimmunity in addition to infectious diseases. Vaccines targeting this diverse group of diseases may need to elicit very different types of immune responses. Recent advances in our understanding of the functions of dendritic cells (DC) and cytokines in orchestrating qualitatively different immune responses has allowed the design of vaccines that can elicit immune responses appropriate for cancer, autoimmunity or infectious organisms. This review will focus on recent advances in the ways DC and cytokines can be used to develop the most appropriate and effective vaccines.
Keywords: Dendritic cells, cytokines, vaccine, immune response, T helper cells
Introduction
The development of vaccines for infectious diseases has been one of the success stories in medicine, and devastating diseases such as smallpox have been eradicated thanks to the widespread use of vaccines. The most successful vaccines consist of live-attenuated organisms such as the vaccines against polio, yellow fever and smallpox. These vaccines were designed empirically and we know little of the immunological mechanisms that characterize the most successful of these vaccines. Today vaccines are being developed not only for the prevention of infectious diseases but also for the treatment and prevention of conditions such as autoimmunity and cancer. The goal of vaccination in the cancer or infectious disease setting is the generation of specific immune responses that can induce tumor/pathogen rejection. Conversely, vaccination against autoimmunity should specifically prevent autoimmune tissue destruction. Clearly the nature of the immune response necessary for the rejection of tumor or pathogens is not the same as that needed to prevent autoimmunity or transplant rejection. It is thought that the induction of type 1 immune responses, characterized by interferon (IFN)-γ production and cytotoxic T cells (CTL), is necessary for efficient tumor rejection [1]. In contrast the induction of type 2 immune responses, characterized by interleukin (IL)-4, and the expansion of T regulatory (Treg) cells is beneficial for the prevention and treatment of autoimmunity [2, 3]. In addition, infectious organisms such as HIV, Plasmodium falciparum and Toxoplasma gondii will not be controlled with the same effector immune responses [4]. HIV vaccines should elicit strong Th1 and CTL responses in order to eliminate infected cells as well as neutralizing antibodies that can prevent infection. Malaria vaccines are complicated by the complex life cycle of the parasite and it may be necessary to induce neutralizing antibodies to the sporozoite stage in order to prevent infection and cell-mediated immunity to target parasites that have invaded the liver [5]. Progress has been made in the identification of epitopes for use in vaccines for many of these conditions [6], but there is now a need to develop new vaccination strategies to ensure that the immune response appropriate for the challenge is induced. It is therefore important to understand in more detail the innate and adaptive responses that are initiated by the most successful of the existing vaccines. A recent study by Querec et al has attempted to do this for the yellow fever vaccine, using the modern day tools of high throughput biological analysis coupled with systems biology and computational modeling [7]. In this study, healthy individuals were vaccinated with the yellow fever vaccine and detailed analysis of the cytokine production, cell surface phenotype and transcriptional activity of peripheral blood mononuclear cells at various time points following vaccination was performed. These studies revealed specific gene expression profiles that were predictive of CD8+ T cell responses and neutralizing antibody responses. Increases in the expression of complement components were found to predict robust CD8+ T cells while the expression of the BLys-BAFF receptor (TNFRSF7) was a key predictor for the B cell responses [7]. These studies indicate that key innate immune responses can predict the generation of effective adaptive T and B cell responses following vaccination. Many of these innate responses involve dendritic cells (DC) and the cytokines they produce. DC are the sentinels of the immune system and express a panoply of pattern recognition receptors (PRR) that recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). These receptors include toll-like receptors (TLR) that can recognize both PAMPs such as LPS [8] and DAMPs such as high mobility group box 1 protein (HMGB1) [9], as well as DAMP-specific receptors such as the receptor for advanced glycation end products (RAGE) [10]. Activation of DC via these receptors promotes their migration to draining lymph nodes, increased antigen presentation to T cells and the elaboration of cytokines that drive the differentiation of T cells down specific effector pathways. In this review we will discuss the features of DC and the cytokines they produce that drive specific immune responses and how this knowledge can be harnessed in the design of effective vaccines against infectious, autoimmune and malignant diseases.
Dendritic cells and the control of the immune response
DCs are professional antigen presenting cells (APC) that are uniquely able to activate naïve T cells. In the steady state conventional (c)DC reside in the peripheral tissues where they sample proteins and particulates from the local environment. DCs express receptors such as TLR that recognize molecules expressed by pathogenic organisms as well as endogenous signals of tissue damage [11]. Signaling through these receptors leads to activation and maturation of the DC, resulting in the downregulation of the antigen uptake machinery, upregulation of molecules important for antigen presentation to T cells and migration of the DC from the tissues to the draining lymph nodes where naïve T cells reside. In the lymph node, antigen-specific naïve CD4+ T cells recognizing antigen on DC will be induced to expand and depending on the signals delivered by DC, will differentiate into various effector T cell types. These include T helper (Th)1, Th2, Th17 and Treg subsets, each of which have distinct functions and can be distinguished by the pattern of cytokines they secrete [12, 13]. Activation of naïve CD8+ T cells often requires cross-presentation of antigens, a function that involves the presentation of soluble proteins in the MHC class I pathway for recognition by CD8+ T cells [14], and distinct cytokine profiles also exist for CD8+ T cells. Several DC features determine the nature of specific T cell responses and these include the mechanism by which the antigen was taken up, the cytokines secreted, the level and type of co-stimulatory molecule expression and the dose of antigen presented, in terms of peptide/MHC complexes (Figure 1) [15, 16]. Some of these features vary by DC subset and others are influenced by the nature of the invading pathogen or antigens that are taken up [17]. Subsets of DC include both cDC and plasmacytoid (p)DC. pDC circulate in the blood and through lymph nodes and provide early responses to viral infection through the secretion of factors such as type 1 IFN [18–20]. Many of these subsets can be distinguished by the expression of receptors that play important roles in antigen uptake, such as FcR, DC inhibitory receptor (DCIR) and CD205, which, as discussed below, can be targeted in vaccine design. Current vaccine strategies are taking advantage of these features to induce the immune response most likely to be efficacious against the target pathogen.
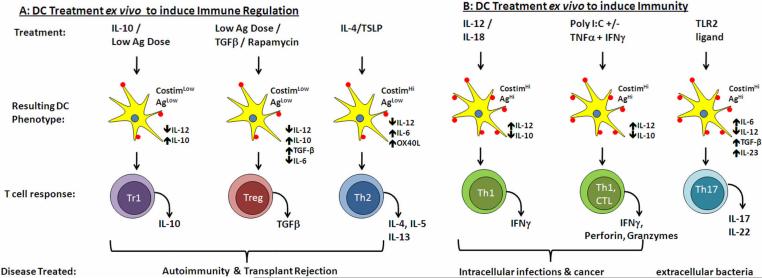
Figure 1.
Examples of ex vivo treatment of DC leading to immune regulation (A) or immunity (B). The treatment can include cytokines such as IL-10, IL-12, IL-4, TLR ligands such as poly I:C, low antigen dose or pharmacological agents such as rapamycin. These treatments change the DC phenotype in terms of co-stimulatory molecules expression and cytokine production as indicated. These result in the differentiation of specific Th subsets such as Tr1, Treg and Th2 (A) or Th1, CTL and Th17 (B).
Cytokines and the induction of effector T cells
Cytokine production, particularly by DC, plays an important role in defining the type of T cell effector response that is induced [16, 21–23]. IL-12, IFN-α and IFN-γ potently induce type 1 immune responses and IL-4 and thymic stromal lymphopoeitin (TSLP) is important for the induction of type 2 immune responses. The differentiation of T cells into IL-17 producing effector cells requires the presence of IL-6 and TGF-β in mouse and IL-1, IL-6, and TGF-β in human [13, 24, 25], whereas the presence of IL-10 and/or TGF-β results in the differentiation of Treg with suppressor function [26, 27]. It was originally thought that distinct DC subsets produced these differing patterns of cytokines [22, 28]. In particular CD8+ CD205+ DC in mice were identified as the subset that produced high levels of IL-12, whereas the production of high levels IFN-α following viral infection was attributed to pDC [18]. However, more recent data have suggested that DC can be polarized to produce different patterns of cytokine by specific PAMPs binding to their receptors [29]. For example engagement of TLR4 by LPS or TLR3 by double stranded RNA leads to high levels of IL-12 production and Th1 differentiation [29]. In contrast engagement of TLR2-TLR1 heterodimers leads to IL-23 production [30, 31] whereas triggering of TLR2-TLR6 heterodimers with zymosan leads to IL-10 production [32]. An additional layer of complexity is provided by the fact that different sets of PRR are expressed by different DC subsets: for example only pDC in the human express TLR7 and TLR9 [18] thus allowing them to respond to viral infections. This is not the case in mouse where TLR7 and TLR9 are expressed by both cDC and pDC, and this needs to be taken into account when testing vaccine strategies in mouse models. Recent studies have suggested that targeting multiple TLR or PRR simultaneously leads to more robust cytokine production by DC [31, 33]. This makes sense since most pathogens express more than one PAMP and would therefore be expected to engage several different PRRs. In addition to triggering multiple PRR optimal cytokine production can be induced when TLR are engaged in the presence of inflammatory cytokines. In work recently published we and others showed that TLR9 engagement by CpG led to high IL-10 and low IL-12 production by murine DC, but the addition of IFN-γ completely inhibited IL-10 production leading to enhanced IL-12 secretion [34, 35]. The presence of DAMPs, such as HMGB1 and uric acid, as indicators of host cell damage also enhance DC maturation and cytokine production. Thus, DC do not become fully activated unless triggered via both a PRR and an inflammatory cytokine or signal of cell damage. The most effective vaccines will likely include TLR and/or PRR ligands that induce DC to adopt the appropriate maturation phenotype and cytokine production profile for the target disease. The choice of these TLR/cytokine combinations will determine the character of the immune response elicited and a few examples are provided in Figure 1.
Vaccine adjuvants to control cytokine production
As discussed above, the engagement of PRR on DC or other APC induces the secretion of cytokines that have profound effects on subsequent adaptive T cell responses. This has been exploited by vaccinologists in the design of novel adjuvants to target specific PRR. In animal studies, complete Freund's adjuvant, which contains killed mycobacteria, provides strong stimulation of multiple PRR and is a powerful adjuvant for type 1 immune responses. However, it is too toxic for use in human vaccines and thus more defined adjuvants have been tested for potential use in man. These include TLR4 or TLR9 agonists such as monophosphoryl lipid A (MPL) or CpG respectively [36, 37]. DNA vaccination has the added advantage of combining the adjuvant properties of bacterial CpG in the plasmids encoding the vaccine antigen. Interestingly recent data have suggested that the adjuvant properties of DNA vaccines are TLR9-independent [38] and involve TANK-binding kinase [39]. However, DNA vaccines have not lived up to their early promise and have not stimulated strong immune responses in many cases. This could be due to the route of administration as well as the localization of the expressed protein antigen [40]. We have shown that gene gun immunization with DNA-coated gold particles targets DC in the skin and in order to elicit strong CTL responses the protein should be expressed either in the cytoplasm or in the membrane. Interestingly when the same constructs were given via the intramuscular route CTL responses were elicited when the protein was secreted but not if the expression was restricted to the cytoplasm [40]. In addition, different patterns of cytokines were induced depending both on the route of vaccination and cellular localization of the expressed protein. DNA vaccines have also been made more effective by co-administering plasmids that express chemokines known to be important in the recruitment of DC [41]. In these studies plasmids expressing macrophage inflammatory protein (MIP)1-α and Flt-3 ligand were combined with an HIV DNA vaccine. This approach increased the migration of DC to the injection site, which resulted in enhanced protective HIV-specific CD4+ and CD8+ T cell responses [41]. Recent studies have also targeted DNA vaccines to DC to further improve efficacy [42]. Thus DNA vaccines can be made more effective by changing the route of vaccine administration, the cellular localization of the expressed protein and by adding other factors such as chemokines and cytokines.
DC as vaccines
Another approach for inducing the appropriate cytokine milieu at the site of vaccination is to generate DC ex vivo and expose them in vitro to antigens in the presence of TLR ligands or maturation cocktails. This approach has been most extensively used in the case of vaccines with tumor antigens [1, 43] with the aim of inducing longstanding anti-tumor cytotoxic responses (Table 1). Multiple approaches have been employed, including pulsing DC with peptide, proteins, tumor lysates or live tumor cells in the presence of various cytokine cocktails. DC have also been transduced with plasmids encoding cytokines such as IL-12 and IL- 18 [44] and infected with viral vectors encoding cytokines or tumor antigens [45]. DC vaccines have also been co-administered with tumor cells transduced with cytokines such as GM-CSF [46]. One of the difficulties associated with developing strong immune responses against tumor cells is that the tumor itself secretes immunosuppressive cytokines such as TGF-β [47] and induces the expansion of myeloid-derived suppressor cells [48], which suppress adaptive and innate immune responses, Vaccination strategies have to overcome this immunosuppressive milieu and the fact that individuals with tumors show reduced ability to respond to vaccines. Another concern with this approach is the fact that DC need to traffic to the draining lymph node in order to stimulate T cell responses. Maturing DC in vitro with various cytokine cocktails, increases their ability to migrate to lymph nodes [49, 50], but DC are not always able deliver the required cytokine signals upon arrival at the lymph node due to DC exhaustion [51]. There have been recent improvements in the cocktails used to mature DC for tumor vaccines [52], and these have been shown to generate mature DC capable of secreting large amounts of IL-12 and to drive potent Th1 responses [53]. Ex vivo generated DC have also been used to vaccinate against infectious diseases such as hepatitis C virus (HCV) [54] and simian immunodeficiency virus (SIV) [55]. In these cases DCs were transduced with mRNA expressing viral proteins, resulting in robust anti-viral immune responses. A recent study examined the ability of DC, infected with the yellow fever vaccine strain, to induce immune responses and found that pretreating the DC with TNF-α protected the DC from the cytopathic effects of the virus. Virus-specific CD8+ T cell responses were induced following co-culture of T cells with the infected DC [56].
Table 1.
Examples of DC-based vaccines used to induce immunity or immune regulation
| DC Vaccines against Cancer | ||||
|---|---|---|---|---|
| Vaccine Approach | Adjuvant or treatment | Target Disease | Outcome | References |
| Ex vivo derived DC given s.c. | Maturation cytokine cocktails, pulsed with tumor antigen | Various cancer types | Improved tumor rejection | [52, 53, 118] |
| Ex vivo derived DC. | Transduced to express cytokines | Melanoma, glioma | Improved Th1 response | [44, 119–121] |
| Irradiated tumor cells | Transduced to express cytokines | Various cancers | Increased DC migration and maturation | [46, 122] |
| Ex vivo derived DC | Transduced to express tumor antigen | Melanoma | Improved Th1 response | [45, 123] |
| Ex vivo derived DC | Express tumor peptide coupled to MHC class I tracking signals | Lymphoma | Enhanced T cell response and tumor rejection | [86] |
| In vivo targeting with peptide coupled to anti-CD205 mAb | Poly I:C | Survivin | Strong Th but no CTL response | [96] |
| In vivo targeting with VLP | Contained tumor antigen HER2Neu | Breast cancer | Prevention of tumor outgrowth | [124] |
| DC derived exosomes | None | Pancreatic cancer | Activate NK cells | [125] |
| DC Vaccines against Autoimmunity and Transplant Rejection | ||||
|---|---|---|---|---|
| Vaccine Approach | Adjuvant or treatment | Target Disease | Outcome | References |
| Ex vivo derived DC | CD86Hi DC selected | Type 1 diabetes | Disease prevention; induction of Th2 and Treg | [71, 72, 77] |
| Ex vivo derived DC | TNF-α (semimature) | EAE and EAT | Induction of Treg | [75, 126] |
| Ex vivo derived DC | TGF-β | EAMG | Disease Prevention. Reduced specific antibody levels. | [61] |
| Ex vivo derived DC | Pharmacological agents (aspirin, rapamycin, PGE2, Vit D3 | Organ transplantation, and autoimmunity | Disease prevention; Treg induction | [62–68] |
| In vivo targeting | Vitamin D3 and mycophenolic acid | Organ transplantation | Prolonged graft survival; reduced DC maturation | [63, 64] |
| In vivo targeting with peptide coupled to anti-CD205 mAb | None | Type 1 diabetes | Prevent disease; induce Treg | [102] |
| In vivo targeting with PLGA beads | Rapamycin | Organ transplantation | reduced DC maturation | [107] |
| DC-derived exosomes | DC transduced with IDO | Collagen induced arthritis | Suppress DTH and arthritis | [117] |
| DC Vaccines against Infectious Diseases | ||||
|---|---|---|---|---|
| Vaccine Approach | Adjuvant or treatment | Target Disease | Outcome | References |
| Ex vivo derived DC | Transduced with viral mRNA | HCV, SIV | Virus-specific T cell response | [54, 55] |
| Ex vivo derived DC | Infected with attenuated virus | Yellow fever | Virus-specific T cell response | [56] |
| Ex vivo derived DC | Express CMV peptide coupled to MHC class I tracking signals | CMV | Enhanced T cell response | [86] |
| DNA vaccine – i.m. injection | MIP1-α and Flt3L expressing plasmid | HIV | Increased DC migration, improved T cell response | [41] |
| In vivo targeting with peptide coupled to anti-CD205 mAb | Anti-CD40 mAb and/or poly I:C | Yersinia Pestis, EBV, L. Major, HIV | Strong Th1 and antibody response | [98, 99, 101, 127] |
| In vivo targeting with peptide coupled to anti-DCIR2 mAb | Poly I:C | L. Major | Th2 response | [101] |
| In vivo targeting with PLGA beads | None | L. monocytogenes | Survive lethal infection | [106] |
| In vivo targeting with VLP | None | HPV, HIV, ebola | DC activation and strong T cell responses | [111–114] |
| DC derived exosomes | None | S. pneumoniae | Survive lethal infection | [116] |
DC vaccines have been also been used in the context of autoimmune disease and transplantation [2, 57]. In these cases the tolerogenic properties of DC are exploited by generating DC that either delete autoreactive T cells or induce Treg cells. In the context of transplantation immature DC appear to be the most effective and many approaches have been utilized to induce and maintain this state of immaturity [58]. These include the use of cytokines such as IL-10 and TGF-β [59–61], pharmacological agents such as aspirin [62], vitamin D3 [63, 64], prostaglandin E2 [65, 66] and rapamycin [67, 68]. DC cultured in rapamycin are phenotypically immature and are also resistant to further maturation stimuli, which has been attributed to the induction of ST2, a negative regulator of TLR signaling [69]. While there has been some success with immature DC in the context of autoimmunity [70] our own studies have suggested that mature DC are better able to prevent autoimmune disease [71]. In these studies DC were generated from bone marrow cultures with GM-CSF and IL-4 and expressed high levels of MHC and co-stimulatory molecules but secreted low levels of IL-12 when stimulated with TLR ligands or CD40L [72, 73]. This phenotype is similar to semi-mature DC that have been described by several groups [74, 75]. We showed that therapeutic DC induced type 2 cytokine production in treated non-obese diabetic (NOD) mice [71, 76] and more recently we observed that semi-mature DC induce expansion of Treg cells [77]. Furthermore we observed that there was an inverse correlation between the induction of Treg by DC and the strength of the TCR signal as measured by signaling via the Akt/mTOR pathway [77]. Interestingly both mature and immature DC could induce Treg expansion provided the appropriate dose of the antigenic peptide was used, and this was observed in three different TCR transgenic systems [77]. The Akt/mTOR signaling pathway has been implicated in the induction or Tregs since it has been shown that inhibition of this pathway results in increased Treg expansion [78, 79]. Indeed low dose antigen has been used in several animal models of autoimmune disease [80, 81] to induce tolerance and this has been attributed to the induction of Treg. Signaling via the Akt/mTor pathway represents the culmination of signals received via various receptors on the T cell surface including TCR, CD28 and cytokine receptors such as IL-6 and IL-2 [82]. Thus it is possible that immature DC are most effective at inducing Treg in the context of transplantation because they would deliver weak stimulation via the TCR to high-affinity alloreactive T cells and induce Treg differentiation, whereas those same T cells would receive strong signals from mature DC which express high levels of MHC and provide additional signaling via co-stimulatory molecules and cytokines to result in the expansion of effector cells rather than Treg. In contrast, autoreactive T cells are usually of low affinity and thus mature DC are required in order to trigger the low level of Akt/mTOR signaling that is optimal for Treg induction (Figure 1).
Targeting DC in vivo
While the generation of DC ex vivo provides an attractive way to control the function and phenotype of DC this approach is costly and time-consuming and is not practical for the widespread use of vaccines that are necessary to protect against infectious diseases. Thus vaccines that target DC in vivo are considered to be optimal [83, 84]. As discussed in some detail above it is necessary to ensure that the appropriate DC subset is targeted with the optimal dose of antigen and that the cytokines produced by those DC will drive differentiation of the desired T cell and B cell responses.
DC subsets can be distinguished by the expression of receptors that play important roles in antigen uptake, such as FcR, DCIR and CD205, and these have been used as vaccine targets (Table 1, Figure 2) All of these receptors direct the antigen to specific intracellular compartments that can influence antigen presentation potential, such as cross-presentation [85]. We have shown that the localization of antigenic protein within the cell is very important for the induction of specific immunes responses [40]. In these experiments DNA vaccination with plasmids that targeted protein production to different cellular compartments was performed, strong CTL responses were induced when the protein was cytoplasmic or transmembrane whereas strong antibody responses were induced when the protein was secreted [40]. In another approach, the direct coupling of antigenic peptides to MHC class I trafficking signals was shown to enhance cross-presentation and CTL responses [86]. Antigen uptake receptors have been shown to be important for cross-presentation of antigen to CD8+ T cells [85]. In the mouse, splenic and lymph node DC that express CD205 are specialized in cross-presentation and thus induce strong CD8+ T cell responses [87–89]. In contrast, DCIR-2+ DC present antigen preferentially to CD4+ T cells and do not effectively cross-present to CD8+ T cells [90]. In human, DCs do not express CD8α and thus the exact human equivalent of the murine CD8+ CD205+ DC has not been clearly defined. A recent study demonstrated that only CD1c+ DC from human peripheral blood or in vitro-generated DC were able to cross-present antigen to CD8+ T cells whereas pDC were unable to do so and only presented antigen to CD4+ T cells [91]. It was interesting to note that, in this study, cross-presentation by CD1c+ DC required that the antigen be complexed with specific antibody, thus allowing uptake by FcγR. Other uptake receptors such as DC-SIGN (CD209), Langerin (CD207) and DCIR are type II proteins of the C-type lectin family [43]. A novel C-type lectin (Clec9A) was recently identified on murine CD8+ DC and pDC, and was also found on a subset of human DC [92]. Pathogens binding to these receptors can also influence DC maturation. For example HIV, CMV and several other viruses bind to CD209 and inhibit DC maturation [43, 93], whereas HIV binding to CD207 results in degradation of the virus and reduced transmission to T cells [94].
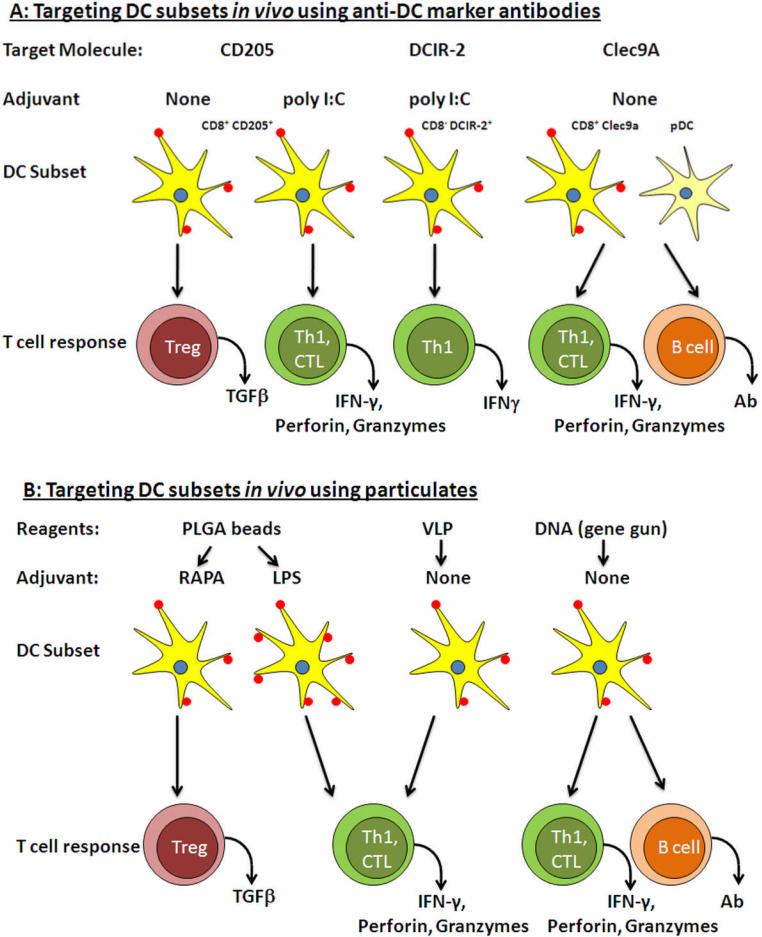
Figure 2.
Examples of in vivo targeting of DC using antibodies to specific DC markers (A) or particulates (B). A. Three potential target molecules are shown along with the expected immune responses generated. In the case of CD205 and DCIR-2 an additional adjuvant such as poly I:C is required in order to induce immunity, whereas targeting Clec9A alone results in potent immune responses. Tolerance can be induced by targeting antigen to CD205 in the absence of adjuvant. B. Three different particulates are shown with the expected immune responses. These responses are influenced by altering the size of the particles and by including adjuvants such as LPS or drugs such as rapamycin. In the case of DNA vaccines immune responses can be influenced by the route of immunization and by the cellular localization of the expressed protein (see text)
Several of these receptors have been targeted in vaccine strategies designed to induce specific immune responses [83]. Approaches have included the coupling of antigenic peptides to antibodies specific for certain receptors or to pathogen associated proteins known to bind specifically to uptake receptors. CD205 has been targeted by several groups [87, 95, 96] and in these studies DNA encoding antigenic peptides has been cloned in frame into the anti-CD205 antibody [95, 96] or the peptide itself coupled to the anti-CD205 antibody using biochemical means [87]. This has proved to be a very efficient way of activating specific T cells and extremely small doses of antigen are required. However, in the absence of any stimulus to activate the DC in situ targeting antigen to CD205 DC either leads to deletion of antigen-specific T cells [95] or the induction of antigen-specific Foxp3+ Treg [97]. The addition of a TLR ligand such as poly I:C [98] or activating antibody such as anti-CD40 [90, 99] was required to induce strong immune responses. Targeting antigens via CD205 in this manner leads to the induction of Th1 responses and effective CD8+ T cells since CD205+ DC are effective at cross-presenting antigen and produce high levels of IL-12 [100]. In contrast targeting antigens, using a similar strategy, to DCIR-2, which is expressed on a different subset of DC leads to the induction of Th2 responses [101]. In both of these cases a DC maturation signal such as poly I:C or anti-CD40 mAb has to be included to induce immunity. This fact has been exploited in the area of autoimmunity and a recent study showed that targeting an islet autoantigen to CD205+ DC led to the deletion of the islet antigen specific T cells [102]. However in the case of the tumor antigen, survivin, even the addition of poly I:C to the survivin-coupled anti-CD205 mAb failed to induce CD8+ T cell responses, although robust CD4+ T cell responses and antibodies were induced [96]. This provides a further example of the challenges facing the development of effective vaccines against tumor antigens.
The recently identified DC marker, Clec9a, can also be used to target DCs in vivo [92], and in this case no other DC stimulus is required. Using a model antigen this study showed that coupling the antigen to an anti-Clec9A antibody was sufficient to induce strong Th1, CTL and B cell responses (Figure 2A) [92]. Other receptors that been targeted for vaccine purposes include CD11b [103] and mannose receptor [104]. In the case of CD11b, investigators took advantage of a CyA, a adenylate cyclase from Bordatella pertussis, that is known to bind to CD11b. Coupling of antigenic peptides to CyA resulted in robust type 1 CD4+ and CD8+ T cell responses in the absence of any additional DC maturation signal [103]. To date most of these approaches have used model antigens, but research is starting in murine models using antigens relevant to infectious disease and autoimmunity (Table 1). As we learn more about the function and distribution of antigen uptake receptors we will be able to take advantage of their properties to design appropriate new vaccines,
DC are phagocytic cells and the development of nanoparticles, such as liposomes and biodegradable polymers has led to a new approach to vaccine design [105] (Figure 2B). Vaccine antigens can be encapsulated within these particles or attached to the surface. A recent study using biodegradable poly(γ-glutamic acid) (PLGA) nanoparticles demonstrated that these particles were taken up by DC in vivo and induced potent humoral and cellular immunes responses [106]. The particles were taken up by several DC subsets in the spleen and induced maturation of the DC, cytokine production and type 1 immune responses. Vaccination using these particles coated with a peptide from Listeria monocytogenes was able to protect mice from lethal infection [106]. Interestingly if the peptide was encapsulated within the particle the mice were not protected [106], suggesting the rate of degradation and release of antigenic peptide is important [105]. These particles can be coated with various DAMPS or PAMPS in order to provide an added adjuvant effect. A similar approach has been used to develop tolerogenic DC by the delivery of rapamycin, an mTOR inhibitor, encapsulated within PLGA nanoparticles [107]. This approach was found to be more efficient than soluble rapamycin in the modulation of DC function [107]. Another approach along these lines has been the use of virus-like particles (VLP) [108], which have been successfully developed for the recently approved human papilloma virus (HPV) vaccine [109]. VLPs consist of the empty capsid of a virus and can be made from the corresponding virus [108], or they can be chimeric and consist of viral or tumor proteins incorporated into a VLP backbone [108]. The size of all of these particles can be varied and it has been shown that distinct DC subsets take up particles based on size [110], such that large particles are taken up by DC at the injection site whereas smaller VLP traffic to the lymph node where they are taken up by resident DC [110]. DC uptake of VLPs results in activation and the stimulation of strong specific T and B cell responses [111–114]. As discussed above this can have an impact on the type of immune response that is elicited. VLP-based vaccines are in development for several infectious diseases and cancer (Table 1).
DC also release exosomes, which are small nano-sized vesicles that contain immunomodulatry cytokines and mediators. In a recent study DC were treated with diphtheria toxoid (DT) resulting in the release of exosomes than express MHC and co-stimulatory molecules. Administration of these DT-induced exosomes in vivo resulted in DT-specific antibody responses [115]. A similar study demonstrated that exosomes from bone-marrow derived DC shared a cross-reactive epitope with a polysaccharide from Streptococcus pneumoniae. Mice immunized with these exosomes were protected from lethal pneumococcal infection [116]. DC-derived exosomes can also be used to in the context of autoimmune disease especially if the exosomes are derived from DC exposed to cytokines such as IL-10. In a recent study exosomes from DC transduced to express indoleamine-2-3-deoxygenase (IDO) were shown to prevent collagen-induced arthritic in mice [117]. It is not entirely understood how exosomes exert their tolerogenic or immunogenic effects but this is likely to prove a fruitful area of research in the future. DC can thus be targeted using particulate vaccines of various types and the immune response that results will depend of a variety of factors such as particle size, location of the antigen in or on the particle, the rate of degradation and the addition of adjuvant molecules that induce DC maturation and activation (Figure 2B).
Conclusions
Early success in vaccine development was based on empiricism and we have limited understanding of how to measure and predict the effectiveness of new vaccines that are being developed. The recent study of the yellow fever vaccine [7] provides an approach by which to assess future vaccine efficacy. It is important to understand the type of immune response required to respond to a particular challenge, be it type 1 immune responses for viral infections and cancer, type 2 responses for parasitic infections, type 17 responses for bacterial and fungal infections or regulatory T cell responses to prevent autoimmunity. DC and the cytokines they produce play a key role in driving these immune responses and can be harnessed to induce an effective immune response against the pathogen or disease of choice. A wide array of tools are being developed to target vaccines to specific DC subsets in order to achieve the desired immune response. Major challenges still remain before these approaches can be widely used. These include: 1) defining the appropriate immune response for a particular challenge in terms of which lymphocytes or antibody types will be effective in each context; 2) defining the appropriate antigenic targets and the dose of the vaccinating antigen in order to induce immunity, or tolerance; 3) identifying the appropriate DC subset that should be targeted by the vaccine, either using ex vivo or in vivo targeting approaches; 4) increasing our understanding of how defined adjuvants work to induce DC maturation and how to combine these for optimal effectiveness; and 5) improving our understanding of the difference between human DC subsets and function and similar populations defined in animal models such as mouse and primate. It is likely that each disease situation and/or pathogen will require a specific approach and the more we can learn about how our most effective vaccines work the better able we will be to design the vaccines of the future.
ACKNOWLEDEMENTS
This work was supported by NIH grant NO1 AI-50018 and ADA grant 1-06-RA-94 to PAM. MST is supported by a training grant from the National Institutes of Health: #5T32 CA82084-10.
REFERENCES
- [1].Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29(3):372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- [2].Morel PA, Feili-Hariri M, Coates PT, Thomson AW. Dendritic cells, T cell tolerance and therapy of adverse immune reactions. Clin. Exp. Immunol. 2003;133(1):1–10. doi: 10.1046/j.1365-2249.2003.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Raimondi G, Turner MS, Thomson AW, Morel PA. Naturally occurring regulatory T cells: recent insights in health and disease. Crit. Rev. Immunol. 2007;27(1):61–95. doi: 10.1615/critrevimmunol.v27.i1.50. [DOI] [PubMed] [Google Scholar]
- [4].Steinman RM. Dendritic cells and vaccines. Proc. (Bayl. Univ. Med. Cent.) 2008;21(1):3–8. doi: 10.1080/08998280.2008.11928346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Targett GA, Greenwood BM. Malaria vaccines and their potential role in the elimination of malaria. Malar. J. 2008;7(Suppl 1):S10. doi: 10.1186/1475-2875-7-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen SW, Van Regenmortel MH, Pellequer JL. Structure-activity relationships in peptide-antibody complexes: implications for epitope prediction and development of synthetic peptide vaccines. Curr. Med. Chem. 2009;16(8):953–964. doi: 10.2174/092986709787581914. [DOI] [PubMed] [Google Scholar]
- [7].Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009;10(1):116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- [9].Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- [10].Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 2007;220(1):60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- [11].Lopez-Bravo M, Ardavin C. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity. 2008;29(3):343–351. doi: 10.1016/j.immuni.2008.08.008. [DOI] [PubMed] [Google Scholar]
- [12].Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129(1):33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- [13].Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24(6):677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [14].Bevan MJ. Cross-priming. Nat. Immunol. 2006;7(4):363–365. doi: 10.1038/ni0406-363. [DOI] [PubMed] [Google Scholar]
- [15].Kadowaki N. Dendritic cells: a conductor of T cell differentiation. Allergol. Int. 2007;56(3):193–199. doi: 10.2332/allergolint.R-07-146. [DOI] [PubMed] [Google Scholar]
- [16].Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- [17].Palucka K, Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 2002;14(4):420–431. doi: 10.1016/s0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- [18].Liu YJ. IPC: Professional Type 1 Interferon-Producing Cells and Plasmacytoid Dendritic Cell Precursors. Annu. Rev. Immunology. 2005;23(1):275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- [19].Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 2007;7(1):19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- [20].Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002;2(3):151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- [21].Manickasingham SP, Edwards AD, Schulz O, Reis e Sousa C. The ability of murine dendritic cell subsets to direct T helper cell differentiation is dependent on microbial signals. Eur. J. Immunol. 2003;33(1):101–107. doi: 10.1002/immu.200390001. [DOI] [PubMed] [Google Scholar]
- [22].Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96(3):1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr. Opin. Immunol. 1994;6(3):458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- [24].Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. U S A. 2008;105(47):18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol. Rev. 2008;226(1):112–131. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J. Clin. Invest. 2004;114(9):1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- [28].Maldonado-Lopez R, de Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189(3):587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pulendran B. Variegation of the Immune Response with Dendritic Cells and Pathogen Recognition Receptors. J. Immunol. 2005;174(5):2457–2465. doi: 10.4049/jimmunol.174.5.2457. [DOI] [PubMed] [Google Scholar]
- [30].Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur. J. Immunol. 2009;39(5):1379–1386. doi: 10.1002/eji.200838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, Consolaro MR, De Marchi M, Giachino D, Robbiano A, Astegiano M, Sambataro A, Kastelein RA, Carra G, Trinchieri G. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 2008;205(6):1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dillon S. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 2006;116(4):916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Flores RR, Diggs KA, Tait LM, Morel PA. IFN-γ negatively regulates CpG-induced IL-10 in bone marrow-derived dendritic cells. J. Immunol. 2007;178(1):211–218. doi: 10.4049/jimmunol.178.1.211. [DOI] [PubMed] [Google Scholar]
- [35].Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, Woodgett JR, Ivashkiv LB. IFN-γ Suppresses IL-10 Production and Synergizes with TLR2 by Regulating GSK3 and CREB/AP-1 Proteins. Immunity. 2006;24(5):563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- [36].Goto Y, Bhatia A, Raman VS, Vidal SEZ, Bertholet S, Coler RN, Howard RF, Reed SG. Leishmania infantum sterol 24-c-methyltransferase formulated with MPL-SE induces cross-protection against L. major infection. Vaccine. 2009;27(21):2884–2890. doi: 10.1016/j.vaccine.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rosa DS, Bastos KR, Bargieri DY, Tzelepis F, Nomizo A, Russo M, Soares IS, Rodrigues MM. Role of interferon-gamma during CpG oligodeoxynucleotide-adjuvanted immunization with recombinant proteins. Vaccine. 2007;25(32):6007–6017. doi: 10.1016/j.vaccine.2007.05.031. [DOI] [PubMed] [Google Scholar]
- [38].Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O, Akira S. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 2006;7(1):40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- [39].Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, Akira S. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451(7179):725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- [40].Morel PA, Falkner D, Plowey J, Falo LD. DNA immunization: altering the cellular localization of expressed protein and the immunization route allows manipulation of the immune response. Vaccine. 2004;22(3–4):447–456. doi: 10.1016/j.vaccine.2003.07.012. [DOI] [PubMed] [Google Scholar]
- [41].Sumida SM, McKay PF, Truitt DM, Kishko MG, Arthur JC, Seaman MS, Jackson SS, Gorgone DA, Lifton MA, Letvin NL, Barouch DH. Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines. J. Clin. Invest. 2004;114(9):1334–1342. doi: 10.1172/JCI22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nchinda G, Kuroiwa J, Oks M, Trumpfheller C, Park CG, Huang Y, Hannaman D, Schlesinger SJ, Mizenina O, Nussenzweig MC, Uberla K, Steinman RM. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J. Clin. Invest. 2008;118(4):1427–1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- [44].Vujanovic L, Ranieri E, Gambotto A, Olson WC, Kirkwood JM, Storkus WJ. IL-12p70 and IL-18 gene-modified dendritic cells loaded with tumor antigen-derived peptides or recombinant protein effectively stimulate specific Type-1 CD4+ T-cell responses from normal donors and melanoma patients in vitro. Cancer Gene Ther. 2006;13(8):798–805. doi: 10.1038/sj.cgt.7700964. [DOI] [PubMed] [Google Scholar]
- [45].He Y, Zhang J, Mi Z, Robbins P, Falo LD., Jr. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J. Immunol. 2005;174(6):3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- [46].Ji Q, Gondek D, Hurwitz AA. Provision of granulocyte-macrophage colony-stimulating factor converts an autoimmune response to a self-antigen into an antitumor response. J. Immunol. 2005;175(3):1456–1463. doi: 10.4049/jimmunol.175.3.1456. [DOI] [PubMed] [Google Scholar]
- [47].MassaguÈ J. TGF-β in Cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, Strijk SP, Ruers TJ, Boerman OC, Oyen WJ, Adema GJ, Punt CJ, Figdor CG. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63(1):12–17. [PubMed] [Google Scholar]
- [50].Aarntzen EH, Figdor CG, Adema GJ, Punt CJ, de Vries IJ. Dendritic cell vaccination and immune monitoring. Cancer Immunol. Immunother. 2008;57(10):1559–1568. doi: 10.1007/s00262-008-0553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 2000;1(4):311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- [52].Lee JJ, Foon KA, Mailliard RB, Muthuswamy R, Kalinski P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J. Leukoc. Biol. 2008;84(1):319–325. doi: 10.1189/jlb.1107737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W, Kalinski P. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol. Immunother. 2009;58(8):1329–1336. doi: 10.1007/s00262-008-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yu H, Babiuk LA, van Drunen Littel-van den Hurk S. Strategies for loading dendritic cells with hepatitis C NS5a antigen and inducing protective immunity. J. Viral Hepat. 2008;15(6):459–470. doi: 10.1111/j.1365-2893.2008.00959.x. [DOI] [PubMed] [Google Scholar]
- [55].Melhem NM, Liu XD, Boczkowski D, Gilboa E, Barratt-Boyes SM. Robust CD4+ and CD8+ T cell responses to SIV using mRNA-transfected DC expressing autologous viral Ag. Eur. J. Immunol. 2007;37(8):2164–2173. doi: 10.1002/eji.200636782. [DOI] [PubMed] [Google Scholar]
- [56].Barba-Spaeth G, Longman RS, Albert ML, Rice CM. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J. Exp. Med. 2005;202(9):1179–1184. doi: 10.1084/jem.20051352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Clare-Salzler MJ, Brooks J, Chai A, Van Herle K, Anderson C. Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J. Clin. Invest. 1992;90(3):741–748. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 2007;7(8):610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- [59].Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105(3):1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- [60].Maynard CL, Hatton RD, Helms WS, Oliver JR, Stephensen CB, Weaver CT. Contrasting roles for all-trans retinoic acid in TGF-β-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J. Exp. Med. 2009;206(2):343–357. doi: 10.1084/jem.20080950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yarilin D, Duan R, Huang YM, Xiao BG. Dendritic cells exposed in vitro to TGF-beta1 ameliorate experimental autoimmune myasthenia gravis. Clin. Exp. Immunol. 2002;127(2):214–219. doi: 10.1046/j.1365-2249.2002.01748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hackstein H, Morelli AE, Larregina AT, Ganster RW, Papworth GD, Logar AJ, Watkins SC, Falo LD, Thomson AW. Aspirin inhibits in vitro maturation and in vivo immunostimulatory function of murine myeloid dendritic cells. J. Immunol. 2001;166(12):7053–7062. doi: 10.4049/jimmunol.166.12.7053. [DOI] [PubMed] [Google Scholar]
- [63].Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 2001;167(4):1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- [64].Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- [65].Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97(11):3466–3469. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- [66].Rieser C, Papesh C, Herold M, Bock G, Ramoner R, Klocker H, Bartsch G, Thurnher M. Differential deactivation of human dendritic cells by endotoxin desensitization: role of tumor necrosis factor-alpha and prostaglandin E2. Blood. 1998;91(9):3112–3117. [PubMed] [Google Scholar]
- [67].Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am. J. Transplant. 2005;5(2):228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- [68].Turnquist HR, Thomson AW. Taming the lions: manipulating dendritic cells for use as negative cellular vaccines in organ transplantation. Curr. Opin. Organ Transplant. 2008;13(4):350–357. doi: 10.1097/MOT.0b013e328306116c. [DOI] [PubMed] [Google Scholar]
- [69].Turnquist HR, Sumpter TL, Tsung A, Zahorchak AF, Nakao A, Nau GJ, Liew FY, Geller DA, Thomson AW. IL-1β-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J. Immunol. 2008;181(1):62–72. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Steinman RM, Hawiger D, Nussenzweig MC. TOLEROGENIC DENDRITIC CELLS*. Annu.l Rev. Immunol. 2003;21(1):685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- [71].Feili-Hariri M, Falkner DH, Morel PA. Regulatory Th2 response induced following adoptive transfer of dendritic cells in prediabetic NOD mice. Eur. J. Immunol. 2002;32(7):2021–2030. doi: 10.1002/1521-4141(200207)32:7<2021::AID-IMMU2021>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- [72].Feili-Hariri M, Dong X, Alber SM, Watkins SC, Salter RD, Morel PA. Immunotherapy of NOD mice with bone marrow-derived dendritic cells. Diabetes. 1999;48(12):2300–2308. doi: 10.2337/diabetes.48.12.2300. [DOI] [PubMed] [Google Scholar]
- [73].Feili-Hariri M, Morel PA. Phenotypic and functional characteristics of BM-derived DC from NOD and non diabetes-prone strains. Clin. Immunol. 2001;98(1):133–142. doi: 10.1006/clim.2000.4959. [DOI] [PubMed] [Google Scholar]
- [74].Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23(9):445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- [75].Menges M, Rossner S, Voigtlander C, Schindler H, Kukutsch NA, Bogdan C, Erb K, Schuler G, Lutz MB. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 2002;195(1):15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Feili-Hariri M, Flores RR, Vasquez AC, Morel PA. Dendritic cell immunotherapy for autoimmune diabetes. Immunol. Res. 2006;36(1–3):167–173. doi: 10.1385/IR:36:1:167. [DOI] [PubMed] [Google Scholar]
- [77].Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J. Immunol. 2009;183(8):4895–4903. doi: 10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008;205(3):565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U S A. 2008;105(22):7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kang HK, Liu M, Datta SK. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J. Immunol. 2007;178(12):7849–7858. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- [81].Kretschmer K, Heng TS, von Boehmer H. De novo production of antigen-specific suppressor cells in vivo. Nat. Protoc. 2006;1(2):653–661. doi: 10.1038/nprot.2006.105. [DOI] [PubMed] [Google Scholar]
- [82].Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 2009;9(5):324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur. J. Immunol. 2009;39(4):931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- [84].Shortman K, Lahoud MH, Caminschi I. Improving vaccines by targeting antigens to dendritic cells. Exp. Mol. Med. 2009;41(2):61–66. doi: 10.3858/emm.2009.41.2.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lin ML, Zhan Y, Villadangos JA, Lew AM. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol. Cell. Biol. 2008;86(4):353–362. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- [86].Kreiter S, Selmi A, Diken M, Sebastian M, Osterloh P, Schild H, Huber C, Tureci O, Sahin U. Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J. Immunol. 2008;180(1):309–318. doi: 10.4049/jimmunol.180.1.309. [DOI] [PubMed] [Google Scholar]
- [87].Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient Targeting of Protein Antigen to the Dendritic Cell Receptor DEC-205 in the Steady State Leads to Antigen Presentation on Major Histocompatibility Complex Class I Products and Peripheral CD8+ T Cell Tolerance. J. Exp. Med. 2002;196(12):1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192(12):1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 2001;166(9):5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- [90].Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential Antigen Processing by Dendritic Cell Subsets in Vivo. Science. 2007;315(5808):107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- [91].Schnurr M, Chen Q, Shin A, Chen W, Toy T, Jenderek C, Green S, Miloradovic L, Drane D, Davis ID, Villadangos J, Shortman K, Maraskovsky E, Cebon J. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105(6):2465–2472. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- [92].Caminschi I, Proietto AI, Ahmet F, Kitsoulis S, Shin Teh J, Lo JCY, Rizzitelli A, Wu L, Vremec D, van Dommelen SLH, Campbell IK, Maraskovsky E, Braley H, Davey GM, Mottram P, van de Velde N, Jensen K, Lew AM, Wright MD, Heath WR, Shortman K, Lahoud MH. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112(8):3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100(5):587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- [94].de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MAWP, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TBH. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 2007;13(3):367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- [95].Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic Cells Induce Peripheral T Cell Unresponsiveness Under Steady State Conditions In Vivo. J. Exp. Med. 2001;194(6):769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Charalambous A, Oks M, Nchinda G, Yamazaki S, Steinman RM. Dendritic Cell Targeting of Survivin Protein in a Xenogeneic Form Elicits Strong CD4+ T Cell Immunity to Mouse Survivin. J. Immunol. 2006;177(12):8410–8421. doi: 10.4049/jimmunol.177.12.8410. [DOI] [PubMed] [Google Scholar]
- [97].Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+CD205+ Splenic Dendritic Cells Are Specialized to Induce Foxp3+ Regulatory T Cells. J. Immunol. 2008;181(10):6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gurer C, Strowig T, Brilot F, Pack M, Trumpfheller C, Arrey F, Park CG, Steinman RM, Munz C. Targeting the nuclear antigen 1 of Epstein-Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood. 2008;112(4):1231–1239. doi: 10.1182/blood-2008-03-148072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, Nussenzweig MC, Piperno AG, Steinman RM. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc. Natl. Acad. Sci. U S A. 2007;104(4):1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Maldonado-Lopez R, Maliszewski C, Urbain J, Moser M. Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(−) dendritic cells to prime Th1/Th2 cells in vivo. J. Immunol. 2001;167(8):4345–4350. doi: 10.4049/jimmunol.167.8.4345. [DOI] [PubMed] [Google Scholar]
- [101].Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 2007;204(5):1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, von Boehmer H, Mahnke K, Buer J. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54(12):3395–3401. doi: 10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- [103].Schlecht G, Loucka J, Najar H, Sebo P, Leclerc C. Antigen targeting to CD11b allows efficient presentation of CD4+ and CD8+ T cell epitopes and in vivo Th1-polarized T cell priming. J. Immunol. 2004;173(10):6089–6097. doi: 10.4049/jimmunol.173.10.6089. [DOI] [PubMed] [Google Scholar]
- [104].Betting DJ, Mu XY, Kafi K, McDonnel D, Rosas F, Gold DP, Timmerman JM. Enhanced immune stimulation by a therapeutic lymphoma tumor antigen vaccine produced in insect cells involves mannose receptor targeting to antigen presenting cells. Vaccine. 2009;27(2):250–259. doi: 10.1016/j.vaccine.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27(12):573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [106].Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M, Baba M. Targeting of antigen to dendritic cells with poly(gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J. Immunol. 2007;178(5):2979–2986. doi: 10.4049/jimmunol.178.5.2979. [DOI] [PubMed] [Google Scholar]
- [107].Jhunjhunwala S, Raimondi G, Thomson AW, Little SR. Delivery of rapamycin to dendritic cells using degradable microparticles. J. Control. Release. 2009;133(3):191–197. doi: 10.1016/j.jconrel.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ramqvist TR, Andreasson K, Dalianis T. Vaccination, immune and gene therapy based on virus-like particles against viral infections and cancer. Expert Opin. Biol. Ther. 2007;7(7):997–1007. doi: 10.1517/14712598.7.7.997. [DOI] [PubMed] [Google Scholar]
- [109].Schmiedeskamp MR, Kockler DR. Human Papillomavirus Vaccines. Ann. Pharmacother. 2006;40(7):1344–1352. doi: 10.1345/aph.1G723. [DOI] [PubMed] [Google Scholar]
- [110].Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008;38(5):1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- [111].Lenz P, Lowy DR, Schiller JT. Papillomavirus virus-like particles induce cytokines characteristic of innate immune responses in plasmacytoid dendritic cells. Eur. J. Immunol. 2005;35(5):1548–1556. doi: 10.1002/eji.200425547. [DOI] [PubMed] [Google Scholar]
- [112].Pinto LA, Viscidi R, Harro CD, Kemp TJ, GarcÌa-PiÒeres AJ, Trivett M, Demuth F, Lowy DR, Schiller JT, Berzofsky JA, Hildesheim A. Cellular immune responses to HPV-18, -31, and -53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology. 2006;353(2):451–462. doi: 10.1016/j.virol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- [113].Tsunetsugu-Yokota Y, Morikawa Y, Isogai M, Kawana-Tachikawa A, Odawara T, Nakamura T, Grassi F, Autran B, Iwamoto A. Yeast-Derived Human Immunodeficiency Virus Type 1 p55gag Virus-Like Particles Activate Dendritic Cells (DCs) and Induce Perforin Expression in Gag-Specific CD8+ T Cells by Cross-Presentation of DCs. J. Virol. 2003;77(19):10250–10259. doi: 10.1128/JVI.77.19.10250-10259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ye L, Lin J, Sun Y, Bennouna S, Lo M, Wu Q, Bu Z, Pulendran B, Compans RW, Yang C. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351(2):260–270. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- [115].Colino J, Snapper CM. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J. Immunol. 2006;177(6):3757–3762. doi: 10.4049/jimmunol.177.6.3757. [DOI] [PubMed] [Google Scholar]
- [116].Colino J, Snapper CM. Dendritic cell-derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice. Infect. Immun. 2007;75(1):220–230. doi: 10.1128/IAI.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bianco NR, Kim SH, Ruffner MA, Robbins PD. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009;60(2):380–389. doi: 10.1002/art.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, Finn OJ, Ramanathan RK. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955–964. [PMC free article] [PubMed] [Google Scholar]
- [119].Knippertz I, Hesse A, Schunder T, Kampgen E, Brenner MK, Schuler G, Steinkasserer A, Nettelbeck DM. Generation of human dendritic cells that simultaneously secrete IL-12 and have migratory capacity by adenoviral gene transfer of hCD40L in combination with IFN-γ. J. Immunother. 2009;32(5):524–538. doi: 10.1097/CJI.0b013e3181a28422. [DOI] [PubMed] [Google Scholar]
- [120].Cao DY, Yang JY, Dou KF, Ma LY, Teng ZH. alpha-fetoprotein and interleukin-18 gene-modified dendritic cells effectively stimulate specific type-1 CD4- and CD8-mediated T-Cell response from hepatocellular carcinoma patients in Vitro. Hum. Immunol. 2007;68(5):334–341. doi: 10.1016/j.humimm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [121].Kuwashima N, Nishimura F, Eguchi J, Sato H, Hatano M, Tsugawa T, Sakaida T, Dusak JE, Fellows-Mayle WK, Papworth GD, Watkins SC, Gambotto A, Pollack IF, Storkus WJ, Okada H. Delivery of dendritic cells engineered to secrete IFN-α into central nervous system tumors enhances the efficacy of peripheral tumor cell vaccines: dependence on apoptotic pathways. J. Immunol. 2005;175(4):2730–2740. doi: 10.4049/jimmunol.175.4.2730. [DOI] [PubMed] [Google Scholar]
- [122].Russell HV, Strother D, Mei Z, Rill D, Popek E, Biagi E, Yvon E, Brenner M, Rousseau R. A phase 1/2 study of autologous neuroblastoma tumor cells genetically modified to secrete IL-2 in patients with high-risk neuroblastoma. J. Immunother. 2008;31(9):812–819. doi: 10.1097/CJI.0b013e3181869893. [DOI] [PubMed] [Google Scholar]
- [123].Lopes L, Fletcher K, Ikeda Y, Collins M. Lentiviral vector expression of tumour antigens in dendritic cells as an immunotherapeutic strategy. Cancer Immunol. Immunother. 2006;55(8):1011–1016. doi: 10.1007/s00262-005-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tegerstedt K, Lindencrona JA, Curcio C, Andreasson K, Tullus C, Forni G, Dalianis T, Kiessling R, Ramqvist T. A Single Vaccination with Polyomavirus VP1/VP2Her2 Virus-Like Particles Prevents Outgrowth of HER-2/neu-Expressing Tumors. Cancer Res. 2005;65(13):5953–5957. doi: 10.1158/0008-5472.CAN-05-0335. [DOI] [PubMed] [Google Scholar]
- [125].Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N. Dendritic Cell-Derived Exosomes Promote Natural Killer Cell Activation and Proliferation: A Role for NKG2D Ligands and IL-15Rα. PLoS ONE. 2009;4(3):e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Verginis P, Li HS, Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J. Immunol. 2005;174(11):7433–7439. doi: 10.4049/jimmunol.174.11.7433. [DOI] [PubMed] [Google Scholar]
- [127].Do Y, Park CG, Kang YS, Park SH, Lynch RM, Lee H, Powell BS, Steinman RM. Broad T cell immunity to the LcrV virulence protein is induced by targeted delivery to DEC-205/CD205-positive mouse dendritic cells. Eur. J. Immunol. 2008;38(1):20–29. doi: 10.1002/eji.200737799. [DOI] [PMC free article] [PubMed] [Google Scholar]